Abstract
Aims
Patients with ST-segment elevation myocardial infarction (STEMI) feature thrombus-rich lesions with large necrotic core, which are usually associated with delayed arterial healing and impaired stent-related outcomes. The use of bioresorbable vascular scaffolds (Absorb) has the potential to overcome these limitations owing to restoration of native vessel lumen and physiology at long term. The purpose of this randomized trial was to compare the arterial healing response at short term, as a surrogate for safety and efficacy, between the Absorb and the metallic everolimus-eluting stent (EES) in patients with STEMI.
Methods and results
ABSORB-STEMI TROFI II was a multicentre, single-blind, non-inferiority, randomized controlled trial. Patients with STEMI who underwent primary percutaneous coronary intervention were randomly allocated 1:1 to treatment with the Absorb or EES. The primary endpoint was the 6-month optical frequency domain imaging healing score (HS) based on the presence of uncovered and/or malapposed stent struts and intraluminal filling defects. Main secondary endpoint included the device-oriented composite endpoint (DOCE) according to the Academic Research Consortium definition. Between 06 January 2014 and 21 September 2014, 191 patients (Absorb [n = 95] or EES [n = 96]; mean age 58.6 years old; 17.8% females) were enrolled at eight centres. At 6 months, HS was lower in the Absorb arm when compared with EES arm [1.74 (2.39) vs. 2.80 (4.44); difference (90% CI) −1.06 (−1.96, −0.16); Pnon-inferiority <0.001]. Device-oriented composite endpoint was also comparably low between groups (1.1% Absorb vs. 0% EES). One case of definite subacute stent thrombosis occurred in the Absorb arm (1.1% vs. 0% EES; P = ns).
Conclusion
Stenting of culprit lesions with Absorb in the setting of STEMI resulted in a nearly complete arterial healing which was comparable with that of metallic EES at 6 months. These findings provide the basis for further exploration in clinically oriented outcome trials.
Keywords: ST elevation myocardial infarction, Bioresorbable scaffold, Optical coherence tomography, Randomized control study
See page 241 for the editorial comment on this article (doi:10.1093/eurheartj/ehv537)
Introduction
Reperfusion therapy by means of primary percutaneous coronary intervention (pPCI) is the standard of care treatment of patients with ST-segment elevation myocardial infarction (STEMI).1 New generation drug-eluting stents (DES) have been shown superior to BMS by reducing the risk of recurrent myocardial infarction, stent thrombosis, and target lesion revascularization.2–6 However, in this clinical setting, thrombotic lesions superimposed on large necrotic core-containing plaques are common histopathological findings which have been shown to delay arterial healing and provoke chronic inflammation. Optical coherence tomography imaging revealed an increased frequency of uncovered and/or malapposed stent struts, residual thrombus, and late pathological remodelling in lesions of STEMI compared with stable coronary artery disease patients during mid- and long-term follow-up.7–9
Implantation of fully bioresorbable stents into STEMI culprit lesions may be advantageous. Specifically, the biodegradation of the stent struts with eventual restoration of vessel physiology, as previously shown after Absorb bioresorbable stent implantation among patients with stable coronary artery disease,10 may prevent the occurrence of late events. Moreover, stents may result in a neo-cap formation11,12 as protective layer shielding underlying large necrotic cores which are usually located in the proximal segment of the large coronary vessels.
However, to date no head-to-head comparison has been performed to assess the early phase of the arterial healing of Absorb implantation in this thrombogenic milieu relative to that of the current gold standard metallic DES.
Therefore, the purpose of the ABSORB-STEMI TROFI II trial was to compare the arterial healing response between the Absorb and the metallic everolimus-eluting stent (EES) in STEMI patients. Furthermore, angiographic and clinical outcomes were compared between groups as exploratory and hypothesis-generating analyses.
Methods
Patients and study design
This is a multicentre, international, randomized, two-arm, single-blind, controlled trial performed in STEMI patients (clinicaltrial.org, NCT01986803). This is an investigator-initiated trial, sponsored by the European Cardiovascular Research Institute (ECRI). Inclusion and exclusion criteria were reported elsewhere.13 Briefly, the study included patients presenting with STEMI with the following ECG criteria: at least 1 mm in two or more standard leads or at least 2 mm in two or more contiguous precordial leads or new left bundle-branch block, within the first 24 h after the symptoms onset requiring emergent PCI with a vessel size ranging between 2.25 and 3.8 mm and following adequate lesion preparation. Main exclusion criteria included cardiogenic shock, severe tortuosity, or calcification, and inadequate vessel size (<2.25 or >3.80 mm). All patients were randomized 1:1 to one of the two treatment arms: Absorb vs. EES (Xience™ Expedition stent). Randomization was performed after establishment of at least TIMI 2 flow after thrombus aspiration and/or pre-dilatation. Written informed consent needed to be obtained in all patients prior to randomization. Randomization was performed by dedicated web-based software. The patients were blinded to the treatment. A total of eight centres in four countries were involved in the trial (Supplementary material online, Appendix). All centres had approval of their Medical Ethics Committee. The study was conducted in compliance with the protocol, the Declaration of Helsinki, BS EN ISO 14155 Part 1 and Part 2, and applicable local requirements. All patients provided written informed consent. Description of the Data and Safety Monitoring Board, Clinical Event Committee may be found in Supplementary material online, Appendix.
