Abstract
Purpose
Age induces a progressive decline in functional reserve and impacts cancer treatments. Telomere attrition leads to tissue senescence. We tested the hypothesis that telomere length (TL) could predict patient vulnerability and outcome with cancer treatment.
Patients and methods
An ancillary study in the Elderly Women GINECO Trial 3 was performed to evaluate the impact of geriatric covariates on survival in elderly advanced ovarian cancer patients receiving six cycles of carboplatin. TL was estimated from peripheral blood at inclusion using standard procedures.
Results
TL (in base pairs) was estimated for 109/111 patients (median 6.1 kb; range [4.5-8.3 kb]). With a cut-off of 5.77 kb, TL discriminated two patient groups, long telomere (LT) and short telomeres (ST), with significantly different treatment completion rates of 0.80 (95%CI [0.71-0.89]) and 0.59 (95%CI [0.41-0.76]), respectively (odds ratio [OR]=2.8, p=0.02). ST patients were at higher risk of serious adverse events (SAE, OR=2.7; p=0.02) and had more unplanned hospital admissions (OR=2.1; p=0.08). After adjustment on FIGO stage, TL shorter than 6 kb was a risk factor of premature death (HR=1.57; p=0.06).
Conclusion
This exploratory study identifies TL as predictive factor of decreased treatment completion, SAE risk, unplanned hospital admissions and OS after adjustment on FIGO stage.
Keywords: telomere, ovarian cancer, elderly, geriatric, prognostic factor
INTRODUCTION
Aging is associated with a progressive decline in the functional reserve of multiple organ systems [1]. Given that the process of aging is heterogeneous, this decline should ideally be assessed individually and care of an elderly person adapted accordingly rather than solely on the basis of chronological age. Such assessments are currently entirely clinical, based on a geriatric evaluation.
During normal ageing, the gradual loss of telomeric DNA in dividing somatic cells contributes to replicative senescence [2]. Importantly, this telomere length dynamics plays an important signaling role in determining cell fate during aging and cancer [3]. There is growing evidence linking pathologic aging to telomere shortening in prospective studies recruiting elderly patients, although there is some controversy associated with this research. Patients with shorter telomeres tend to develop more functional disabilities [4], have increased cognitive loss [5], higher cardiovascular morbidity [6], more degenerative diseases [7] and higher mortality [8].
In an oncologic context, the impact of aging on a patient's survival is challenged by the nature of the tumor itself, which in turn means a differential impact of geriatric covariates on overall survival (OS). In 1997, the French National Group of Investigators for the Study of Ovarian and Breast Cancer (GINECO) established a research program focused on the treatment of ovarian cancer in elderly women. The feasibility of carboplatin-cyclophosphamide and standard carboplatin AUC5-paclitaxel protocols in patients over 70 years of age was demonstrated in two studies [9,10], with treatment completion rates of 76% and 68% respectively [10]. A multivariate analysis performed in a non-randomized retrospective review of these trials reported a significant negative impact of various geriatric covariates on survival [10]. A prospective trial, the Elderly Woman GINECO Trial 3, was thus initiated to evaluate the impact of geriatric covariates on survival in elderly patients with advanced ovarian cancer treated with six cycles of carboplatin AUC5. A geriatric vulnerability score (GVS) was developed which segregates patients into two groups with significantly different outcomes in terms of treatment completion rates and risk of treatment toxicities (serious and severe adverse events, unplanned hospital admissions, [11]).
An ancillary study was envisaged in the original design of the GINECO Trial 3 with a working hypothesis that telomere biology influences patients' future outcomes and may correlate with a clinical geriatric assessment. The results reported here pinpoint an association between short TL, treatment tolerance and completion in ovarian cancer patients.
RESULTS
An overall decrease in telomere length with age in the patient cohort used in this study
Duplicate telomere length (TL) distribution measurements were performed on blood samples from 109 of the 111 patients included between August 2007 and January 2010. Patient characteristics and outcome of the geriatric assessment are shown in Table 1. Median follow-up was 16.4 months (range 0.2-49.6).
