Abstract
Background and Purpose
Tumors involving the cerebellopontine angle (CPA) pose a diagnostic challenge due to their diverse manifestations. Head impulse tests (HITs) have been used to evaluate vestibular function, but few studies have explored the head impulse gain of the vestibulo-ocular reflex (VOR) in patients with a vestibular schwannoma. This study tested whether the head impulse gain of the VOR is an indicator of the size of a unilateral CPA tumor.
Methods
Twenty-eight patients (21 women; age=64±12 years, mean±SD) with a unilateral CPA tumor underwent a recording of the HITs using a magnetic search coil technique. Patients were classified into non-compressing (T1-T3) and compressing (T4) groups according to the Hannover classification.
Results
Most (23/28, 82%) of the patients showed abnormal HITs for the semicircular canals on the lesion side. The bilateral abnormality in HITs was more common in the compressing group than the non-compressing group (80% vs. 8%, Pearson's chi-square test: p<0.001). The tumor size was inversely correlated with the head impulse gain of the VOR in either direction.
Conclusions
Bilaterally abnormal HITs indicate that a patient has a large unilateral CPA tumor. The abnormal HITs in the contralesional direction may be explained either by adaptation or by compression and resultant dysfunction of the cerebellar and brainstem structures. The serial evaluation of HITs may provide information on tumor growth, and thereby reduce the number of costly brain scans required when following up patients with CPA tumors.
Keywords: vertigo, vestibulo-ocular reflex, cerebellopontine-angle tumor, head impulse test, flocculus
INTRODUCTION
Head impulse tests (HITs) can evaluate the vestibulo-ocular reflex (VOR) elicited by high-velocity and high-acceleration stimuli.1 The findings of HITs are typically abnormal during head rotation toward the lesion side in the presence of peripheral vestibulopathy,1,2 but are mostly normal in central vestibular disorders.3,4 However, HIT findings may be abnormal bilaterally in unilateral lesions involving the vestibular nucleus or cerebellar flocculus.3,5
Tumors involving the cerebellopontine angle (CPA) pose a diagnostic challenge due to their diverse manifestations.6,7 Various tests have been used to evaluate the audiovestibular function in the presence of a CPA tumor, including vestibular schwannomas.8,9 Even though HITs have been used to evaluate vestibular function in various peripheral and central disorders involving the vestibular system,10,11,12 few studies have explored the head impulse gain of the VOR in patients with a vestibular schwannoma.9,13,14 A previous study found that the VOR gains decreased during ipsilesional HITs, and that there was a correlation between the gain and tumor size in vestibular schwannoma.9 The present study was prompted by a serendipitous observation of bilaterally positive HIT findings in a patient with chronic dizziness and imbalance that were due to a large CPA tumor compressing the cerebellum and brainstem. The tested hypothesis was that large CPA tumors compressing the cerebellum and brainstem can cause abnormal HIT findings in both directions, while small tumors that do not impinge on the cerebellum or brainstem would be associated with abnormal HIT findings (if any) only on the lesion side. Confirmation of this hypothesis would mean that HIT findings may be a useful biologic marker for the size of CPA tumors, and hence also for managing these patients.
METHODS
Subjects
From 2013 to 2015, 33 patients with a unilateral CPA tumor confirmed by brain MRI were initially recruited. After excluding five patients who had received gamma-knife surgery before the evaluation (n=4) or with a previous history of otologic dysfunction (n=1), 28 patients (21 women; age=64±12 years, mean±SD) were finally included in this study. The radiological diagnoses included vestibular schwannoma in 21 patients, meningioma in 6, and metastasis in the remaining patient. Four patients underwent surgical resection of the tumor after the evaluation, which resulted in a pathologic confirmation of vestibular schwannoma.
Bedside neurotologic examination
All of the patients were evaluated for spontaneous and gaze-evoked nystagmus (GEN) with fixation. Spontaneous nystagmus without visual fixation was also observed on a monitor without fixation using video Frenzel goggles (SLMED, Seoul, Korea).15 Bedside HITs were performed manually by rapidly rotating the head by ~20° in the planes of six semicircular canals (SCCs). HIT findings were considered abnormal if a corrective saccade supplemented the inadequate slow phase in the plane of the SCC that was stimulated.1
Recording of HITs
Head and eye movements were also quantified during HITs using a magnetic search coil technique in a 70-cm cubic search coil frame (Skalar, Delft, The Netherlands).16 A scleral annulus ring (CHRONOS VISION, Berlin, Germany) was placed on the subject's left eye after anesthetizing the conjunctiva with 0.5% proparacaine hydrochloride (Alcon, Seoul, Korea). A second coil was fixed at the center of the forehead. The eye and head positions were calibrated using a gimbal that could be rotated independently around the three orthogonal axes. In addition to in vitro calibration using the gimbal system, we also used horizontal and vertical fixation spots (deviating from the center by 10° in all directions) for in vivo eye calibration. The patients were instructed to fixate on a red target placed 1.2 m in front of them. Each head impulse was a passive, unpredictable, low-amplitude, and high-acceleration head rotation in the plane of the horizontal semicircular canal (HC), left anterior semicircular canal (AC), right posterior semicircular canal (PC), right AC, or left PC while the patient sat upright.17 Eye and head position signals were digitized at 200 Hz using an analog-to-digital converter (EZAD, Seoul, Korea) and were displayed on a computer screen to allow the eye motion to be monitored in real time during the tests. Digitized data were analyzed using MATLAB software (version R2011b, The MathWorks, Natick, MA, USA).16
At least seven impulses were delivered in each direction. The VOR gains were measured for individual trials as the ratio of the mean eye velocity divided by the mean head velocity during a 40-ms window centered at the time of peak head acceleration.18,19 Reference data were obtained from 20 healthy subjects (14 women; age=44±12 years, age range=24-66 years) with no history of vestibular or neurologic disorders. We defined abnormal HIT findings when the mean VOR gain was less than the mean minus 2 SDs of the control data (i.e., <0.70 for the HC, <0.70 for the AC, and <0.74 for the PC).
