Abstract
Background and Purpose
A substantial proportion of patients with atrial fibrillation (AF) are not treated optimally; however, the inappropriateness of drug therapy has never been evaluated before or after a stroke event. We investigated the adherence to guidelines for therapy in AF patients hospitalized with acute ischemic stroke (AIS) before stroke onset and at discharge, with the aim of identifying the factors associated with inappropriate therapy.
Methods
AIS patients with AF hospitalized within 7 days of onset were identified from a prospective nine-center stroke registry database. Two cohorts were defined: patients diagnosed with AF prior to the stroke event (admission cohort) and patients diagnosed with AF at discharge from hospital (discharge cohort). Any of the following conditions were regarded as nonadherence to guidelines in this study: use of anticoagulant or nonuse of antithrombotics with CHADS2 score=0, nonuse of antithrombotics with CHADS2 score=1, or nonuse of anticoagulant with CHADS2 score ≥2.
Results
Overall, 406 patients were enrolled in the admission cohort and 518 in the discharge cohort. The rates of nonadherence before a stroke event and at discharge were 77.8% and 33.3%, respectively. These rates varied widely for both cohorts, with interhospital differences being statistically significant. Multivariable analysis revealed that old age, stroke history, and congestive heart failure were associated with nonadherence before stroke. At discharge, males, coronary heart disease, inappropriate antithrombotic use before stroke, and functional disability at discharge were associated with nonadherence.
Conclusions
This study shows that antithrombotic use in AIS patients with AF might be not optimal before and after stroke in Korea.
Keywords: atrial fibrillation, drug utilization review, cerebral infarction, guideline adherence
INTRODUCTION
The heterogeneity of stroke risk in patients with atrial fibrillation (AF) is well known, with the stroke incidence reportedly ranging from 0.7% to 14.2% per year.1 A need for risk stratification schemes resulted in the introduction of a point system in 2001 called the CHADS2 score. Because this system was simple to use and widely validated, most current clinical practice guidelines recommend its application in determining the use of antithrombotics in patients with AF.2,3,4,5
Despite the clear benefits of antithrombotic therapy for stroke prevention,6,7,8 a substantial proportion of patients with AF do not receive optimal treatment.9,10,11,12 Recent studies performed at the national level found that nearly 40% of patients with AF were on warfarin therapy when they experienced a stroke.13,14 Furthermore, a nonnegligible proportion of patients with AF are not treated optimally even after they experience stroke. A national survey from Israel found that 39% of high-risk AF patients were taking an anticoagulant when they presented with acute ischemic stroke, and 62% were discharged with anticoagulant.14 However, despite the importance of the CHADS2 score in daily practice, no previous study has evaluated the appropriate use of antithrombotics according to the CHADS2 score in AF patients who present with acute ischemic stroke in Korea. Taking advantage of a nationwide multicenter stroke registry database in Korea,15 we aimed to describe the inappropriateness of antithrombotic use in hospitalized stroke patients with AF before and after stroke, and to elucidate factors associated with inappropriate pharmacological therapy in these patients.
METHODS
Study subjects
A consecutive series of patients with ischemic stroke hospitalized at participating centers between April 2008 and March 2009 were identified in the prospective registry database of the Clinical Research Center for Stroke-5th division (CRCS-5).15 Nine stroke centers (including academic and regional hospitals) belonging to the CRCS-5 participated in this study (Supplementary Fig. 1 in the online-only Data Supplement). The CRCS-5 registry began to collect data on the demographics, stroke characteristics, vascular risk factors, etiological work-up, in-hospital management, and functional status at discharge of hospitalized stroke cases in April 2008. Approval was obtained from the institutional review boards at all participating centers for the collection of anonymized clinical data without patients' consent for the purpose of monitoring and improving the quality of stroke care. We received further approval for the collection of additional data and the use of the registry database for this study.
Among the registered patients, those with a history of AF or were newly diagnosed with AF were enrolled in this study. Patients who had mitral stenosis, prosthetic cardiac valves, or acute myocardial infarction within 4 weeks of stroke onset were excluded.