Table 1. Patient and disease characteristics and geriatric assessment.
| N of patients (%) | |
|---|---|
| Median age in years (range) | 78 (70-93) |
| ≥80 years | 44 (40.3) |
| Performance status (ECOG) ≥2 | 51 (46.8) |
| Tumor assessment | |
| FIGO stage IV | 38 (34.9) |
| Complete primary cytoreduction | 18 (16.5) |
| Geriatric assessment | |
| ≥3 comorbidities | 26 (23.9) |
| N comedications | |
| 1-3 | 32 (29.4) |
| 4-6 | 44 (40.4) |
| ≥7 | 30 (27.5) |
| Functional assessment | |
| ADL score <6 | 60 (55.0) |
| IADL score <25 | 76 (69.7) |
| Nutritional assessment | |
| Albuminemia <35 g/L | 64 (58.8) |
| BMI <21 kg/m. | 24 (22.0) |
| Lymphocyte count <1 G/L | 27 (24.8) |
| Psychocognitive assessment | |
| MMS score <25 | 32 (29.4) |
| HADS score >14 | 40 (36.7) |
| GDS score >10 | 39 (35.6) |
ADL: Activities of Daily Living; IADL: Instrumental Activities of Daily Living; BMI: Body Mass Index; ECOG: Eastern Cooperative Oncology Group; GDS: Geriatric Depression Scale; HADS: Hospital Anxiety and Depression Scale; MMS: Mini-Mental Scale
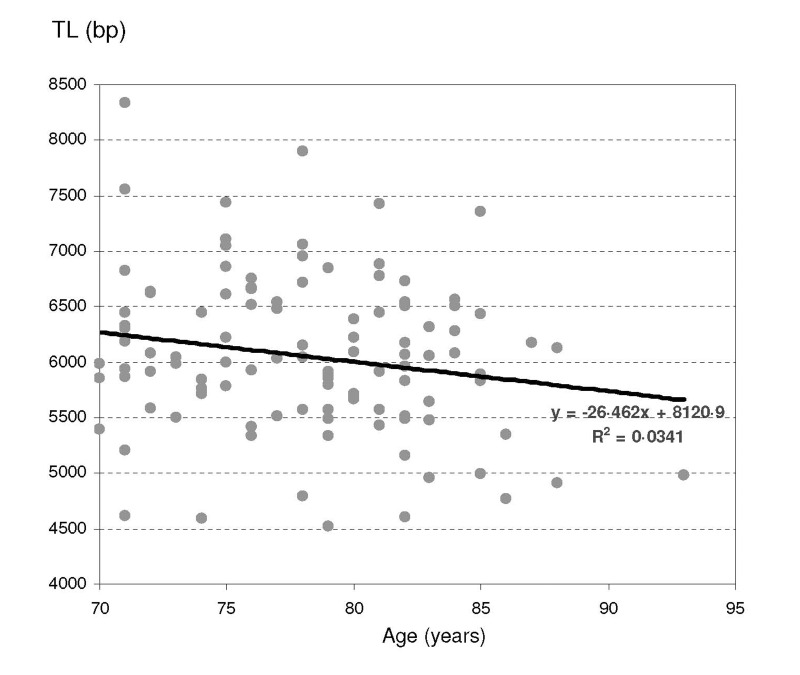
TL ranged from 4.52 to 8.33 kilobases (kb) with a mean of 6.05 kb (SD 0.71 kb). A weak inverted linear correlation with age was demonstrated, with every 1-year increase in age associated with a 26-base pair decrease in mean TL, with a R. ratio of 0.0341 (Figure 1).
Figure 1. Telomere length repartition according to age.
Longer TL in patients that completed their treatment and exhibited a better tolerance
Since treatment completion is considered a meaningful short-term marker of patient outcome, we analyzed for a possible correlation between TL distribution and the fact that patients completed their treatment. In order to analyze variations in the non-gaussian distributions of human TL, we segregated the patients according to TL quartiles (Table 2). Patients with telomeres longer than 6.54 kb had a 52% higher chance of treatment completion than those with telomeres shorter than 5.77 kb (95%CI: 1.00-2.32, P=0.04). Thus, we can identify two groups of patients with different treatment completion rates according to their TL distribution: 80% (95%CI: 71% to 89%) in the group with telomeres longer than 5.77 kb (LT group) versus 59% (95%CI: 41% to 76%) in the group with shorter telomeres (ST group, P=0.02).
Table 2. Association between telomere length parameters and patient outcomes.
| Telomere length | Odds ratio for treatment completion (95%CI) | P-value | Hazard ratio for death (95%CI) (adjusted for FIGO stage) | P-value | |
|---|---|---|---|---|---|
| TL mean | 6.05 kb | 0.56 (0.22-1.27) | 0.15 | 1.42 (0.88-2.29) | 0.15 |
| TL median | 6.00 kb | 0.50 (0.21-1.2) | 0.11 | 1.57 (0.98-2.51) | 0.06 |
| TL quartiles | 1.50 (1.01-2.23) | 0.04 | 0.82 (1.67-1.01) | 0.07 | |
| <5.77 kb | 0.36 (0.15-0.87) | 0.02 | 1.49 (0.91-2.44) | 0.12 | |
| 5.77-6.06 kb | 1.60 (0.54-4.74) | 0.38 | 1.02 (0.59-1.77) | 0.94 | |
| 6.06-6.54 kb | 1.28 (0.46-3.58) | 0.64 | 0.97 (0.55-1.72) | 0.91 | |
| >6.54 kb | 2.08 (0.65-6.67) | 0.19 | 0.62 (0.34-1.14) | 0.12 |
Using the same cut-off of 5.77 kb, TL segregates the same two groups (i.e., ST vs LT) as having different outcomes in terms of tolerance. Serious adverse events were significantly more frequent in the ST group, with an odds ratio of 2.7 (P=0.02). Unplanned hospital admissions and grade 3-4 non-hematological toxicity also tended to be more frequent (Table 3). No significant difference between TL groups could be identified in terms of hematological toxicity, however blood cell counts were only routinely evaluated 1 day prior to chemotherapy.
