Abstract
Spinal epidural abscesses (SEAs) are unusual bacterial infections, with possible devastating neurologic sequelae. Despite abundance of case series in adults, reports in children are scanty.
We describe a spontaneous SEA due to methicillin susceptible Staphylococcus aureus (MSSA) in a previously healthy 15-year old male, and we perform a literature review regarding management of pediatric SEAs without risk factors, from 2001 to 2014.
We found a total of 12 cases (8 males, average age 9.6 years). Clinical presentation was mainly fever, back pain and elevation of inflammation markers. All cases were initially misdiagnosed. Lumbar puncture was performed in 36% of patients. Etiological diagnosis was obtained in 8 cases. MSSA was isolated in 4 patients, methicillin-resistant S. aureus in 1 patient, and S. aureus with unknown susceptibility patterns in 2 cases. The average of therapy duration was 6 weeks. Patients’ spine was always evaluated by gadolinium-enhanced magnetic resonance imaging; most abscesses were localized at thoracic and lumbar area, without osteomyelitis. In 8 cases, laminectomy and/or abscess drainage were performed in association with medical therapy; 3 cases were successfully treated with antimicrobial therapy only; no data were available in one case. A good outcome was obtained in all patients, except a reported residual headache and paraspinal pain lasting for 3 years.
The rarity and the possible differential diagnosis can lead to underestimate SEA occurrence in children without risk factors. It seems therefore essential to maintain a high attention to pediatric SEAs. A prompt diagnosis and adequate therapy are essential prognostic factors for remission.
Keywords: Spinal epidural abscess, Management, Staphylococcus aureus, Children
Introduction
Spinal epidural abscesses (SEAs) are unusual bacterial infections requiring prompt diagnosis and management to prevent devastating neurologic sequelae.
They represent about 7% of vertebral infections and usually occur in subjects with predisposing underlying diseases or conditions such as diabetes mellitus, chronic renal failure, cancer, advanced age, immunodeficiency, alcoholism, intravenous drug abuse, cauda equina, holocord syndrome, neurosurgery, spinal anesthesia and acupuncture, mucocutaneous trauma, or by spreading of a known infection localized at other sites.
Bacteria gain access to the epidural space through contiguous spread (primary SEA) or by hematogenous dissemination (secondary SEA); the source of infection is not identified in 20–40% of cases [1], [2], [3], [4].
The most common causative agent is Staphylococcus aureus, both methicillin-susceptible (MSSA) or methicillin resistant (MRSA), accounting for 50–90% of cases, followed by streptococci (8–17%) and Gram negative bacteria (10–17%) [1], [4], [5].
SEAs have an insidious onset of pain and a progressively worsening clinical picture characterized by fever and elevation of the indices of inflammation.
Four clinical stages have been described: stage 1 – lumbar pain, fever and local tenderness; stage 2 – radicular pain, nuchal rigidity and changes in the reflexes; stage 3 – sensory and motor abnormalities, with motor weakness and bowel and bladder dysfunction; stage 4 – paralysis with permanent sequelae. Alterations are reversible up to stage 4. It is therefore essential to achieve an early diagnosis, start effective antimicrobial therapy and, if required, proceed with a prompt neurosurgical intervention [2], [3].
Several case series of spinal epidural abscesses in adults have been reported in the scientific literature, whereas reports in children are scanty. We describe a spontaneous SEA due to MSSA in a 15-year-old boy without risk factors, and have performed a review of publications in the scientific literature regarding management of pediatric SEAs published in the last 14 years. Two previous reviews, including reports on patients with risk factors, reported cases up to calendar year 2000 [6], [7]. In the present work, we analyzed reports and reviews from pediatric patients published from 2001 to 2014 which, as in our case, did not present risk factors.