MRI and tumor grading
The CPA tumors were graded according to the Hannover classification20 as follows: T1, purely intrameatal; T2, intra-extrameatal; T3, filling the cerebellopontine cistern or reaching the brainstem; and T4, compressing the brainstem or severely dislodging the brainstem and compressing the fourth ventricle. We also measured the tumor size according to the maximum diameter on T1-weighted axial or coronal images. The grading and measurements of tumor size were conducted by a neurosurgeon who was blinded to the results of the vestibular function tests.
Other neurotologic tests
Patients also underwent video-oculographic recording of eye movements, bithermal caloric tests, audiometry, and recording of the cervical and ocular vestibular-evoked myogenic potentials (VEMPs) and brainstem auditory evoked potentials (BAEP). Detailed descriptions of the protocols for these tests are available elsewhere.8,21,22
Statistical analyses
Data from all patients were recoded in terms of ipsilesional and contralesional responses according to the side of the CPA tumor as determined by MRI. The t-test was used to compare the VOR gain during HITs for each SCC, the tumor size between the compressing and non-compressing groups, and patients with bilaterally abnormal HIT findings and those with unilaterally abnormal or normal HIT findings. The chi-square test was used to compare abnormalities in other neurotologic tests between the groups of bilaterally abnormal HITs and unilaterally abnormal or normal HITs. The correlation between the head impulse VOR gain and tumor size was determined using Pearson correlation. All of the tests were performed using SPSS statistical software (version 20.0, SPSS, Chicago, IL, USA), and a probability value p<0.05 was considered indicative of statistical significance.
Standard protocol approval and patient consents
All of the experiments followed the tenets of the Declaration of Helsinki, and this study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB No. B-1112/141-003). All of the included subjects provided written informed consent.
RESULTS
Clinical features
Most of the patients (24/28, 86%) received the initial evaluation primarily due to dizziness or imbalance, and 15 of them also reported auditory symptoms that included hearing loss (n=7) or tinnitus (n=11). However, 16 of 24 them showed unilateral hearing loss of more than 26 dB. Two of the remaining eight patients showed asymmetric high-frequency hearing loss, one showed asymmetric low-frequency hearing loss, and only five patients had a normal audiogram: four with nonvestibular symptoms including headache and one with hemifacial spasm. The duration of symptoms varied from 2 weeks to 10 years (median=4 months, interquartile range=1-12 months).
Fifteen patients were classified into the compressing group (T4), while 13 were assigned to the non-compressing group (T1-T3). The maximum tumor diameter across all patients ranged from 0.2 cm to 5.4 cm (2.3±1.5 cm; median=2.8 cm, interquartile range=0.8-3.3 cm). The tumor was larger in the compressing group than the non-compressing group (3.6±0.7 cm vs. 0.9±0.7 cm, t-test: p<0.001).
HIT findings
HIT findings were abnormal for at least 1 SCC in 23 (23/28, 82%) patients. All 15 patients in the compressing group showed abnormal HIT findings either only to the lesion side (n=3, 20%) or in both directions (n=12, 80%). In contrast, only 8 (62%) of the 13 patients in the non-compressing group showed abnormal HIT findings to the lesion side only (n=7, 54%) or in both directions (n=1, 8%), while the remaining 5 (38%) patients showed normal HIT findings bilaterally (Table 1).
Table 1. Fingdings in the patients.
| Pt | Age/Sex | Side | Grade | IHC | IAC | IPC | CHC | CAC | CPC | SN | GEN | CP | BAEP | cVEMP | oVEMP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 63/F | L | T4 | 0.26* | 0.39* | 0.55* | 0.58* | 0.57* | 0.54* | - | + | I | I | I | I |
| 2 | 77/M | L | T4 | 0.00* | 0.16* | 0.36* | 0.30* | 0.62* | 0.47* | - | + | I | I | I | I |