We assessed the appropriateness of antithrombotic use based on the CHAD2 score at two time points: at stroke onset and at discharge. The assessment at stroke onset was performed for patients who were diagnosed with AF prior to the stroke event (admission cohort) and the assessment was completed at discharge for the admission cohort plus for those patients who were newly diagnosed with AF after stroke onset (discharge cohort). Patients who died during hospitalization were included in the admission cohort but not the discharge cohort.
CHADS2 score and inappropriate use of antithrombotic therapy
We defined nonadherence to reference guidelines as 'inappropriateness'. The inappropriateness of antithrombotic therapy was defined based on the CHADS2 score.16 The CHADS2 scale is scored by assigning 1 point for congestive heart failure (CHF), hypertension, age >74 years, and diabetes mellitus (DM), and 2 points for a history of stroke or transient ischemic attack. Information on these factors except CHF was obtained directly from the registry database. CHF was defined through an additional review of medical records as a left-ventricle ejection fraction of ≤35% on echocardiography3 or International Classification of Diseases-10 codes of I50.0 or I50.9 in the insurance claim database. We graded CHADS2 scores of 0, 1, and ≥2 as low, moderate, and high risk, respectively.5,17 The nonadherence to guidelines for antithrombotic therapy was determined using the Clinical Practice Guidelines for Stroke from the Clinical Research Center for Stroke as reference guidelines, because theses have been widely used in Korea and are accredited by the Korean Stroke Society and the Korean Neurological Association.4 According to these guidelines, inappropriate treatment was defined as the use of an oral anticoagulant (OAC) or nonuse of an antiplatelet in the low-risk group, neither antiplatelet use nor OAC therapy in the moderate-risk group, and nonuse of any OAC in the high-risk group. Aspirin, clopidogrel, ticlopidine, triflusal, and cilostazol were classified as antiplatelet drugs.
Statistical analysis
Continuous variables are presented as mean and standard deviation or median (interquartile range) values, whereas categorical variables are expressed as absolute values (percentages). The distributions of CHADS2 scores and the antithrombotics used were analyzed in the admission and discharge cohorts. The patient characteristics were compared according to the inappropriateness of antithrombotic use in the admission and discharge cohorts. The distributions of inappropriate use at stroke onset and at discharge were analyzed according to the participating centers.
Multivariable logistic regression analysis identified the correlates of inappropriate therapy use. Variables with p values of <0.2 in bivariate analyses were selected for adjustment in multivariable models. We used different models for analyzing the admission and discharge cohorts. The modified Rankin Scale (mRS) was used to assess prestroke disability for the admission cohort models. Inappropriate drug use at stroke onset applied at the discharge cohort model. The National Institutes of Health Stroke Scale score at admission was not chosen as a covariate due to a possibility of multicollinearity with the discharge mRS score. After the first round of multivariable analyses (model 1 for the admission cohort and model 3 for the discharge cohort), hospital was added as a variable to examine its effect on inappropriate therapy use (models 2 and 4). Only variables showing p<0.05 in models 1 and 3 were selected as covariates in models 2 and 4, respectively. The results of multivariable analysis were summarized as odds ratios, 95% confidence intervals, and p values. For these results, the reference category for hospital was selected by the number of patients and inappropriate therapy use of each hospital in each cohort. The global Wald test was used for the overall association of the hospital with inappropriate therapy use in each cohort.
All statistical analyses were performed using SPSS (version 19.0, SPSS Inc., Chicago, IL, USA). A two-sided p value of <0.05 was used as the cutoff for statistical significance.
RESULTS
Over a 12-month period, 3,240 patients were hospitalized at the participating centers for acute ischemic stroke; of these, 591 patients were diagnosed with AF and were eligible for inclusion in this study. Twenty-nine patients were excluded from the study due to the presence of prosthetic cardiac valves (n=3), mitral stenosis (n=22), or a recent myocardial infarction event (n=4). Of the remaining 562 patients who were enrolled for this study, 406 (72.2%) were known to have AF prior to the stroke event and were analyzed as the admission cohort, while 518 (92.2%) were assigned to the discharge cohort since 44 patients (7.8%) had died during the hospitalization period.