A review of the English literature was performed by an exhaustive Pubmed search for case reports and reviews, with publication date January 2001–December 2014, using the following terms: “spinal subdural abscess”, “spinal epidural abscess”, “spontaneous subdural abscess”, “spontaneous epidural abscess”, “spontaneous spinal epidural empyema”. The exclusion criteria were: (i) adult population (age ≥18 years), (ii) incomplete clinical or age information or undistinguishable data between pediatric and adult patients, (iii) tubercular spinal epidural abscesses, and (iv) any underlying disease in the medical or surgical history. A manual review of the papers found by the above search method was performed to verify inclusion and exclusion criteria and to exclude cases with risk factors for hematogenous spreading (e.g. impetigo in chickenpox, cat scratch, mucocutaneous trauma) and/or underling predisposing diseases (e.g. cauda equina, holocord syndrome, neurosurgery).
Case
In June 2013, a 15-year-old male was referred to the Emergency Department of Siena University Hospital, Tuscany, Italy, from another regional hospital with a provisional diagnosis of meningitis. He reported a history of fever, headache and back pain, mainly in the lumbar-sacral region, during the previous 3 days. No previous trauma, nor minor or major surgery was reported by the boy or his parents.
His past medical history was unremarkable, but he received a anti group C meningococcal conjugate vaccine dose together with a anti diphtheria-tetanus booster dose 2 weeks before admission; seven days of myalgias and low grade fever (maximum 37.5 °C) with spontaneous resolution were reported to after those immunizations.
On admission the patient was conscious complaining of headache and lumbar pain with bilateral leg weakness; on physical examination his BMI was 17.3 blood pressure 110/70 mmHg, heart rate 92/min, respiratory rate 20/min, body temperature 36.3 °C (he had received 1000 mg of acetaminophen one hour before admission); he had a stiff neck with a lumbar pain arising while attempting to flex his neck, and presence of a bilateral straight leg raise sign, there was not any focal tenderness on palpation of the patient's spine. Neurological examination did not demonstrate any motor or sensory deficit, but the patient was not able to walk due to the severe pain. The rest of physical exam was completely negative. Laboratory exams showed: leukocytes 11,300 × 109/L (86.3% neutrophils), C-reactive protein (CRP) 10.2 mg/dl (upper normal value, upper limit of normal, ULN 0.5), procalcitonin 0.18 ng/ml (ULN 0.5), PT 71% (normal range 80–120%), INR 1.24.
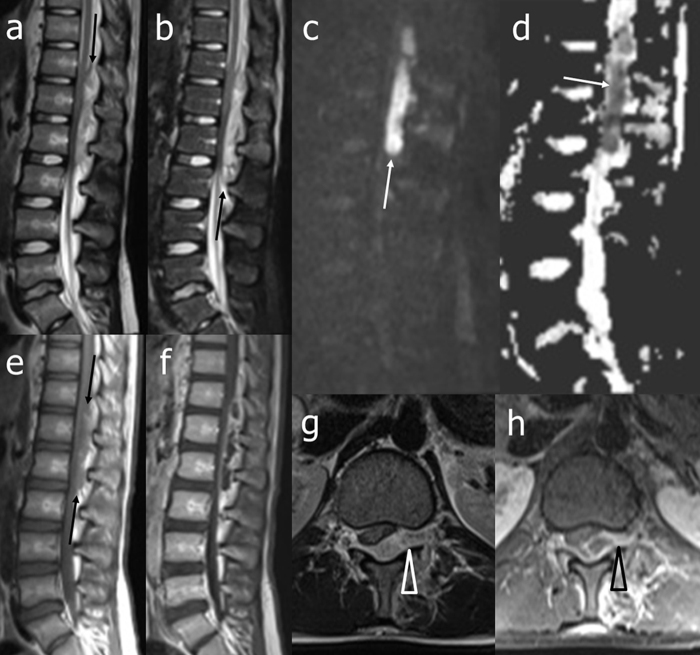
Brain computer assisted tomography (CT) was normal. Three sets of blood cultures were collected and lumbar puncture was performed. Cerebrospinal fluid (CSF) examination revealed a clear fluid with pleocytosis (138 leukocytes/mm3, 67% polymorphs), glucose concentration 67 mg/dl (blood glucose 61 mg/dl), protein concentration 145.3 mg/dl (normal range 20–40 mg/dl). Empirical treatment with intravenous (iv) ceftriaxone 2 g bid and iv acyclovir 500 mg tid was prescribed and the patient was transferred to the Infectious Disease ward. The day after admission, lumbar pain was progressively worsening and lower and upper limbs weakness appeared. No peripheral nerve conduction deficits were detected on electromyography and no immunoglobulins type G in the alkaline region of CSF on isoelectrofocusing were revealed. Urgent gadolinium-enhanced cerebral and spinal magnetic resonance imaging (MRI) disclosed a large epidural purulent collection in the posterior and median-paramedian left spinal canal, extending from T11 to L2, with mass effect on spinal nerve roots (Fig. 1).