Study endpoints
Study endpoints have been previously described.13 In brief, the primary endpoint is the optical frequency domain imaging (OFDI)-derived healing score (HS) assessed at 6 months. The HS is based on four stent-related characteristics: presence of intraluminal mass (assigned a weight of ‘4’); presence of both malapposed and uncovered struts (assigned a weight of ‘3’); presence of uncovered struts alone (assigned a weight of ‘2’); and presence of malapposed struts alone (assigned a weight of ‘1’).14,15 Precise definitions of the HS components are reported in Supplementary material online, Appendix. In brief, a low HS indicates a favourable healing process without intraluminal luminal defect, malapposition or uncovered struts, etc., whereas a high HS reflects a poor healing process with remnant thrombus, uncovered and/or malapposed struts.
For the OFDI endpoint analysis, stent area and derived measures are based on the abluminal stent contour.13,16 Secondary clinical endpoints included device-oriented composite endpoint (DOCE: composite of cardiac death, target vessel myocardial infarction (MI), or clinically driven target lesion revascularization, TLR) at 1, 6, and 36 months; the individual components of DOCE; device and procedural success, all-cause death; any myocardial infarction; non-clinically driven TLR; clinically indicated and non-clinically driven target vessel revascularization; stent thrombosis according to Academic Research Consortium definition17; angina status classified according to Canadian Society of Cardiology class at 6-month follow-up. Reinfarction is defined according to the Third Universal Definition of MI as evidence of myocardial necrosis in a clinical setting consistent with acute MI.18,19 Device success was defined as the implantation of the assigned study device with a post-procedure residual stenosis <30%.13 Procedure success was defined as device success without the occurrence of any component of the DOCE.13 All clinical events were adjudicated by an independent Clinical Event adjudication Committee. Clinical follow-up was scheduled at 1, 6, 12, 24, and 36 months. Angiographic follow-up was scheduled at 6 months.
Percutaneous coronary intervention procedure
Primary PCI was performed according to standard practice.2 As per protocol, manual thrombectomy was mandatory to reduce thrombus burden. The decision to perform pre- or post-dilatation was left to the operator's discretion. If these techniques were performed, specific rules had to be followed during the procedure as well as for stent sizing.13 Stent implantation was carried out following current standards.13,20 The Absorb stent was available in the diameters of 2.5, 3.0, and 3.5 mm and in the lengths of 8, 12, 18, and 28 mm. It was recommended to use similar sizes for the EES (Xience Xpedition™) stent. Anticoagulation was achieved either by the use of bivalirudin or unfractionated heparin. The use of GPIIb/IIIa antagonists was at the operator's discretion. It was recommended that patients received a loading dose of aspirin and a P2Y12 inhibitor pre-procedure, followed by dual antiplatelet therapy for at least 12 months.
Angiographic and optical frequency domain imaging analysis
Angiographic endpoints at 6 months included percent diameter stenosis, minimal lumen diameter (MLD), late lumen loss, and binary restenosis. All angiographic endpoints were assessed for the in-segment, in-device, proximal, and distal region. Optical frequency domain imaging endpoints were assessed at 6 months and included all individual components of the HS (see above), the mean and minimal stent and diameter, area and volume, the frequency of incomplete strut apposition including area and volume, the percentage of uncovered struts, the mean neointima thickness, and neointimal hyperplasia area on top of the strut and interstrut, and volume, the mean flow area and volume and intraluminal defect area and volume.13
Optical frequency domain imaging assessment of the stented coronary segment was performed using the TERUMO Lunawave console and the FastView catheter. Angiography and OFDI recordings were sent to an independent Core Laboratory (Cardialysis B.V.) for off-line analysis. It was not possible to blind the analysts to the device type based on the characteristic appearance of Absorb and EES stent struts. Taking into account, the difference in optical properties of cobalt chromium and polylactide, OFDI analysis was performed using comparative methods.16
Angiographic or OFDI data from patients returning for any repeat angiography within 14 days after the index procedure were not used in calculation of the HS and Quantitative coronary angiography (QCA), since the need for repeat revascularization in this period was not related to neointimal healing but rather to an acute response of the lesion to the procedure. This is consistent with methods used in the resolute AC trial.21
Statistical analysis
The primary endpoint and all imaging-based (OFDI and angiography) findings were analysed based on the as-treated population, which consists of all patients whose culprit lesion have been treated with the assigned study device. Clinical outcomes analyses were based on the intention-to-treat population.