Exactly half of the patients had not received any antithrombotic drug therapy when they experienced a stroke despite having a history of AF (Table 1). In this admission cohort, 21% (n=86) had taken OACs, but 90% of these patients were receiving an anticoagulant at a subtherapeutic level [prothrombin time international normalization ratio (INR) <2.0]. Inappropriate use of antithrombotic medications was found in 87.5% of the low-risk group (CHADS2 score=0, n=32), in 63.7% of the moderate-risk group (CHADS2 score=1, n=91), and in 77.4% of the high-risk group (CHADS2 score ≥2, n=283). We did not identify any correlation between higher CHADS2 score and greater likelihood of patients receiving the appropriate antithrombotic drug (p=0.26, Mantel-Haenszel chi-square test for trend) (Table 1). Of the patients with a CHADS2 score of 0, 28.1% were treated with OACs, while only 22.5% of the patients with a CHADS2 score of ≥2 were taking OACs. Overall, 6.7% of the admission cohort (n=27) took both antiplatelet and OAC agents.
Table 1. Distribution of CHADS2 scores and antithrombotic use in the admission and discharge cohorts.
| CHADS2 score | Admission cohort (n=406) | Discharge cohort (n=518) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | AP | OAC | Either | IU | Overall | AP | OAC | Either | IU | |
| 0 | 32 | 5 (15.6) | 9 (28.1) | 13 (40.6) | 28 (87.5) | |||||
| 1 | 91 | 25 (27.5) | 13 (14.3) | 33 (36.3) | 58 (63.7) | |||||
| 2 | 116 | 38 (32.8) | 19 (16.4) | 53 (45.7) | 97 (83.6) | 68 | 22 (32.4) | 52 (76.5) | 60 (88.2) | 16 (23.5) |
| 3 | 71 | 21 (29.6) | 16 (22.5) | 35 (49.3) | 55 (77.5) | 156 | 59 (37.8) | 117 (75.0) | 141 (90.4) | 39 (25.0) |
| 4 | 70 | 41 (58.6) | 17 (24.3) | 48 (68.6) | 53 (75.7) | 206 | 86 (41.7) | 123 (59.7) | 182 (88.3) | 83 (40.3) |
| 5 | 19 | 11 (57.9) | 8 (42.1) | 16 (84.2) | 11 (57.9) | 76 | 29 (38.2) | 49 (64.5) | 67 (88.2) | 27 (35.5) |
| 6 | 7 | 3 (42.9) | 4 (57.1) | 5 (71.4) | 3 (42.9) | 12 | 6 (50.0) | 8 (66.7) | 11 (91.7) | 4 (33.3) |
| Total | 406 | 144 (35.5) | 86 (21.2) | 203 (50.0) | 305 (75.1) | 518 | 202 (39.0) | 349 (67.4) | 461 (89.0) | 169 (32.6) |
Data are number of patients (%) values.
AP: antiplatelet, Either: AP or/and OAC, IU: inappropriate medication user, OAC: oral anticoagulant.
The patient characteristics did not differ significantly between appropriate and inappropriate users in the admission cohort (Table 2).
Table 2. Patient characteristics according to the inappropriateness of antithrombotic use in the admission and discharge cohorts.
| Admission cohort | Discharge cohort | |||||
|---|---|---|---|---|---|---|
| AU | IU | p* | AU | IU | p* | |
| Age, years | 71.3±10.0 | 73.2±9.8 | 0.10 | 772.2±10.2 | 74.7±10.5 | 0.01 |
| Male | 52 (51.5) | 149 (48.9) | 0.65 | 164 (47.0) | 85 (50.3) | 0.48 |
| CHF | 28 (27.7) | 59 (19.3) | 0.08 | 62 (17.8) | 33 (19.5) | 0.63 |
| Hypertension | 74 (73.3) | 218 (71.5) | 0.73 | 245 (70.2) | 122 (72.2) | 0.64 |
| DM | 21 (20.8) | 87 (28.5) | 0.13 | 77 (22.1) | 52 (30.8) | 0.03 |
| History of stroke/TIA | 42 (41.6) | 97 (31.8) | 0.07 | 97 (27.8) | 55 (32.5) | 0.27 |
| Ever smoked | 25 (24.8) | 78 (25.6) | 0.87 | 80 (22.9) | 51 (30.2) | 0.08 |
| CHD | 18 (17.8) | 45 (14.8) | 0.46 | 53 (15.2) | 35 (20.7) | 0.12 |
| NIHSS score at admission | 6 (3-14) | 12 (5-18) | <0.01 | |||
| IU at admission | 258 (73.9) | 153 (90.5) | <0.01 | |||
| Prestroke mRS score | 0.06 | |||||
| 0 to 2 | 89 (88.1) | 286 (93.8) | ||||
| 3 to 5 | 12 (11.9) | 19 (6.2) | ||||
| Discharge mRS score | <0.01 | |||||
| 0 to 2 | 171 (49.0) | 34 (20.1) | ||||
| 3 to 5 | 178 (51.0) | 135 (79.9) | ||||
Data are number (%), mean±SD, or median (interquartile range) values.
