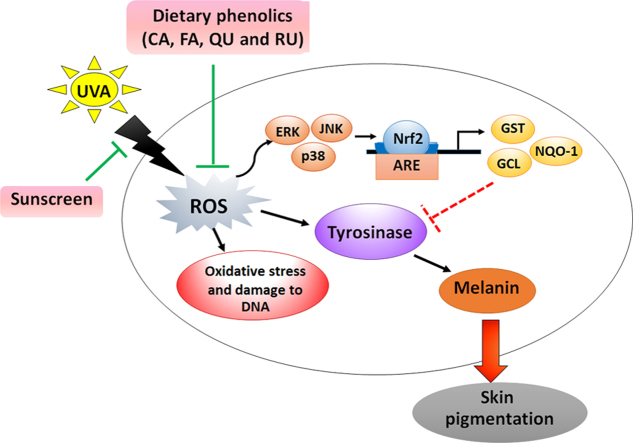
Abstract
Dietary phenolics may play a protective role in UV-mediated skin pigmentation through their antioxidant and UV-absorbing actions. In this study, we investigated whether genetic silencing of Nrf2, regulating the transcription of antioxidant genes, affected melanogenesis in primary human epidermal melanocytes (HEMn) and B16F10 melanoma cells subjected to UVA (8 J/cm2) exposure. Then, we explored the antimelanogenic actions of phenolics; caffeic acid (CA) and ferulic acid (FA) providing partial UVA protection; quercetin (QU) and rutin (RU) providing strong UVA protection and; avobenzone (AV), an efficient UVA filter, in association with modulation of Nrf2-mediated antioxidant defenses in response to UVA insults in B16F10 cells. Upon oxidative insults, Nrf2 silencing promoted melanogenesis in both HEMn and B16F10 cells irradiated with UVA. Stimulation of melanogenesis by UVA correlated with increased ROS and oxidative DNA damage (8-OHdG), GSH depletion as well as a transient downregulation of Nrf2 nuclear translocation and of Nrf2-ARE signaling in B16F10 cells. All test compounds exerted antimelanogenic effects with respect to their abilities to reverse UVA-mediated oxidative damage as well as downregulation of Nrf2 activity and its target antioxidants (GCLC, GST and NQO1) in B16F10 cells. In conclusion, defective Nrf2 may promote melanogenesis under UVA irradiation through oxidative stress mechanisms. Compounds with antioxidant and/or UVA absorption properties could protect against UVA-induced melanogenesis through indirect regulatory effect on Nrf2-ARE pathway.
Abbreviations: ARE, antioxidant response element; AV, avobenzone; CA, caffeic acid; CDNB, 1-chloro-2,4-dinitrobenzene; DCPIP, 2,6-dichloroindophenol; DMEM, dulbecco’s modified Eagle medium; DPBS, dulbecco’s phosphate buffered saline; DTNB, (5,5′-dithio-bis-2-(nitrobenzoic acid); FA, ferulic acid; γ-GCL, γ-glutamate cysteine ligase; γ-GCLC, γ-glutamate cysteine ligase catalytic subunit; γ-GCLM, γ-glutamate cysteine ligase modifier subunit; GSH, glutathione; GSSG, glutathione reductase; GST, glutathione S-transferase; H2DCFDA, non-fluorescent dichlorofluorescein; HEMn, primary human epidermal melanocytes; NQO1, NAD(P)H quinone oxidoreductase1; Nrf2, nuclear factor E2-related factor 2; 8-OHdG, 8-hydroxy-2’–deoxyguanosine; QU, quercetin; RNAi, RNA interference; ROS, reactive oxygen species; RU, rutin; siCtrl, non-silencing siRNA controls; siNrf2, siRNA against Nrf2; siRNA, small-interfering RNA; UVA, ultraviolet A
Keywords: Phenolics, Antioxidant, Ultraviolet A, Melanogenesis, Nuclear factor E2-related factor 2 (Nrf2)
Graphical abstract
Highlights
-
•
Depletion of Nrf2 could stimulate melanogenesis under UVA-mediated oxidative stress.
-
•
UVA caused time-course changes of Nrf2 activity and its target antioxidants.
-
•
Phenolics could inhibit UVA-induced melanogenesis through modulation of Nrf2 pathway.
1. Background
Oxidative stress induced by ultraviolet A (UVA) radiation has been recognized to play a crucial role in physiological and biological stress responses including dysregulation of melanogenesis in melanocytes and/or melanoma cells [1], [2]. Whereas melanin production primarily regulated by tyrosinase plays a beneficial role in protecting the skin against damaging effects of UV radiation, excessive formation of melanin could be harmful, in particular following UV exposure [3], [4].
UVA exposure has been demonstrated to play a crucial role in increased melanogenesis partly through induction of oxidative stress and impairment of antioxidant defense in melanocytes and/or melanoma cells [5], [6], improvement of antioxidant defense system to cope with the overwhelmed oxidative stress could thus be one of effective and safe approaches to inhibit melanogenesis and photodamaged skin. Nuclear factor E2-related factor 2 (Nrf2), an important transcription factor controlling the antioxidant response in various tissues including the skin, has been reported to play a beneficial role in cellular function and integrity by protecting skin cells including melanocytes against oxidative insults particularly from UV exposure [7], [8], [9], [10], [11], [12]. Attempts have thus been made to develop effective photoprotective agents targeting Nrf2.
Diet- and plant-derived phytochemicals have been proposed as good candidates for effective and safe photoprotective agents possibly due to their antioxidant and UV-absorbing properties [13], [14]. Phytochemicals having antioxidant properties including caffeic acid (CA), ferulic acid (FA), quercetin (QU) and rutin (RU) found abundantly in plant-based diets and beverages as well as sunscreen agents have been reported to exert photoprotective and depigmenting actions [15], [16], [17], [18], [19], [20], [21]. In this study, we therefore aimed to investigate antimelanogenic effects of compounds with different antioxidant and UVA blocking properties in correlation to UVA-mediated modulation of Nrf2-ARE signaling pathway and its downstream antioxidants including γ-glutamate cysteine ligase (γ-GCL), the rate-limiting enzyme for GSH synthesis, glutathione S-transferase (GST) and NAD(P)H quinone oxidoreductase 1 (NQO1). At first, we examined whether depletion of Nrf2 using small-interfering RNA-mediated silencing of Nrf2 affected UVA-induced melanogenesis in primary human epidermal melanocytes (HEMn) and B16F10 melanoma cells. In addition, UVA irradiation was suggested to induce photodamaged skin through activation of MAPK signaling in association with oxidative stress responses in various types of skin cells [22], [23]. Thus, the role of MAPK signaling as upstream mediators that could regulate Nrf2 nuclear translocation in response to UVA irradiation was also evaluated in this study. Then, we explored the underlying mechanisms of dietary phenolics; CA and FA having ability to partially (approximately 30-50%) absorb UVA ray [21] (Supplementary Table 1); QU and RU having strong UVA absorption properties as well as; AV, an efficient UVA filter which does not possess antioxidant activity, in protecting B16F10 cells against UVA-induced melanogenesis in association with inhibition of oxidative stress and oxidative DNA damage (8-hydroxy-2’–deoxyguanosine; 8-OHdG) through modulation of Nrf2-ARE signaling and its downstream antioxidants.
2. Materials and methods
2.1. Cell cultures and treatment
Primary human epidermal melanocytes (HEMn) (Lonza, Basel, Switzerland) were grown in Medium 254 (#M-254-500) supplemented with human melanocyte growth supplement (HMGS) according to the manufacturer's instructions. B16F10 mouse melanoma cells (ATCC, Rockville, Md, USA), a gift from Assoc. Prof. Wajjwalku, Faculty of Veterinary Medicine, Kasetsart University, were grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin (100 units/ml)/streptomycin (100 μg/ml). All cells were maintained at 37 οC in a humidified air of 5% CO2 (PCO2=40 Torr) (a Forma Scientific CO2 Water Jacketed Incubator).
To test whether cellular oxidative stress modulate melanogenesis, B16F10 cells were treated with H2O2 (up to 500 µM) without UVA irradiation for 30 min and with l-buthionine-(S,R)-sulfoximine; BSO (up to 500 µM), an inhibitor of gamma-glutamylcysteine synthetase, the first enzyme involved in GSH synthesis, for 24 h prior to UVA irradiation. To evalulate the effects of phenolics on melanogenesis induced by UVA through Nrf2-dependent antioxidant responses, cells were treated with test compounds (up to 30 µM) in Dulbecco’s phosphate buffered saline (DPBS) for 30 min before exposure to a single dose of UVA radiation (8 J/cm2) and, to achieve a UVA dose required, the UV intensity was evaluated as previously described [2], [21]. The dose of UVA and concentrations of phenolics employed in this study were non-cytotoxic to both HEMn and B16F10 cells. To demonstrate an involvement of MAPK pathway in Nrf2 nuclear translocation, B16F10 cells were pretreated with 1 µM of specific ERK inhibitor (U0126), JNK inhibitor (SP600125) and p38 inhibitor (SB203580) in serum-free medium for 1 h prior to UVA (8 J/cm2) irradiation and then harvested at 1 h after irradiation for western blot analysis of nuclear/cytosolic Nrf2 ratio. After UVA irradiation, cells were washed, further incubated in serum-free medium and harvested at different time points as indicated in Results. The UVA source was a xenon arc lamp (Dermalight ultrA1; Hoenle, Martinsried, Germany).