Fig. 1.
Magnetic resonance imaging of the thoraco-lumbar spine at diagnosis: unenhanced T2-weighted (a), short tau inversion recovery (STIR) (b), diffusion-weighted (DW) (c), apparent diffusion coefficient (ADC) map (d), and T1-weighted (e) sagittal and T2-weighted axial (g) images, and gadolinium-enhanced T1-weighted sagittal (f) and axial (h) images. At the thoracolumbar junction, a posterior median-left paramedian epidural collection compressing the caudal spinal cord, conus medullaris, and upper cauda equina is clearly evident. The lesion shows irregular signal intensity on unenhanced conventional sequences (a, b, and e, black arrows), restricted diffusion on DW images and ADC map (c, d, white arrows), and peripheral gadolinium-enhancement (f, h). Note also that the lesion shows extent toward the left intervertebral foramen (g, h, arrow head). STIR image does not show associated abnormal signal intensity of the bone marrow.
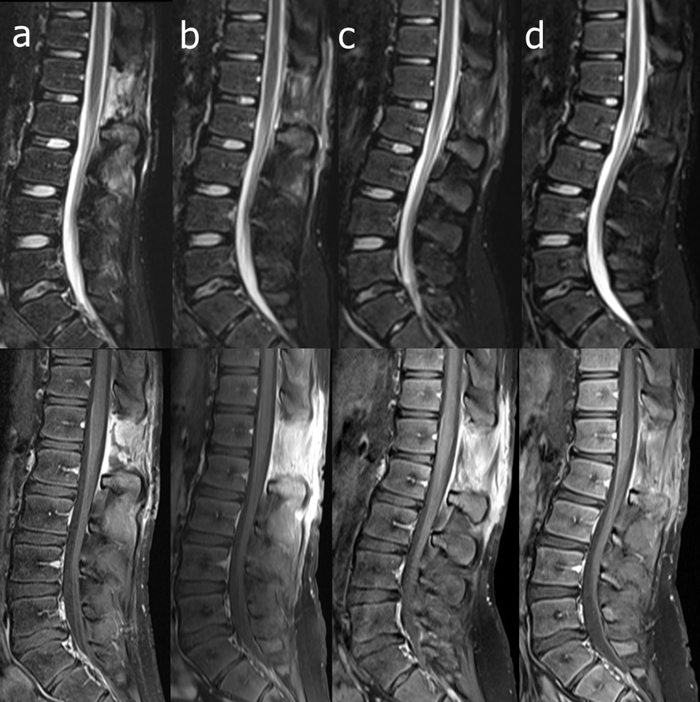
With a diagnosis of SEA evolving in stage 3, in the evening of the 2nd day from admission, neurosurgeons performed an L2 laminectomy and abscess drainage. Antimicrobial treatment was modified with discontinuation of ceftriaxone and acyclovir, and empirical switch to meropenem 2 g tid and vancomycin 1 g bid., choosing a wider spectra coverage due to the aforementioned neurological deterioration and to the large amount of obtained purulent material, Histological analysis of the surgical tissue samples showed chronic purulent inflammation, without neoplastic changes. Abscess culture revealed MSSA; the strain was also fully susceptible to all tested antimicrobials. On the basis of the above in vitro susceptibility results, the treatment was modified again with iv ceftriaxone 2 g bid and iv clindamycin 900 mg tid Negative results were obtained from blood cultures, CSF culture and polymerase chain reaction on CSF for Borrelia spp., mycobacterial and viral genomes; serum agglutination tests for typhoid and brucellosis were negative; no congenital or acquired immunodeficiency was revealed. Normal values of CRP and white blood cell count were obtained respectively on the 7th and 18th day; remission of the fever was obtained within 72 h after admission and a progressive improvement of the clinical condition was observed. Lumbar pain disappeared and the patient underwent rehabilitation with a complete recovery. On the 21st day, the patient was discharged from hospital with the final diagnosis of SEA by MSSA and with the advice to continue antimicrobial therapy with iv ceftriaxone 2 g daily and oral clindamycin 600 mg tid Lumbar spinal MRI, performed approximately one month after surgery (Fig. 2a, b), showed complete resolution of the abscess and gadolinium-enhancing postoperative reactive changes in the fascial muscular planes. Heart and abdomen ultrasound and chest radiography were negative.
