Abstract
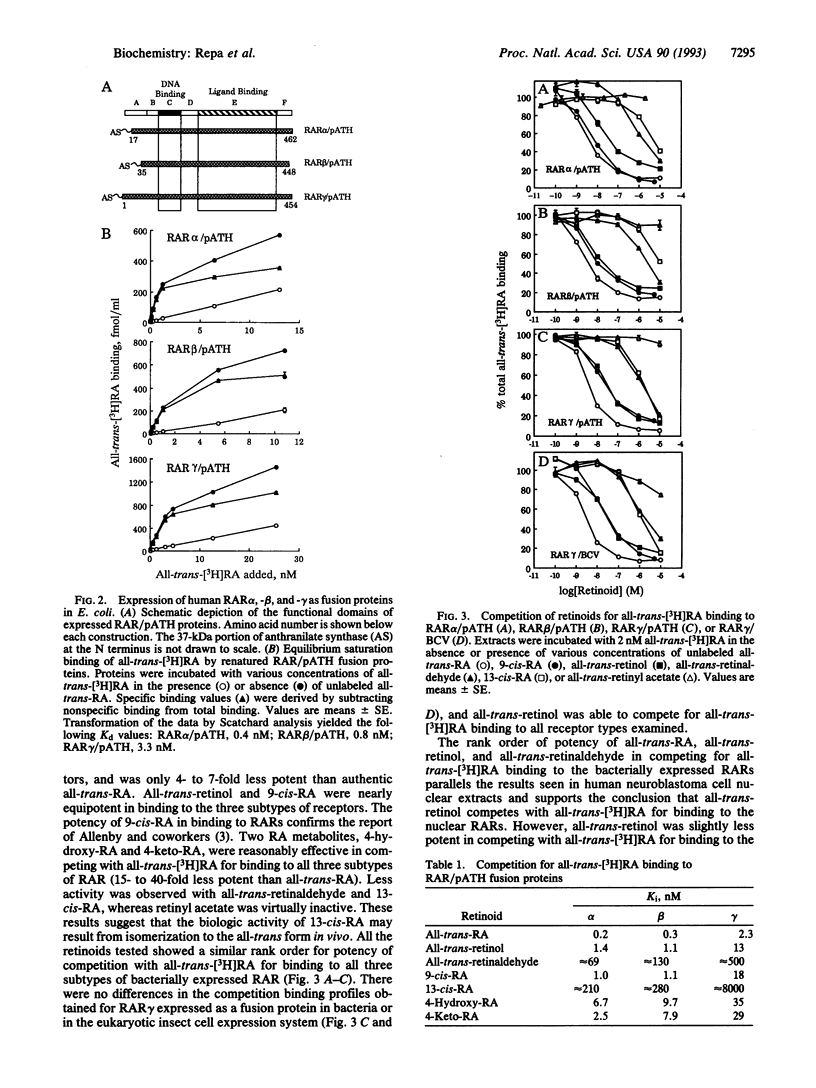
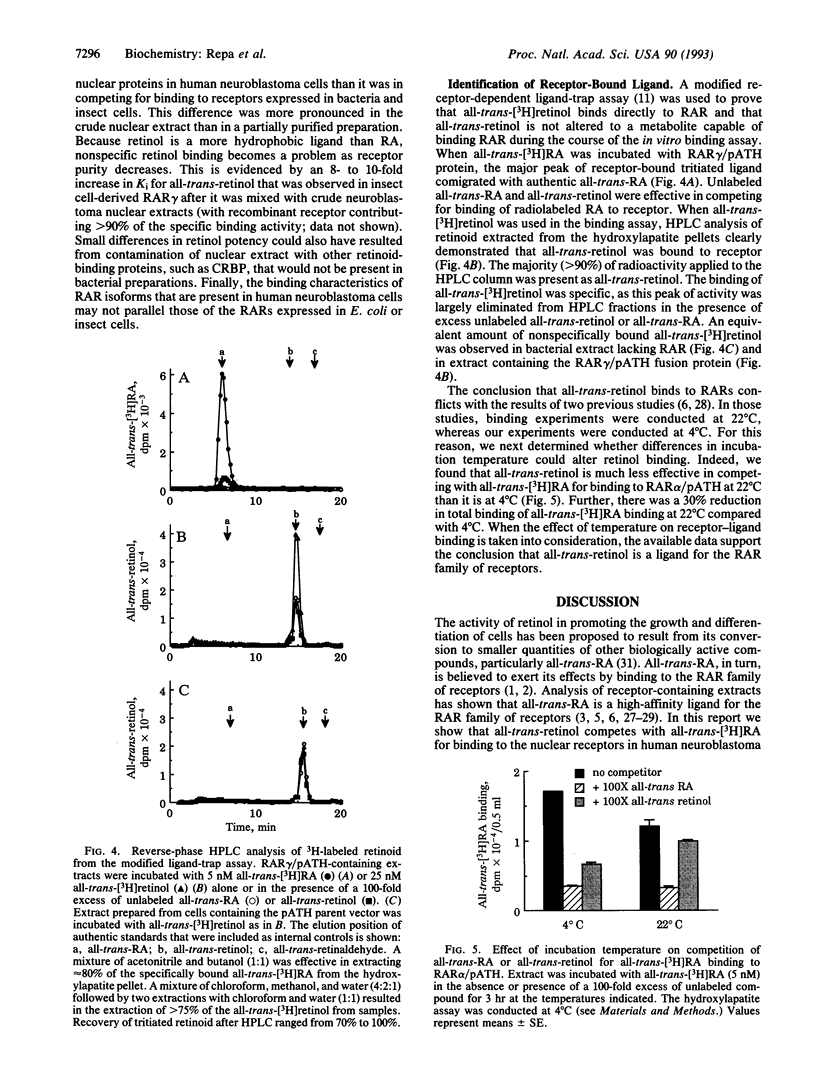
Competition of all-trans-retinol and all-trans-retinaldehyde with 3H-labeled all-trans-retinoic acid (RA) for binding to retinoic acid receptors (RARs) was examined in human neuroblastoma cell nuclear extracts. All-trans-retinol was 35-fold less potent than all-trans-RA, whereas all-trans-retinaldehyde was 500-fold less active in binding to the nuclear receptors. To confirm that all-trans-retinol binds to RARs, experiments were carried out with RARs alpha, beta, and gamma expressed as bacterial fusion proteins. All-trans-retinol was only 4- to 7-fold less potent than all-trans-RA in binding to all three RAR subtypes. The all-trans-retinol binding observed was not the result of metabolism of retinol to RA or some other active compound during the binding experiment. Retinyl acetate was virtually inactive in competition binding experiments, while very slight activity was observed with 13-cis-RA and all-trans-retinaldehyde. Significant competition occurred with 4-hydroxy-RA and 4-keto-RA, which were 15- to 40-fold less potent than all-trans-RA. The 9-cis isomer of RA was equipotent with all-trans-retinol in these studies. These results suggest that all-trans-retinol cannot be excluded as a physiologically significant ligand for RAR-mediated gene expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allenby G., Bocquel M. T., Saunders M., Kazmer S., Speck J., Rosenberger M., Lovey A., Kastner P., Grippo J. F., Chambon P. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):30–34. doi: 10.1073/pnas.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrook D., Lernhardt E., Pfahl M. A new retinoic acid receptor identified from a hepatocellular carcinoma. Nature. 1988 Jun 16;333(6174):669–672. doi: 10.1038/333669a0. [DOI] [PubMed] [Google Scholar]
- Boylan J. F., Gudas L. J. Overexpression of the cellular retinoic acid binding protein-I (CRABP-I) results in a reduction in differentiation-specific gene expression in F9 teratocarcinoma cells. J Cell Biol. 1991 Mar;112(5):965–979. doi: 10.1083/jcb.112.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan J. F., Gudas L. J. The level of CRABP-I expression influences the amounts and types of all-trans-retinoic acid metabolites in F9 teratocarcinoma stem cells. J Biol Chem. 1992 Oct 25;267(30):21486–21491. [PubMed] [Google Scholar]
- Cavey M. T., Martin B., Carlavan I., Shroot B. In vitro binding of retinoids to the nuclear retinoic acid receptor alpha. Anal Biochem. 1990 Apr;186(1):19–23. doi: 10.1016/0003-2697(90)90565-q. [DOI] [PubMed] [Google Scholar]
- Clagett-Dame M., Verhalen T. J., Biedler J. L., Repa J. J. Identification and characterization of all-trans-retinoic acid receptor transcripts and receptor protein in human neuroblastoma cells. Arch Biochem Biophys. 1993 Feb 1;300(2):684–693. doi: 10.1006/abbi.1993.1095. [DOI] [PubMed] [Google Scholar]