Sample size was calculated assuming a mean HS of 9.0 in the Absorb stent group with an SD of 9.4. These data were based on an OFDI analysis of the ABSORB cohort B1 in stable patients.22 The HS of the EES was anticipated to be similar as the one observed with the Absorb (with similar SD) based on a historical 13 months data showing an HS of 10.8 (SD 15.3).23 With a non-inferiority margin of 4.5 points, a one-sided significance level (α) of 0.05 and an attrition rate of 20%, 190 patients would provide 90% power to confirm non-inferiority.
For the superiority analysis of the primary endpoint the 95% two-sided confidence interval for the difference in the mean HS between Absorb and EES arms, was calculated using the Students t-distribution. To eliminate the influence of outliers, a sensitivity analysis was performed using the logarithm of the HS augmented with 1. A test of normality was performed; in case the normality hypothesis did not hold, the non-parametric Wilcoxon–Mann–Whitney test was performed.
Continuous variables were presented as mean and standard deviation or medians and interquartile ranges whenever appropriate. The variable means were evaluated by a two-sample t-test; differences between treatments with the corresponding 95% confidence interval were presented. Categorical variables were summarized by frequencies and percentages. Dichotomous variables were evaluated by Fisher's exact test. Categorical variables with more than two categories were evaluated by Mantel Haenszel Rank Score test.
Role of the funding source
The trial was designed by the principal investigators. The trial was sponsored by the ECRI and supported with unrestricted grants from TERUMO EUROPE N.V. and ABBOTT VASCULAR. The sponsor funded an independent data management and analysis centre (Cardialysis, Rotterdam, Netherlands) for database management and all statistical analyses. The grant givers were not involved in the conduct of the trial, data management, data analysis, drafting of the manuscript, and did not participate in the decision to submit the manuscript for publication. The three PIs had full access to the study data. The corresponding author had full responsibility for the decision to submit the report for publication.
Results
Patients
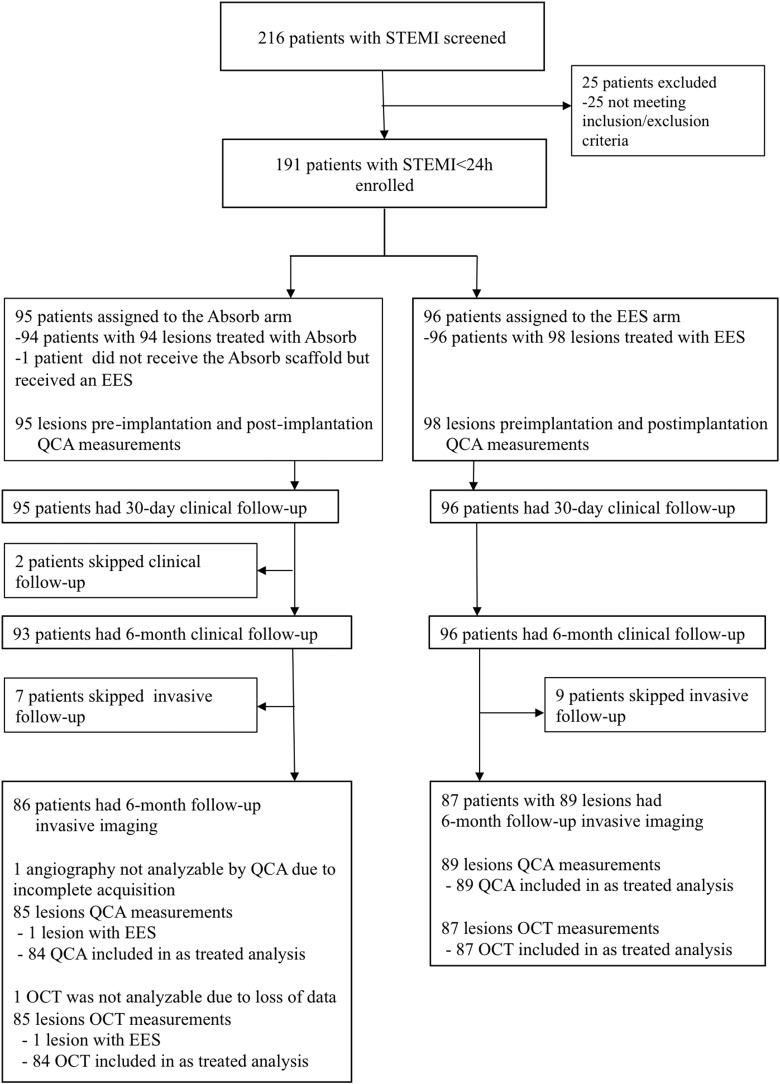
Of 2055 patients admitted with STEMI and requiring primary PCI in the participating centres during the recruitment period, 216 were screened and 191 patients were finally included in the trial (Figure 1) and randomly assigned to undergo treatment with Absorb (95 patients) or EES (96 patients). Baseline clinical characteristics were comparable between the two study groups (Table 1). Smoking followed by dyslipidaemia were the most commonly encountered coronary risk factors (72 and 60%, respectively). Diabetes mellitus was found in 17% of patients. Time from symptom onset to treatment, door-to-balloon time, and total ischaemia time were comparable between the two groups. Procedural characteristics were presented in Table 