| 3 | 76/M | R | T4 | 0.00* | 0.43* | 0.41* | 0.22* | 0.53* | 0.53* | - | + | B | I | ? | ? |
| 4 | 56/F | L | T4 | 0.00* | 0.22* | 0.25* | 0.43* | 0.69* | 0.41* | - | + | I | I | I | I |
| 5 | 74/F | R | T4 | 0.13* | 0.23* | 0.37* | 0.66* | 0.60* | 0.36* | C | + | I | ? | B | B |
| 6 | 77/F | R | T4 | 0.13* | 0.45* | 0.31* | 0.19* | 0.48* | 0.30* | C | + | B | B | B | I |
| 7 | 71/M | R | T4 | 0.00* | 0.25* | 0.10* | 0.52* | 0.54* | 0.19* | C | + | I | ? | ? | ? |
| 8 | 79/M | R | T4 | 0.22* | 0.61* | 0.25* | 0.60* | 0.88 | 0.48 | C | + | ? | I | B | B |
| 9 | 68/F | R | T4 | 0.00* | 0.54* | 0.29* | 0.52* | 0.86 | 0.44* | - | - | I | I | I | I |
| 10 | 60/F | L | T4 | 0.00* | 0.61* | 0.48* | 0.56* | 0.82 | 0.76 | - | + | I | ? | I | I |
| 11 | 71/F | R | T4 | 0.32* | 0.71 | 0.63* | 0.74 | 0.97 | 0.65* | C | + | I | I | I | I |
| 12 | 63/F | R | T4 | 0.41* | 0.70 | 0.47* | 0.78 | 0.91 | 0.69* | - | + | I | I | I | I |
| 13 | 43/F | L | T4 | 0.68* | 0.84 | 0.82 | 0.75 | 0.85 | 0.88 | - | - | N | N | I | N |
| 14 | 46/F | R | T4 | 0.52* | 0.75 | 0.97 | 1.03 | 1.03 | 1.09 | - | - | I | I | I | I |
| 15 | 66/F | R | T4 | 0.61* | 0.80 | 0.94 | 0.81 | 0.89 | 0.86 | - | - | I | I | N | N |
| M (SD) |
0.22 (0.23) |
0.51 (0.22) |
0.48 (0.25) |
0.58 (0.22) |
0.75 (0.17) |
0.58 (0.24) |
|||||||||
| 16 | 75/M | L | T3 | 0.14* | 0.70 | 0.39* | 0.71 | 0.84 | 0.49* | - | - | I | I | I | I |
| 17 | 47/F | L | T1 | 0.39* | 0.55* | 0.63* | 0.74 | 0.83 | 0.83 | - | - | I | I | I | I |
| 18 | 62/F | L | T2 | 0.42* | 0.68* | 0.60* | 0.72 | 0.76 | 0.86 | - | - | ? | ? | ? | ? |
| 19 | 53/M | R | T2 | 0.23* | 0.69* | 0.99 | 0.76 | 0.80 | 0.84 | C | - | I | N | N | I |
| 20 | 59/F | R | T3 | 0.57* | 0.84 | 0.36* | 0.87 | 0.97 | 0.75 | C | - | I | I | I | I |
| 21 | 31/M | L | T1 | 0.69* | 0.79 | 0.57* | 0.77 | 0.77 | 0.78 | C | - | I | N | I | N |
| 22 | 79/F | R | T2 | 0.64* | 0.94 | 0.66* | 0.76 | 0.89 | 0.83 | - | - | N | ? | B | N |
| 23 | 56/F | R | T1 | 0.47* | 0.74 | 0.79 | 0.84 | 0.91 | 0.81 | - | - | I | I | B | I |
| 24 | 71/F | R | T1 | 0.86 | 0.96 | 1.01 | 0.99 | 0.85 | 0.97 | - | - | I | ? | ? | ? |
| 25 | 69/F | L | T1 | 0.93 | 1.04 | 0.92 | 0.88 | 0.94 | 0.93 | - | - | ? | N | N | N |
| 26 | 67/F | R | T1 | 0.88 | 0.97 | 1.06 | 0.86 | 0.99 | 0.95 | - | - | ? | ? | ? | ? |
| 27 | 73/F | R | T2 | 0.86 | 0.84 | 0.78 | 0.83 | 0.89 | 0.84 | - | - | N | ? | N | N |
| 28 | 72/F | L | T2 | 0.71 | 0.85 | 0.77 | 0.85 | 0.88 | 0.87 | - | - | N | N | N | N |
| M (SD) |
0.60 (0.14) |
0.81 (0.22) |
0.73 (0.08) |
0.81 (0.08) |
0.87 (0.07) |
0.83 (0.11) |
*Abnormal values.
B: bilateral, BAEP: brainstem auditory evoked potentials, C: contralesional, CAC: contralesional anterior semicircular canal, CHC: contralesional horizontal semicircular canal, CP: caloric paresis, CPC: contralesional posterior semicircular canal, cVEMP: cervical vestibular evoked myogenic potentials, GEN: gaze-evoked nystagmus, I: ipsilesional, IAC: ipsilesional anterior semicircular canal, IHC: ipsilesional horizontal semicircular canal, IPC: ipsilesional posterior semicircular canal, L: left, N: normal, oVEMP: ocular vestibular evoked myogenic potentials, R: right, SN: spontaneous nystagmus, ?: not done.
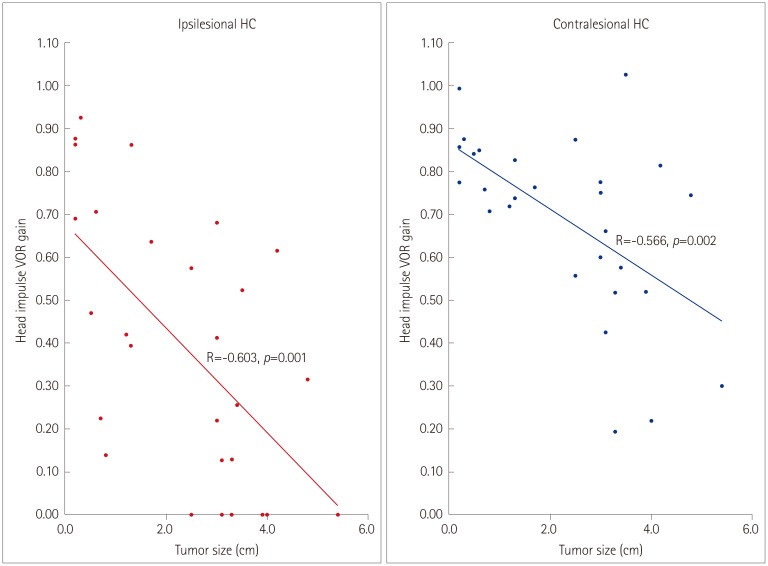
The bilateral abnormality in HIT findings was more common in the compressing group than the non-compressing group (80% vs. 8%, Pearson's chi-square test: p<0.001) (Fig. 1). Moreover, the head impulse VOR gains for all six SCCs were significantly lower in the compressing group than the non-compressing group (t-test: p<0.05) (Fig. 2). The patients with bilaterally abnormal HIT findings showed head impulse VOR gains for all SCCs that were significantly lower than those observed in patients with normal or only ipsilesionally abnormal HIT findings (t-test: p<0.01). The tumor size was inversely correlated with the head impulse VOR gain for all SCCs except the contralesional AC (Fig. 3).