- Crettaz M., Baron A., Siegenthaler G., Hunziker W. Ligand specificities of recombinant retinoic acid receptors RAR alpha and RAR beta. Biochem J. 1990 Dec 1;272(2):391–397. doi: 10.1042/bj2720391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame M. C., Pierce E. A., DeLuca H. F. Identification of the porcine intestinal 1,25-dihydroxyvitamin D3 receptor on sodium dodecyl sulfate/polyacrylamide gels by renaturation and immunoblotting. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7825–7829. doi: 10.1073/pnas.82.23.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerick R. J., Zile M., DeLuca H. F. Formation of retinoic acid from retinol in the rat. Biochem J. 1967 Feb;102(2):606–611. doi: 10.1042/bj1020606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorella P. D., Napoli J. L. Expression of cellular retinoic acid binding protein (CRABP) in Escherichia coli. Characterization and evidence that holo-CRABP is a substrate in retinoic acid metabolism. J Biol Chem. 1991 Sep 5;266(25):16572–16579. [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Kagechika H., Shudo K. Expression of retinoic acid receptor genes and the ligand-binding selectivity of retinoic acid receptors (RAR's). Biochem Biophys Res Commun. 1990 Feb 14;166(3):1300–1307. doi: 10.1016/0006-291x(90)91007-f. [DOI] [PubMed] [Google Scholar]
- Heyman R. A., Mangelsdorf D. J., Dyck J. A., Stein R. B., Eichele G., Evans R. M., Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992 Jan 24;68(2):397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- Keidel S., Rupp E., Szardenings M. Recombinant human retinoic acid receptor alpha. Binding of DNA and synthetic retinoids to the protein expressed in Escherichia coli. Eur J Biochem. 1992 Mar 15;204(3):1141–1148. doi: 10.1111/j.1432-1033.1992.tb16739.x. [DOI] [PubMed] [Google Scholar]
- Koerner T. J., Hill J. E., Myers A. M., Tzagoloff A. High-expression vectors with multiple cloning sites for construction of trpE fusion genes: pATH vectors. Methods Enzymol. 1991;194:477–490. doi: 10.1016/0076-6879(91)94036-c. [DOI] [PubMed] [Google Scholar]
- Krust A., Kastner P., Petkovich M., Zelent A., Chambon P. A third human retinoic acid receptor, hRAR-gamma. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5310–5314. doi: 10.1073/pnas.86.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin A. A., Sturzenbecker L. J., Kazmer S., Bosakowski T., Huselton C., Allenby G., Speck J., Kratzeisen C., Rosenberger M., Lovey A. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature. 1992 Jan 23;355(6358):359–361. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- Martin B., Bernardon J. M., Cavey M. T., Bernard B., Carlavan I., Charpentier B., Pilgrim W. R., Shroot B., Reichert U. Selective synthetic ligands for human nuclear retinoic acid receptors. Skin Pharmacol. 1992;5(1):57–65. doi: 10.1159/000211018. [DOI] [PubMed] [Google Scholar]
