Abstract
Metabolic and bioenergetic dysfunction are associated with oxidative stress and thought to be a common underlying mechanism of chronic diseases such as atherosclerosis, diabetes, and neurodegeneration. Recent findings support an emerging concept that circulating leukocytes and platelets can act as sensors or biomarkers of mitochondrial function in patients subjected to metabolic diseases. It is proposed that systemic stress-induced alterations in leukocyte bioenergetics are the consequence of several factors including reactive oxygen species. This suggests that oxidative stress mediated changes in leukocyte mitochondrial function could be used as an indicator of bioenergetic health in individuals. To test this concept, we investigated the effect of the redox cycling agent, 2,3 dimethoxynaphthoquinone (DMNQ) on the bioenergetic profiles of monocytes isolated from healthy human subjects using the extracellular flux analyzer. In addition, we tested the hypothesis that the bioenergetic health index (BHI), a single value that represents the bioenergetic health of individuals, is dynamically sensitive to oxidative stress in human monocytes. DMNQ decreased monocyte ATP-linked respiration, maximal respiration, and reserve capacity and caused an increase in proton leak and non-mitochondrial respiration compared to monocytes not treated with DMNQ. The BHI was a more sensitive indicator of the DMNQ-dependent changes in bioenergetics than any individual parameter. These data suggest that monocytes are susceptible to oxidative stress mediated by DMNQ and this can be accurately assessed by the BHI. Taken together, our findings suggest that the BHI has the potential to act as a functional biomarker of the impact of systemic oxidative stress in patients with metabolic disorders.
Abbreviations: AA, antimycin A; BHI, bioenergetic health index; DMNQ, 2,3 dimethoxynapthoquinone; DPI, diphenyleneiodonium chloride; FCCP, carbonyl cyanide p-[trifluoromethoxy]-phenyl-hydrazone; XF, extracellular flux; OCR, oxygen consumption rate; ROS, reactive oxygen species
Keywords: BHI, DMNQ, Monocytes, Bioenergetics, And oxidative stress
Graphical abstract
Highlights
-
•
DMNQ (2,3 dimethoxynapthoquinone) inhibits mitochondrial function in human monocytes.
-
•
The BHI (Bioenergetic Health Index) measures DMNQ mediated oxidative stress.
-
•
The BHI is more sensitive to oxidative stress than each bioenergetic parameter.
1. Introduction
Mitochondria are both a source and target for oxidative stress and the failure to maintain mitochondrial quality by the appropriate balance of biogenesis and autophagy ultimately leads to cell death [1], [2], [3], [4]. Many of the chronic diseases which afflict aging populations, such as diabetes and atherosclerosis, are associated with both increased systemic oxidative stress and mitochondrial dysfunction [1], [3], [5], [6]. Translation of these ideas to the clinic in diseases such as diabetes and cardiac surgery has resulted in the hypothesis that circulating platelets or monocytes can serve as bioenergetic biomarkers of the systemic exposure to metabolic stressors or pro-inflammatory cytokines [7], [8], [9], [10], [11]. Indeed, a broad range of environmental, dietary and epidemiological studies have shown distinct patterns of cytokine profiles in patients with chronic inflammatory diseases such as cancer, diabetes, obesity, and metabolic syndrome [12], [13], [14], [15]. Another study reported that human mononuclear cells isolated from Type 2 diabetic patients have alterations in mitochondrial morphology, mitochondrial mass and membrane potential [16]. These data highlight how measurement of cellular bioenergetics in leukocytes and platelets can act as a surrogate index of mitochondrial function in several pathologies including Alzheimer's disease or in some cases can be directly related to underlying pathologies such as autoimmune diseases [17], [18], [19]. Since mitochondria are particularly susceptible to oxidative stress, we hypothesized that the bioenergetics in circulating monocytes would exhibit a dose dependent change in response to oxidants.
We have recently developed methods to assess the changing dynamics of bioenergetic function in leukocytes and platelets from human subjects [20], [21], [22]. Importantly, we have determined that both glycolysis and oxidative phosphorylation are functionally distinct in platelets, monocytes, neutrophils and lymphocytes which suggests that their response to oxidative stress will also differ [23]. Mitochondrial cellular function can be defined using a stress test in which the addition of oxidative phosphorylation inhibitors can cause alterations in a cell's oxygen consumption rate (OCR) and bioenergetic profile [24], [25]. The mitochondrial parameters from the stress test include ATP linked respiration, proton leak, maximal respiration, the reserve capacity and non-mitochondrial respiration [1]. Each of these parameters are all uniquely sensitive to different free radicals or oxidants. The interactive nature of these parameters has led us to propose that an integrated single value known as the Bioenergetic Health Index (BHI) can be used as a measurement of changing bioenergetic health in human subjects for translational medicine [21]. Specifically, this integrated value could be useful for prognostic or diagnostic value. We have previously shown that the BHI is significantly depressed in monocytes isolated from the post-operative pericardial fluid of patients undergoing cardiac surgery [26] and this is associated with the highly oxidative environment of the pericardial fluid following surgery [11].