*p calculated using Pearson's chi-square test, Student's t-test, or the Mann-Whitney U test.
AU: appropriate medication user, CHD: coronary heart disease, CHF: congestive heart failure, DM: diabetes mellitus, IU: inappropriate medication user, NIHSS: National Institutes of Health Stroke Scale, mRS: modified Rankin Scale, TIA: transient ischemic attack.
Eighty-nine percent of patients with AF were discharged on antithrombotic medication (Table 1), 67.4% on OACs, and 39.0% on antiplatelet drugs. Ninety patients (17.4%) were discharged on both antiplatelet therapy and OACs. The CHADS2 scores in the discharge cohort ranged from 2 to 6, with those having a CHADS2 score of 4 constituting the largest proportion (39.8%), followed by CHADS2 scores of 3, 5, 2, and 6 in 30.1%, 14.7%, 13.1%, and 2.3%, respectively. The proportion of the discharge cohort with inappropriate use was 32.6%, and this increased with the CHADS2 score (p=0.01, Mantel-Haenszel chi-square test for trend) (Table 1).
Comparisons of patient characteristics between the appropriate and inappropriate users in the discharge cohort revealed that the inappropriate users were more likely to be older, have DM, have a more severe stroke at presentation, be receiving inappropriate therapy before the stroke, and be disabled (mRS score=3-5) at discharge (Table 2).
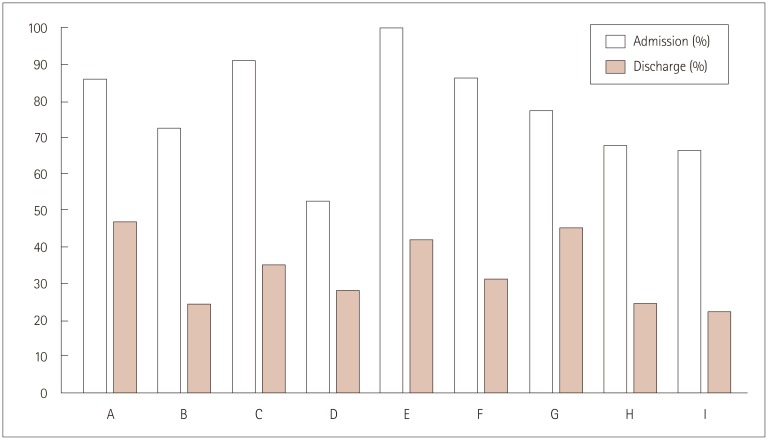
The proportions of inappropriate use differed significantly among the participating centers at stroke onset (p<0.01) and at discharge (p=0.03), ranging from 52.4% to 100.0% in the admission cohort and from 22.2% to 46.9% in the discharge cohort (Fig. 1).
Fig. 1. Inappropriateness of antithrombotic use among participating centers in the admission and discharge cohorts.
Multivariable analyses excluding the hospital variable (models 1 and 3) revealed that age was an independent predictor of inappropriate medications at stroke onset, while age, smoking, inappropriate medication at admission, and discharge mRS score were independent predictors at discharge (Table 3). In the admission cohort, the statistical significance of age in inappropriate medication use disappeared when the analysis was adjusted to include the hospital variable (model 2). However, in the discharge cohort, the statistical significance of all the variables (p<0.05) in model 3 remained significant even after adding the hospital variable to the model (to produce model 4).