2. Thrombectomy was attempted in 90% of the patients and pre-dilatation in half of the population without differences between groups. Post-dilatation was more frequently performed in the Absorb arm (50.5 vs. 25.5%, P < 0.001). On average, a total of 1·2 stents were implanted at the culprit lesion with a median total length of 18.0 mm in both groups. Mean nominal diameter was larger in the Absorb arm (3.25 vs. 3.12 mm; P = 0.005). No differences were observed in the antiplatelet and anticoagulation regimens between groups. Most patients (43%) received the combination of aspirin and ticagrelor loading doses before the intervention. Overall, the combination of unfractionated heparin with IIb/IIIa inhibitors accounted for 37.6% of patients, unfractionated heparin alone 35.6%, and bivalirudin alone 8.4%. Device and procedure success rates were both 95.8% in the Absorb arm and 100% in the EES arm; P = 0.06 and P = 0.06, respectively. This difference was related to the higher frequency of post-procedure residual stenosis in excess of >30% in the Absorb arm, 3 vs. 0 patients in the EES arm.
Figure 1.
Flow chart of the study. A total of 216 patients were screened during the recruitment period. Of them 191 were included in the trial. At 6 months, clinical follow-up was obtained in 189 patients and optical frequency domain imaging analysis was available in 171 lesions.
Table 1.
Baseline characteristics
| Absorb, N = 95 | EES, N = 96 | Difference (95% CI) | |
|---|---|---|---|
| Male, n (%) | 73 (76.8) | 84 (87.5) | −10.7% [−21.4%, 0.1%] |
| Age (years), mean ± SD | 59.1 ± 10.7 | 58.2 ± 9.6 | 0.9 [−2.0, 3.8] |
| Current smoking, n (%) | 46 (48.4) | 47 (49.5) | −1.1% [−15.3%, 13.2%] |
| Previous smoking, n (%) | 22 (23.2) | 22 (23.2) | 0.0% [−12.0%, 12.0%] |
| Diabetes mellitus, n (%) | 18 (18.9) | 14 (14.7) | 4.2% [−6.4%, 14.8%] |
| Insulin dependent, n (%) | 5 (5.3) | 3 (3.2) | 2.1% [−3.6%, 7.8%] |
| Non-insulin dependent, n (%) | 13 (13.7) | 11 (11.6) | 2.1% [−7.3%, 11.5%] |
| Hypertension, n (%) | 41 (44.1) | 35 (36.5) | 7.6% [−6.3%, 21.6%] |
| Hypercholesterolaemia, n (%) | 60 (63.8) | 55 (57.3) | 6.5% [−7.3%, 20.4%] |
| Previous stroke, n (%) | 1 (1.1) | 1 (1.0) | 0.0% [−2.9%, 2.9%] |
| Previous myocardial infarction, n (%) | 2 (2.1) | 3 (3.1) | −1.0% [−5.5%, 3.5%] |
| Previous PCI, n (%) | 4 (4.2) | 3 (3.1) | 1.1% [−4.2%, 6.4%] |
| Chronic obstructive pulmonary disease, n (%) | 3 (3.2) | 3 (3.1) | 0.0% [−4.9%, 5.0%] |
| Body mass index (kg/mm2), mean ± SD | 27.0 ± 4.1 | 27.7 ± 4.2 | −0.7 [−1.9, 0.5] |
| Killip class | |||
| Class 1, n (%) | 90 (94.7) | 93 (96.9) | −2.1% [−7.8%, 3.5%] |
| Class 2, n (%) | 4 (4.2) | 3 (3.1) | 1.1% [−4.2%, 6.4%] |
| Class 3, n (%) | 1 (1.1) | 0 (0.0) | 1.1% [−1.0%, 3.1%] |
| Class 4, n (%) | 0 (0.0) | 0 (0.0) | |
| Onset of symptoms to FMC (min), mean ± SD | 115 ± 154 | 132 ± 165 | −17 [−62, 29] |
| Median (Q1, Q3) | 52 (26, 140) | 64 (31, 165) | |
| Onset of symptoms to thrombectomy/pre-dilatation (min), mean ± SD | 247 ± 209 | 257 ± 209 | −9 [−69, 50] |