In the present study, we tested whether the response of monocytes isolated from healthy human subjects show a differential response to oxidative stress produced by 2, 3 dimethoxynapthoquinone (DMNQ). DMNQ is a redox cycling agent that generates both superoxide and hydrogen peroxide intracellularly in a concentration dependent manner [27]. These two oxidants are known to be generated in a broad range of pathological conditions associated with inflammation due to the activation of the NADPH oxidases [28], [29], [30]. The concept that cells isolated from patients may exhibit increased susceptibility to the oxidants generated by DMNQ has recently been tested in subjects with autistic disorder [31]. Specifically, it was determined that lymphoblastoid cells from a subgroup of children with autistic disorder had increased mitochondrial dysfunction due to DMNQ mediated oxidative stress compared to another subgroup of children with autistic disorder. In the present study, we examined the impact of varying concentrations of DMNQ in monocytes isolated from healthy subjects. Additionally, we evaluated the utility of the BHI to detect mitochondrial dysfunction induced by DMNQ in monocytes. Interestingly, the BHI was suppressed with increasing DMNQ concentrations and was found to be a more sensitive parameter than individual parameters derived from the mitochondrial stress test. Our findings suggest that the BHI is dynamically sensitive to acute oxidative stress in human monocytes and could be a potential functional biomarker of oxidative stress in patients suffering from metabolic disorders.
2. Materials and methods
2.1. Materials
DMNQ (2,3 dimethoxynapthoquinone) was purchased from Enzo Life Sciences (Farmingdale, NY, USA). RPMI was obtained from Life Technologies (Grand Island, NY, USA). Materials for the extracellular flux assays were from Seahorse Biosciences (North Billerica, MA, USA). BSA, Oligomycin, FCCP (carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone), Antimycin A and diphenyleneiodonium chloride (DPI) were all purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Human subjects
The work described here has been carried out in accordance with the Declaration of Helsinki. All study protocols for the collection and handling of human samples were reviewed and approved by the Institutional Review Board at the University of Alabama at Birmingham. Written informed consent was obtained from all participants. Blood samples (~24 ml) were collected from healthy control subjects (no medications or known pathological conditions, n=4–11, 3 males, 8 females, age 30.7±3.6 years) in vacutainers (BD Biosciences, Franklin Lakes, NJ, USA) containing 1.5 ml ACD solution.
2.3. Monocyte isolation from blood
All samples were processed as described previously [20], [22]. In brief, the platelet-rich plasma and buffy coat were separated by centrifugation at 500 g for 10 min. The buffy coat was diluted with RPMI media before being applied to a histopaque density gradient to collect peripheral blood mononuclear cells. Afterwards, antibody bead selection and a series of centrifugations were implemented to collect CD14+ monocytes before determining cell counts using the Bio-Rad TC20 automated cell counter (Bio-Rad Laboratories, Hercules, CA, USA). The typical yield of cells from ~24 ml of blood was 5.7±1.0×106 cells.
2.4. Cellular bioenergetics analysis and the Bioenergetic Health Index (BHI)
The Seahorse Biosciences XF96 extracellular flux analyzer was used to determine the effects of the oxidative stressor, DMNQ, on cellular bioenergetics in human monocytes. Purified monocytes (150,000 cells/well) were resuspended in XF assay buffer (DMEM with 1 mM pyruvate, 5.5 mM D-glucose, 4 mM L-glutamine, pH 7.4) and plated in Cell-Tak (BD Biosciences, Franklin Lakes, NJ, USA) coated assay plates as we have recently described [22]. Cells were pretreated with DMNQ (0.05, 0.1, 0.2, 1 and 5 µM) for 1 h at 37 °C before measuring the oxygen consumption rate (OCR). Dimethyl sulfoxide (DMSO) was used as a vehicle control. The mitochondrial stress test was performed by injecting oligomycin (0.5 µg/mL), FCCP (0.6 µM), and antimycin A (10 µM) sequentially into the cellular media. From this assay we calculated the following parameters: ATP-linked respiration, proton leak respiration, maximal OCR, reserve capacity, and non-mitochondrial respiration [1], [25]. The Bioenergetic Health Index (BHI) was calculated using the following formula: BHI=(ATP-linked×reserve capacity)/(proton leak×non-mitochondrial) [21]. In additional experiments, monocytes were treated with DPI (1, 5, and 10 µM) for 15 min prior to being treated with 0.2 µM DMNQ for 1 h and performing the mitochondrial stress test.
2.5. Statistical analysis
The statistical analysis was performed using GraphPad Prism software (La Jolla, CA, USA). Data is presented as mean±standard error of the mean (SEM). Individual measurements were comprised of 5–6 technical replicates and analyzed as a group by taking the mean of the bioenergetic parameters for each individual. A p value less than 0.05 was considered statistically significant. The statistical significance was determined using a two-tailed paired Student's t-test or ANOVA with Tukey post-hoc test for data with more than two groups as appropriate.