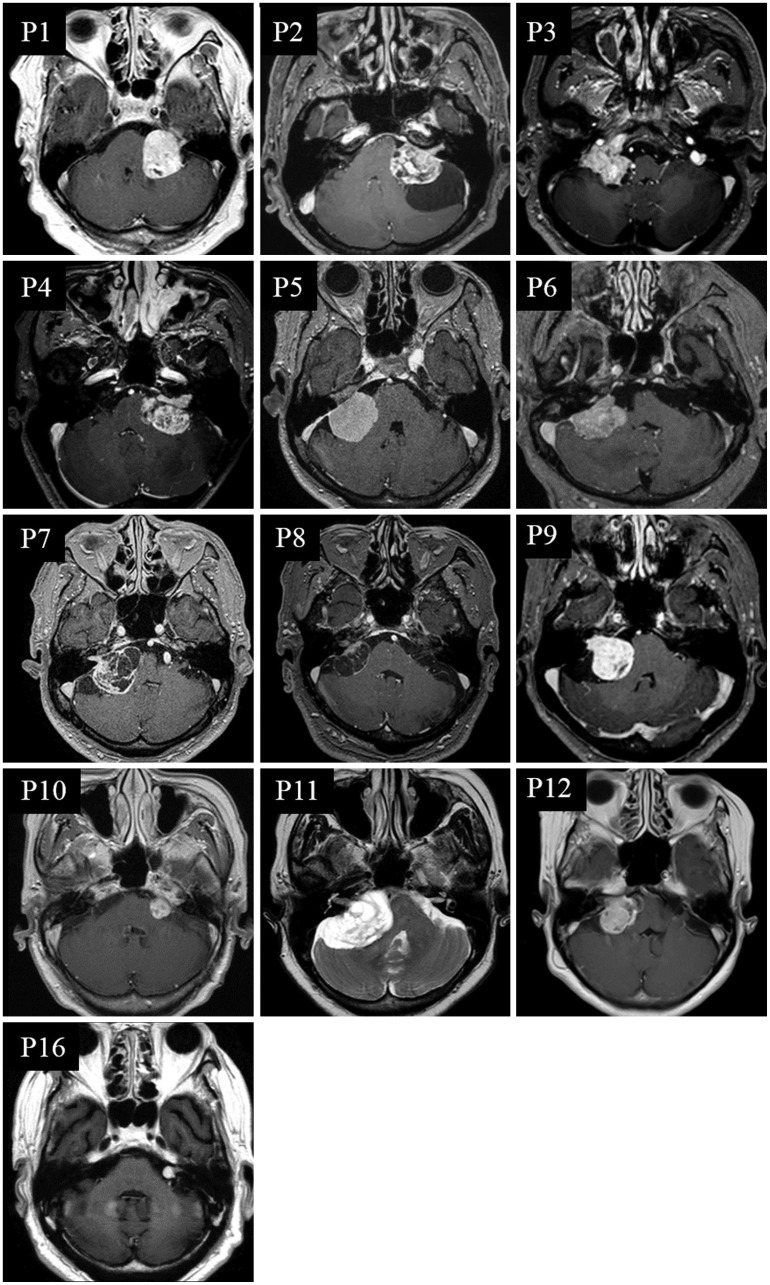
Fig. 1. MRI of patients with bilaterally abnormal head impulse test (HIT) findings. Twelve patients (P1-P12) belong to the compressing group, and only one (P16) is from the non-compressing group.
Fig. 2. Polar plots of the vestibulo-ocular reflex (VOR) gains during head impulses for all six semicircular canals (SCCs) show decreased gains for SCCs except the contralesional anterior SCC in the compressing group. In contrast, only the gains for ipsilesional horizontal and posterior SCCs are reduced in the non-compressing group. The head impulse VOR gains for all six SCCs are significantly lower in the compressing group than the non-compressing group (t-test: p<0.05). CA: contralesional anterior SCC, CH: contralesional horizontal SCC, CP: contralesional posterior SCC, IA: ipsilesional anterior SCC, IH: ipsilesional horizontal SCC, IP: ipsilesional posterior SCC.
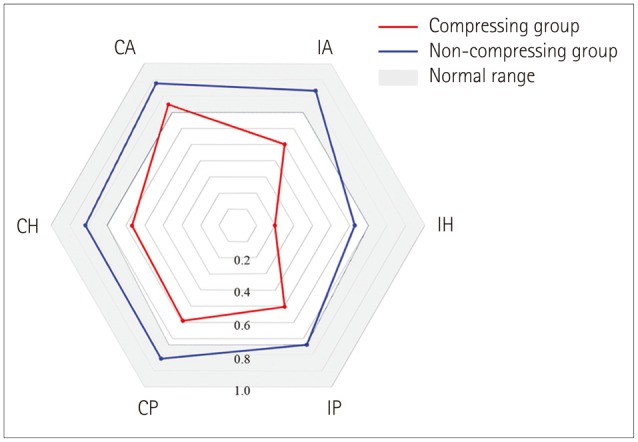
Fig. 3. Correlation between tumor size and gain of the vestibulo-oclar reflex (VOR) during Head impulse tests. HC: horizontal semicircular canal.
Correlation between the abnormality in HITs and other neurotologic findings
Other neurotologic findings included contralesional spontaneous nystagmus (8/28, 29%), GEN (11/28, 39%), caloric paresis (20/24, 83%), abnormal BAEP (14/20, 70%), abnormal cervical VEMPs (18/23, 78%), and abnormal ocular VEMPs (16/23, 70%) (Table 1).