| Median (Q1, Q3) | 177 (132, 285) | 185 (130, 299) | |
| FMC to thrombectomy/pre-dilatation (min), mean ± SD | 138 ± 145 | 133 ± 84 | 5 [−29, 39] |
| Median (Q1, Q3) | 108 (85, 139) | 115 (81, 144) | |
| Infarct-related target lesions | N = 95 | N = 98 | |
| Right coronary artery, n (%) | 44 (46.3) | 44 (44.9) | 1.4% [−12.6%, 15.5%] |
| Left anterior descending artery, n (%) | 34 (35.8) | 41 (41.8) | −6.0% [−19.8%, 7.7%] |
| Left circumflex artery, n (%) | 17 (17.9) | 13 (13.3) | 4.6% [−5.6%, 14.9%] |
| Grade of perfusion (TIMI) | |||
| TIMI 0, n (%) | 60 (63.2) | 61 (62.9) | 0.3% [−13.4%, 13.9%] |
| TIMI 1, n (%) | 3 (3.2) | 3 (3.1) | 0.1% [−4.9%, 5.0%] |
| TIMI 2, n (%) | 8 (8.4) | 13 (13.4) | −5.0% [−13.8%, 3.8%] |
| TIMI 3, n (%) | 24 (25.3) | 20 (20.6) | 4.6% [−7.2%, 16.5%] |
EES, everolimus-eluting stent; CI, confidence interval; PCI, percutaneous coronary intervention; FMC, first medical contact; TIMI, thrombolysis in myocardial infarction; Q1, first quartile; Q3, third quartile; N refers to number of patients or lesions with data available.
Table 2.
Procedural details
| Absorb | EES | Difference (95% CI) | P | |
|---|---|---|---|---|
| Number of lesions | 95 | 98 | ||
| Thrombectomy | 0.19 | |||
| Successful thrombectomy, n (%) | 77 (81.1) | 72 (73.5) | 7.6 [−4.2, 19.4] | |
| Unsuccessful thrombectomy, n (%) | 12 (12.6) | 12 (12.2) | 0.4 [−8.9, 9.7] | |
| No attempt, n (%) | 6 (6.3) | 14 (14.3) | −8.0 [−16.5, 0.5] | |
| Mode of stenting: | 0.51 | |||
| Direct stenting, n (%) | 42 (44.2) | 48 (49.0) | −4.8 [−18.8, 9.3] | |
| Pre-dilatation, n (%) | 53 (55.8) | 50 (51.0) | 4.8 [−9.3, 18.8] | |
| Maximum pressure (atm), mean ± SD | 14.1 ± 3.8 | 13.3 ± 3.0 | 0.8 [−0.6, 2.1] | 0.27 |
| Number of study devices, mean ± SD | 1.2 ± 0.4 | 1.1 ± 0.4 | 0.0 [−0.1, 0.2] | 0.54 |
| Nominal length of stent, mean ± SD | 20.6 ± 5.8 | 20.7 ± 6.7 | −0.1 [−1.8, 1.5] | 0.86 |
| Nominal diameter of stent, mean ± SD | 3.25 ± 0.30 | 3.12 ± 0.37 | 0.13 [0.04, 0.22] | 0.005 |
| Post-dilatation performed, n (%) | 48 (50.5) | 25 (25.5) | 25.0 [11.8, 38.3] | <0.001 |
| Use of non-compliant balloon, n (%) | 43 (89.6) | 13 (52.0) | 37.6 [16.2, 59.0] | <0.001 |
| Diameter of post-dilatation balloon (mm), mean ± SD | 3.51 ± 0.34 | 3.29 ± 0.62 | 0.22 [−0.01, 0.44] | 0.11 |
| Maximum pressure (atm), mean ± SD | 15.8 ± 3.4 | 18.6 ± 3.9 | −2.9 [−4.6, −1.1] | 0.002 |
| Post-procedural grade of perfusion (TIMI) | 0.50 | |||
| TIMI 0, n (%) | 0 (0.0) | 0 (0.0) | ||
| TIMI 1, n (%) | 0 (0.0) | 0 (0.0) | ||
| TIMI 2, n (%) | 0 (0.0) | 2 (2.0) | −2.0 [−4.8, 0.8] | |
| TIMI 3, n (%) | 95 (100.0) | 96 (98.0) | 2.0 [−0.8, 4.8] | |
| Device success, n (%) | 91 (95.8) | 98 (100.0) | −4.2 [−8.2, −0.2] | 0.057 |
| Number of patients | 95 | 96 | ||
| Medication before procedure | ||||
| ASA loading, n (%) | 95 (100) | 96 (100) | ||
| Ticagrelor, n (%) | 42 (44.2) | 41 (42.7) | 1.5 [−12.6, 15.6] | 0.83 |
| Prasugrel, n (%) | 18 (18.9) | 26 (27.1) | −8.1 [−20.0, 3.7] | 0.18 |
| Clopidogrel, n (%) | 36 (37.9) | 29 (30.2) | 7.7 [−5.7, 21.1] | 0.26 |