Table 3. TL repartition of vulnerability criteria and clinical end points between.
| Observed risk:short/long telomere group | 95% CI | P-value | |
|---|---|---|---|
| Treatment completion | 0.36 | 0.15-0.87 | 0.020 |
| Serious Adverse Events | 2.69 | 1.17-6.19 | 0.019 |
| Unplanned hospital admissions | 2.14 | 0.92-4.95 | 0.076 |
| Grade ≥ 3 non-hematological toxicity | 2.04 | 0.88-4.71 | 0.095 |
| Grade ≥ 3 hematological toxicity | 1.32 | 0.58-3.00 | 0.51 |
| Geriatric vulnerability parameters: | |||
| ADL score < 6 | 1.78 | 0.77-4.12 | 0.17 |
| IADL score < 25 | 1.31 | 0.53-3.22 | 0.56 |
| HADS score >14 | 1.89 | 0.82-4.33 | 0.13 |
| Albuminemia <35 g/L | 1.27 | 0.55-2.90 | 0.57 |
| Lymphocytes <1 × 109/L | 1.76 | 0.71-4.36 | 0.22 |
| Geriatric vulnerability score | |||
| GVS ≥3 | 2.06 | 0.90-4.70 | 0.08 |
Correlation between TL distribution and overall survival
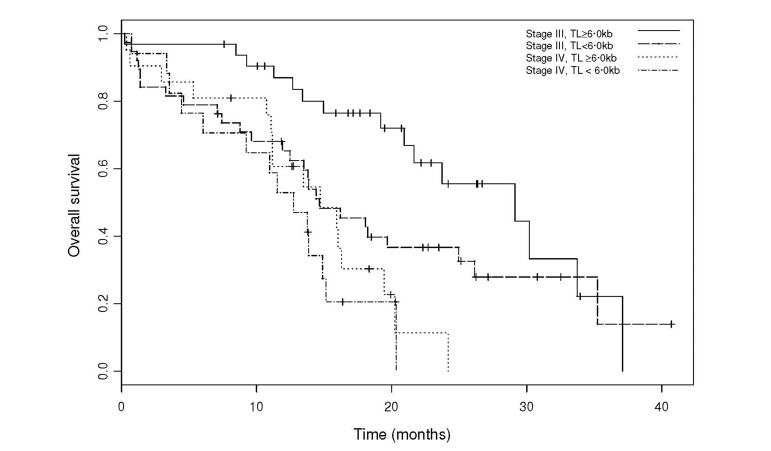
A survival analysis using Cox proportional hazards was conducted in order to evaluate the impact of telomere length on survival (Figure 2). Reasons for death were the most frequently related to cancer progression (105 patients), 2 patients died from treatment toxicity (septic shock), 4 from other reasons (colic perforation: 1; pulmonary embolism: 1, suspicion of pulmonary embolism: 1, major depression and denutrition: 1). After adjustment on FIGO stage (IV versus III), TL less than 6.00 kb was identified as a risk factor for premature death, with an HR of 1.57 (95%CI: 0.98 to 2.51, P=0.06). This pejorative trait staid robust in different models including FIGO stage and age [HR = 1.58 (95%CI: 0.99 to 2.53, P=0.06)], FIGO stage and GVS . 3 [HR = 1.56 (95%CI: 0.97 to 2.49, P=0.07)] and FIGO stage, GVS . 3 and age [HR = 1.57 (95%CI: 0.98 to 2.53, P=0.06)] (Supplementary Table 1).
Figure 2. Overall survival by TL groups, adjusted for FIGO stage.
A tendency towards correlation between TL and geriatric vulnerability parameters
We then tested the correlation between TL groups and geriatric vulnerability groups using the Geriatric Vulnerability Score [11]. Patients displaying at least three geriatric vulnerability parameters had a 2.94-fold higher risk of mortality in a univariate analysis (95%CI 1.79-4.84, P <0.0001) and this was 2.89-fold in a multivariate analysis after adjustment for FIGO stage (95%CI 1.74-4.78, P<0.0001). Despite the absence of a significant correlation with patients' characteristics except age (Supplementary Table 2) and any of the individual geriatric vulnerability parameters, TL groups and geriatric vulnerability groups showed a tendency towards correlation (P=0.08, Table 3).