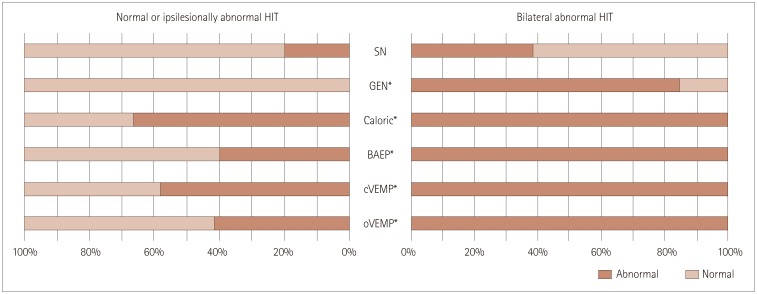
Patients with bilaterally abnormal HIT findings showed GEN (85% vs. 0%, Pearson's chi-square test: p<0.001), caloric paresis (100% vs. 67%, Pearson's chi-square test: p=0.032), and abnormalities of BAEP (100% vs. 40%, Fisher's exact test: p=0.029), cervical VEMPs (100% vs. 58%, Fisher's exact test: p=0.037), and ocular VEMPs (100% vs. 42%, Fisher's exact test: p=0.005) more frequently than those with normal or only ipsilesionally abnormal HIT findings (Fig. 4).
Fig. 4. Comparison of other neurotologic findings. Patients with bilaterally abnormal Head impulse tests (HIT) findings show gaze-evoked nystagmus (GEN), caloric paresis, and abnormalities of brainstem auditory evoked potentials (BAEP), cervical vestibular evoked myogenic potentials (cVEMPs), and ocular vestibular evoked myogenic potentials (oVEMPs) more frequently than those with normal or only ipsilesionally abnormal HIT findings (chi-square, *p<0.05). SN: spontaneous nystagmus.
Description of the index case (patient 1)
A 62-year-old woman presented with dizziness and unsteadiness that had first appeared 1 year previously. Her unsteadiness worsened when walking up or down the stairs. She had suffered from severe vertigo and left-side hearing loss 8 months previously, and had a diagnosis of labyrinthitis from another hospital.
An examination revealed small right-beating spontaneous nystagmus with a clockwise torsional component both with and without fixation (Supplementary Video 1 in the online-only Data Supplement). GEN was evident in both lateral gazes, with rebound nystagmus upon resuming the straight-ahead gaze (Supplementary Video 1 in the online-only Data Supplement). The spontaneous right-beating nystagmus increased after horizontal head-shaking, when applying vibration stimuli to either mastoid process or the forehead, and after hyperventilation. Saccades were hypermetric to the left. Horizontal and vertical smooth pursuits were impaired in both directions. Bedside HIT findings were positive in both horizontal directions, but more so to the left (Supplementary Video 2 in the online-only Data Supplement).
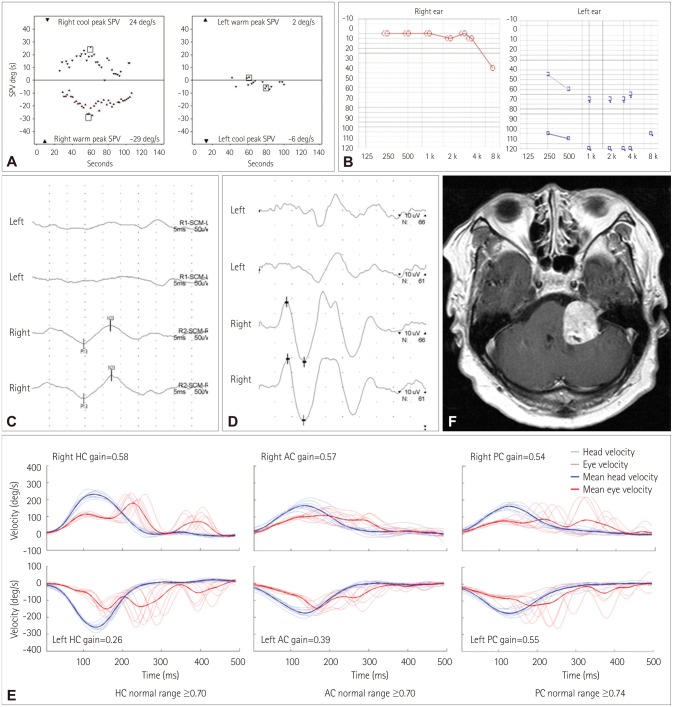
The patient also showed a left-side caloric paresis of 74% (Fig. 5A) and complete hearing loss in the left ear (Fig. 5B). No BAEP, cervical VEMP, or ocular VEMP could be detected when stimulating the left ear (Fig. 5C and D). Recording of HITs using a magnetic search coil system documented that the VOR gain was decreased for all six SCCs (Fig. 5E). MRI disclosed a large tumor (3.4×3.2 cm) in the left CPA compressing the cerebellum and brainstem (Fig. 5F), which was confirmed as a schwannoma after resection.
Fig. 5. Neurotologic findings in the index case (patient 1). A: Bithermal caloric tests show a left-side caloric paresis of 74%. B: Pure-tone audiometry shows complete hearing loss on the left side. No cervical (C) or ocular (D) vestibular-evoked myogenic potentials can be evoked when stimulating the left ear. MRI reveals a large tumor (3.4×3.2 cm) involving the left cerebellopontine angle (E). F: Performing HITs using a magnetic search coil technique show decreased gains for all six semicircular canals. AC: anterior semicircular canal, HC: horizontal semicircular canal, PC: right posterior semicircular canal.