For preparation of cell lysate, cells were harvested and resuspended in lysis buffer consisted of 50 mM Tris-HCl, 10 mM ethylene diaminetetraacetic acid (EDTA), 1% (v/v) Triton X-100, phenylmethylsulfonyl fluoride (PMSF) (100 mg/ml) and pepstatin A (1 mg/ml) in DMSO and leupeptin (1 mg/ml) in H2O, pH 6.8. The lysed cells were centrifuged at 10,000 rpm for 10 min at 4 °C and the total lysates were collected and either assayed immediately or stored frozen at −80 °C.
2.2. Silencing of Nrf2 via RNA interference (RNAi)
A combination of four gene-specific small-interfering RNA (siRNA) against human Nrf2 (NM_006164) was used (FlexiTube GeneSolution GS4780 for NFE2L2, Qiagen; Cat.#:1027416). HEMn and B16F10 cells were transfected with 5 nM siRNA against Nrf2 (siNrf2) or equal molar non-silencing siRNA controls (siCtrl, Qiagen; Cat.#:1022076) for 48 h. These siRNAs were earlier complexed with liposome carrier (HiPerFect Transfection Reagent, Qiagen; Cat.#: 301705) at 0.08 μL/ng siRNA concentration by incubating mixture for 5–10 min at room temperature in serum-free culture medium. At 48 h post-transfection, cells were then washed with DPBS and subjected to UVA irradiation, following which melanin content, tyrosinase activity and protein were determined. Cells appeared normal morphologically and did not differ from untransfected cells in cell viability. At 48 h post-transfection, all siRNAs were verified to ensure achieving functional and specific silencing by evaluating mRNA and protein levels of Nrf2 and known Nrf2 target genes including GCLC, GCLM, GST and NQO1 before employment in all experiments. To evaluate melanogenic response of Nrf2-depleted cells to UVA irradiation, HEMn and B16F10 cells transfected with Nrf2-siRNA or non-silencing negative control siRNA (siCtrl) were irradiated with 8 J/cm2 of UVA and harvested at 1 h post-irradiation for determination of melanin content and tyrosinase activity and at 24 h post-irradiation for tyrosinase protein expression.
2.3. Melanin content assay
An evaluation of melanin production was performed as described previously [19]. Cells were harvested at 1 h after UV radiation (8 J/cm2) and the cell pellets were solubilized in 1 N NaOH for 1 h to dissolve melanin, which was then measusred spectrophotometrically at 475 nm. The melanin content (μg/mg protein) was calculated by comparison to a standard curve derived using synthetic melanin.
2.4. Tyrosinase activity assay
The rate of l-DOPA oxidation was measured to assess cellular tyrosinase activity at 1 h following exposure to a UVA dose of 8 J/cm2. The assay was performed as previously described by Shin et al. [12]. Briefly, 20 mM L-DOPA used as the substrates was added to each lysate in a 96-well plate and absorbance of dopachrome formation was measured spectrophotometrically at 475 nm every 10 min for 1 h at 37 °C by a spectrophotometer. The tyrosinase activity (unit/mg protein) was calculated by comparison to a standard curve using tyrosinase (2034 U/mg).
2.5. Measurement of intracellular glutathione content
GSH level was spectrophotometrically measured using glutathione reductase (GR): (5,5′-dithio-bis-2-(nitrobenzoic acid) (DTNB) enzymatic recycling method following the kit protocol from Sigma-Aldrich (MO, US). The assay is based on conversion of glutathione disulfide (GSSG) to GSH by GR in the presence of NADPH and GSH oxidation by the sulfhydryl reagent DTNB to produce the yellow TNB (5′-thio-2-nitrobenzoic acid) measured at 412 nm. The rate of TNB production is directly proportional to this recycling reaction in turn directly proportional to the concentration of GSH. The GSH level was calculated by comparing the valued obtained with a standard curve of GSH and was expressed in nmol/mg protein.
2.6. Measurement of glutathione-S-transferase activity
GST activity was measured following the kit protocol from Cayman chemical (Ann Arbor, MI). The assay is based on GST-catalyzed conjugation of GSH to 1-chloro-2,4-dinitrobenzene (CDNB) as a substrate. The GS-DNB conjugate was determined spectrophotometrically at 340 nm immediately and every 30 s for 10 min. 10 μl of 100 mM CDNB was added to start the reaction of 20 μl of sample or positive control GST with 20 μl of 200 mM GSH in 150 μl of assay buffer (100 mM potassium phosphate, pH 6.5, containing 0.1% Triton X-100). One unit of GST activity is defined as the amount of enzyme that catalyzes 1 nmol of GS-DNB conjugate/min and the results were expressed as nmol/min/mg protein).
2.7. Measurement of NQO1 activity
B16F10 cells were harvested at 4 h post-irradiation, and NQO1 activity in cell lysates was measured using 2,6-dichloroindophenol (DCPIP) as a substrate as previously described [24]. The assay was based on the activities for NAD(P)H-dependent reduction of DCPIP at 600 nm and the reaction was specifically inhibited by dicumarol. Briefly, reactions contained 25 mM Tris-HCl, pH 7.4, 0.17 mg/ml bovine serum albumin, 0.2 mM NADH and sample. 80 µM DCPIP was added to initiate the reactions and the NQO1 activity was measured as the dicumarol inhibitable reduction in absorbance at 600 nm. The NQO1 activity was expressed as nmole DCPIP reduced/min/mg protein.
2.8. Determination of protein content
Protein concentration was measured using the Bio-Rad Protein Assay Kit (Bio-Rad, Germany) and bovine serum albumin (BSA) was used as protein standard.
2.9. Determination of intracellular oxidant formation by flow cytometry
The assay is based on oxidation of non-fluorescent dichlorofluorescein (H2DCFDA) by intracellular ROS to fluorescent 2,7-DCF. After UVA irradiation, cells were washed and incubated with serum-free DMEM for 30 min. Then, cells were incubated in DPBS with 5 μM H2DCFDA at 37 °C for 30 min and analyzed by flow cytometery using a fluorescence activated cell sorter (FACS-calibur).
2.10. Protein preparation and western blot analysis
Protein extraction
Western blotting were carried out using whole cell extracts for detection of tyrosinase, GCLC, GST and NQO1 protein expressions and cytosolic, and nuclear extracts for Nrf2 levels. Whole cells were extracted by incubation for 10 min at 4 °C with RIPA (radioimmunoprecipitation assay) buffer containing 10% NP40, 5 M NaCl, 1 M HEPES (pH 7.4), 0.5 M EDTA (pH 8.0) and proteinase inhibitor cocktail. Cytosolic and nuclear extraction were prepared using a commercial kit according to the manufacturer’s instructions (Sigma). Cells were washed with DPBS and collected in micro-centrifuge tubes. Cell pellets were suspended in 100 µl of hypotonic lysis buffer containing 0.01 M DTT and proteinase inhibitor cocktail. Cells were incubated on ice for 15 min and lysed in ice-cold cytosolic extraction buffer containing 10% IGEPAL CA-630. Lysate mixtures were centrifuged at 11,000 rpm for 1 min at 4 °C, and the supernatant was collected as the cytosolic extract. Nuclear pellets were suspended in 60 µl of nuclear extraction buffer (20 mM HEPES, pH 7.9, 1.5 mM MgCl, 0.42 M NaCl, 0.2 mM EDTA, and 25% (v/v) Glycerol) containing 0.01 M DTT and proteinase inhibitor cocktail. The mixtures were incubated on ice with intermittent vortexing for 15–30 min. Then, extracts were centrifuged at 14,000 rpm for 10 min and the supernatant was collected as the nuclear extract.
Western blotting
Protein concentrations were quantified using the Bradford method (Bio-Rad, Germany).
The proteins were resolved by a 10% (w/v) SDS-PAGE gel and transferred to a nitrocellulose membrane. The membranes were blocked with 5% skim milk (Tris-buffer saline containing 0.1% (v/v) Tween 20 and 5% (w/v) skim milk) for 1.5 h and then incubated overnight at 4 °C with the primary antibody against tyrosinase (ab178676; Abcam, Cambridge, MA, USA) (1:10000), Nrf2 (sc-722; Santa Cruz Biotechnology, Santa Cruz, CA) (1:2000), GCLC (ab53179; Abcam, Cambridge, MA, USA) (1:2000), GST (sc-459; Santa Cruz Biotechnology, Santa Cruz, CA) (1:1000) and NQO1 (ab34173; Abcam, Cambridge, MA, USA) (1:2000) in 5% skim milk. The membranes were washed 3 times with a PBS solution of 0.1% (v/v) Tween 20 for 30 min and incubated for 1.5 h at room temperature with the the HRP-conjucated secondary antibodies (ab6789 for anti-mouse and ab6721 for anti-rabbit HRP labeled secondary antibody; Abcam, Cambridge, MA, USA) (1:2000) in 5% skim milk. Immunoreactivity is detected using the Bio-Rad Clarity western ECL (Bio-Rad). Protein bands were imaged using an ImageQuant LAS 4000 digital imaging system (GE Healthcare, UK) and the integrated optical density of the bands was analyzed by the Image-J software version 1.45 s (National institutes of health, USA). The protein expressions were normalized to expression of loading controls; α-Tubulin (ab7291; Abcam, Cambridge, MA, USA) (1:5000) for whole cell proteins or cytosol Nrf2 and TATA binding protein (TBP) (ab818; Abcam, Cambridge, MA, USA) (1:2000) for nuclear Nrf2. Cells treated with sulforaphane (an Nrf2 activator; 10 μM) was used as positive control for Nrf2 nuclear translocation.