DISCUSSION
This analysis was planned as an ancillary study in the prospective multicentric Elderly Woman GINECO Trial 3. The working biological hypothesis - identifying TL as a putative prognostic biomarker in elderly cancer patients - was based on the pooled results of two prior studies, Elderly Woman GINECO Trials 1 and 2. In a retrospective multivariate analysis of overall survival (OS), several factors - FIGO stage IV, use of paclitaxel, age, emotional disorders and lymphopenia - were significantly associated with an increased risk of premature death ([10] and unpublished data). Moreover, a significant correlation was also shown between emotional disorders and lymphopenia. TL shortening, previously shown to correlate with age [13,14], lifestyle stress [15] and survival [10], is considered to be both an actor and a witness of the pathologic aging process. The myeloid skewing of hematological progenitors that accompanies aging [16] and telomere dysfunction [17,18] could explain why there is an increasing amount of clinical data proposing lymphopenia as a marker of pathologic aging [19].
In previous epidemiological cohorts, a correlation was observed between TL and lifespan [8,20,21], aging associated diseases [7], and OS in healthy subjects [22]. These findings are of limited clinical use since associations only appear when large cohorts are investigated and the predictive value on an individual basis is poor. TL in blood leucocytes has also gained considerable interest as a potential biomarker of cancer risk, and direct measurement of TL and telomerase activity in tumors are considered to be cancer prognosis markers [23, 24].
To our knowledge, this study is the first to investigate the impact of TL on treatment feasibility as an individual risk factor. In spite of a relatively small patient sample size (111 patients), we were able to control several putative biased errors typically associated with TL measurement in clinical trials [25–27]. All patients were female, post-menopausal, almost exclusively Caucasian and fell into a narrow age bracket (70 to 93 years). According our biologic working model, the impact of TL shortening was expected to be challenged by the competition between tumor-related and host-related covariates on patients' outcomes. Due to the context of the trial, the study did not include any external reference, as for example TL samples from non-cancer elderly patients, that would have evaluated the impact of the tumor itself on TL.
Despite these constraints, in this particular oncologic context of ovarian cancer, our results reveal a clear correlation between TL and patient immediate outcomes. Indeed, TL distribution identified a subgroup of elderly patients with short telomeres who have a lower probability of completing treatment and a higher risk of severe adverse events and unplanned hospitalization This subgroup partially overlaps with patients identified as vulnerable according to the GVS [28]. However, the translation to clinical practice of these results might be difficult for the following reasons. Firstly and as usually for TL analyses, the cut-off between short and long telomeres were made a posteriori, being highly dependent on the technical conditions and the population studied. Moreover, different cut-offs separated the immediate outcomes (treatment completion, severe adverse events, unplanned hospital admissions) and the risk of premature death. Secondly, TL remained less discriminating than the GVS, based on simple clinical tests and routine bioassays, for immediate outcomes and survival. Finally, TL was estimated using the gold standard technique, namely the mean length terminal restriction fragments. Even if this technique is highly feasible, it is time-consuming and difficult to implement in routine analysis. In this respect, many of large epidemiological cohort analyses have preferred an alternative method of TL estimation, namely quantitative PCR [29,30]. However, this alternative technique suffers from a number of technical disadvantages, notably a high coefficient of variation and a lack of good reference standards, making it difficult to evaluate absolute TL [31]. Studies in mouse models have revealed that the number of dysfunctional telomeres is a more accurate factor than mean telomere length in the evolution of tissue pathology during aging [32]. Thus, evaluation of blood markers of dysfunctional telomeres [33] appear to be a promising method for telomere biology analysis in clinical trials [34, 35].
In conclusion, our study demonstrates a correlation between telomere length and patient outcomes in an oncogeriatric context [34]. This finding opens the way for future work aimed at identifying telomere biomarkers which can be implemented in routine practice for outcome prediction as well as on the evaluation of the impact of cancer treatment - mainly chemotherapy - on biomarkers of aging.
MATERIALS AND METHODS
Study design
The Elderly Woman GINECO Trial 3 was an open-label phase II multicentric trial approved by the Independent Ethics Committee of Lyon University Hospital (EUDRACT No. 2006-005504-13). The study design, population and assessments have been described elsewhere [1]. Written informed consent was obtained from each patient and included authorization for collection of a blood sample for TL measurement. Patients were treated with up to six cycles of carboplatin AUC5 (5 mg/mL/min for 30 min every 3 weeks). In this ancillary study, TL at inclusion was evaluated, along with the impact of TL on patient outcome and the correlation between TL and geriatric covariates.