Fig. 2.
Magnetic resonance imaging follow-up by STIR (upper line) and gadolinium-enhanced fat-suppressed T1-weighted (lower line) sagittal images obtained 24 days after surgery (a) and one month (b), three months (c), and seven months (d) later. Besides complete removal and absence of relapse of the epidural abscess, a clearcut reduction of gadolinium-enhancing reactive changes is evident. Note also the progressive development of kyphosis with fulcrum at L1–L2 level, together with increase of lumbar lordosis.
At monthly follow-up examinations, white blood cells and CRP were constantly normal but the patient complained general asthenia and mild occasional lumbar pain and the same antimicrobial regimen with ceftriaxone and clindamycin was prolonged for further 4 weeks.
Two months after hospital discharge, spinal lumbar MRI (Fig. 2c) showed reduction of reactive changes and the patient complained persistent mild lumbar pain during subsequent clinical evaluation; amoxicillin/clavulanate 1 g tid plus rifampicin 600 mg per daily orally was prescribed during the subsequent 6 months.
After total 8 months of antimicrobial treatment, lumbar spinal MRI showed further reduction of reactive changes (Fig. 2d), despite development of thoracolumbar kyphosis and lumbar lordosis. Antimicrobial therapy was finally stopped. No symptoms neither sequelae were reported in the subsequent 1 year clinical follow up.
Discussion
Including our report, we found a total of 12 pediatric SEA cases without predisposing factors: 8 were males, average age was 9.6 years [range 16 days–17 years]. The patient described by Prasad and De Vere [15], masquerading as an acute abdomen, was excluded from subsequent analysis, due to lack of complete clinical information (paper submitted as “Visual Diagnosis”). In the remaining 11 patients, clinical presentation was: fever and back pain, with elevation of CSF cell counts (average, 15,196 cells/mm3) and CRP levels, except in a single case. Lumbar puncture was performed in 4/11 cases (36.3%), with an abnormal result of CSF's cell count in 3 cases. Responsible microorganisms were identified in 8 cases (72%): in 1 case a group A beta-hemolytic Streptococcus was isolated from a purulent collection, in the remaining 7 cases S. aureus was cultured from purulent collection (n = 4), from blood (n = 2) or from both sites (n = 1). MSSA was reported in 4 patients, MRSA in 1 patient, while in the remaining 2 cases drug susceptibility was not reported. In one of these two latter cases, even if not specified in the original paper, a presumptive diagnosis of MSSA can be inferred based on response to antimicrobial therapy (cefazolin, cephalexin). The average of therapy duration was 6 weeks. In four cases the duration was not specified.