3. Results
3.1. DMNQ alters cellular bioenergetics in monocytes from healthy subjects
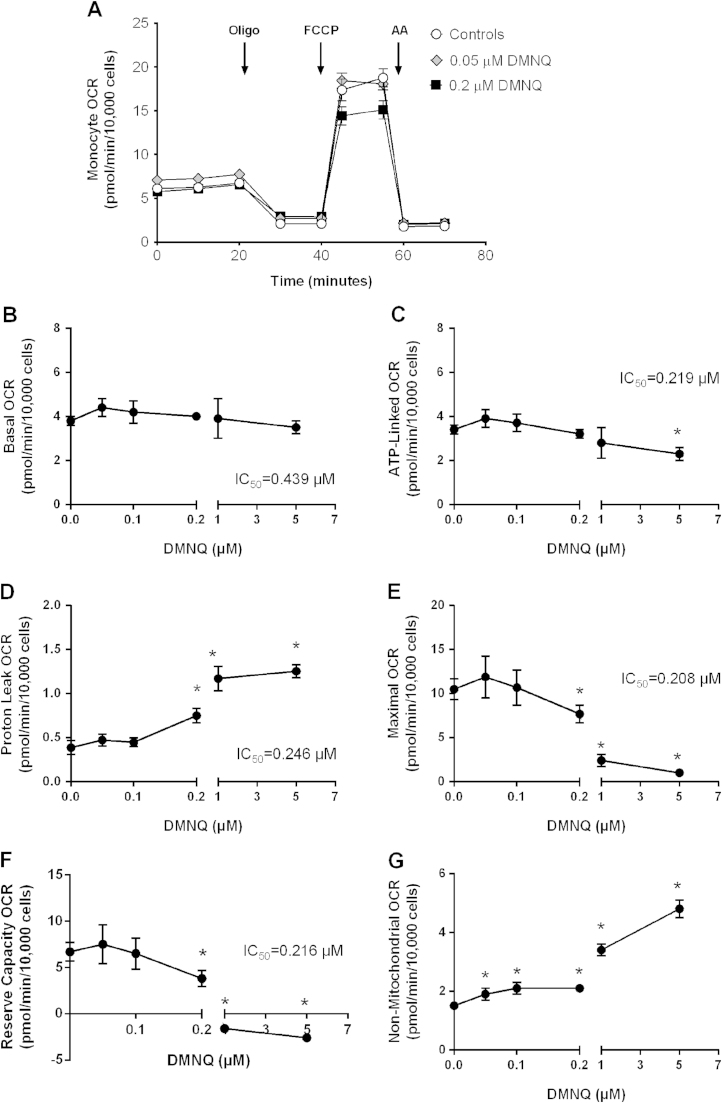
To investigate the sensitivity of monocyte mitochondrial function to acute oxidative stress, we utilized the redox cycling agent, DMNQ. In these series of experiments, monocytes from healthy subjects were pre-treated with varying concentrations of DMNQ (0.05, 0.1, 0.2, 1 and 5 µM) or vehicle control for 1 h before assessing cellular bioenergetics using the mitochondrial stress test. Fig. 1A illustrates a representative profile of monocytes from a single individual treated with DMNQ (0.05 and 0.2 µM) or vehicle control for 1 h. DMNQ had no effect on basal OCR over the first 20 min of the assay. Next, the complex V inhibitor, oligomycin, was injected onto the cells and caused a rapid decline in the OCR in both control and DMNQ treated groups (0.05 and 0.2 µM) (Fig. 1A). The remaining respiration or proton leak was similar between controls and monocytes treated with 0.05 µM DMNQ. However, 0.2 µM DMNQ increased proton leak. To assess maximal respiration, FCCP was injected after 40 min. FCCP stimulated maximal respiration in both controls and monocytes treated with 0.05 µM DMNQ. In contrast, cells treated with 0.2 µM DMNQ had decreased FCCP stimulated maximal respiration compared to control cells. Lastly, antimycin A was injected onto the cells after 60 min to measure non-mitochondrial respiration. Antimycin A significantly decreased OCR to the same extent in all groups (Fig. 1A).
Fig. 1.
The effect of DMNQ on monocyte mitochondrial function. Monocytes from healthy subjects were seeded (150,000 cells/well) on Cell-Tak coated Seahorse XF 96-well plates. Cells were pretreated with DMNQ (0.05, 0.1, 0.2, 1 and 5 µM) or vehicle control (DMSO) for 1 h prior to measuring the basal respiration and OCR following oligomycin (Oligo), FCCP, and antimycin A (AA) injections. (A) Representative OCR traces in monocytes from one individual treated with vehicle or 0.05 and 0.2 µM DMNQ. Results are mean±SEM, n=5–6 technical replicates per group. The effect of DMNQ on (B) basal; (C) ATP-Linked; (D) proton leak; (E) maximal; (F) reserve capacity; and (G) non-mitochondrial OCR from n=4–8 healthy subjects. Results are mean±SEM, *p<0.05 compared to controls not treated with DMNQ.
The effect of DMNQ (0.05, 0.1, 0.2, 1 and 5 µM) on each bioenergetic parameter was determined using the bioenergetic profile from a number of healthy subjects and plotted as a function of DMNQ concentration (Fig. 1B–G). Basal respiration was calculated by subtracting the initial OCR from the OCR following Antimycin A injection. Increasing DMNQ concentrations had no effect on basal respiration (Fig. 1B). Next, ATP-linked respiration was determined by subtracting oligomycin stimulated OCR from the basal OCR. The highest DMNQ concentration (5 µM) caused a significant decline in ATP-linked respiration compared to controls. Interestingly, lower doses of DMNQ had no effect on proton leak compared to controls; whereas, 1 and 5 µM DMNQ increased proton leak significantly compared to control monocytes (Fig. 1D). Maximal respiration was determined after the addition of the mitochondrial uncoupler FCCP and was inhibited by DMNQ with an IC50 of 0.208 µM (Fig. 1E). Exposure to higher concentrations of DMNQ (0.2, 1, and 5 µM) inhibited maximal respiration (Fig. 1E). As a result, DMNQ significantly decreased the reserve capacity in 0.2, 1 and 5 µM DMNQ treated groups (Fig. 1F). The reserve capacity was calculated by subtracting basal from maximal OCR. Lastly, the non-mitochondrial respiration was determined. As shown in Fig. 1G, non-mitochondrial respiration increased significantly with increasing DMNQ concentrations. Out of all of the parameters, non-mitochondrial respiration was the first parameter to show a significant change at the lowest DMNQ concentration. This was expected due to the oxygen consumption associated with the generation of superoxide and hydrogen peroxide mediated by DMNQ [32].