2.11. Quantitative real-time reverse transcriptase-polymerase chain reaction for measurement of mRNA expression
Total RNA was isolated using the illustra RNAspin Mini RNA Isolation Kit (GE Healthcare, UK). Reverse transcription was carried out with 1 μg of total RNA using the Improm-II reverse transcriptase (Promega, Medison, USA) under the conditions described in the kit manual. Reactions were performed in triplicate for each sample in the ABI Prism 7500 Real Time PCR System (Applied Biosystems, USA) under the following amplification conditions: 95 °C for 10 min, 40 cycles of 95 °C for 15 s, 60 °C for 40 s, and 72 °C for 40 s. Real-time RT-PCR was performed in a total volume of 25 μl of reaction mixtures containing 5 μl cDNA template with FastStart universal SYBR Green Master (ROX) and 10 μM concentrations of primers. Primers for PCR were designed using the Primer Express software version 3.0 (Applied Biosystems, USA). Sequences of PCR primer sets of genes studied were shown in Table 1. The mRNA level was normalized with reference to the amount of housekeeping gene transcripts (GAPDH mRNA). The mean Ct from mRNA expression in cDNA from each sample was compared with the mean Ct from GAPDH determinations from the same cDNA samples.
Table 1.
Sequences (in 5′-3′ direction) of primers used in this study.
| Primer | Sequences | Product size (bp) | GeneBank |
|---|---|---|---|
| Nrf2 (sense) | TTCTGTTGCTCAGGTAGCCCCTCA | 161 | NM_006164.4 |
| Nrf2 (antisense) | GTTTGGCTTCTGGACTTGG | ||
| GCLC (sense) | GCTGTCTTGCAGGGAATGTT | 160 | NM_001498.2 |
| GCLC (antisense) | ACACACCTTCCTTCCCATTG | ||
| GCLM (sense) | TTGGAGTTGCACAGCTGGATT | 200 | NM_002061.2 |
| GCLM (antisense) | TGGTTTTACCTGTGCCCACTG | ||
| GST (sense) | CCTGTACCAGTCCAATACCATCCT | 72 | NM_000852.3 |
| GST (sense) | TCCTGCTGGTCCTTCCCATA | ||
| NQO-1 (sense) | ATGACAAAGGACCCTTCCGGAGTAA | 245 | NM_000903.2 |
| NQO-1 (antisense) | ATTCTCCAGGCGTTTCTTCCATCCT | ||
| GAPDH (sense) | CCT CCA AAA TCA AGT GGG GCG ATG | 150 | NM_002046.3 |
| GAPDH (antisense) | CGA ACA TGG GGG CAT CAG CAG A |
2.12. Determination of Nrf2-ARE transcriptional activity
Transcriptional activity of Nrf2-ARE was determined using the Cignal™ Antioxidant Response Reporter (luc) Kit (SABiosciences, Qiagen, USA). B16F10 cells were transfected in 24-well plate for 16 h with an Nrf2-responsive firefly luciferase reporter plasmid and a control plasmid constitutively expressing Renilla luciferase (SABiosciences, Qiagen) in Lipofectamine® LTX & Plus Reagent (Invitrogen, USA) according to the manufacturer’s instructions. The transfected cells were pretreated with CA, QU and AV (up to 30 µM) for 30 min before exposure to UVA (8 J/cm2). Cells were washed, further incubated in serum-free medium and harvested at 1 h after UVA irradiation. The firefly and Renilla luciferase activities were measured using a Dual-Glo Luciferase Assay Kit (Promega, USA) in a luminometer (FLUOstar Omega, BMG labtech, Germany). Firefly luciferase activity was normalized to Renilla luciferase activity to account for transfection efficiency,.
2.13. 8-hydroxydeoxyguanosine (8-OHdG) analysis
DNA was isolated using DNA extraction kit (Geneaid, UKAS) according to the protocol’s instruction and the RNA-free DNA obtained was used to determine 8-OHdG levels using Oxiselect oxidative DNA damage ELISA kit (cell Biolabs, San Diego, CA) according to manufacturer’s instructions.
2.14. Statistical analysis
Data are expressed as means±standard deviation of the mean (SD) of at least three separate experiments (n≥3) performed on different days using freshly prepared reagents. The significance of non-irradiated controls or individual treatment groups in comparison to the UVA-irradiated groups was evaluated by independent t-test (Student’s; 2 populations) or one-way analysis of variance (ANOVA) followed by Tukey or Dunnett tests, where appropriate, using Prism (GraphPad Software Inc., San Diego, CA).
3. Results
3.1. SiRNA knockdown of Nrf2 in HEMn and B16F10 cells enhanced melanogenesis in response to UVA irradiation
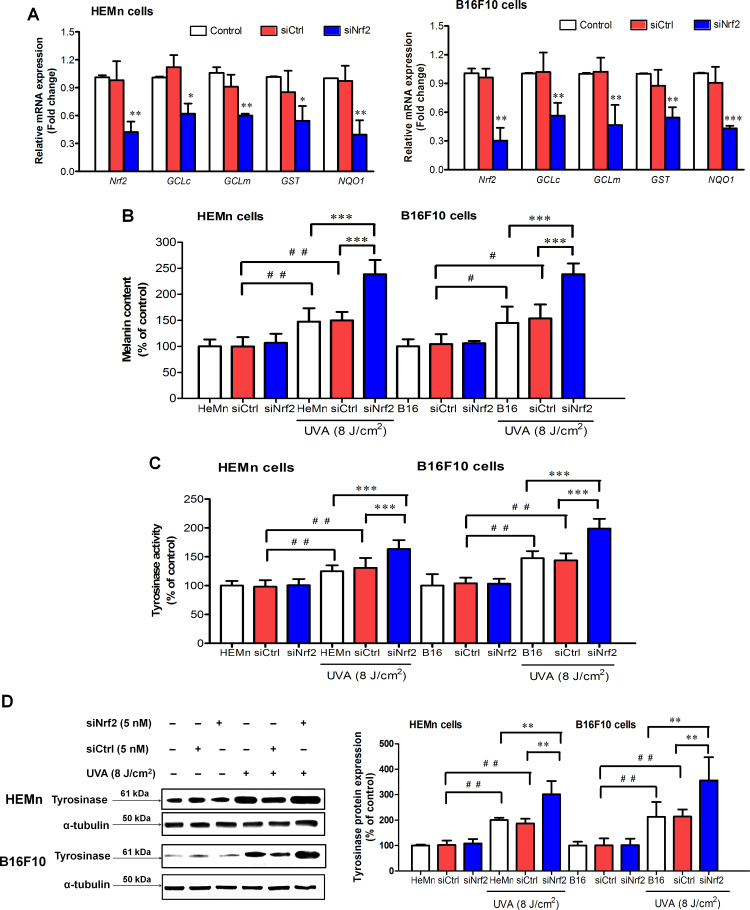
To verify efficacy of siRNA against Nrf2, mRNA levels of Nrf2 and its target antioxidants and Nrf2 protein of transfected HEMn and B16F10 cells were evaluated by real-time RT-PCR (Fig. 1A) and western blot (Supplementary Figure 1), respectively, at 48 h after transfection with either Nrf2-siRNA or siCtrl. Fig. 1A showed a pronounced reduction of Nrf2 mRNA by ∼70% and mRNA levels of its target antioxidants including GCL and GST by ∼50% as well as NQO1 by ∼60% in both HEMn and B16F10 cells compared with untransfected and siCtrl cells. Nrf2-siRNA used in this study thus efficiently reduced Nrf2 and its downstream target genes and protein levels in both HEMn and B16F10 cells. Expression of Nrf2 and its target genes and protein levels in cells transfected with siCtrl were not different from the untransfected cells.
Fig. 1.
Effects of Nrf2 knockdown on melanogenesis in HEMn and B16F10 cells in response to UVA irradiation. (A) HEMn and B16F10 cells were transfected with 5 nM Nrf2-siRNA (siNrf2) or non-silencing siRNA control (siCtrl) for 48 h. mRNA levels of Nrf2 and its target antioxidants (GCLC, GCLM, GST and NQO1) of HEMn and B16F10 cells transfected with siNrf2 were evaluated by real-time RT-PCR. *P<0.05; **P<0.01; ***P<0.001 versus siCtrl-transfected cells. (B) Melanin content and (C) tyrosinase activity were measured in HEMn and B16F10 cells transfected with siNrf2 or siCtrl at 1 h following UVA (8 J/cm2) irradiation. (D) Tyrosinase protein expression was measured in HEMn and B16F10 cells transfected with siNrf2 or siCtrl at 24 h post-irradiation. Data was expressed as mean±SD. The statistical significance of differences was evaluated by one-way ANOVA followed by Dunnett’s test. #P<0.05; ##P<0.01 versus siCtrl-transfected cells without UVA irradiation. **P<0.01; ***P<0.001 versus siNrf2-transfected cells irradiated with UVA.