All the patients, were initially misdiagnosed as: back pain, meningitis (even in absence of the classical meningeal syndrome), acute myelitis, diskitis, cord compression by a neoplastic mass and septic arthritis; patients’ spine was always evaluated by gadolinium-enhanced MRI. Most abscesses (N = 10/11) were localized at the thoracic and lumbar area, without signs of osteomyelitis.
In 8/11 cases, laminectomy and abscess drainage were performed in association with effective medical therapy; only in two cases, both without an aetiological diagnosis, treatment was successful with antimicrobial therapy only [13], [14]. A good outcome, defined as a complete recovery, was obtained in all patients, with the exception of the report of Rook et al. [9] who described residual headache and paraspinal pain lasting for 3 years. Complete data of the reviewed literature reports are summarized in Table 1, Table 2.
Table 1.
Clinical, instrumental and laboratoristic findings in the reviewed SEAs cases.
| Patient | Year of study [reference] |
Gender, age | Spine level | Locationa | Symptoms at admission | Primary Diagnosisa |
CSFc | WBC/mmc (% PMN) |
CRP Levelb | Positive blood culturea |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2001 [8] |
F, 7 weeks | T10–12 | N/A | Flaccid paraplegia | N/A | N/P | Normal | Normal | N/A |
| 2 | 2011 [9] |
M, 15 years | T3–T8 | Right Postero-lateral | Right scapular pain, fever, chills with night sweats, headache, photophobia | Right rhomboid muscle strain with spasm, acute febrile illness | Normal | N/A | 84.5 mg/L (n.v. <10) | Yes |
| 3 | 2011 [10] |
F, 11 years | T11–L4 | Posterior | Fever, lumbar pain | Back pain | N/P | 13,770 (82%) | 27.2 mg/dl (n.v. <0.5) | No |
| 4 | 2012 [11] |
M, 16 days | C1/2 – lumbosacral | Posterior+ Right side (plusParaspinal collection C3–T4, right pleural space and right kidney collection) |
Fever, nervousness | N/A | 180 PMN, 9900 red blood cells | 21,200 (43%) | 232 g/L (n.v. N/A) | Yes |
| 5 | 2012 [12] |
M, 15 years | L2–L3 | Posterior | Urinary retention, Backpain, Fever | Low back pain and Not specified urinary retention | N/P | 18,600 (70%) | ++ (value N/A) | N/A |
| 6 | 2013 [13] |
M, 13 years | C7–T1 | Posterior, on both sides | Transient fever, neck and upper back pain, tingling sensation in hands and feet, urine incontinence, abdominal distension, inability to sit and walk | Acute myelitis, diskitis, meningitis | 800 cells/mmc, 2% PMN, glucose 21 mg/dl | 7,100 (N/A) | ++ (value N/A) | No |
| 7 | 2013 [14] |
M, 1.2 years | L3–L4 | Posterior | Refusal to walk, irritability, weakness | N/A | N/P | 13,800 (N/A) | N/A | No |
| 8 | 2013 [14] |
M, 3 years | T1–L2 | Posterior | Fever, stomachache | N/A | N/P | 20,000 (N/A) | 82.2 mg/L | Yes |
| 9 | 2013 [14] |
F, 17 years | L1–L4 | Posterior | Fever, nausea, vomiting | N/A | N/P | 15,800 (N/A) | 182 mg/L | No |
| 10 | 2013 [Present report] | M, 15 years | T11–L2 | Left and posterior | Fever, headache, back pain | Meningitis, myelitis | 138 cells/mmc, 67% PMN, glucose 67 mg/dl, proteins 145.30 mg/dl | 11,300 (86.3%) | 10.20 mg/dl (n.v. <0.5) | No |
| 11 | 2014 [16] |
M, 21 months | L4–L5 | Right into the spinal canal | Fever, refuse to walk | Septic arthritis | N/P | N/A | 200.1 mg/L | No |
N/A, not available.
CRP, C-reactive protein; n.v., normal value.