Table 3. Multivariable analysis of the inappropriateness of antithrombotic use.
| Admission cohort | Discharge cohort | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| Age, 10 years | 1.29 | (1.02-1.62) | 0.032 | 1.35 | (1.06-1.72) | 0.016 | 1.26 | (1.02-1.55) | 0.029 | 1.31 | (1.06-1.62) | 0.014 |
| CHF | 0.59 | (0.35-1.00) | 0.051 | - | - | - | ||||||
| DM | 1.64 | (0.94-2.85) | 0.082 | - | 1.27 | (0.82-1.98) | 0.291 | - | ||||
| History of stroke/TIA | 0.67 | (0.41-1.10) | 0.114 | - | - | - | ||||||
| Prestroke mRS score | ||||||||||||
| 0 to 2 | Ref. | |||||||||||
| 3 to 5 | 0.50 | (0.22-1.13) | 0.097 | - | - | - | ||||||
| Ever smoked | - | 2.06 | (1.29-3.29) | 0.003 | 2.04 | (1.27-3.28) | 0.003 | |||||
| CHD | - | 1.55 | (0.93-2.61) | 0.096 | - | |||||||
| IU at admission | - | 3.50 | (1.93-6.34) | <0.001 | 3.00 | (1.62-5.56) | 0.001 | |||||
| Poor outcome at discharge (mRS score=3-5) | - | 3.90 | (2.48-6.13) | <0.001 | 3.89 | (2.44-6.19) | <0.001 | |||||
| Hospital | <0.001* | 0.350* | ||||||||||
| A | - | 1.61 | (0.50-5.13) | - | 1.32 | (0.63-2.77) | ||||||
| B | - | 0.72 | (0.27-1.90) | - | 0.55 | (0.26-1.18) | ||||||
| C | - | 3.09 | (1.13-8.42) | - | Ref | |||||||
| D | - | 0.30 | (0.13-0.70) | - | 0.67 | (0.34-1.33) | ||||||
| E | - | 8.21 | (0.41-163.55) | - | 1.06 | (0.36-3.13) | ||||||
| F | - | 1.62 | (0.41-6.35) | - | 0.72 | (0.28-1.87) | ||||||
| G | - | Ref | - | 1.12 | (0.56-2.23) | |||||||
| H | - | 0.54 | (0.18-1.56) | - | 0.62 | (0.26-1.46) | ||||||
| I | - | 0.53 | (0.20-1.36) | - | 0.54 | (0.24-1.22) | ||||||
Model 1: Adjusted for age, CHF, DM, history of stroke/TIA, and mRS score at admission. Model 2: Adjusted for age and hospital. Model 3: Adjusted for age, DM, smoking, history of CHD, IU at admission, and mRS score at discharge. Model 4: Adjusted for age, smoking, IU at admission, mRS score at discharge, and hospital.
*p calculated using the global Wald test for the overall association of the hospital variable with inappropriate therapy use.
CHD: coronary heart disease, CHF: congestive heart failure, DM: diabetes mellitus, IU: inappropriate medication user, mRS: modified Rankin Scale, TIA: transient ischemic attack.
DISCUSSION
This study has shown that stroke prevention therapy might not be optimal in AF patients, not only when patients experience a stroke but also when they are discharged from acutecare hospitals in Korea.
The CHADS2 score was used for stratifying the stroke risk of patients with AF, since it is advocated in several international guidelines.2,3,5 However, clinical practices generally do not appear to follow these guidelines. In a recent single-hospital study from Australia, 71% of patients with AF were aware of the presence of AF prior to the stroke event, and only 31% of them were receiving OAC therapy when they experienced the stroke.18 In a study from Italy, 78% of patients were diagnosed with AF before a stroke event, but only 22% were taking OACs and 37% had received no antithrombotic therapy when they experienced the stroke.19 Another study targeting stroke patients with AF, who are ideal candidates for warfarin, showed that only 40% were on warfarin and 29% were on no antithrombotic medications.13 Our results are consistent with these findings. About 70% of our patients with AF were diagnosed prior to the stroke event, but the rate of optimal treatment was only 25% in these patients with known AF at the time of the stroke event. OAC therapy was prescribed in <30% of patients at medium or high risk as defined by CHADS2 scores. Half of the patients were not receiving any antithrombotic agent at the time of stroke presentation in this study, which is higher than the rates found in previous studies.13,19 This high proportion might be at least partly attributable to many patients with contraindications to antithrombotic therapy for various reasons (e.g., high bleeding risk, allergy, and recent surgery) not being excluded from this study.