| Medication during procedure | 0.74 | |||
| Heparin only, n (%) | 31 (32.6) | 37 (38.5) | −5.9 [−19.5, 7.6] | |
| Bivalirudin only, n (%) | 7 (7.4) | 9 (9.4) | −2.0 [−9.9, 5.8] | |
| GP IIb/IIIa only, n (%) | 1 (1.1) | 2 (2.1) | −1.0 [−4.5, 2.5] | |
| Heparin and bivalirudin, n (%) | 18 (18.9) | 13 (13.5) | 5.4 [−5.0, 15.8] | |
| Heparin and GP IIb/IIIa, n (%) | 37 (38.9) | 35 (36.5) | 2.5 [−11.3, 16.2] | |
| Bivalirudin and GP IIb/IIIa, n (%) | 0.0% (0/95) | 0.0% (0/96) | ||
| Procedure success, n (%) | 91 (95.8) | 96 (100.0) | −4.2 [−8.2, −0.2] | 0.059 |
EES, everolimus-eluting stent; CI, confidence interval; atm, atmosphere; TIMI, thrombolysis in myocardial infarction.
Pre- and post-procedural QCA analyses are presented in Table 3. Pre-, post-procedural MLD, and acute gain were similar in both groups. Cardiac biomarkers (peak values) were comparable between groups (Supplementary material online, Figures SA1–SA4 and appendix). No clinical events occurred during hospitalization in either group. At discharge, aspirin and ticagrelor constituted the most frequently prescribed dual antiplatelet regimen (55%) followed by aspirin and prasugrel (35%).
Table 3.
Quantitative coronary angiography (as treated analysis)
| Absorb, N = 94 | EES, N = 98 | Difference (95% CI) | P | |
|---|---|---|---|---|
| Pre-procedure | ||||
| Lesion length (mm), mean ± SD | 12.88 ± 6.94 | 13.41 ± 7.40 | −0.53 [−2.64, 1.59] | 0.62 |
| Reference diameter (mm), mean ± SD | 2.86 ± 0.48 | 2.76 ± 0.51 | 0.09 [−0.05, 0.24] | 0.91 |
| MLD (mm), mean ± SD | 0.29 ± 0.43 | 0.28 ± 0.43 | 0.01 [−0.11, 0.14] | 0.84 |
| %DS, mean ± SD | 89.5 ± 15.1 | 89.9 ± 15.4 | −0.4 [−4.7, 4.0] | 0.86 |
| Post-procedure | ||||
| Device length (mm), mean ± SD | 21.41 ± 9.86 | 21.16 ± 9.77 | 0.26 [−2.54, 3.05] | 0.86 |
| In-device reference diameter (mm), mean ± SD | 2.88 ± 0.40 | 2.85 ± 0.47 | 0.02 [−0.10, 0.15] | 0.73 |
| In-device MLD (mm), mean ± SD | 2.46 ± 0.33 | 2.46 ± 0.40 | −0.00 [−0.11, 0.10] | 0.94 |
| In-device %DS, mean ± SD | 14.1 ± 6.8 | 13.4 ± 5.5 | 0.7 [−1.0, 2.5] | 0.43 |
| In-device acute gain (mm), mean ± SD | 2.16 ± 0.52 | 2.21 ± 0.56 | −0.04 [−0.20, 0.11] | 0.57 |
| At 6 months | N = 84 | N = 89 | ||
| In-device MLD (mm), mean ± SD | 2.29 ± 0.37 | 2.38 ± 0.41 | −0.09 [−0.21, 0.03] | 0.13 |
| In-device reference diameter (mm), mean ± SD | 2.76 ± 0.37 | 2.79 ± 0.44 | −0.03 [−0.15, 0.10] | 0.68 |
| In-device %DS, mean ± SD | 17.3 ± 7.4 | 14.5 ± 9.3 | 2.8 [0.3, 5.3] | 0.028 |
| In-device late loss (mm), mean ± SD | 0.17 ± 0.24 | 0.08 ± 0.28 | 0.09 [0.01, 0.17] | 0.024 |
| In-device binary restenosis, n (%) | 0 | 1 (1.1) | −1.1 [−3.3, 1.1] | 1.00 |
| In-segment late loss (mm), mean ± SD | 0.14 ± 0.28 | 0.06 ± 0.29 | 0.07 [−0.01, 0.16] | 0.09 |
| In-segment binary restenosis, n (%) | 0 | 1 (1.1) | −1.1 [−3.3, 1.1] | 1.00 |
EES, everolimus-eluting stent; CI, confidence interval; SD, standard deviation, N refers to number of lesions with data available; MLD, minimal lumen diameter; %DS, percentage diameter stenosis.