CSF, cerebrospinal fluid; N/P, lumbar puncture not performed.
Table 2.
Etiology, therapy and outcome in the reviewed SEAs cases.
| Patient | Year of study [reference] |
Etiologya | Source of isolate | Osteomyelitisa | Surgery | Empiric therapy | Targeted therapyb | Duration of therapyb | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2001 [8] |
Staphylococcus aureus | Pus | Absent | T9–T12 laminectomy | N/A | Not specified iv anti-staphylococcal antibiotics | N/A | No deficit after 18 months follow up |
| 2 | 2011 [9] |
MSSA | Blood | Absent | Thoracic laminectomy-drainage | Cefotaxime | Ceftriaxone | 4 weeks | Residual headache and paraspinal pain for subsequent 3 years |
| 3 | 2011 [10] |
MSSA | Pus | Absent | Drainage | Vancomycin + Ceftriaxone | Clindamycin+ Ceftriaxone iv then Clindamycin + Cefuroxime per os |
7.5 weeks | No deficit |
| 4 | 2012 [11] |
MSSA | Pus and Blood | Absent | Right sided laminectomy drainage | Ampicillin + Gentamicin Then Vancomycin + Meropenem |
Flucloxacillin + Ampicillin | 8 weeks (Note: E. faecalis from urine and renal abscess) |
No deficit |
| 5 | 2012 [12] |
Staphylococcus aureus | Pus | N/A | L2–L3 laminectomy drainage | N/A | Cefazolin Then Cephalexin |
6 weeks | No deficit |
| 6 | 2013 [13] |
Unknown | – | Absent | None | Ceftriaxone + Vancomycin | N/A | 6 weeks | No deficit |
| 7 | 2013 [14] |
Unknown | – | Absent | None | Nafcillin, Vancomycin Then Clindamycin (+Vancomycin on readmission) |
N/A | N/A | Standing with assistance on discharge |
| 8 | 2013 [14] |
MRSA | Blood | Absent | Left laminectomy T5–L2 | Clindamycin iv+ Vancomycin iv+ Rifampin iv |
Clindamycinperos + Rifampinperos | N/A | No deficit |
| 9 | 2013 [14] |
Unknown | – | Absent | None | Daptomycin iv+ Doripenem iv+ Doxycycline ThenDoxycycline + Doripenem |
N/A | N/A | No deficit |
| 10 | 2013 [Present report] | MSSA | Pus | Absent | L2 Laminectomy-abscess drainage | Vancomycin + meropenem | Ceftriaxone + Clindamycin iv, Then amoxicillin/clavulanate + rifampicin per os | 8 months | No deficit |
| 11 | 2014 [16] |
Group A beta-hemolytic Streptococcus | Pus | Absent | L4–L5, Partial S1 Laminectomy and drainage |
N/A | Ceftriaxone | 6 weeks | No deficit |
MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillin resistant Staphylococcus aureus; where not specified: not reported.
N/A, Not available.
To the best of our knowledge, this case report is one of the rare descriptions of pediatric SEA, without underlying risk factors. In a previous literature review covering reports from 1980 to 2000 [6], 12 pediatric patients with SEA without any risk factors were found: no complications or associated osteomyelitis were reported and a favorable outcome was obtained after medical (n = 1/12, 8%) or combined surgical plus medical (n = 11/12, 92%) therapy. MSSA was the predominant detected pathogen (n = 7/12, 58%) [6].
According to our review of recent literature from 2001 to 2014, SEA in pediatric age is confirmed to be very rarely reported, especially in the absence of predisposing risk factors: in the last 14 years only 12 cases were described.
Osteomyelitis complication was never reported. A combined surgical and medical therapy was usually performed with favorable outcome and MSSA was confirmed to be the main aetiological agent. Despite this prominent etiology, given the potential for serious sequelae, anti-MRSA therapy should be considered mandatory in the empiric antimicrobial regimen for pediatric SEA in areas with high rates of MRSA, awaiting microbiological identification and drug susceptibility results.