The effects of Nrf2 on melanogenesis were examined in HEMn and B16F10 cells with and without UVA exposure. UVA irradiation led to a significant induction of melanin content and tyrosinase activity as well as a substantial upregulation of tyrosinase protein in untransfected HEMn and B16F10 cells and siCtrl-transfected cells. Our findings indicated that partial knockdown of Nrf2 significantly stimulated melanin content (Fig. 1B) as well as activity (Fig. 1C) and protein expression (Fig. 1D) of tyrosinase in both HEMn and B16F10 cells compared to siCtrl-transfected cells in response to UVA exposure. Nevertheless, when the cells were not exposed to UVA, levels of melanogenesis in both HEMn and B16F10 cells treated with siNrf2 were comparable to those in siCtrl-transfected cells.
3.2. The test phenolics inhibited UVA-induced melanin content as well as tyrosinase activity and protein expression in B16F10 cells
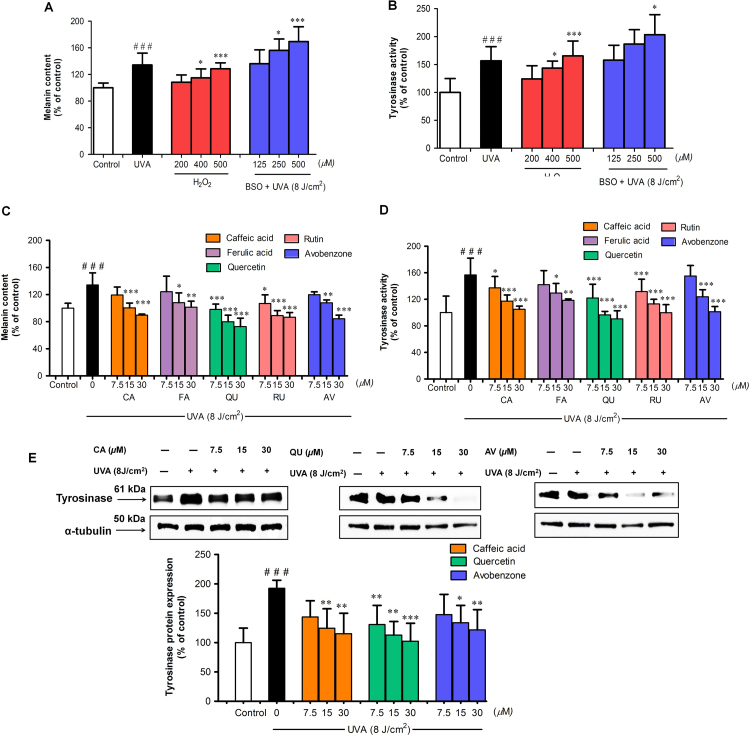
Since our findings suggested that Nrf2 could play a role in melanogenesis upon UVA challenge, we then examined whether antimelanogenic mechanisms of antioxidant phenolics with different UVA blocking properties involved modulation of Nrf2-mediated antioxidant responses. Our data showed that pattern of melanogenesis in response to UVA exposure was similar between Nrf2-depleted HEMn and B16F10 cells. B16F10 cells could therefore be used for further assessment of antimelanogenic effects of test compounds in association with modulation of Nrf2. We evaluated whether cellular oxidative stress affected melanogenesis, which was enhanced in Nrf2-depleted B16F10 cells in response to UVA irradiation, and observed that the treatment of B16F10 cells with H2O2 alone for 30 min and with BSO for 24 h prior to UVA exposure led to a pronounced induction of melanin content (Fig. 2A) and tyrosinase activity (Fig. 2B).
Fig. 2.
The protective effects of CA, FA, QU, RU and AV on UVA-induced melanogenesis in B16F10 cells. (A) Melanin content and (B) tyrosinase activity of B16F10 cells pretreated with H2O2 alone for 30 min and with BSO for 24 h prior to UVA (8 J/cm2) exposure. (C) Melanin and (D) tyrosinase activity of cells pretreated with test compounds prior to UVA (8 J/cm2) exposure. Levels of melanin content and tyrosinase activity were measured at 1 h after UVA irradiation. (E) Tyrosinase protein level was examined by western blot analysis at 24 h after UVA irradiation. Data was represented as the means±SD from at least three independent experiments. The statistical significance of differences between the control and irradiated cells was evaluated by Student’s t test and between UVA-irradiated and compounds-treated cells by one-way analysis of variance (ANOVA) with Dunnett’s multiple comparison test. ###P<0.001 versus unirradiated control cells. *P<0.05; **P<0.01; ***P<0.001 versus untreated cells exposed to UVA.
Our study then assessed inhibitory effects of phenolics with partial UVA absorption properties; CA and FA, phenolics with strong UVA absorption properties; QU and RU, and AV; an effectient chemical UVA filter, at non-toxic concentrations on UVA-dependent melanogenesis in B16F10 cells. A drastic augmentation in melanin production (Fig. 2C), tyrosinase activity, (Fig. 2D) and tyrosinase protein expression (Fig. 2E) was found in B16F10 cells irradiated with a UVA dose of 8 J/cm2. Nevertheless, treatment with test compounds before UV irradiation led to a pronounced inhibition of melanin production and tyrosinase activity. Based on the IC30 values, the rank order of test compounds’ abilities to inhibit UVA-mediated melanin content and tyrosinase activitiy was QU>RU≈CA≈AV>FA (Table 2), suggesting that CA produced greater inhibitory effect than FA and QU than RU. Thus, CA, QU and AV, a UVA sunscreen, were chosen for further experiments. Our findings demonstrated that all test compounds suppressd tyrosinase protein expression in UVA-irradiated B16F10 cells compared to irradiated cells in the absence of test compounds. Moreover, QU was found to yield the highest activity against UVA-induced melanogenesis.
Table 2.
IC30 values of the test compounds for inhibition of tyrosinase activity and melanin content in B16F10 cells exposed to UVA irradiation.
| Test compounds |
IC30(μM) |
|
|---|---|---|
| Inhibition of melanin content | Inhibition of tyrosinase activity | |
| Caffeic acid | 17.54±4.8** | 24.1±6.2*** |
| Ferulic acid | >30 | >30 |
| Quercetin | 7.8±1.4 | 10.1±3.1 |
| Rutin | 15.31±4.7* | 18.56±4.2* |
| Avobenzone | 21.94±6.2*** | 24.25±5.9*** |
Data are mean±SD from at least three independent experiments. The statistical significance of differences in the IC30 values for different compounds was evaluated by one-way ANOVA followed by Tukey’s post hoc test.
p<0.05;
p<0.01;
p<0.001 compared with IC30 values of QU
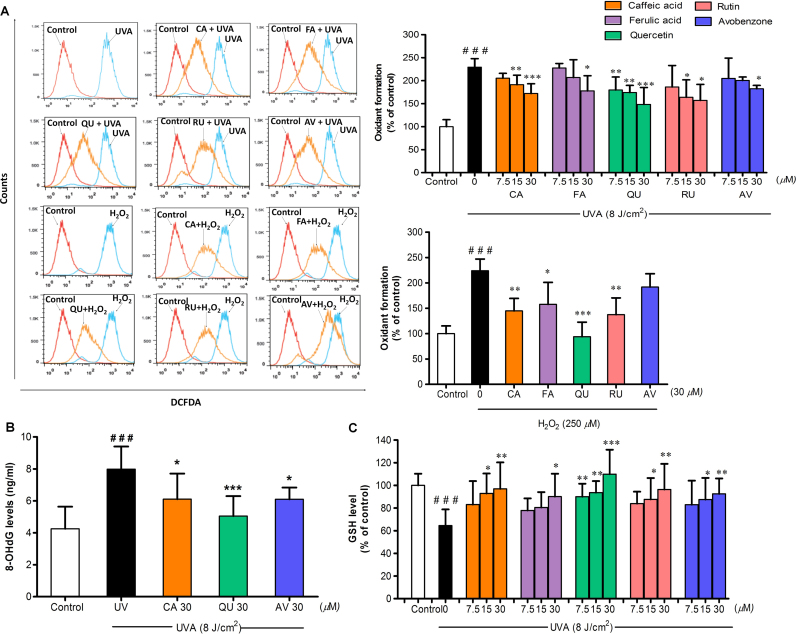
3.3. The test phenolics inhibited UVA-induced ROS formation, 8-OHdG DNA damage and GSH depletion in B16F10 cells
To examine whether antimelanogenic effects of test compounds associated with their inhibitory actions against UVA-mediated cellular oxidative stress, we determined formation of ROS and 8-OHdG, a well-recognized oxidative damage biomarker of skin photodamage [25], as well as level of GSH, considered an indicative of cellular oxidative stress, in response to a UVA challenge. At 1 h following irradiation, UVA irradiation substantially induced ROS formation and depleted GSH level in irradiated B16F10 cells as compared to non-irradiated cells, although the presence of all test phenolics and AV reversed UVA-mediated ROS production (Fig. 3A) and GSH reduction (Fig. 3C) as compared to irradiated B16F10 cells without treatment with test compounds. Additionally, treatment with CA, QU and AV was observed to markedly suppress 8-OHdG formation in B16F10 cells exposed to UVA irradiation (Fig. 3B). We also evaluated the protective effects of CA, QU and AV against increased 8-OHdG levels induced by H2O2 challenge and observed that CA and QU but not AV were able to inhibit H2O2-mediated 8-OHdG formation, suggesting that AV exerted photoprotective effects against oxidative DNA damage probably through sunscreen action but not antioxidant action.