Patient population
Eligible patients were ≥70 years old, with a life expectancy ≥3 months, and histologically or cytologically proven epithelial FIGO stage III-IV ovarian cancer. Cytology consistent with ovarian cancer was considered sufficient if associated with both a CA125 rise and a radiological pelvic mass. Patients were considered ineligible if they had any prior malignancy except basal cell carcinoma or carcinoma in situ of the cervix or urinary bladder, prior chemo- or radiotherapy, serious medical or psychiatric illness that might affect treatment, major disturbance of hepatic parameters (alanine aminotransferase or aspartate aminotransferase >3 times the upper limit of normal, total bilirubin >2 times the upper limit of normal), severe renal insufficiency (creatinine clearance <30 mL/min), or abnormal hematological parameters (neutrophils <1.5 × 109/L, platelets <100 × 109/L). Patients with planned interval debulking surgery were also excluded.
Assessments
A multidimensional pre-inclusion geriatric assessment was performed at baseline. Data concerning the patient's medical charts, nutrition, functionality and an extensive psychocognitive assessment were collected, including comorbities, comedications, body mass index (BMI), serum albumin levels, and functional scores for Activities of Daily Living (ADL), Instrumental ADL (IADL), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and the Mini-Mental Scale (MMS).
Patient outcomes of treatment completion rate (defined as receiving six courses of chemotherapy without premature discontinuation for death, treatment toxicity or tumor progression), survival, serious adverse events, unplanned hospital admissions, and grade ≥3 toxicities were recorded.
The GVS was calculated for each patient as described previously [11]. This score is the addition of the following geriatric vulnerability parameters: ADL score <6, IADL score <25, HADS score >14, albuminemia <35 g/L, and lymphopenia <1 × 109/L.
Measurement of telomere length
A blood sample was collected at inclusion. DNA extraction was performed within 14 days using the PAXgene Blood DNA System (PreAnalytix GmbH, Hombrechtikon, Switzerland) according to the manufacturer's instructions. DNA integrity was assessed by electrophoresis on 1.0% agarose gels. DNA samples (4μg) were digested overnight with the restriction digest set Hinf1 (33 U) /Rsa1 (33 U) (New Englands Biolabs, France) and resolved using field inversion electrophoresis (FIGE) on FIGE Mapper System (Bio-Rad Life Science, France). Briefly, samples were precipited, resuspended in 12μl of H20 and run on 1% pulse field grade agarose gel (20cm × 13cm) containing 0.5X TBE at room temperature for 13h. The switch time ramp was between 0.1 and 0.5s (linear shape) with forward and reverse voltages of 160 and 100 V, respectively. A combination of two DNA molecular weight size standards was run on each gel : l Mix Marker 19 that spans 48.5 – 1.5kb and MassRuler™ DNA Ladder mix that spans 10 – 0.08kb (Thermo Scientific Molecular Biology Inc., France) and used to establish a standard curve (molecular size as a function of migration distance). Digested DNA were blotted to N+ Hybond membrane (GE Healthcare, France) by capillary transfer using SCC 20× transfer buffer and then UV cross-linked. Hybridization was carried out overnight at 65°C in hybridization buffer (0.5M NaPO4 pH7.2, 7% SDS, 0.1% BSA, 1mM EDTA) containing a digoxigenin (DIG)-labeled probe specific for telomeric repeats (400 bp of repeated 5.-T2AG3-3. motif). Membranes were then washed twice at room temperature in 2X SSC, 0.1% SDS (5min) and twice at 50°C in 0.2X SSC, 0.1% SDS (25min). Chemiluminescence detection was carried out according to TeloTAGGG Telomere Length Assay (Roche Applied Science, France) instructions. Telomeric Restriction Fragments (TRF) chemiluminescence signals were captured using a LAS-3000 Imager (FujiFilm Life Science, France) and images were processed using ImageJ software (http://rsb.info.nih.gov/ij/). The optical densities (OD) versus mean TRF length were calculated according to the formula (∑ ODi/ ∑(ODi/MWi), where ODi is the chemiluminescent signal and MWi is the length of the TRF at position i ([13] and Supplementary Figure 1). Measurements were performed on each sample at least twice in different gels and the mean was used for statistical analyses. Pearson's correlation coefficient for duplicates was 0.74, with an average coefficient of variation for pair sets of 7.3%. The laboratory conducting the TL measurement was blinded to all patient characteristics.