An interesting matter of discussion is the possible source of staphylococcal bacteremia in pediatric patients without risk factors: as nares and skin are the primary colonization sites, these should be considered the primary sources. In our case report, no previous trauma could be linked to a bacteremia by microorganisms colonizing the skin, not even acupuncture procedures as previously reported for adult patients [19].
The required duration of antimicrobial therapy remains an issue: a mean duration of 6 weeks was reported in the reviewed cases; the previous most recent review [14] suggested a 4–6 weeks regimen. In the case reported herein, antimicrobial therapy was indeed prolonged until 8 months, with a successful outcome and without side effects. The adopted regimen and its duration can be matter of discussion: despite rapid fever remission and normalization of CRP, clinicians chose this long lasting dual regimen due to unusual presentation in an otherwise healthy adolescent and to long term persistence of mild referred back pain. It has been previously stated that a prolonged medical therapy is advisable in cases without surgical interventions and its duration should depend on the level of immune competence, clinical improvement and response to treatment demonstrated by subsequent reduction of inflammation at MRI [14], [17]. In the only previous report describing residual 3 years of headache and paraspinal pain [9], the duration of therapy was the shortest described (4 weeks).
It should be noted that MRI follow-up is reported to overestimate inflammatory changes, mainly when bone involvement is present: MRI follow-up has been usually reported as unnecessary in spondylodiscitis when the clinical and laboratory abnormalities respond to treatment [18]. Despite the absence of specific statements in the literature, this should be reasonably valid also for patients with SEA. The patient with SEA reported herein did not show a bone involvement, however he complained of a long lasting low back pain. MRI follow-up showed complete removal of the abscess, followed by regular reduction of normal postoperative reactive findings, and development of thoracolumbar kyphosis together with increased lumbar lordosis.
The case described herein, along with the reviewed literature, underline the diagnostic complexity of this condition, particularly at its onset. Many clinical features may be non-specific for SEA and the classical ones (fever, back pain and neurological deficits) can be incompletely present at the early stages. Headache and neck stiffness are rarely reported, but their presence, as described also in our patient, can contribute to misdiagnosis: in 100% of the reviewed cases, a diagnosis other than SEA was postulated on admission and a lumbar puncture was therefore frequently performed, with possible dangerous consequences depending on SEA location.
In conclusion, it seems essential to maintain a high index of suspicion for SEA in children: the rarity and the possible differential diagnosis on the basis of the early signs and symptoms can lead clinicians to underestimate its occurrence, but an as prompt as possible diagnosis is an essential prognostic factor, allowing an immediate medical and surgical intervention before the development of non-reversible sequelae.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Tunkel A.R. Subdural empyema, epidural abscess and suppurative intracranial trombophlebitis. In: Mandell G.L., Bennet J.E., Dolin R., editors. Principle and practice of infectious diseases. 7th ed. Elsevier; Philadelphia: 2010. pp. 1281–1283. [Google Scholar]
- 2.Sendi P., Bregenzer T., Zimmerli W. Spinal epidural abscess in clinical practice. QJM. 2008;101:1–12. doi: 10.1093/qjmed/hcm100. [DOI] [PubMed] [Google Scholar]