At discharge, about 67% of the patients were taking an anticoagulant at an appropriate level in this study. A single-hospital-based study from Taiwan found that only 55% of stroke patients with AF received warfarin at discharge,20 and a hospital-based study from Australia produced similar results.18 Conversely, the rate of OAC therapy at discharge was reported to be over 86% in the Adherence eValuation After Ischemic stroke Longitudinal (AVAIL) registry of North America.21 Direct comparisons of warfarin use between previous studies are limited by the indications and contraindications for warfarin differing among these studies. In the present study, 57 patients (11%) did not receive any antithrombotic agent at discharge and 21% were on antiplatelet agents only. It might be valid to assume that those discharged with no antithrombotic medications were also contraindicated for OAC therapy.
Conversely, 28% of those in the low-risk group at presentation were taking OACs in this study. Current guidelines recommend antiplatelet medications for this group.4,5 Half of those in the low-risk groups in a single hospital-based study from USA,22 a prospective study from Geneva,23 the Euroheart survey,24 and a large Japanese registry study25 received warfarin. It has been claimed that the apparent overprescribing of OACs might be attributable to other indications such as electrical cardioversion (ECV) or radiofrequency ablation (RFA) not being taken into account.23,26 However, the present study focused on the incidence of patients with AF presenting with acute ischemic stroke. The risk of stroke after ECV or RFA is known to be low,27 and the proportion of patients with ECV- or RFA-associated stroke was assumed to be extremely small in the present study. Therefore, most patients in our low-risk group were taking inappropriate OACs at an inadequate intensity when they experienced a stroke. The reasons why they were taking OACs should be further investigated in a future study.
This study showed that the factors associated with inappropriateness of antithrombotic use differed between before and after the stroke event. Inappropriate use at presentation was associated with the hospital variable rather than with patient characteristics. Previous studies have noted that the factors affecting antithrombotic use were advanced age, gender, and stroke history.19,23,28 At discharge, inappropriate use was associated with advanced age, smoking, poor functional status, and inappropriate use at presentation. One Taiwan study found that warfarin use was related to age and stroke history.20 OAC use was associated with OAC use prior to the stroke event and age, and differed between countries in the Stroke and Atrial Fibrillation Ensemble II reanalysis.29 However, region and hospital type were not associated with OAC use in the AVAIL registry.21 The present study revealed a clear interhospital variability of inappropriate use at presentation, which might reflect regional variability (Fig. 1). This interhospital variability decreased at discharge, for which there are several possible explanations. First, inappropriate prescriptions might have been corrected during hospitalization. Second, most of the admitted stroke patients were managed by stroke neurologists during hospitalization in the participating centers of this study, but the physicians who managed these patients prior to the stroke event may not have had up-to-date information on the guidelines for preventing stroke. Third, the treatment before the stroke event might have been influenced more by regional factors (e.g., urban vs. rural area, and socioeconomic status).
Several limitations should be noted when interpreting the results of this study. First, this study may have been affected by the limitations that are common to retrospective studies, such as selection bias. Second, we did not obtain details of the inappropriateness of medications, and so some of the patients in the inappropriate group might have received the correct medication, such as antiplatelet medication instead of OAC in the high-risk group in cases of high bleeding risk, poor functional status, or unavailable INR monitoring. Readers should therefore exercise caution when interpreting the causes of the inappropriateness. Third, the CHA2DS2-VASc is new popular AF scheme that makes it clearer to decide the appropriate antithrombotic agent. However, we did not investigate the appropriateness of treatment according to CHA2DS2-VASc because adequate data were not available. Fourth, all of the guidelines are not recommended equally. According to antithrombotic therapy and the prevention of thrombosis in the 9th edition from American College of Chest Physicians,30 the best medication in low-risk patients does not include antithrombotic therapy. This indicates that using a different guideline in our trial could have produced different results. Lastly, because this study did not focus on the anticoagulation status of the patient, but rather on the nonadherence to guidelines for medication at hospital admission and at discharge, not limiting the study to patients with AF might be a better approach when exploring the real-world status.
Acknowledgements
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI10C2020).