DISCUSSION
The patients in this study with audiovestibulopathy due to unilateral CPA tumors frequently showed abnormal HITs in both directions, especially when the tumors were large enough to compress the cerebellum and brainstem. Furthermore, the head impulse VOR gain was inversely correlated with the tumor size.
Large CPA tumors compressing the cerebellum mostly manifest as chronic unilateral audiovestibulopathy and cerebellar dysfunction including GEN, dysmetria, and imbalance. Our patients showed typical findings of unilateral audiovestibulopathy, which comprised hearing loss, caloric paresis, abnormal head impulse, and absent BAEP, cervical VEMPs, and ocular VEMPs. However, abnormal HITs to the contralesional side were an unexpected finding.
The abnormal HITs to the contralesional side in our patients with a unilateral CPA tumor may be explained by compression of the cerebellum or the brainstem by the tumor and resultant dysfunction of the cerebellar flocculus or vestibular nucleus. Indeed, recent studies have documented decreased head impulse VOR gains in both directions in patients with a unilateral lesion restricted to the flocculus or the vestibular nucleus.23,24 The flocculus is involved in the modulation of the VOR. A previously reported patient with an isolated unilateral floccular infarction showed increased horizontal VOR gains during a low-frequency rotatory chair test, but bilaterally decreased VOR gains during horizontal HITs.24 Thus, the flocculus appears to suppress the horizontal VOR during low-frequency stimulation while enhancing it during high-frequency stimulation. This differential modulation of the VOR according to stimulation frequency may contribute to extending the upper range of velocities and accelerations of the head to which the VOR can accurately respond, despite the presence of inhibitory saturation due to Ewald's second law.25 Indeed, bilateral flocculectomy in monkeys increased the VOR gain for low-frequency stimulation, but decreased the gain during high-frequency stimulation.26,27 Since the floccular projections to the vestibular nuclei are known to be ipsilateral,28 the abnormal HIT findings in the contralesional direction may be explained by reciprocal interneuron connections between the vestibular nuclei,29 connections via the inhibitory and excitatory floccular target neurons in the ipsilesional vestibular nucleus,30 or an adaptive action of the contralateral flocculus.
Previously reported patients with isolated vestibular nuclear infarction also showed reduced VOR gains during HITs in both directions, but these reductions were greater for the lesion side.23,24 The decreased head impulse VOR gains for the contralesional SCCs in such patients may be an adaptive mechanism, probably involving the inhibitory interneurons within the vestibular nuclei.23
The symptoms and signs of both the cerebellar dysfunction and bilateral vestibulopathy observed in our index case (patient 1) were similar to those observed in patients with the cerebellar ataxia with bilateral vestibulopathy (CABA) syndrome31 or cerebellar ataxia, neuropathy, and vestibular areflexia syndrome (CANVAS).32 However, the markedly asymmetrical nature of the audiovestibular dysfunction documented in laboratory tests contrast with those that are typically observed in CABA syndrome or CANVAS.
Since the medial vestibular nucleus and flocculus constitute the neural integrator for horizontal eye motion, dysfunction of these structures would give rise to GEN. Especially, Bruns' nystagmus refers to the combination of vestibular nystagmus with a constant slow phase velocity during contralateral gaze and GEN with a decreasing slow-phase velocity during ipsilateral gaze, and this has been considered a pathognomonic sign of a large CPA tumor compressing the cerebellum.33,34 Indeed, 39% of our patients showed GEN or Bruns' nystagmus, but only in those with bilaterally abnormal HIT findings. This also supports dysfunction of the flocculus or the vestibular nucleus as a mechanism underlying the abnormal HIT findings in the contralesional direction in our patients with large CPA tumors. Thus, along with GEN or Bruns' nystagmus, bilaterally abnormal HIT findings should be considered a sign of a large CPA tumor compressing the cerebellum and brainstem.
The increasing rate of detecting small vestibular schwannomas and the improving understanding of the natural course of this tumor has resulted in conservative treatment being the main strategy for managing small-to-medium-sized vestibular schwannomas.35 This conservative management strategy necessitates the close monitoring of symptoms and tumor growth.36 The determination of tumor growth has mostly depended on brain imaging, since the tests that were previously adopted for evaluating audiovestibular function were found to be of limited use for monitoring the growth of a vestibular schwannoma.37,38 In contrast, the head impulse VOR gains, both the ipsilesional and contralesional ones, were strongly correlated with the size of CPA tumors in the present study. Thus, HITs may play an important role in predicting tumor growth and reducing the number of costly brain scans when following up patients with CPA tumors. Serial studies of the correlation between the head impulse VOR gain and tumor growth in individual patients will further elucidate the role of HITs in monitoring CPA tumors. We used a magnetic search coil technique to perform the HITs in this study, but employing a video-based system would enhance the applicability of HITs without reducing the diagnostic accuracy given the recent developments in video-based equipment.39
In conclusion, patients with a unilateral CPA tumor may show abnormal HIT findings on both the contralesional and ipsilesional sides, and bilaterally abnormal HIT findings indicate the presence of a large unilateral CPA tumor compressing the cerebellum and brainstem. Abnormal HIT findings on the contralesional side may be explained by dysfunction of the flocculus or the vestibular nucleus due to compression by the tumor. HIT findings may be a useful biologic marker for the size of unilateral CPA tumors, and hence also for following up patients with these tumors.
Acknowledgements
This study was supported by a grant of Korea Medical Devices Industrial Cooperative Association and Small & Medium Business Administration (08-2011-065).
Footnotes
Conflicts of Interest: Dr. J-S Kim serves as an Associate Editor of Frontiers in Neuro-otology and on the editorial boards of the Journal of Clinical Neurology, Frontiers in Neuro-ophthalmology, Journal of Neuro-ophthalmology, and Journal of Vestibular Research, and received research support from SK Chemicals, Co. Ltd. The other authors have nothing to disclose.