- McCormick A. M., Napoli J. L., DeLuca H. F. High-pressure liquid chromatography of vitamin A metabolites and analogs. Methods Enzymol. 1980;67:220–233. doi: 10.1016/s0076-6879(80)67030-x. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Napoli J. L., Posch K. C., Burns R. D. Microsomal retinal synthesis: retinol vs. holo-CRBP as substrate and evaluation of NADP, NAD and NADPH as cofactors. Biochim Biophys Acta. 1992 Apr 8;1120(2):183–186. doi: 10.1016/0167-4838(92)90267-h. [DOI] [PubMed] [Google Scholar]
- Nau H. Correlation of transplacental and maternal pharmacokinetics of retinoids during organogenesis with teratogenicity. Methods Enzymol. 1990;190:437–448. doi: 10.1016/0076-6879(90)90050-b. [DOI] [PubMed] [Google Scholar]
- Petkovich M. Regulation of gene expression by vitamin A: the role of nuclear retinoic acid receptors. Annu Rev Nutr. 1992;12:443–471. doi: 10.1146/annurev.nu.12.070192.002303. [DOI] [PubMed] [Google Scholar]
- Reddy A. P., Chen J. Y., Zacharewski T., Gronemeyer H., Voorhees J. J., Fisher G. J. Characterization and purification of human retinoic acid receptor-gamma 1 overexpressed in the baculovirus-insect cell system. Biochem J. 1992 Nov 1;287(Pt 3):833–840. doi: 10.1042/bj2870833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig W. J., Spengler B. A., Chesa P. G., Old L. J., Biedler J. L. Coordinate changes in neuronal phenotype and surface antigen expression in human neuroblastoma cell variants. Cancer Res. 1987 Mar 1;47(5):1383–1389. [PubMed] [Google Scholar]
- Ross A. C. Cellular metabolism and activation of retinoids: roles of cellular retinoid-binding proteins. FASEB J. 1993 Feb 1;7(2):317–327. doi: 10.1096/fasebj.7.2.8440409. [DOI] [PubMed] [Google Scholar]
- Ross T. K., Prahl J. M., Herzberg I. M., DeLuca H. F. Baculovirus-mediated expression of retinoic acid receptor type gamma in cultured insect cells reveals a difference in specific DNA-binding behavior with the 1,25-dihydroxyvitamin D3 receptor. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10282–10286. doi: 10.1073/pnas.89.21.10282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willhite C. C., Wier P. J., Berry D. L. Dose response and structure-activity considerations in retinoid-induced dysmorphogenesis. Crit Rev Toxicol. 1989;20(2):113–135. doi: 10.3109/10408448909017906. [DOI] [PubMed] [Google Scholar]
- Williams D., Gorski J. Equilibrium binding of estradiol by uterine cell suspensions and whole uteri in vitro. Biochemistry. 1974 Dec 31;13(27):5537–5542. doi: 10.1021/bi00724a013. [DOI] [PubMed] [Google Scholar]
- Yang N., Schüle R., Mangelsdorf D. J., Evans R. M. Characterization of DNA binding and retinoic acid binding properties of retinoic acid receptor. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3559–3563. doi: 10.1073/pnas.88.9.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]