3.2. DMNQ decreases the BHI in monocytes from healthy subjects
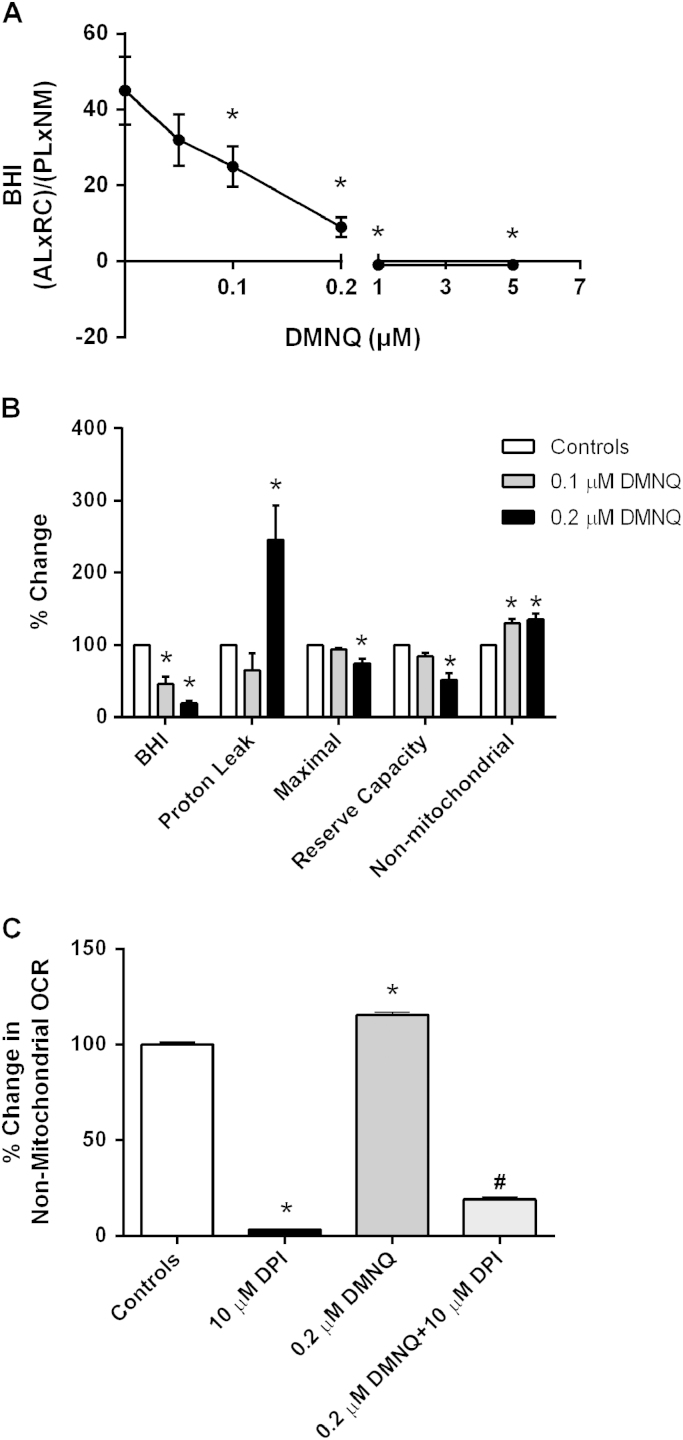
The effect of DMNQ on the BHI in healthy control subject monocytes was established. As shown in Fig. 2A, increasing concentrations of DMNQ caused a significant decline in the BHI. In particular, 0.1–0.2 µM DMNQ decreased BHI significantly compared to monocytes not treated with DMNQ (45±8.99 controls vs. 25± 5.37 or 9± 2.63 for 0.1 and 0.2 µM respectively). The BHI was further depleted below zero with higher concentrations of DMNQ (−1± 0.31 or −1± 0.16 for 1 and 5 µM DMNQ respectively). The effect of DMNQ on the BHI and certain bioenergetic parameters in monocytes was calculated. OCR rates were compared on a % basis to allow comparison of the DMNQ-dependent changes in BHI, proton leak, maximal, reserve capacity, and non-mitochondrial respiration. As shown in Fig. 2B, 0.1 and 0.2 µM DMNQ caused a 55% and 80% decrease in the BHI respectively. Importantly, these changes in BHI occurring at 0.1 µM DMNQ were not associated with changes in proton leak, maximal, or reserve capacity. However, it did cause a 30% increase in non-mitochondrial respiration in monocytes. In contrast, 0.2 µM DMNQ caused a significant increase in proton leak and a decrease in both maximal and reserve capacity (25% and 50% respectively) compared to control cells not treated with DMNQ. Interestingly, 0.2 µM DMNQ did not further increase non-mitochondrial respiration. To evaluate whether NADPH oxidases may be responsible for increased non-mitochondrial respiration, cells were treated with DPI, an NAPDH oxidase inhibitor. As shown in Fig. 2C, pretreating the cells with increasing concentrations of DPI (1, 5, and 10 µM) caused a significant decrease in DMNQ mediated non-mitochondrial respiration. These data suggest that the BHI and individual parameters are dynamically sensitive to oxidative stress mediated by DMNQ.
Fig. 2.
The effect of DMNQ on monocyte BHI. Healthy subject monocytes were seeded (150,000 cells/well) on Cell-Tak coated Seahorse XF 96-well plates and pretreated with DMNQ (0.05, 0.1, 0.2, 1 and 5 µM) or vehicle control for 1 h prior to measuring mitochondrial function. (A) The effect of increasing DMNQ concentrations on the BHI of monocytes from n=4–8 healthy subjects. (B) Percent change in BHI, proton leak, maximal, reserve capacity, and non-mitochondrial OCR in DMNQ treated cells (0.1 and 0.2 µM) compared to controls. (C) The effect of DPI, a NADPH oxidase inhibitor (10 µM), on non-mitochondrial respiration in monocytes treated with 0.2 µM DMNQ. Results are mean±SEM from 2–3 healthy subjects, *p<0.05 compared to controls not treated with DMNQ and #p<0.05 compared to cells treated with 0.2 µM DMNQ.