Outcomes
Optical frequency domain imaging data are summarized in Table 4. Representative cases are presented in Figures 2 and 3. The primary endpoint (HS) amounted to 1.74 [2.39] in the Absorb arm vs. 2.80 [4.44] in the EES arm (difference −1.06, 90% CI −1.96 to −0.16; Pnon-inferiority < 0.001), reaching the pre-specified non-inferiority criterion. Subsequent superiority testing resulted in a trend favouring Absorb (Psuperiority = 0.053). The difference in HS was driven by higher frequencies of malapposed struts and uncovered and malapposed struts in the EES compared with Absorb. The cumulative frequency distribution curves of the HS are shown in Figure 4.
Table 4.
Six-month qualitative and quantitative optical coherence tomography analysis (as treated analysis)
| Absorb, N = 84 | EES, N = 87 | Difference (95% CI) | P | |
|---|---|---|---|---|
| Healing score, mean ± SD | 1.74 ± 2.39 | 2.80 ± 4.44 | −1.06 [−2.14, 0.02] | <0.001* 0.053** |
| Healing score, median (Q1, Q3) | 0.90 (0.00, 3.00) | 1.04 (0.00, 3.85) | ||
| % Volume of intraluminal mass | ||||
| Mean ± SD | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 [−0.0, 0.0] | 0.39 |
| Median (Q1, Q3) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | ||
| % Uncovered and malapposed struts | ||||
| Mean ± SD | 0.0 ± 0.1 | 0.1 ± 0.4 | −0.1 [−0.2, −0.0] | 0.036 |
| Median (Q1, Q3) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | ||
| % Covered and malapposed struts | ||||
| Mean ± SD | 0.6 ± 1.2 | 1.5 ± 2.9 | −0.9 [−1.6, −0.2] | 0.011 |
| Median (Q1, Q3) | 0.0 (0.0, 0.9) | 0.2 (0.0, 2.3) | ||
| % Covered and apposed struts | ||||
| Mean ± SD | 99.4 ± 1.0 | 99.1 ± 1.8 | 0.2 [−0.2, 0.7] | 0.27 |
| Median (Q1, Q3) | 99.9 (99.2, 100.0) | 100.0 (99.1, 100.0) | ||
| % Uncovered and apposed struts | ||||
| Mean ± SD | 0.5 ± 1.0 | 0.5 ± 1.6 | −0.0 [−0.4, 0.4] | 0.96 |
| Median (Q1, Q3) | 0.0 (0.0, 0.8) | 0.0 (0.0, 0.3) | ||
| Region length (mm) | ||||
| Mean ± SD | 23.1 ± 10.6 | 23.5 ± 10.6 | −0.4 [−3.6, 2.8] | 0.78 |
| Median (Q1, Q3) | 18.3 (17.3, 27.4) | 19.1 (17.8, 28.0) | ||
| Abluminal stent area (mm2) | ||||
| Mean ± SD | 8.73 ± 1.73 | 8.19 ± 2.04 | 0.53 [−0.04, 1.11] | 0.07 |
| Median (Q1, Q3) | 8.60 (7.52, 9.95) | 8.05 (6.36, 9.68) | ||
| Abluminal minimal stent area (mm2) | ||||
| Mean ± SD | 7.30 ± 1.69 | 7.04 ± 1.88 | 0.26 [−0.28, 0.80] | 0.34 |
| Median (Q1, Q3) | 7.12 (6.00, 8.78) | 7.17 (5.40, 8.29) | ||
| ISA area | ||||
| Mean ± SD | 0.04 ± 0.11 | 0.11 ± 0.34 | −0.07 [−0.15, 0.01] | 0.07 |
| Median (Q1, Q3) | 0.00 (0.00, 0.03) | 0.00 (0.00, 0.08) | ||
| Mean intraluminal mass area (mm2) | ||||
| Mean ± SD | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 [−0.00, 0.00] | 0.33 |