Statistical analyses
The sample size of 110 patients was calculated on the basis of the primary objective of the main part of the study (to confirm the impact of psychogeriatric covariates on OS) as reported elsewhere [12]. TL was analyzed both as a continuous ordinal variable and a categorical variable. For the former analysis, non-parametric two-sample Wilcoxon rank-sum tests were performed to evaluate the impact of TL on patient outcome. For the latter analysis, TL was transformed into quartiles, categorized into TL groups (shorter vs longer) and introduced as a dichotomous trait into linear regression models and Cox's proportional-hazards regression models. Different cut-offs were used based on the outcome under consideration. Survival curves were estimated using the Kaplan-Meier method and OS models were adjusted for FIGO stage (IV versus III). Odd ratios (ORs) and hazard ratios (HRs), 95% confidence intervals (CIs), and p values (P) were calculated. Analyses were performed using R statistical package (R Foundation for Statistical Computing, Austria) and Splus, version 6.2 (Insightful Corp., WA, USA).
SUPPLEMENTARY MATERIAL TABLES AND FIGURE
Acknowledgments
The authors would like to acknowledge Douglas Micheau-Bonnier, Nicolas Gane, and Benedicte Votan from the GINECO study office and the Delegation a la Recherche Clinique et a l'Innovation of the Hospices Civils de Lyon. We also thank the following investigators who participated in the trial: Pr. H. Cure (Institut Jean Godinot, Reims), Dr J. Salvat (Hopitaux du Leman, Thonon-les-Bains), Dr M. Combe (Centre Hospitalier du Mans, Le Mans), Dr. M.C. Kaminsky (Centre Alexis Vautrin-Brabois, Vandoeuvre-les-Nancy), Dr. I. Ray-Coquard (Centre Leon Berard, Lyon), Dr. G. Yazbek, (Institut Jean Godinot, Reims), Dr. J. Meunier (Centre Hospitalier Regional d'Orleans, Orleans), Dr. J. Cretin (Clinique Bonnefon, Ales), Dr. L. Chauvenet, (Hopital Hotel-Dieu, Paris), Dr J. Provencal (Centre Hospitalier de la region d'Annecy, Pringy), Dr. M. Fabbro (Hopital Arnaud de Villeneuve, Montpellier), Dr. M.N. Certain (Centre Hospitalier d'Auxerre, Auxerre), Dr. J.P. Guastalla (Centre Leon Berard, Lyon), Dr. S. Kalla (Groupe Hospitalier Saint-Joseph, Paris), Pr. J. Alexandre (Hopital Hotel-Dieu, Paris), Dr. E. Legouffe (Clinique de Valdegour, Nimes), Dr. F. Savinelli (Groupe Hospitalier Saint-Joseph, Paris), Dr. J.M. Tigaud (Hopital Hotel-Dieu, Paris), Dr. R. Largillier (Centre Azureen de Cancerologie, Mougins), Pr. O. Gisserot (Hopital d'Instruction des Armees Sainte-Anne, Toulon), Dr. C. Ligeza-Poisson (Clinique Mutualiste de l'Estuaire, Saint-Nazaire), Dr. G. Deplanque (Groupe Hospitalier Saint-Joseph, Paris), Dr. P. Deguiral (Clinique Mutualiste de l'Estuaire, Saint-Nazaire), Dr. E. Luporsi (Centre Alexis Vautrin-Brabois, Vandoeuvre-les-Nancy), Dr. F. Priou (Centre Hospitalier Departemental Les Oudairies, La Roche-sur-Yon), Dr. F. Rousseau (Institut Paoli Calmettes, Marseille), Dr. A. Le Rol (Hopital Perpetuel Secours, Levallois-Perret). We thank Sarah MacKenzie, PhD for language editing (funded by ARCAGY-GINECO).
Footnotes
Funding
This work was supported by research grants from the French Ministry of Health (Projet Hospitalier de Recherche Clinique) and La Fondation de France and promoted by the Hospices Civils de Lyon. EG Lab was supported by "La Ligue Nationale Contre le Cancer" (Équipe labellisée), the "Institut National du Cancer" (EG Lab: INCa, TELOFUN program) and the "Agence Nationale de la Recherche" (ANR, TELOREP program). CF held fellowships from "la Région Rhônes-Alpes", the "Association de Recherche contre le Cancer" and Astra-Zeneca.
Conflict of interest statement
The authors declare no conflicts of interest.