Fig. 3.
The protective effects of CA, FA, QU, RU and AV on UVA-induced oxidative damage and GSH depletion. (A) Oxidant formation was examined in B16F10 cells pretreated with test compounds at 1 h after UVA irradiation (8 J/cm2) and H2O2 (250 µM) treatment. Determination of DCFDA was performed by flow cytometry and data was expressed as a percentage of control (100%, non-irradiated and untreated cells). (B) 8-OHdG and (C) GSH levels were determined at 1 h after UVA irradiation. Data was expressed as mean±SD from at least three independent experiments. The statistical significance of differences between the control and UVA or H2O2-treated cells was evaluated by Student’s t test and between UVA or H2O2-treated and compounds-treated cells by one-way ANOVA followed by Dunnett’s test. ###P<0.001 versus unirradiated control cells. *P<0.05; **P<0.01; ***P<0.001 versus untreated cells subjected to UVA or H2O2.
In addition, all test compounds at the highest concentrations (30 μM) used did not substantially affect melanin content, tyrosinase activity and oxidant formation in the cells without UVA irradiation (Supplementary Figure 2).
3.4. CA, QU and AV inhibited UVA-mediated downregulation of nuclear Nrf levels and Nrf2-ARE transcriptional activity in B16F10 cells
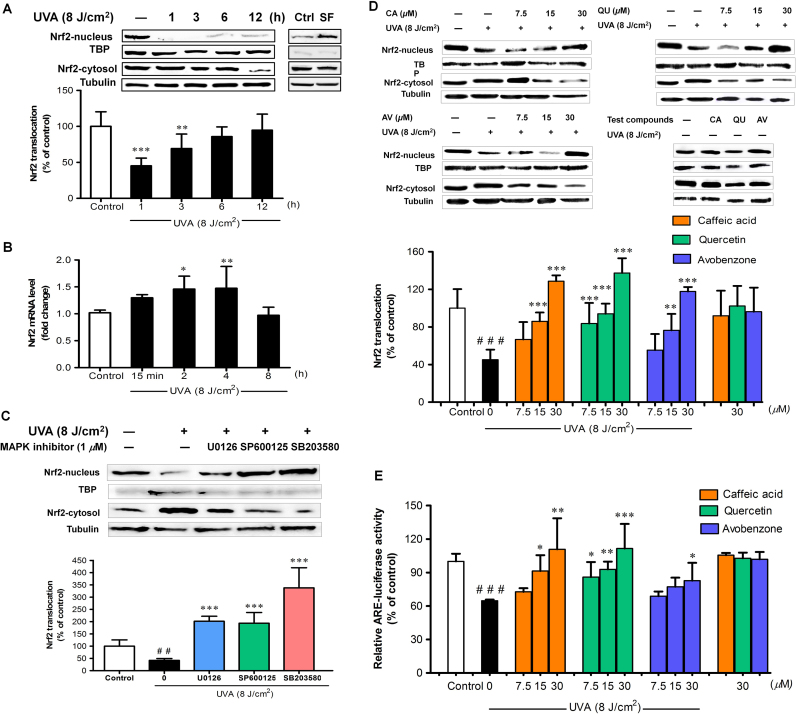
Nrf2, which binds to the ARE, is crucial for transcriptional regulation of antioxidant enzymes. We further examined whether antimelanogenic effects of test phenolics involved modulation of Nrf2 nuclear accumulation and its transcriptional activity, we assessed nuclear/cytosolic Nrf2 ratio indicating nuclear translocation of Nrf2 and ARE luciferase activity. As shown in Fig. 4A, UVA (8 J/cm2) irradiation was found to mediate time-dependent alterations in Nrf2 nuclear accumulation. A pronounced decrease in nuclear/cytosolic Nrf2 ratio in irradiated cells was observed at 1 h following UVA exposure compared to non-irradiated cells, although a substantial recovery in the nuclear/cytosolic Nrf2 ratio was detected by 6 h in irradiated cells compared to non-irradiated control cells. Moreover, while exposure to UVA resulted in a marked decline in Nrf2 nuclear translocation and transactivation at 1 h following irradiation, time-dependent elevation of Nrf2 mRNA levels was observed in irradiated cells at 2 and 4 h and a decline to the basal level was found by 8 h after UVA exposure (Fig. 4B).
Fig. 4.
The protective effects of CA, QU and AV on UVA-suppressed Nrf2 nuclear translocation and Nrf2-ARE transcriptional activity. (A) Time-dependent effects of UVA (8 J/cm2) on Nrf2 nuclear translocation in B16F10 cells harvested at various times after UVA exposure. Western blot was performed to determine Nrf2 nuclear translocation at 1, 3, 6 and 12 h post-irradiation. Nrf2 was detected at 68 kDa, TATA binding protein (TBP), the loading control for nuclear protein, at 37 kDa and and α-Tubulin, the loading control for cytosol protein, at 50 kDa. Cells treated with 10 µM of sulforaphane (SF) for 6 h was used as positive control. (B) Nrf2 mRNA was assessed at 15 min, 2, 4 and 8 h after UVA exposure. *P<0.05; **P<0.01; ***P<0.001 versus unirradiated control cells. (C) Nrf2 nuclear accumulation was detected in B16F10 cells pretreated with 1 µM of ERK inhibitor (U0126), JNK inhibitor (SP600125) and p38 inhibitor (SB203580) for 1 h before UV exposure and harvested at 1 h post-irradiation. Protection against UVA-dependent reduced (D) nuclear translocation of Nrf2 and (E) Nrf2-ARE activity by test compounds was examined in B16F10 cells at 1 h after UVA irradiation. Nrf2 activity was determined by a dual luciferase assay after transfection of the cells with ARE luciferase reporter construct. The data are represented as means±SD of three independent experiments. The statistical significance of differences between the control and irradiated cells was evaluated by Student’s t test and between UVA-irradiated and compounds-treated cells by one-way analysis of variance (ANOVA) with Dunnett’s multiple comparison test. ###P<0.001 versus unirradiated control cells. *P<0.05; **P<0.01; *** P< 0.001 versus untreated cells exposed to UVA.
Since MAPK signaling pathway was suggested to modulate Nrf2 at protein levels, to further support above findings indicating downregulation of Nr2 nuclear accumulation at 1 h post-irradiation, we tested the effect of specific inhibitors of ERK, JNK and p38 MAPK pathways on Nrf2 nuclear translocation in response to UVA irradiation. Induction of phosphorylated-MAPK protein levels including pERK, pJNK and pp38 was observed within 5 min and remained increased for 30 min following UVA irradiation (Supplementary Figure 3). Our findings demonstrated that nuclear to cytosolic Nrf2 ratios were markedly enhanced by blocking the ERK, JNK and p38 pathway with U0126, SP600125 and SB203580 at non-cytotoxic concentrations of 1 μM in irradiated B16F10 cells compared to irradiated cells without MAPK inhibitors (Fig. 4C).
Protective effects of CA, QU and AV on UVA-induced downregulation of Nrf2 nuclear accumulation and its transcriptional activity were further evaluated. In consistent with Nrf2 nuclear translocation data, exposure of B16F10 cells to UVA resulted in reduction of ARE luciferase activity at 1 h post-irradiation. Treatment with test compounds prior to UVA irradiation reversed UVA-mediated downregulation of nuclear/cytosolic Nrf2 ratio (Fig. 4D) and ARE luciferase activity (Fig. 4E) as compared to irradiated B16F10 cells without compound treatment. In addition, test phenolics at the highest concentration did not affect nuclear Nrf2 levels (Fig. 4D) and Nrf2-ARE activity in non-irradiated cells (Fig. 4E).
3.5. CA, QU and AV inhibited UVA-mediated mRNA downregulation of γ-GCLC, γ-GCLM, GST and NQO1 in B16F10 cells
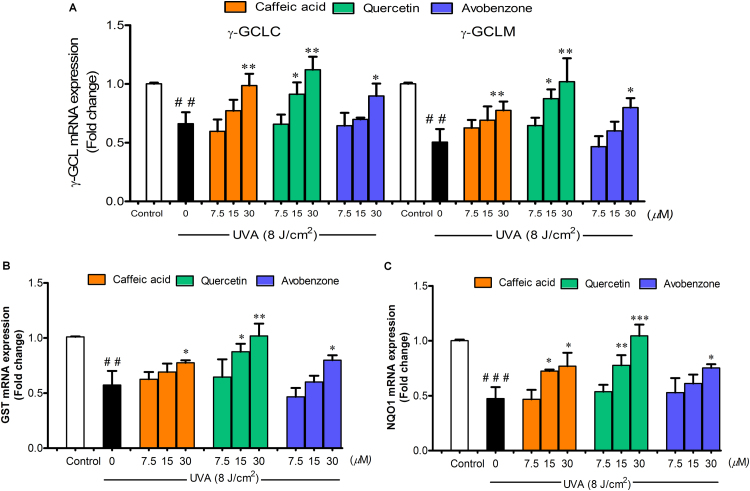
We further investigated whether the their inhibitory effects involved the transcriptional modulation of Nrf2 target genes. As shown in Fig. 5, UVA caused a significant decline in mRNA levels of γ-GCLC and γ-GCLM (Fig. 5A), GST (Fig. 5B) and NQO1 (Fig. 5C) at 2 h post-irradiation compared to non-irradiated cells, although pretreatment with test compounds was able to reverse UVA-mediated downregulation of all genes studied.