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
Supplementary Materials
The online-only Data Supplement is available with this article at http://dx.doi.org/10.3988/jcn.2016.12.1.34.
Locations of participating centers.
References
- 1.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 2.Singer DE, Albers GW, Dalen JE, Fang MC, Go AS, Halperin JL, et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 Suppl):546S–592S. doi: 10.1378/chest.08-0678. [DOI] [PubMed] [Google Scholar]
- 3.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–e354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 4.Clinical Research Center for Stroke. Clinical Practice Guidelines for Stroke. Revision. Seoul: Clinical Research Center for Stroke; 2013. Available from: http://www.stroke.or.kr/image/CPGStrok(English)20130730.pdf. [Google Scholar]
- 5.Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/ American Stroke Association. Stroke. 2011;42:517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 6.ACTIVE Writing Group of the ACTIVE Investigators. Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903–1912. doi: 10.1016/S0140-6736(06)68845-4. [DOI] [PubMed] [Google Scholar]
- 7.van Walraven C, Hart RG, Singer DE, Laupacis A, Connolly S, Petersen P, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta-analysis. JAMA. 2002;288:2441–2448. doi: 10.1001/jama.288.19.2441. [DOI] [PubMed] [Google Scholar]
- 8.van Walraven C, Hart RG, Connolly S, Austin PC, Mant J, Hobbs FD, et al. Effect of age on stroke prevention therapy in patients with atrial fibrillation: the atrial fibrillation investigators. Stroke. 2009;40:1410–1416. doi: 10.1161/STROKEAHA.108.526988. [DOI] [PubMed] [Google Scholar]
- 9.Kowey PR, Reiffel JA, Myerburg R, Naccarelli GV, Packer DL, Pratt CM, et al. Warfarin and aspirin use in atrial fibrillation among practicing cardiologist (from the AFFECTS Registry) Am J Cardiol. 2010;105:1130–1134. doi: 10.1016/j.amjcard.2009.11.047. [DOI] [PubMed] [Google Scholar]
- 10.Friberg L, Hammar N, Ringh M, Pettersson H, Rosenqvist M. Stroke prophylaxis in atrial fibrillation: who gets it and who does not? Report from the Stockholm Cohort-study on Atrial Fibrillation (SCAF-study) Eur Heart J. 2006;27:1954–1964. doi: 10.1093/eurheartj/ehl146. [DOI] [PubMed] [Google Scholar]
- 11.Monte S, Macchia A, Pellegrini F, Romero M, Lepore V, D'Ettorre A, et al. Antithrombotic treatment is strongly underused despite reducing overall mortality among high-risk elderly patients hospitalized with atrial fibrillation. Eur Heart J. 2006;27:2217–2223. doi: 10.1093/eurheartj/ehl208. [DOI] [PubMed] [Google Scholar]
- 12.Hylek EM, D'Antonio J, Evans-Molina C, Shea C, Henault LE, Regan S. Translating the results of randomized trials into clinical practice: the challenge of warfarin candidacy among hospitalized elderly patients with atrial fibrillation. Stroke. 2006;37:1075–1080. doi: 10.1161/01.STR.0000209239.71702.ce. [DOI] [PubMed] [Google Scholar]
- 13.Gladstone DJ, Bui E, Fang J, Laupacis A, Lindsay MP, Tu JV, et al. Potentially preventable strokes in high-risk patients with atrial fibrillation who are not adequately anticoagulated. Stroke. 2009;40:235–240. doi: 10.1161/STROKEAHA.108.516344. [DOI] [PubMed] [Google Scholar]
- 14.Schwammenthal Y, Bornstein NM, Goldbourt U, Koton S, Schwartz R, Koren-Morag N, et al. Anticoagulation remains underused in prevention of stroke associated with atrial fibrillation: insights from two consecutive national surveys. Int J Cardiol. 2011;152:356–361. doi: 10.1016/j.ijcard.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Kim BJ, Han MK, Park TH, Park SS, Lee KB, Lee BC, et al. Current status of acute stroke management in Korea: a report on a multicenter, comprehensive acute stroke registry. Int J Stroke. 2014;9:514–518. doi: 10.1111/ijs.12199. [DOI] [PubMed] [Google Scholar]