3.3. The BHI is more sensitive to DMNQ than individual mitochondrial parameters
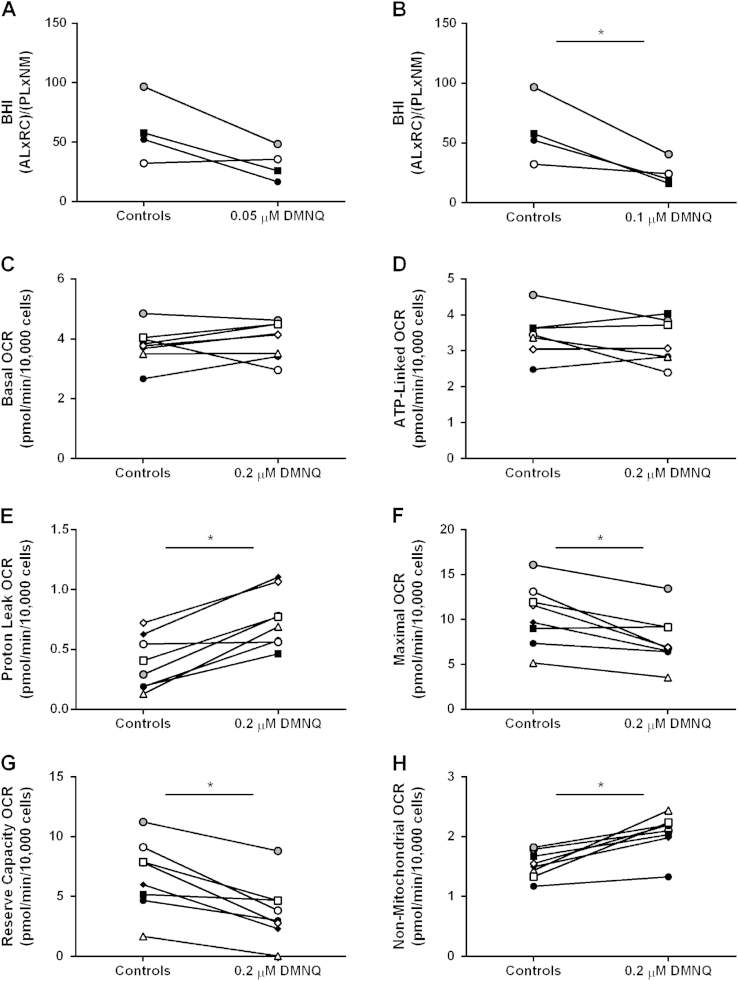
To test the possibility that different healthy subjects may exhibit differential DMNQ-dependent sensitivity to BHI and mitochondrial parameters, a more detailed analysis was performed based on individual profiles. As shown in Fig. 3A and B, the lowest DMNQ (0.05 µM, 0.1 µM) concentrations significantly decreased the BHI in three of the four healthy subjects while one subject was more resistant (Fig. 3A and B). The sensitivity of each mitochondrial parameter to 0.2 µM DMNQ treatment in monocytes from eight individuals was next determined. Both basal and ATP-linked respiration showed only minor variation with DMNQ treatment amongst individuals (Fig. 3C and D). However, proton leak was significantly increased to approximately the same extent in seven of the eight participants (Fig. 3E). Both the maximal respiration and reserve capacity was significantly decreased with 0.2 µM DMNQ treatment in 7 of the 8 subjects (Fig. 3F and G). Lastly, DMNQ significantly elevated non-mitochondrial respiration to the same extent in 7 of the 8 subjects suggesting that the extent of redox cycling does not change significantly between individuals but should be independently assessed (Fig. 3H).
Fig. 3.
The sensitivity of the BHI and individual mitochondrial parameters to DMNQ. Healthy subject monocytes were seeded (150,000 cells/well) on Cell-Tak coated Seahorse XF 96-well plates and pretreated with DMNQ (0.05, 0.1, and 0.2 µM) or vehicle control for 1 h prior to measuring mitochondrial function. (A) The BHI of individual healthy subject monocytes before and after 0.05 or 0.1 µM DMNQ treatment. (B) The sensitivity of each mitochondrial parameter to 0.2 µM DMNQ treatment in monocytes was determined. Results are mean±SEM, n=5–6 technical replicates per group, *p<0.05 compared to controls not treated with DMNQ. n=4–8 healthy subjects.
4. Discussion
Mitochondrial function has been shown to be impaired in leukocytes and platelets in systemic pathologies such as sepsis and fibromyalgia [33], [34]. The mechanisms responsible for these impairments are thought to be influenced by reactive oxygen species (ROS). Understanding these mechanisms further could be useful to develop diagnostic or prognostic assays to clinically benefit patients with systemic diseases. The purpose of this study was to determine whether acute oxidative stress generated by the redox cycling agent, DMNQ, alters cellular bioenergetics in monocytes from healthy subjects. In addition, we tested whether these mitochondrial changes could be detected by the BHI, a single value that represents mitochondrial function in cells. We have previously determined that DMNQ alters cellular bioenergetics in neuronal and endothelial cells [32], [35], and more recently in lymphocytes [36]. We opted to focus on monocytes in the current study because they are vital to the innate immune system and play an important role in the resolution of inflammation caused by tissue injury or infection [37]. They also rely on mitochondrial metabolism to carry out cellular processes such as differentiation and phagocytosis to mitigate inflammation. Lastly, they are involved in the pathogenesis of a number of systemic diseases such as atherosclerosis, stroke, and rheumatoid arthritis [38].