REFERENCES
- 1.Balducci L, Extermann M. Management of cancer in the older person: A practical approach. Oncologist. 2000;5:224–237. doi: 10.1634/theoncologist.5-3-224. [DOI] [PubMed] [Google Scholar]
- 2.Gilson E, Geli V. How telomeres are replicated. Nat Rev Mol Cell Biol. 2007;8:825–838. doi: 10.1038/nrm2259. [DOI] [PubMed] [Google Scholar]
- 3.Ye J, Renault VM, Jamet K, Gilson E. Transcriptional outcome of telomere signalling. Nat Rev Genet. 2014;15:491–503. doi: 10.1038/nrg3743. [DOI] [PubMed] [Google Scholar]
- 4.Risques RA, Arbeev KG, Yashin AI, Ukraintseva SV, Martin GM, Rabinovitch PS, Oshima J. Leukocyte telomere length is associated with disability in older u.S. Population. J Am Geriatr Soc. 2010;58:1289–1298. doi: 10.1111/j.1532-5415.2010.02948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yaffe K, Lindquist K, Kluse M, Cawthon R, Harris T, Hsueh WC, Simonsick EM, Kuller L, Li R, Ayonayon HN, Rubin SM, Cummings SR. Telomere length and cognitive function in community-dwelling elders: Findings from the health abc study. Neurobiol Aging. 2011;32:2055–2060. doi: 10.1016/j.neurobiolaging.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epel ES, Merkin SS, Cawthon R, Blackburn EH, Adler NE, Pletcher MJ, Seeman TE. The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging (Albany NY) 2008;1:81–88. doi: 10.18632/aging.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atzmon G, Cho M, Cawthon RM, Budagov T, Katz M, Yang X, Siegel G, Bergman A, Huffman DM, Schechter CB, Wright WE, Shay JW, Barzilai N, Govindaraju DR, Suh Y. Evolution in health and medicine sackler colloquium: Genetic variation in human telomerase is associated with telomere length in ashkenazi centenarians. Proc Natl Acad Sci U S A. 2010;1:1710–1717. doi: 10.1073/pnas.0906191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 9.Freyer G, Geay JF, Touzet S, Provencal J, Weber B, Jacquin JP, Ganem G, Tubiana-Mathieu N, Gisserot O, Pujade-Lauraine E. Comprehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: A gineco study. Ann Oncol. 2005;16:1795–1800. doi: 10.1093/annonc/mdi368. [DOI] [PubMed] [Google Scholar]
- 10.Trédan O, Geay JF, Touzet S, Delva R, Weber B, Cretin J, Provencal J, Martin J, Pujade-Lauraine E, Freyer G. Carboplatin cyclophosphamide or carboplatin pacitaxel in elderly with advanced ovarian cancer? Analysis of two consecutive trials from the gineco. Annals of Oncology. 2007;18:256–262. doi: 10.1093/annonc/mdl400. [DOI] [PubMed] [Google Scholar]
- 11.Falandry C, Weber B, Savoye A-M, Tinquaut F, Tredan O, Sevin E, Stefani L, Savinelli F, Atlassi M, Salvat J, Pujade-Lauraine E, Freyer G. Development of a geriatric vulnerability score in elderly patients with advanced ovarian cancer treated with first-line carboplatin: A gineco prospective trial. Ann Oncol. 2013;24:2808–2813. doi: 10.1093/annonc/mdt360. [DOI] [PubMed] [Google Scholar]
- 12.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 13.Counter CM. The roles of telomeres and telomerase in cell life span. Mutat Res. 1996;366:45–63. doi: 10.1016/s0165-1110(96)90006-8. [DOI] [PubMed] [Google Scholar]
- 14.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE, Scadden DT. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16ink4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 16.Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- 17.Ju Z, Jiang H, Jaworski M, Rathinam C, Gompf A, Klein C, Trumpp A, Rudolph KL. Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment. Nat Med. 2007;13:742–747. doi: 10.1038/nm1578. [DOI] [PubMed] [Google Scholar]
- 18.Cretel E, Veen I, Pierres A, Binan Y, Robert P, Loundou AD, Baumstarck-Barrau K, Hubert AM, Bongrand P, Heim M. [immune profile of elderly patients admitted in a geriatric short care unit] Rev Med Interne. 2011;32:275–282. doi: 10.1016/j.revmed.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Martin-Ruiz C, Jagger C, Kingston A, Collerton J, Catt M, Davies K, Dunn M, Hilkens C, Keavney B, Pearce SH, Elzen WP, Talbot D, Wiley L, Bond J, Mathers JC, Eccles MP, Robinson L, James O, Kirkwood TB, von Zglinicki T. Assessment of a large panel of candidate biomarkers of ageing in the newcastle 85+ study. Mech Ageing Dev. 2011;132:496–502. doi: 10.1016/j.mad.