Supplementary Materials
The online-only Data Supplement is available with this article at http://dx.doi.org/10.3988/jcn.2016.12.1.65.
Patient showing spontaneous right-beating nystagmus without fixation, and gaze-evoked nystagmus during lateral gazes.
Bedside head impulse test findings were positive in both horizontal directions, but more so to the left.
References
- 1.Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45:737–739. doi: 10.1001/archneur.1988.00520310043015. [DOI] [PubMed] [Google Scholar]
- 2.Jeong SH, Kim HJ, Kim JS. Vestibular neuritis. Semin Neurol. 2013;33:185–194. doi: 10.1055/s-0033-1354598. [DOI] [PubMed] [Google Scholar]
- 3.Kattah JC, Talkad AV, Wang DZ, Hsieh YH, Newman-Toker DE. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. 2009;40:3504–3510. doi: 10.1161/STROKEAHA.109.551234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee H. Isolated vascular vertigo. J Stroke. 2014;16:124–130. doi: 10.5853/jos.2014.16.3.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baek SH, Choi JY, Jung JM, Kwon do Y, Park MH, Choi J, et al. Abnormal head impulse test in a unilateral cerebellar lesion. J Clin Neurol. 2015;11:279–282. doi: 10.3988/jcn.2015.11.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hitselberger WE. Tumors of the cerebellopontine angle in relation to vertigo. Arch Otolaryngol. 1967;85:539–541. doi: 10.1001/archotol.1967.00760040541013. [DOI] [PubMed] [Google Scholar]
- 7.Lin C, Gong Q, Zuo W, Zhang R, Zhou A. The clinical characteristics and treatment for sudden sensorineural hearing loss with vestibular schwannoma. Eur Arch Otorhinolaryngol. 2015;272:839–842. doi: 10.1007/s00405-014-2885-x. [DOI] [PubMed] [Google Scholar]
- 8.Choi KD, Kim JS, Kim HJ, Koo JW, Kim JH, Kim CY, et al. Hyperventilation-induced nystagmus in peripheral vestibulopathy and cerebellopontine angle tumor. Neurology. 2007;69:1050–1059. doi: 10.1212/01.wnl.0000271378.54381.6a. [DOI] [PubMed] [Google Scholar]
- 9.Taylor RL, Kong J, Flanagan S, Pogson J, Croxson G, Pohl D, et al. Prevalence of vestibular dysfunction in patients with vestibular schwannoma using video head-impulses and vestibular-evoked potentials. J Neurol. 2015;262:1228–1237. doi: 10.1007/s00415-015-7697-4. [DOI] [PubMed] [Google Scholar]
- 10.Newman-Toker DE, Kattah JC, Alvernia JE, Wang DZ. Normal head impulse test differentiates acute cerebellar strokes from vestibular neuritis. Neurology. 2008;70(24 Pt 2):2378–2385. doi: 10.1212/01.wnl.0000314685.01433.0d. [DOI] [PubMed] [Google Scholar]
- 11.Newman-Toker DE, Saber Tehrani AS, Mantokoudis G, Pula JH, Guede CI, Kerber KA, et al. Quantitative video-oculography to help diagnose stroke in acute vertigo and dizziness: toward an ECG for the eyes. Stroke. 2013;44:1158–1161. doi: 10.1161/STROKEAHA.111.000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mangabeira Albernaz PL, Zuma E. The video head impulse test. Acta Otolaryngol. 2014;134:1245–1250. doi: 10.3109/00016489.2014.942439. [DOI] [PubMed] [Google Scholar]
- 13.Hirvonen M, Aalto H, Petteri Hirvonen T. Motorized head impulse rotator in patients with vestibular schwannoma. Acta Otolaryngol. 2008;128:1215–1220. doi: 10.1080/00016480801908027. [DOI] [PubMed] [Google Scholar]
- 14.Blödow A, Blödow J, Bloching MB, Helbig R, Walther LE. Horizontal VOR function shows frequency dynamics in vestibular schwannoma. Eur Arch Otorhinolaryngol. 2015;272:2143–2148. doi: 10.1007/s00405-014-3042-2. [DOI] [PubMed] [Google Scholar]
- 15.Huh YE, Kim JS. Bedside evaluation of dizzy patients. J Clin Neurol. 2013;9:203–213. doi: 10.3988/jcn.2013.9.4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi KD, Oh SY, Kim HJ, Kim JS. The vestibulo-ocular reflexes during head impulse in Wernicke's encephalopathy. J Neurol Neurosurg Psychiatry. 2007;78:1161–1162. doi: 10.1136/jnnp.2007.121061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halmagyi GM, Aw ST, Cremer PD, Curthoys IS, Todd MJ. Impulsive testing of individual semicircular canal function. Ann N Y Acad Sci. 2001;942:192–200. doi: 10.1111/j.1749-6632.2001.tb03745.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Todd M, Halmagyi GM, Aw S. Head impulse gain and saccade analysis in pontine-cerebellar stroke and vestibular neuritis. Neurology. 2014;83:1513–1522. doi: 10.1212/WNL.0000000000000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehnen N, Aw ST, Todd MJ, Halmagyi GM. Head impulse test reveals residual semicircular canal function after vestibular neurectomy. Neurology. 2004;62:2294–2296. doi: 10.1212/wnl.62.12.2294. [DOI] [PubMed] [Google Scholar]