Fig. 5.
The protective effects of CA, QU and AV on UVA-mediated downregulation of Nrf2 target genes. (A) γ-GCL (γ-GCLC and γ-GCLM), (B) GST and (C) NQO1 mRNA expressions were assessed by real-time RT-PCR analysis at 2 h after UVA irradiation in B16F10 cells pretreated with test compounds. The statistical significance of differences between the control and irradiated cells was evaluated by Student’s t test and between UVA-irradiated and compounds-treated cells by one-way analysis of variance (ANOVA) with Dunnett’s multiple comparison test. ##P<0.01; ###P<0.001 versus unirradiated control cells. *P<0.05; **P<0.01; ***P<0.001 versus untreated cells exposed to UVA.
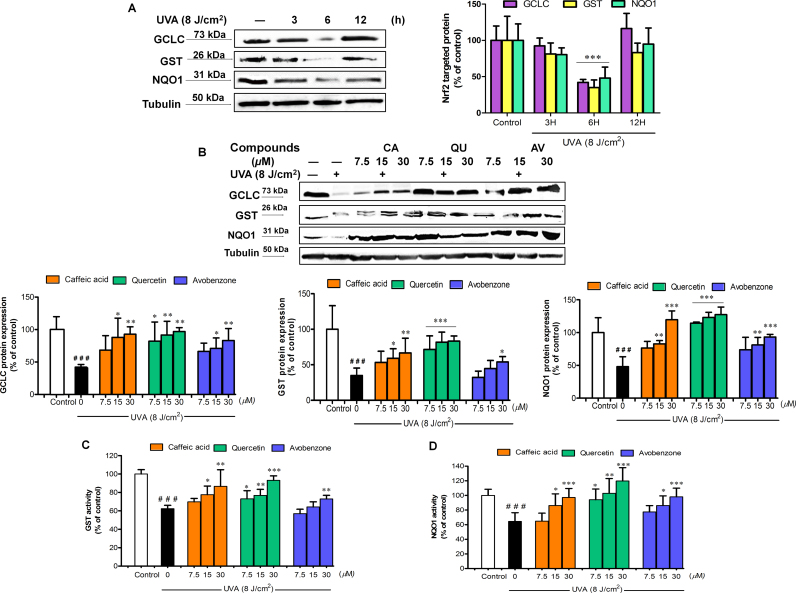
3.6. CA, QU and AV inhibited UVA-induced decreased protein expression and activity of Nrf2 target antoxidants in B16F10 cells
We further assessed whether test compounds also have abilities to modulate Nrf2-mediated antioxidant response. In accordance with results showing time-dependent effect of UVA irradiation on Nrf2 nuclear accumulation, UVA irradiation was observed to result in time-course changes in protein expression of Nrf2 target antioxidants including GCLC, GST and NQO1 (Fig. 6A). A marked reduction of GCLC, GST and NQO1 protein expressions in UVA-irradiated cells was observed at 6 h post-irradiation as compared to non-irradiated cells, although a substantial restoration in the antioxidant protein levels was detected by 12 h. To investigate whether the test compounds could restore UVA-mediated impairment of Nrf2 target antioxidants, we therefore determined whether test compounds were able to abrogate UVA-mediated reduction of antioxidant protein levels and activities at 4 h post-irradiation. As shown in Fig. 6B, while a decline in GCLC, GST and NQO1 protein expressions as well as in enzyme activities of GST and NQO1 in response to UVA exposure was observed, treament with test compounds prior to UVA challenge provided a concentration-dependent induction in GST (Fig. 6C) and NQO1 (Fig. 6D) activities in irradiated cells. In addition, pretreatment with all phenolics did not affect activities of the antioxidant enzymes studied in non-irradiated cells (Supplementary Figure 4).
Fig. 6.
The protective effects of test compounds on UVA-mediated downregulation of Nrf2 target protein and antioxidant enzyme activities. (A) Time-dependent effects of UVA (8 J/cm2) on Nrf2 target proteins (GCLC, GST and NQO1) in B16F10 cells were determined by Western blot analysis at 3, 6 and 12 h post-irradiation. ***P<0.001 versus unirradiated control cells. (B) At 6 h after UVA irradiation, Nrf2 target proteins were examined in B16F10 cells pretreated with test compounds (CA, QU and AV) at 7.5–30 µM. GCLC, GST, NQO1 and the loading control (α-Tubulin) were detected at 73, 25, 31 and 50 kDa, respectively. Enzyme activities of (C) GST and (D) NQO1 in B16F10 cells pretreated with test compounds (CA, QU and AV) at 6 h after UVA irradiation. The statistical significance of differences between the control and irradiated cells was evaluated by Student’s t test and between UVA-irradiated and compounds-treated cells by one-way analysis of variance (ANOVA) with Dunnett’s multiple comparison test. ###P<0.001 versus unirradiated control cells. *P<0.05; **P<0.01; ***P<0.001 versus untreated cells exposed to UVA.
4. Discussion
Nrf2 is a master regulator of antioxidant and cytoprotective genes in response to environmental insults including UV irradiation and hence plays a beneficial role in protection against photooxidative stress in the skin cells including melanocytes [10], [11], although whether modulation of Nrf2 by phytochemicals can protect against UVA-dependent melanogenesis has not been reported. Our findings suggested that while genetic silencing of Nrf2 without UVA challenge did not affect melanogenesis, an immediate increase in melanin content as well as tyrosinase activity and protein expression was observed in Nrf2-depleted HEMn and B16F10 cells in response to UVA exposure. It is possible that, under oxidative insults induced by UVA, defective Nrf2 may promote melanogenesis through increased ROS formation and depleted GSH level. Our observations are in agreement with a previous study suggesting a protective role of Nrf2 in melanogenesis [12].
UV irradiation and alpha-melanocyte-stimulating hormone can cause an immediate stimulation of tyrosinase activity and increase biosynthesis of melanin through transcriptional and translational upregulation of tyrosinase possibly through ROS-related signaling [26], [27], [28]. Our findings indicated that UVA irradiation was able to stimulate melanin production as well as activity and protein level of tyrosinase in association with oxidative stress, indicated by enhanced formation of ROS and 8-OHdG as well as GSH depletion.
A number of studies suggested that phytochemicals having antioxidant activities produced benefical effects against photodamage and photocarcinogenesis of the skin through promotion of Nrf2 [29], [30], [31], [32]. We addressed whether antimelanogenic mechanisms of dietary phytochemicals with different UVA-absorbing properties involved modulation of Nrf2-regulated antioxidant defenses. CA and FA having partial UVA absorption properties, QU and RU with strong UVA absorption properties as well as AV, which possessed UVA blocking but not antioxidant properties, were shown to suppress UVA-induced melanogenesis in correlation with abrogation of ROS and oxidative DNA damage formation as well as GSH loss in B16F10 cells. We further evaluated the effects of UVA challenge on Nrf2-ARE signaling and its downstream antioxidants and observed that a single dose of UVA irradiation led to time-dependent alterations of nuclear accumulation of Nrf2 and its target antioxidant proteins including GCLC, GST and NQO1 in B16F10 cells. While UVA-mediated downregulation of Nrf2 was not achieved at transcriptional level, a decrease in Nrf2 nuclear translocation and its transcriptional activity occurred as early as 1 h post-irradiation. Additionally, UVA was shown to cause a pronounced downregulation of mRNA and protein expressions of its target antioxidants (GCLC, GST and NQO1), although a recovery of nuclear Nrf2 level and protein levels of its target antioxidants was observed at later time points. Control of Nrf2 nuclear translocation crucial for its function in Nrf2 antioxidant response pathway is complicated. While nuclear accumulation of Nrf2 was enhanced by UVB at low dose in mouse hepatoma, human skin fibroblast and keratinocyte cells as well as by UVA in dermal fibroblasts after 2 h, a high dose of UVB irradiation was found to diminish nuclear translocation of Nrf2 [33]. Moreover, depletion of peroxiredoxin I, which can be induced by oxidative stimuli, was observed to reduce Nrf2 expression, leading to increased sensitivity to UVA exposure in mouse embryonic fibroblasts (MEF) [34]. In response to ROS-mediated DNA damage, activation of p53, which played a vital role in stimulation of apoptosis, led to suppression of Nrf2-dependent transcription of antioxidant response genes [35].
Furthermore, oxidative stress could either upregulate or downregulate Nrf2 nuclear translocation through upstream signaling kinases. We then investigated the role of MAPK signaling in UVA-mediated Nrf2 nuclear translocation in B16F10 cells and found that, under oxidative stress induced by UVA radiation, suppression of ERK, JNK and p38 MAPK pathways resulted in diminished nuclear/cytosolic ratio of Nrf2 in response to UVA challenge in B16F10 cells. Downregulation of Nrf2 nuclear accumulation was possibly attributed to UVA-dependent upregulation of phosphorylated-MAPK pathways. Previous studies also observed that interference with kinase signaling pathways including ERK and p38 MAPK was shown to cause reduction of Nrf2 nuclear translocation and its transcriptional activity in microglia, neuronal cells and cardiac cells [36], [37], [38] and homocysteic acid was found to induce oxidative stress through downregulation of Nrf2 pathways in association with increased JNK phosphorylation in HT-22 neuronal cells [39]. Taken together with our findings showing that oxidative stress could either upregulate or downregulate Nrf2 through upstream signaling kinases, oxidative insults may play a dual role in control of Nrf2 that is dependent on cell types, intensity of oxidative stimuli, time after exposure to stress, basal Nrf2 level and Keap-1 function.