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Fitzpatrick AL, Kronmal RA, Kimura M, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Hardikar S, Aviv A. Leukocyte telomere length and mortality in the cardiovascular health study. J Gerontol A Biol Sci Med Sci. 2011;66:421–429. doi: 10.1093/gerona/glq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Njajou OT, Hsueh WC, Blackburn EH, Newman AB, Wu SH, Li R, Simonsick EM, Harris TM, Cummings SR, Cawthon RM. Association between telomere length, specific causes of death, and years of healthy life in health, aging, and body composition, a population-based cohort study. J Gerontol A Biol Sci Med Sci. 2009;64:860–864. doi: 10.1093/gerona/glp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gertler R, Rosenberg R, Stricker D, Friederichs J, Hoos A, Werner M, Ulm K, Holzmann B, Nekarda H, Siewert JR. Telomere length and human telomerase reverse transcriptase expression as markers for progression and prognosis of colorectal carcinoma. J Clin Oncol. 2004;22:1807–1814. doi: 10.1200/JCO.2004.09.160. [DOI] [PubMed] [Google Scholar]
- 23.Umehara N, Ozaki T, Sugihara S, Kunisada T, Morimoto Y, Kawai A, Nishida K, Yoshida A, Murakami T, Inoue H. Influence of telomerase activity on bone and soft tissue tumors. J Cancer Res Clin Oncol. 2004;130:411–416. doi: 10.1007/s00432-004-0553-z. [DOI] [PubMed] [Google Scholar]
- 24.Bischoff C, Graakjaer J, Petersen HC, Jeune B, Bohr VA, Koelvraa S, Christensen K. Telomere length among the elderly and oldest-old. Twin Res Hum Genet. 2005;8:425–432. doi: 10.1375/183242705774310079. [DOI] [PubMed] [Google Scholar]
- 25.Lee DC, Im JA, Kim JH, Lee HR, Shim JY. Effect of long-term hormone therapy on telomere length in postmenopausal women. Yonsei Med J. 2005;46:471–479. doi: 10.3349/ymj.2005.46.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisenberg DT, Salpea KD, Kuzawa CW, Hayes MG, Humphries SE. Substantial variation in qpcr measured mean blood telomere lengths in young men from eleven european countries. Am J Hum Biol. 2011;23:228–231. doi: 10.1002/ajhb.21126. [DOI] [PubMed] [Google Scholar]
- 27.Freyer G, Weber BE, Lebrun-Jezekova D, Tinquaut F, Tredan O, Sevin E, Stefani L, Pujade-Lauraine E, Falandry C. Development of a geriatric vulnerability score (gvs) in elderly advanced ovarian cancer (aoc) patients (pts) treated in first line: A prospective gineco trial. J Clin Oncol. 2012;30 doi: 10.1093/annonc/mdt360. Abstr 9079. [DOI] [PubMed] [Google Scholar]
- 28.Cawthon RM. Telomere measurement by quantitative pcr. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative pcr method. Nucleic Acids Res. 2009;37:e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimura M, Stone RC, Hunt SC, Skurnick J, Lu X, Cao X, Harley CB, Aviv A. Measurement of telomere length by the southern blot analysis of terminal restriction fragment lengths. Nat Protoc. 2010;5:1596–1607. doi: 10.1038/nprot.2010.124. [DOI] [PubMed] [Google Scholar]
- 31.Hemann MT, Strong MA, Hao LY, Greider CW. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107:67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 32.Jiang H, Schiffer E, Song Z, Wang J, Zurbig P, Thedieck K, Moes S, Bantel H, Saal N, Jantos J, Brecht M, Jeno P, Hall MN, Hager K, Manns MP, Hecker H, Ganser A, Dohner K, Bartke A, Meissner C, Mischak H, Ju Z, Rudolph KL. Proteins induced by telomere dysfunction and DNA damage represent biomarkers of human aging and disease. Proc Natl Acad Sci U S A. 2008;105:11299–11304. doi: 10.1073/pnas.0801457105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falandry C, Gilson E, Rudolph KL. Are aging biomarkers clinically relevant in oncogeriatrics? Crit Rev Oncol Hematol. 2013;85:257–265. doi: 10.1016/j.critrevonc.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Augereau A, T'Kint de Roodenbeke C, Simonet T, Bauwens S, Horard B, Callanan M, Leroux D, Jallades L, Salles G, Gilson E, Poncet D. Telomeric damage in early stage of chronic lymphocytic leukemia correlates with shelterin dysregulation. Blood. 2011;118:1316–1322. doi: 10.1182/blood-2010-07-295774. [DOI] [PubMed] [Google Scholar]
- 35.Falandry C, Weber B, Savoye AM, Tinquaut F, Tredan O, Sevin E, Stefani L, Savinelli F, Atlassi M, Salvat J, Pujade-Lauraine E, Freyer G. Development of a geriatric vulnerability score in elderly patients with advanced ovarian cancer treated with first-line carboplatin: A gineco prospective trial. Ann Oncol. 2013;2013:2022. doi: 10.1093/annonc/mdt360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.