We determined that DMNQ lowered monocyte cellular bioenergetics in a concentration dependent manner compared to control cells not treated with DMNQ. In particular, DMNQ lowered ATP-linked respiration, maximal respiration, and depleted reserve capacity. Monocytes treated with higher DMNQ concentrations (1 and 5 µM) had a reserve capacity below zero and this suggests that DMNQ diminished the monocyte's ability to respond to cellular bioenergetic demand. The current data suggests that DMNQ could be causing poor mitochondrial respiratory complex integrity or substrate availability in these cells. It is clear that DMNQ also caused increased ROS generation as evidenced by the elevation in non-mitochondrial respiration at all concentrations. This alone can affect respiratory complex integrity or substrate availability. As expected, non-mitochondrial ROS increases with DMNQ concentration and this is inhibited by DPI. This is consistent with a role of flavoproteins in promoting redox cycling by DMNQ [27].
Importantly, we identified for the first time that the BHI is dynamically sensitive to oxidative stress mediated by DMNQ in monocytes from healthy human subjects. The individual subjects tested in this study showed highly significant changes in BHI when analyzed as a group and similar responses to DMNQ for specific parameters although analysis of individual responses suggest that significant personal differences could emerge in studies with larger populations. This study was not designed to determine whether differential responses to BHI are associated with age, gender or ethnicity. To determine the association of BHI to these demographic factors, samples with wider representation will be investigated in future studies.
The findings from these studies support the concept that the BHI can be used to monitor the extent of disease in individuals affected by pathologies influenced by oxidative stress such as atherosclerosis, diabetes, and neurodegeneration. We determined that concentrations as low as 0.1 µM DMNQ decreases BHI in monocytes and progressively decreased with increasing DMNQ concentrations. The BHI is an integrated measure of mitochondrial function and as such should be more sensitive than any of the individual parameters that can be derived from the mitochondrial stress test. The parameters that comprise the BHI include proton leak, reserve capacity, ATP linked respiration and what we have termed non-mitochondrial respiration [21]. The inclusion of non-mitochondrial respiration in the calculation of the BHI is based upon the established literature which shows mitochondria are both a source and target for the effects of ROS and RNS [39]. Experimentally, this parameter is defined as the value for OCR which remains after antimycin inhibition and may differ between cell types. Potential contributors include the cytochrome P450s, cyclooxygenases, NADPH oxidases and possibly intra-mitochondrial sources of ROS. We found that in monocytes, a substantial proportion of the non-mitochondrial OCR is sensitive to low concentrations of DPI suggesting that a flavoprotein is a major contributor. Clearly, this is an area warranting further investigation.
In the current protocol, the redox cycling of DMNQ is making a significant contribution as we have shown previously in endothelial cells [32], [40]. Importantly, we found that although both maximal respiration and reserve capacity showed significant decreases to 0.2 µM DMNQ, the BHI exhibited a significant decrease at 0.1 µM. To take into account whether non-mitochondrial respiration was primarily responsible for the decline observed in BHI, we evaluated the significance of each parameter on the BHI. We noted that at lower DMNQ concentrations (0.05 µM), proton leak, reserve capacity, or non-mitochondrial respiration was responsible for the decline in BHI in certain individuals (data not shown). However, non-mitochondrial respiration appeared to be a major factor for decreased BHI at higher DMNQ concentrations (0.1 and 0.2 µM) (data not shown). This is important to note because in pathologies where ROS are involved, either of the parameters could drive a decline in BHI based on the severity of oxidative stress. Moreover, it appears that the BHI detected these responses more accurately than any individual parameter alone. These findings highlight the potential of the BHI to be a sensitive predictor of mitochondrial dysfunction in patients.
In conclusion, these data illustrate that DMNQ negatively affects cellular bioenergetics in monocytes and that the BHI is highly sensitive to this stressor. The influence of DMNQ on the BHI in human monocytes could model the response observed in patients with pathologies where oxidative stress is exacerbated. We developed the BHI to assess the bioenergetic health of cells and to use this information to potentially monitor an individual's disease progression or response to treatment. The data presented here supports the emerging recognition of the important link between mitochondria and oxidative stress in monocytes in human subjects. In this study, DMNQ has been used as a prototypical example of a redox cycling compound to expose cells to controlled levels of intracellular ROS. It is important to note that many therapeutics, particularly anti-cancer agents, promote oxidative stress and in some cases this may contribute to unwanted side effects. The techniques we describe here could be applied to precision medicine by determining the susceptibility of patients to the pro-oxidant effects of therapeutics prior to administration and monitoring bioenergetic health during treatment. It is likely that this approach could also be extended to other cell types that can be isolated from human blood. For example, it has been shown that platelets from sickle cell disease, a known condition in which oxidative stress is increased, show a profound decrease in the key parameters of the mitochondrial stress test [41]. A further application of this approach has been reported in cells isolated from patients with autistic disorder in which DMNQ was used as a stressor ex-vivo [31]. This experimental paradigm potentially makes it possible to predict the susceptibility of a condition associated with oxidative stress. Future studies will determine whether cellular bioenergetics and the BHI are altered in patients affected by systemic disorders and whether these parameters could be a potential biomarker to monitor disease progression or susceptibility in individuals.
Conflict of interests
VDU receives funding from Seahorse Biosciences. However, the data presented in this manuscript was not supported by this resource.
Acknowledgments
This work was supported by the O’Brien Center P30 DK079337 and the UAB Blue Sky award (VDU), NIH DK054468-S1 (TM) and funds from the UAB Faculty Development Grant Program, Office of the Provost (TM).