This study and our previous findings suggested that a transient downregulation of nuclear Nrf2 and its downstream antioxidants could be an early response to a single UV dose and adaptive response to oxidative stress leads to restoration of antioxidant defenses at translational and transcriptional levels at later time points, probably through upregulation of Nrf2 in order to maintain redox balance [6].
We further determined the effects of test compounds on UVA-mediated diminished downstream antioxidant enzymes through modulation of Nrf2 in B16F10 cells. Our results suggested that test compounds (CA, QU and AV) could potentially reverse downregulation of Nrf2 nuclear translocation and Nrf2-ARE activity at 1 h following UVA irradiation in B16F10 cells. Findings from this study also indicated that pretreatment with CA, QU and AV could restore UVA-mediated reduction of Nrf2 target antioxidant genes and proteins including GCLC, GST and NQO1 and the corresponding enzyme activites in B16F10 cells. Previous studies using cultured skin fibroblasts, keratinocytes and reconstructed human skin model demonstrated that treatment with several electrophiles or phytochemicals for longer period (up to 4–48 h) caused upregulation and activation of Nrf2 in association with the photoprotective actions Electrophilic compounds known as selective and potent Nrf2 activators have been widely investigated and suggested to play a role in protecting the skin against environmental stressors [40], [41], [42], [43], [44]. Nevertheless, our study demonstrated that test compounds did not provide a direct regulatory effect on Nrf2 because treatment with test compounds alone for 30 min did not significantly affect ROS formation nor Nrf2 nuclear translocation and transcriptional activity detected at later time-points in non-irradiated cells.
Therefore, we proposed here that antioxidant and UVA blocking compounds could potentially provide an early protection against UVA-induced oxidative stress in correlation with enhanced melanogenesis, probably through indirect regulation of Nrf2-ARE pathway. Moreover, QU was shown to yield the inhibitory effects at lower doses than those of other compounds in all the experiments performed in this study, indicating that, QU, a powerful antioxidant having strong UVA absorption property, may produce the greatest protective effects on UVA-mediated melanogenesis, oxidative damage and downregulation of Nrf2 and its downstream antioxidants. Hence, abilities to reverse impaired Nrf2 signaling pathway is probably associated with antioxidant potentials of photoprotective agents.
Redox regulation of pigmentation through Nrf2-regulated antioxidant responses is complex. Impaired Nrf2-ARE signaling associated with melanocyte degeneration by oxidative stress that could be implicated in depigmentation should also be taken into account [11]. Additionally, Nrf2 is tightly regulated in the cytosol by Keap-1 and further studies are thus needed concerning the effects of phytochemicals on function of Keap-1 in regulation of melanogenesis in response to UV irradiation.
In summary, depletion of Nrf2 could stimulate melanogenesis under UVA irradiation possibly through oxidative stress mechanisms. In this respect, targeting Nrf2-mediated antioxidant defenses may be a potential strategy for prevention and inhibition of skin hyperpigmentation. Test phenolics exhibited antimelanogenic effect in correlation with their antioxidant potentials against UVA-mediated downregulation of Nrf2 and its downstream antioxidants in B16F10 cells. Indirect modulation of Nrf2-ARE pathway to promote redox balance by photoprotective compounds with antioxidant or sunscreen actions may provide a pharmacological insight for protection against photooxidative damage and hyperpigmentation.
Conflict of interest
The authors have no conflicts of interest to declare.
Funding sources
This work was supported by Thailand Research Fund (Grant no. RSA5580012); “Mahidol University” Grant; and the “Chalermphrakiat” Grant, Faculty of Medicine Siriraj Hospital, Mahidol University.
Ackonwledgements
We are grateful to Dr. Siwanon Jirawatnotai, Department of Pharmacology and Dr. Naravat Poungvarin, Department of Clinical Pathology, Faculty of Medicine Siriraj Hospital, Mahidol University, for technical assistance and advice.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2015.12.006.
Appendix A. Supplementary material
Supplementary material
References
- 1.Hu Z.M., Zhou Q., Lei T.C., Ding S.F., Xu S.Z. Effects of hydroquinone and its glucoside derivatives on melanogenesis and antioxidation: Biosafety as skin whitening agents. J. Dermatol. Sci. 2009;55:179–184. doi: 10.1016/j.jdermsci.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Panich U., Tangsupa-a-nan V., Onkoksoong T., Kongtaphan K., Kasetsinsombat K., Akarasereenont P., Wongkajornsilp A. Inhibition of UVA-mediated melanogenesis by ascorbic acid through modulation of antioxidant defense and nitric oxide system. Arch. Pharm. Res. 2011;34:811–820. doi: 10.1007/s12272-011-0515-3. [DOI] [PubMed] [Google Scholar]
- 3.Maddodi N., Setaluri V. Role of UV in cutaneous melanoma. Photochem. Photobiol. 2008;84:528–536. doi: 10.1111/j.1751-1097.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 4.Swalwell H., Latimer J., Haywood R.M., Birch-Machin M.A. Investigating the role of melanin in UVA/UVB- and hydrogen peroxide-induced cellular and mitochondrial ROS production and mitochondrial DNA damage in human melanoma cells. Free Radic. Biol. Med. 2012;52:626–634. doi: 10.1016/j.freeradbiomed.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Baldea I., Mocan T., Cosgarea R. The role of ultraviolet radiation and tyrosine stimulated melanogenesis in the induction of oxidative stress alterations in fair skin melanocytes. Exp. Oncol. 2009;31:200–208. [PubMed] [Google Scholar]
- 6.Panich U., Onkoksoong T., Limsaengurai S., Akarasereenont P., Wongkajornsilp A. UVA-induced melanogenesis and modulation of glutathione redox system in different melanoma cell lines: the protective effect of gallic acid. J. Photochem. Photobiol. B. 2012;108:16–22. doi: 10.1016/j.jphotobiol.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Slocum S.L., Kensler T.W. Nrf2: control of sensitivity to carcinogens. Arch. Toxicol. 2011;85:273–284. doi: 10.1007/s00204-011-0675-4. [DOI] [PubMed] [Google Scholar]
- 8.López-Camarillo C., Ocampo E.A., Casamichana M.L., Pérez-Plasencia C., Alvarez-Sánchez E., Marchat L.A. Protein kinases and transcription factors activation in response to UV-radiation of skin: implications for carcinogenesis. Int. J. Mol. Sci. 2012;13:142–172. doi: 10.3390/ijms13010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wölfle U., Seelinger G., Bauer G., Meinke M.C., Lademann J., Schempp C.M. Reactive molecule species and antioxidative mechanisms in normal skin and skin aging. Skin Pharmacol. Physiol. 2014;27:316–332. doi: 10.1159/000360092. [DOI] [PubMed] [Google Scholar]
- 10.Schäfer M., Werner S. Nrf2-A regulator of keratinocyte redox signaling. Free Radic. Biol. Med. 2015;88:243–252. doi: 10.1016/j.freeradbiomed.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Jian Z., Li K., Song P., Zhu G., Zhu L., Cui T., Liu B., Tan L., Wang X., Wang G., Gao T., Li C. Impaired activation of the Nrf2-ARE signaling pathway undermines H2O2-induced oxidative stress response: a possible mechanism for melanocyte degeneration in vitiligo. J. Investig. Dermatol. 2014;134:2221–2230. doi: 10.1038/jid.2014.152. [DOI] [PubMed] [Google Scholar]
- 12.Shin J.M., Kim M.Y., Sohn K.C., Jung S.Y., Lee H.E., Lim J.W., Kim S., Lee Y.H., Im M., Seo Y.J., Kim C.D., Lee J.H., Lee Y., Yoon T.J. Nrf2 negatively regulates melanogenesis by modulating PI3K/Akt signaling. PloS One. 2014;9:e96035. doi: 10.1371/journal.pone.0096035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichols J.A., Katiyar S.K. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korać R.R., Khambholja K.M. Potential of herbs in skin protection from ultraviolet radiation. Pharmacogn. Rev. 2011;5:164–173. doi: 10.4103/0973-7847.91114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Potapovich A.I., Lulli D., Fidanza P., Kostyuk V.A., De Luca C., Pastore S., Korkina L.G. Plant polyphenols differentially modulate inflammatory responses of human keratinocytes by interfering with activation of transcription factors NFkappaB and AhR and EGFR-ERK pathway. Toxicol. Appl. Pharmacol. 2011;255:138–149. doi: 10.1016/j.taap.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Liu D., Hu H., Lin Z., Chen D., Zhu Y., Hou S., Shi X. Quercetin deformable liposome: preparation and efficacy against ultraviolet B induced skin damages in vitro and in vivo. J. Photochem. Photobiol. B. 2013;127:8–17. doi: 10.1016/j.jphotobiol.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Moyal D., Chardon A., Kollias N. UVA protection efficacy of sunscreens can be determined by the persistent pigment darkening (PPD) method (Part 2) Photodermatol. Photoimmunol. Photomed. 2000;16:250–255. doi: 10.1034/j.1600-0781.2000.160603.x. [DOI] [PubMed] [Google Scholar]