- 3.Karikari I.O., Powers C.J., Reynolds R.M., Mehta A.I., Isaacs R.E. Management of spontaneous spinal epidural abscess:a single center 10 years experience. Neurosurgery. 2009;65:919–923. doi: 10.1227/01.NEU.0000356972.97356.C5. [DOI] [PubMed] [Google Scholar]
- 4.Darouiche R.O. Spinal epidural abscess. N Engl J Med. 2006;355:2012–2020. doi: 10.1056/NEJMra055111. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerer S.M., Conen A., Müller A.A., Sailer M., Taub E., Flückiger U. Spinal epidural abscess: aetiology, predisponent factors and clinical outcomes in a 4 year prospective study. Eur Spine J. 2011;20:2228–2234. doi: 10.1007/s00586-011-1838-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auletta J.J., John C.C. Spinal epidural abscess in children: a 15 year experience and review of the literature. Clin Infect Dis. 2001;32:9–16. doi: 10.1086/317527. [DOI] [PubMed] [Google Scholar]
- 7.Sandler A.L., Thompson D., Goodrich J.T., van Aalst J., Kolatch E., El Khashab M. Infections of the spinal subdural space in children: a series of 11 contemporary cases and review of all published reports A multinational collaborative effort. Child Nerv Syst. 2013;29:105–117. doi: 10.1007/s00381-012-1916-4. [DOI] [PubMed] [Google Scholar]
- 8.Tang K., Xenos C., Sgouros S. Spontaneous spinal epidural abscess in a neonate. With a review of the literature. Child Nerv Syst. 2001;17:629–631. doi: 10.1007/s003810100477. [DOI] [PubMed] [Google Scholar]
- 9.Rook J.L., Duffey D., DeRoos S. A case of autonomically mediated pain due to spinal epidural abscess in an adolescent female. Pediatr Emerg Care. 2011;27:530–532. doi: 10.1097/PEC.0b013e31821d86d5. [DOI] [PubMed] [Google Scholar]
- 10.Mantadakis E., Birbilis T., Michailidis L., Souftas V., Chatzimichael A. Spinal epidural abscess in young girl without risk factors. Eur J Pediatr. 2011;170:945–948. doi: 10.1007/s00431-011-1437-2. [DOI] [PubMed] [Google Scholar]
- 11.Hazelton B., Kesson A., Prelog K., Carmo K., Dexter M. Epidural abscess in a neonate. J Pediatr Child Health. 2012;48:E132–E135. doi: 10.1111/j.1440-1754.2011.02078.x. [DOI] [PubMed] [Google Scholar]
- 12.Sales G.J., Tabrizi A., Elmi A., Soleimanpour J., Gavidel E. Adolescence spinal epidural abscess with neurological symptoms: case report, a lesson to be re-learnt. Med J Islam Repub Iran. 2013;27:38–41. [PMC free article] [PubMed] [Google Scholar]
- 13.Pathak A., Singh P., Gehlot P., Dhaneria M. Spinal epidural abscess treated with antibiotics alone. BMJ Case Rep. 2013 doi: 10.1136/bcr-2013-009285. published online 30 April 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawkins M., Bolton M. Pediatric spinal epidural abscess: a 9 year institutional review and review of the literature. Pediatrics. 2013;132:e1680–e1685. doi: 10.1542/peds.2012-3805. [DOI] [PubMed] [Google Scholar]
- 15.Prasad M., De Vere N. Spinal epidural abscess masquerading as an acute abdomen. Pediatr Neurol. 2014;50:540–541. doi: 10.1016/j.pediatrneurol.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 16.Harris T.J., Seamon J.P. Spontaneous spinal epidural abscess in a 21-month-old child. Am J Emerg Med. 2014;32:1558. doi: 10.1016/j.ajem.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 17.Siddiq F., Chowfin A., Tight R., SahmounA.E., Smego R.A., Jr. Medical vs. surgical management of spinal epidural abscess. Arch Intern Med. 2004;164:2409–2412. doi: 10.1001/archinte.164.22.2409. [DOI] [PubMed] [Google Scholar]
- 18.Grados F., Lescure F.X., Senneville E., Flipo R.M., Schmit J.L., Fardellone P. Suggestions for managing pyogenic (non-tuberculous) discitis in adults. Jt Bone Spine. 2007;74:133–139. doi: 10.1016/j.jbspin.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Yu H.J., Lee K.E., Kang H.S., Roh S.Y. Teaching neuroimages: multiple epidural abscesses after acupuncture. Neurology. 2013;80:e169. doi: 10.1212/WNL.0b013e31828c2f1d. [DOI] [PMC free article] [PubMed] [Google Scholar]