References
- 1.Hill B.G., Benavides G.A., Lancaster J.R., Jr., Ballinger S., Dell’Italia L., Jianhua Z., Darley-Usmar V.M. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol. Chem. 2012;393:1485–1512. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giordano S., Darley-Usmar V., Zhang J. Autophagy as an essential cellular antioxidant pathway in neurodegenerative disease. Redox Biol. 2014;2:82–90. doi: 10.1016/j.redox.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apostolova N., Blas-Garcia A., Esplugues J.V. Mitochondria sentencing about cellular life and death: a matter of oxidative stress. Curr. Pharm. Des. 2011;17:4047–4060. doi: 10.2174/138161211798764924. [DOI] [PubMed] [Google Scholar]
- 4.Higdon A.N., Benavides G.A., Chacko B.K., Ouyang X., Johnson M.S., Landar A., Zhang J., Darley-Usmar V.M. Hemin causes mitochondrial dysfunction in endothelial cells through promoting lipid peroxidation: the protective role of autophagy. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H1394–H1409. doi: 10.1152/ajpheart.00584.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blake R., Trounce I.A. Mitochondrial dysfunction and complications associated with diabetes. Biochim. Biophys. Acta. 2013;1840(4):1404–1412. doi: 10.1016/j.bbagen.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Rains J.L., Jain S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011;50:567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srinivasan S., Yeh M., Danziger E.C., Hatley M.E., Riggan A.E., Leitinger N., Berliner J.A., Hedrick C.C. Glucose regulates monocyte adhesion through endothelial production of interleukin-8. Circ. Res. 2003;92:371–377. doi: 10.1161/01.RES.0000061714.74668.5C. [DOI] [PubMed] [Google Scholar]
- 8.Yamagishi S.I., Edelstein D., Du X.L., Brownlee M. Hyperglycemia potentiates collagen-induced platelet activation through mitochondrial superoxide overproduction. Diabetes. 2001;50:1491–1494. doi: 10.2337/diabetes.50.6.1491. [DOI] [PubMed] [Google Scholar]
- 9.Avila C., Huang R.J., Stevens M.V., Aponte A.M., Tripodi D., Kim K.Y., Sack M.N. Platelet mitochondrial dysfunction is evident in type 2 diabetes in association with modifications of mitochondrial anti-oxidant stress proteins. Exp. Clin. Endocrinol. Diabetes: Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2012;120:248–251. doi: 10.1055/s-0031-1285833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo X., Wu J., Du J., Ran J., Xu J. Platelets of type 2 diabetic patients are characterized by high ATP content and low mitochondrial membrane potential. Platelets. 2009;20:588–593. doi: 10.3109/09537100903288422. [DOI] [PubMed] [Google Scholar]
- 11.Kramer P.A., Chacko B.K., Ravi S., Johnson M.S., Mitchell T., Barnes S., Arabshahi A., Dell’Italia L.J., George D.J., Steele C., George J.F., Darley-Usmar V.M., Melby S.J. Hemoglobin-associated oxidative stress in the pericardial compartment of postoperative cardiac surgery patients. Lab. Investig. J. Tech. Methods Pathol. 2015;95:132–141. doi: 10.1038/labinvest.2014.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvehus M., Buren J., Sjostrom M., Goedecke J., Olsson T. The human visceral fat depot has a unique inflammatory profile. Obesity. 2010;18:879–883. doi: 10.1038/oby.2010.22. [DOI] [PubMed] [Google Scholar]
- 13.Estep J.M., Baranova A., Hossain N., Elariny H., Ankrah K., Afendy A., Chandhoke V., Younossi Z.M. Expression of cytokine signaling genes in morbidly obese patients with non-alcoholic steatohepatitis and hepatic fibrosis. Obes. Surg. 2009;19:617–624. doi: 10.1007/s11695-009-9814-x. [DOI] [PubMed] [Google Scholar]
- 14.Slattery M.L., Fitzpatrick F.A. Convergence of hormones, inflammation, and energy-related factors: a novel pathway of cancer etiology. Cancer Prev. Res. 2009;2:922–930. doi: 10.1158/1940-6207.CAPR-08-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosa J.S., Heydari S., Oliver S.R., Flores R.L., Pontello A.M., Ibardolaza M., Galassetti P.R. Inflammatory cytokine profiles during exercise in obese, diabetic, and healthy children. J. Clin. Res. Pediatr. Endocrinol. 2011;3:115–121. doi: 10.4274/jcrpe.v3i3.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Widlansky M.E., Wang J., Shenouda S.M., Hagen T.M., Smith A.R., Kizhakekuttu T.J., Kluge M.A., Weihrauch D., Gutterman D.D., Vita J.A. Altered mitochondrial membrane potential, mass, and morphology in the mononuclear cells of humans with type 2 diabetes. Transl. Res.: J. Lab. Clin. Med. 2010;156:15–25. doi: 10.1016/j.trsl.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosconi L., de Leon M., Murray J., E L., Lu J., Javier E., McHugh P., Swerdlow R.H. Reduced mitochondria cytochrome oxidase activity in adult children of mothers with Alzheimer's disease. J. Alzheimer's Dis. 2011;27:483–490. doi: 10.3233/JAD-2011-110866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onyango I., Khan S., Miller B., Swerdlow R., Trimmer P., Bennett P., Jr. Mitochondrial genomic contribution to mitochondrial dysfunction in Alzheimer’s disease. J. Alzheimer's Dis. 2006;9:183–193. doi: 10.3233/jad-2006-9210. [DOI] [PubMed] [Google Scholar]
- 19.Perl A., Hanczko R., Doherty E. Assessment of mitochondrial dysfunction in lymphocytes of patients with systemic lupus erythematosus. Methods Mol. Biol. 2012;900:61–89. doi: 10.1007/978-1-60761-720-4_4. [DOI] [PubMed] [Google Scholar]