- 16.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 17.Hart RG, Pearce LA. Current status of stroke risk stratification in patients with atrial fibrillation. Stroke. 2009;40:2607–2610. doi: 10.1161/STROKEAHA.109.549428. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad O, Ahmad KE, Dear KB, Harvey I, Hughes A, Lueck CJ. Atrial fibrillation and anticoagulation in a stroke unit population. Intern Med J. 2009;39:752–756. doi: 10.1111/j.1445-5994.2008.01878.x. [DOI] [PubMed] [Google Scholar]
- 19.Gandolfo C, Balestrino M, Burrone A, Del Sette M, Finocchi C. Stroke due to atrial fibrillation and the attitude to prescribing anticoagulant prevention in Italy. A prospective study of a consecutive stroke population admitted to a comprehensive stroke unit. J Neurol. 2008;255:796–802. doi: 10.1007/s00415-008-0615-2. [DOI] [PubMed] [Google Scholar]
- 20.Sun MC, Hsiao PJ. In-hospital case management to increase anticoagulation therapy for stroke patients with atrial fibrillation: a hospital-based registry. J Formos Med Assoc. 2013;112:263–268. doi: 10.1016/j.jfma.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Lopes RD, Shah BR, Olson DM, Zhao X, Pan W, Bushnell CD, et al. Antithrombotic therapy use at discharge and 1 year in patients with atrial fibrillation and acute stroke: results from the AVAIL Registry. Stroke. 2011;42:3477–3483. doi: 10.1161/STROKEAHA.111.625392. [DOI] [PubMed] [Google Scholar]
- 22.Srivastava A, Hudson M, Hamoud I, Cavalcante J, Pai C, Kaatz S. Examining warfarin underutilization rates in patients with atrial fibrillation: detailed chart review essential to capture contraindications to warfarin therapy. Thromb J. 2008;6:6. doi: 10.1186/1477-9560-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meiltz A, Zimmermann M, Urban P, Bloch A Association of Cardiologists of the Canton of Geneva. Atrial fibrillation management by practice cardiologists: a prospective survey on the adherence to guidelines in the real world. Europace. 2008;10:674–680. doi: 10.1093/europace/eun086. [DOI] [PubMed] [Google Scholar]
- 24.Nieuwlaat R, Capucci A, Lip GY, Olsson SB, Prins MH, Nieman FH, et al. Antithrombotic treatment in real-life atrial fibrillation patients: a report from the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2006;27:3018–3026. doi: 10.1093/eurheartj/ehl015. [DOI] [PubMed] [Google Scholar]
- 25.Atarashi H, Inoue H, Okumura K, Yamashita T, Kumagai N, Origasa H J-RHYTHM Registry Investigators. Present status of anticoagulation treatment in Japanese patients with atrial fibrillation: a report from the J-RHYTHM Registry. Circ J. 2011;75:1328–1333. doi: 10.1253/circj.cj-10-1119. [DOI] [PubMed] [Google Scholar]
- 26.Barnes GD, Kaatz S, Winfield J, Gu X, Haymart B, Kline-Rogers E, et al. Warfarin use in atrial fibrillation patients at low risk for stroke: analysis of the Michigan Anticoagulation Quality Improvement Initiative [MAQI (2)] J Thromb Thrombolysis. 2014;37:171–176. doi: 10.1007/s11239-013-0934-8. [DOI] [PubMed] [Google Scholar]
- 27.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123:e269–e367. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 28.Partington SL, Abid S, Teo K, Oczkowski W, O'Donnell MJ. Pre-admission warfarin use in patients with acute ischemic stroke and atrial fibrillation: the appropriate use and barriers to oral anticoagulant therapy. Thromb Res. 2007;120:663–669. doi: 10.1016/j.thromres.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Deplanque D, Leys D, Parnetti L, Schmidt R, Ferro J, de Reuck J, et al. Secondary prevention of stroke in patients with atrial fibrillation: factors influencing the prescription of oral anticoagulation at discharge. Cerebrovasc Dis. 2006;21:372–379. doi: 10.1159/000091546. [DOI] [PubMed] [Google Scholar]
- 30.You JJ, Singer DE, Howard PA, Lane DA, Eckman MH, Fang MC, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e531S–e575S. doi: 10.1378/chest.11-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Locations of participating centers.