- 18.Thangboonjit W., Limsaeng-u-rai S., Pluemsamran T., Panich U. Comparative evaluation of antityrosinase and antioxidant activities of dietary phenolics and their activities in melanoma cells exposed to UVA. Siriraj Med. J. 2014;66:5–10. [Google Scholar]
- 19.Panich U., Kongtaphan K., Onkoksoong T., Jaemsak K., Phadungrakwittaya R., Thaworn A., Akarasereenont P., Wongkajornsilp A. Modulation of antioxidant defense by Alpinia galanga and Curcuma aromatica extracts correlates with their inhibition of UVA-induced melanogenesis. Cell Biol. Toxicol. 2010;26:103–116. doi: 10.1007/s10565-009-9121-2. [DOI] [PubMed] [Google Scholar]
- 20.Panich U., Pluemsamran T., Tangsupa-a-nan V., Wattanarangsan J., Phadungrakwittaya R., Akarasereenont P., Laohapand T. Protective effect of AVS073, a polyherbal formula, against UVA-induced melanogenesis through a redox mechanism involving glutathione-related antioxidant defense, BMC Complement. Altern. Med. 2013;13:159–168. doi: 10.1186/1472-6882-13-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pluemsamran T., Onkoksoong T., Panich U. Caffeic acid and ferulic acid inhibit UVA-induced matrix metalloproteinase-1 through regulation of antioxidant defense system in keratinocyte HaCaT cells. Photochem. Photobiol. 2012;88:961–968. doi: 10.1111/j.1751-1097.2012.01118.x. [DOI] [PubMed] [Google Scholar]
- 22.Bickers D.R., Athar M. Oxidative stress in the pathogenesis of skin disease. J. Invest. Dermatol. 2006;126:2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 23.Siegel D., Kepa J.K., Ross D. Biochemical and genetic analysis of NAD(P)H:quinone oxidoreductase 1 (NQO1) Curr. Protoc. Toxicol. 2007 doi: 10.1002/0471140856.tx0422s32. Unit 4.22. [DOI] [PubMed] [Google Scholar]
- 24.Kato M., Iida M., Goto Y., Kondo T., Yajima I. Sunlight exposure-mediated DNA damage in young adults. Cancer Epidemiol. Biomark. Prev. 2011;20:1622–1628. doi: 10.1158/1055-9965.EPI-11-0228. [DOI] [PubMed] [Google Scholar]
- 25.Jian J., Pelle E., Yang Q., Pernodet N., Maes D., Huang X. Iron sensitizes keratinocytes and fibroblasts to UVA-mediated matrix metalloproteinase-1 through TNF-alpha and ERK activation. Exp. Dermatol. 2011;20:249–254. doi: 10.1111/j.1600-0625.2010.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Legros F., Coel J., Doyen A., Hanson P., Van Tieghem N., Vercammen-Grandjean A., Fruhling J., Lejeune F.J. Alpha-Melanocyte-stimulating hormone binding and biological activity in a human melanoma cell line. Cancer Res. 1981;41:1539–1544. [PubMed] [Google Scholar]
- 27.Peng H.Y., Lin C.C., Wang H.Y., Shih Y., Chou S.T. The melanogenesis alteration effects of Achillea millefolium L. essential oil and linalyl acetate: involvement of oxidative stress and the JNK and ERK signaling pathways in melanoma cells. Int. J. Mol. Sci. 2014;11:1082–1089. doi: 10.1371/journal.pone.0095186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zi S.X., Ma H.J., Li Y., Liu W., Yang Q.Q., Zhao G., Lian S. Oligomeric proanthocyanidins from grape seeds effectively inhibit ultraviolet-induced melanogenesis of human melanocytes in vitro. Int. J. Mol. Med. 2009;23:197–204. [PubMed] [Google Scholar]
- 29.Andreadi C.K., Howells L.M., Atherfold P.A., Manson M.M. Involvement of Nrf2, p38, B-Raf, and nuclear factor-kappaB, but not phosphatidylinositol 3-kinase, in induction of hemeoxygenase-1 by dietary polyphenols. Mol. Pharmacol. 2006;69:1033–1040. doi: 10.1124/mol.105.018374. [DOI] [PubMed] [Google Scholar]
- 30.Chun K.S., Kundu J., Kundu J.K., Surh Y.J. Targeting Nrf2-Keap1 signaling for chemoprevention of skin carcinogenesis with bioactive phytochemicals. Toxicol. Lett. 2014;229:73–84. doi: 10.1016/j.toxlet.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Filip A., Daicoviciu D., Clichici S., Mocan T., Muresan A., Postescu I.D. Photoprotective effects of two natural products on ultraviolet B-induced oxidative stress and apoptosis in SKH-1 mouse skin. J. Med. Food. 2011;14:761–766. doi: 10.1089/jmf.2010.0142. [DOI] [PubMed] [Google Scholar]
- 32.Paredes-Gonzalez X., Fuentes F., Su Z.Y., Kong A.N. Apigenin reactivates Nrf2 anti-oxidative stress signaling in mouse skin epidermal JB6 P+ cells through epigenetics modifications. AAPS J. 2014;16:727–735. doi: 10.1208/s12248-014-9613-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirota A., Kawachi Y., Itoh K., Nakamura Y., Xu X., Banno T., Takahashi T., Yamamoto M., Otsuka F. Ultraviolet A irradiation induces NF-E2-related factor 2 activation in dermal fibroblasts: protective role in UVA-induced apoptosis. J. Investig. Dermatol. 2005;124:825–832. doi: 10.1111/j.0022-202X.2005.23670.x. [DOI] [PubMed] [Google Scholar]
- 34.Ito T., Kimura S., Seto K., Warabi E., Kawachi Y., Shoda J., Tabuchi K., Yamagata K., Hasegawa S., Bukawa H., Ishii T., Yanagawa T. Peroxiredoxin I plays a protective role against UVA irradiation through reduction of oxidative stress. J. Dermatol. Sci. 2014;74:9–17. doi: 10.1016/j.jdermsci.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Faraonio R., Vergara P., Di Marzo D., Pierantoni M.G., Napolitano M., Russo T., Cimino F. p53 suppresses the Nrf2-dependent transcription of antioxidant response genes. J. Biol. Chem. 2006;281:39776–39784. doi: 10.1074/jbc.M605707200. [DOI] [PubMed] [Google Scholar]
- 36.Correa F., Ljunggren E., Mallard C., Nilsson M., Weber S.G., Sandberg M. The Nrf2-inducible antioxidant defense in astrocytes can be both up- and down-regulated by activated microglia: involvement of p38 MAPK. Glia. 2011;59:785–799. doi: 10.1002/glia.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandberg M., Patil J., D’Angelo B., Weber S.G., Mallard C. Nrf2-regulation in brain health and disease: implication of cerebral inflammation. Neuropharmacology. 2014;79:298–306. doi: 10.1016/j.neuropharm.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan Y., Ichikawa T., Li J., Si Q., Yang H., Chen X., Goldblatt C.S., Meyer C.J., Li X., Cai L., Cui T. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress–induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes. 2011;60:625–633. doi: 10.2337/db10-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan M., Ouyang Y., Jin M., Chen M., Liu P., Chao X., Chen Z., Chen X., Ramassamy C., Gao Y. R. Pi, Downregulation of Nrf2/HO-1 pathway and activation of JNK/c-Jun pathway are involved in homocysteic acid-induced cytotoxicity in HT-22 cells. Toxicol. Lett. 2013;223:1–8. doi: 10.1016/j.toxlet.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 40.Benedict A.L., Knatko E.V., Dinkova-Kostova A.T. The indirect antioxidant sulforaphane protects against thiopurine-mediated photooxidative stress. Carcinogenesis. 2012;33:2457–2466. doi: 10.1093/carcin/bgs293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tao S., Justiniano R., Zhang D.D., Wondrak G.T. The Nrf2-inducers tanshinone I and dihydrotanshinone protect human skin cells and reconstructed human skin against solar simulated UV. Redox Biol. 2013;1:532–541. doi: 10.1016/j.redox.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wondrak G.T., Cabello C.M., Villeneuve N.F., Zhang S., Ley S., Li Y., Sun Z., Zhang D.D. Cinnamoyl-based Nrf2-activators targeting human skin cell photo-oxidative stress. Free Radic. Biol. Med. 2008;45:385–395. doi: 10.1016/j.freeradbiomed.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lieder F., Reisen F., Geppert T., Sollberger G., Beer H.D., auf dem Keller U., Schäfer M., Detmar M., Schneider G., Werner S. , Identification of UV-protective activators of nuclear factor erythroid-derived 2-related factor 2 (Nrf2) by combining a chemical library screen with computer-based virtual screening. J. Biol. Chem. 2012;287:33001–33013. doi: 10.1074/jbc.M112.383430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sirerol J.A., Feddi F., Mena S., Rodriguez M.L., Sirera P., Aupí M., Pérez S., Asensi M., Ortega A., Estrela J.M. Topical treatment with pterostilbene, a natural phytoalexin, effectively protects hairless mice against UVB radiation-induced skin damage and carcinogenesis. Free. Radic. Biol. Med. 2015;85:1–11. doi: 10.1016/j.freeradbiomed.2015.03.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material