- 20.Kramer P.A., Chacko B.K., Ravi S., Johnson M.S., Mitchell T., Darley-Usmar V.M. Bioenergetics and the oxidative burst: protocols for the isolation and evaluation of human leukocytes and platelets. J. Vis. Exp. 2014;85:1–9. doi: 10.3791/51301. e51301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chacko B.K., Kramer P.A., Ravi S., Benavides G.A., Mitchell T., Dranka B.P., Ferrick D., Singal A.K., Ballinger S.W., Bailey S.M., Hardy R.W., Zhang J., Zhi D., Darley-Usmar V.M. The Bioenergetic Health Index: a new concept in mitochondrial translational research. Clin. Sci. 2014;127:367–373. doi: 10.1042/CS20140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chacko B.K., Kramer P.A., Ravi S., Johnson M.S., Hardy R.W., Ballinger S.W., Darley-Usmar V.M. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Lab. Investig. J. Tech. Methods Pathol. 2013;93:690–700. doi: 10.1038/labinvest.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kramer P.A., Ravi S., Chacko B., Johnson M.S., Darley-Usmar V.M. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: Implications for their use as bioenergetic biomarkers. Redox Biol. 2014;2:206–210. doi: 10.1016/j.redox.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brand M.D., Nicholls D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dranka B.P., Benavides G.A., Diers A.R., Giordano S., Zelickson B.R., Reily C., Zou L.Y., Chatham J.C., Hill B.G., Zhang J.H., Landar A., Darley-Usmar V.M. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Radic. Biol. Med. 2011;51:1621–1635. doi: 10.1016/j.freeradbiomed.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kramer P.A., Chacko B.K., George D.J., Zhi D., Wei C.C., Dell’Italia L.J., Melby S.J., George J.F., Darley-Usmar V.M. Decreased Bioenergetic Health Index in monocytes isolated from the pericardial fluid and blood of post-operative cardiac surgery patients. Biosci. Rep. 2015;35(4) doi: 10.1042/BSR20150161. pii. e00237 (1-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe N., Forman H.J. Autoxidation of extracellular hydroquinones is a causative event for the cytotoxicity of menadione and DMNQ in A549-S cells. Arch. Biochem. Biophys. 2003;411:145–157. doi: 10.1016/s0003-9861(02)00716-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 29.Paravicini T.M., Touyz R.M. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care. 2008;31(2):S170–S180. doi: 10.2337/dc08-s247. [DOI] [PubMed] [Google Scholar]
- 30.Panday A., Sahoo M.K., Osorio D., Batra S. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015;12:5–23. doi: 10.1038/cmi.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose S., Frye R.E., Slattery J., Wynne R., Tippett M., Pavliv O., Melnyk S., James S.J. Oxidative stress induces mitochondrial dysfunction in a subset of autism lymphoblastoid cell lines in a well-matched case control cohort. PloS One. 2014;9:e85436. doi: 10.1371/journal.pone.0085436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dranka B.P., Hill B.G., Darley-Usmar V.M. Mitochondrial reserve capacity in endothelial cells: The impact of nitric oxide and reactive oxygen species. Free Radic. Biol. Med. 2010;48:905–914. doi: 10.1016/j.freeradbiomed.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belikova I., Lukaszewicz A.C., Faivre V., Damoisel C., Singer M., Payen D. Oxygen consumption of human peripheral blood mononuclear cells in severe human sepsis. Crit. Care Med. 2007;35:2702–2708. doi: 10.1097/01.ccm.0000295593.25106.c4. [DOI] [PubMed] [Google Scholar]
- 34.Cordero M.D., De Miguel M., Moreno Fernandez A.M., Carmona Lopez I.M., Garrido Maraver J., Cotan D., Gomez Izquierdo L., Bonal P., Campa F., Bullon P., Navas P., Sanchez Alcazar J.A. Mitochondrial dysfunction and mitophagy activation in blood mononuclear cells of fibromyalgia patients: implications in the pathogenesis of the disease. Arthritis Res. Ther. 2010;12:R17. doi: 10.1186/ar2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider L., Giordano S., Zelickson B.R., M, S. J. G.,A.B., Ouyang X., Fineberg N., Darley-Usmar V.M., Zhang J. Differentiation of SH-SY5Y cells to a neuronal phenotype changes cellular bioenergetics and the response to oxidative stress. Free Radic. Biol. Med. 2011;51:2007–2017. doi: 10.1016/j.freeradbiomed.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kramer P.A., Prichard L., Chacko B., Ravi S., Overton E.T., Heath S.L., Darley-Usmar V. Inhibition of the lymphocyte metabolic switch by the oxidative burst of human neutrophils. Clin. Sci. 2015;129:489–504. doi: 10.1042/CS20140852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ravi S., Mitchell T., Kramer P.A., Chacko B., Darley-Usmar V.M. Mitochondria in monocytes and macrophages-implications for translational and basic research. Int. J. Biochem. Cell Biol. 2014;53:202–207. doi: 10.1016/j.biocel.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J., Zhang L., Yu C., Yang X.F., Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark. Res. 2014;2:1. doi: 10.1186/2050-7771-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dranka B.P., Benavides G.A., Diers A.R., Giordano S., Zelickson B.R., Reily C., Zou L., Chatham J.C., Hill B.G., Zhang J., Landar A., Darley-Usmar V.M. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Radic. Biol. Med. 2011;51:1621–1635. doi: 10.1016/j.freeradbiomed.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cardenes N., Corey C., Geary L., Jain S., Zharikov S., Barge S., Novelli E.M., Shiva S. Platelet bioenergetic screen in sickle cell patients reveals mitochondrial complex V inhibition, which contributes to platelet activation. Blood. 2014;123:2864–2872. doi: 10.1182/blood-2013-09-529420. [DOI] [PMC free article] [PubMed] [Google Scholar]