- 20.Samii M, Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): hearing function in 1000 tumor resections. Neurosurgery. 1997;40:248–260. doi: 10.1097/00006123-199702000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Kim HJ, Lee JH, Kim JS. Ocular vestibular evoked myogenic potentials to head tap and cervical vestibular evoked myogenic potentials to air-conducted sounds in isolated internuclear ophthalmoplegia. Clin Neurophysiol. 2014;125:1042–1047. doi: 10.1016/j.clinph.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Choi KD, Oh SY, Kim HJ, Koo JW, Cho BM, Kim JS. Recovery of vestibular imbalances after vestibular neuritis. Laryngoscope. 2007;117:1307–1312. doi: 10.1097/MLG.0b013e31805c08ac. [DOI] [PubMed] [Google Scholar]
- 23.Kim HJ, Lee SH, Park JH, Choi JY, Kim JS. Isolated vestibular nuclear infarction: report of two cases and review of the literature. J Neurol. 2014;261:121–129. doi: 10.1007/s00415-013-7139-0. [DOI] [PubMed] [Google Scholar]
- 24.Park HK, Kim JS, Strupp M, Zee DS. Isolated floccular infarction: impaired vestibular responses to horizontal head impulse. J Neurol. 2013;260:1576–1582. doi: 10.1007/s00415-013-6837-y. [DOI] [PubMed] [Google Scholar]
- 25.Baloh RW, Honrubia V, Konrad HR. Ewald's second law re-evaluated. Acta Otolaryngol. 1977;83:475–479. doi: 10.3109/00016487709128874. [DOI] [PubMed] [Google Scholar]
- 26.Zee DS, Yamazaki A, Butler PH, Gücer G. Effects of ablation of flocculus and paraflocculus of eye movements in primate. J Neurophysiol. 1981;46:878–899. doi: 10.1152/jn.1981.46.4.878. [DOI] [PubMed] [Google Scholar]
- 27.Lisberger SG, Miles FA, Zee DS. Signals used to compute errors in monkey vestibuloocular reflex: possible role of flocculus. J Neurophysiol. 1984;52:1140–1153. doi: 10.1152/jn.1984.52.6.1140. [DOI] [PubMed] [Google Scholar]
- 28.Büttner-Ennever JA. Patterns of connectivity in the vestibular nuclei. Ann N Y Acad Sci. 1992;656:363–378. doi: 10.1111/j.1749-6632.1992.tb25222.x. [DOI] [PubMed] [Google Scholar]
- 29.Galiana HL, Outerbridge JS. A bilateral model for central neural pathways in vestibuloocular reflex. J Neurophysiol. 1984;51:210–241. doi: 10.1152/jn.1984.51.2.210. [DOI] [PubMed] [Google Scholar]
- 30.Shin M, Moghadam SH, Sekirnjak C, Bagnall MW, Kolkman KE, Jacobs R, et al. Multiple types of cerebellar target neurons and their circuitry in the vestibulo-ocular reflex. J Neurosci. 2011;31:10776–10786. doi: 10.1523/JNEUROSCI.0768-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bronstein AM, Mossman S, Luxon LM. The neck-eye reflex in patients with reduced vestibular and optokinetic function. Brain. 1991;114(Pt 1A):1–11. [PubMed] [Google Scholar]
- 32.Migliaccio AA, Halmagyi GM, McGarvie LA, Cremer PD. Cerebellar ataxia with bilateral vestibulopathy: description of a syndrome and its characteristic clinical sign. Brain. 2004;127(Pt 2):280–293. doi: 10.1093/brain/awh030. [DOI] [PubMed] [Google Scholar]
- 33.Croxson GR, Moffat DA, Baguley D. Bruns bidirectional nystagmus in cerebellopontine angle tumours. Clin Otolaryngol Allied Sci. 1988;13:153–157. doi: 10.1111/j.1365-2273.1988.tb00756.x. [DOI] [PubMed] [Google Scholar]
- 34.Lloyd SK, Baguley DM, Butler K, Donnelly N, Moffat DA. Bruns' nystagmus in patients with vestibular schwannoma. Otol Neurotol. 2009;30:625–628. doi: 10.1097/MAO.0b013e3181a32bec. [DOI] [PubMed] [Google Scholar]
- 35.Gait C, Frew EJ, Martin TP, Jowett S, Irving R. Conservative management, surgery and radiosurgery for treatment of vestibular schwannomas: a model-based approach to cost-effectiveness. Clin Otolaryngol. 2014;39:22–31. doi: 10.1111/coa.12205. [DOI] [PubMed] [Google Scholar]
- 36.Jethanamest D, Rivera AM, Ji H, Chokkalingam V, Telischi FF, Angeli SI. Conservative management of vestibular schwannoma: predictors of growth and hearing. Laryngoscope. 2015;125:2163–2168. doi: 10.1002/lary.25159. [DOI] [PubMed] [Google Scholar]
- 37.Tutar H, Duzlu M, Göksu N, Ustün S, Bayazit Y. Audiological correlates of tumor parameters in acoustic neuromas. Eur Arch Otorhinolaryngol. 2013;270:437–441. doi: 10.1007/s00405-012-1954-2. [DOI] [PubMed] [Google Scholar]
- 38.van Leeuwen JP, Cremers CW, Thewissen NP, Harhangi BS, Meijer E. Acoustic neuroma: correlation among tumor size, symptoms, and patient age. Laryngoscope. 1995;105(7 Pt 1):701–707. doi: 10.1288/00005537-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 39.MacDougall HG, Weber KP, McGarvie LA, Halmagyi GM, Curthoys IS. The video head impulse test: diagnostic accuracy in peripheral vestibulopathy. Neurology. 2009;73:1134–1141. doi: 10.1212/WNL.0b013e3181bacf85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient showing spontaneous right-beating nystagmus without fixation, and gaze-evoked nystagmus during lateral gazes.
Bedside head impulse test findings were positive in both horizontal directions, but more so to the left.