| Median (Q1, Q3) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | ||
| Mean neointimal hyperplasia area (mm2) | ||||
| Mean ± SD | 1.52 ± 0.38 | 1.35 ± 0.54 | 0.17 [0.03, 0.31] | 0.018 |
| Median (Q1, Q3) | 1.47 (1.25, 1.76) | 1.22 (1.01, 1.59) | ||
| Mean Lumen area (mm2) | ||||
| Mean ± SD | 7.06 ± 1.79 | 7.02 ± 2.01 | 0.04 [−0.53, 0.62] | 0.89 |
| Median (Q1, Q3) | 6.78 (5.81, 8.16) | 6.90 (5.41, 8.40) | ||
| Minimal Lumen area (mm2) | ||||
| Mean ± SD | 5.40 ± 1.75 | 5.53 ± 1.87 | −0.13 [−0.67, 0.42] | 0.65 |
| Median (Q1, Q3) | 5.01 (3.98, 6.64) | 5.59 (4.13, 6.96) | ||
| Mean Flow area (mm2) | ||||
| Mean ± SD | 7.05 ± 1.78 | 7.01 ± 2.00 | 0.04 [−0.53, 0.61] | 0.89 |
| Median (Q1, Q3) | 6.76 (5.81, 8.15) | 6.90 (5.40, 8.40) | ||
| Minimal flow area (mm2) | ||||
| Mean ± SD | 5.40 ± 1.75 | 5.53 ± 1.87 | −0.13 [−0.67, 0.42] | 0.65 |
| Median (Q1, Q3) | 5.01 (3.98, 6.64) | 5.59 (4.11, 6.96) | ||
| Abluminal stent volume (mm3) | ||||
| Mean ± SD | 199 ± 90 | 191 ± 96 | 7 [−21, 35] | 0.61 |
| Median (Q1, Q3) | 177 (138, 238) | 168 (130, 243) | ||
| Neointimal hyperplasia volume (mm3) | ||||
| Mean ± SD | 35.5 ± 20.9 | 31.8 ± 19.7 | 3.7 [−2.4, 9.8] | 0.24 |
| Median (Q1, Q3) | 29.0 (23.2, 41.5) | 25.8 (17.2, 40.0) | ||
| Incomplete strut apposition volume (mm3) | ||||
| Mean ± SD | 0.8 ± 1.9 | 1.8 ± 4.2 | −1.0 [−2.0, −0.0] | 0.048 |
| Median (Q1, Q3) | 0.0 (0.0, 0.6) | 0.0 (0.0, 2.0) | ||
| Lumen volume (mm3) | ||||
| Mean ± SD | 160 ± 73 | 164 ± 85 | −4 [−28, 20] | 0.76 |
| Median (Q1, Q3) | 141 (108, 202) | 141 (112, 191) | ||
| Flow volume (mm3) | ||||
| Mean ± SD | 160 ± 73 | 164 ± 85 | −4 [−28, 20] | 0.76 |
| Median (Q1, Q3) | 141 (108, 202) | 141 (112, 191) | ||
| % volume obstruction | ||||
| Mean ± SD | 17.9 ± 4.8 | 16.9 ± 6.2 | 0.9 [−0.7, 2.6] | 0.27 |
| Median (Q1, Q3) | 17.8 (14.0, 21.2) | 16.3 (12.6, 21.1) | ||
| Mean strut coverage (mm) | ||||
| Mean ± SD | 0.11 ± 0.03 | 0.09 ± 0.05 | 0.02 [0.01, 0.03] | <0.001 |
| Median (Q1, Q3) | 0.10 (0.09, 0.13) | 0.07 (0.05, 0.10) | ||
| Maximal strut coverage (mm) | ||||
| Mean ± SD | 0.32 ± 0.12 | 0.34 ± 0.19 | −0.01 [−0.06, 0.03] | 0.56 |
| Median (Q1, Q3) | 0.30 (0.24, 0.39) | 0.30 (0.21, 0.38) | ||
| ISA distance (mm) | ||||
| Mean ± SD | 0.39 ± 0.15 | 0.32 ± 0.16 | 0.07 [−0.00, 0.15] | 0.057 |
| Median (Q1, Q3) | 0.39 (0.29, 0.48) | 0.33 (0.23, 0.41) | ||
| Mean lumen diameter (mm) | ||||
| Mean ± SD | 2.97 ± 0.38 | 2.95 ± 0.43 | 0.02 [−0.11, 0.14] | 0.8 |
| Median (Q1, Q3) | 2.93 (2.72, 3.22) | 2.96 (2.62, 3.27) | ||
CI, confidence interval; Q1, first quartile; Q3, third quartile; N refers to number of lesions with data available, ISA, incomplete strut apposition.
*P-value for non-inferiority test.
**P-value for superiority test.
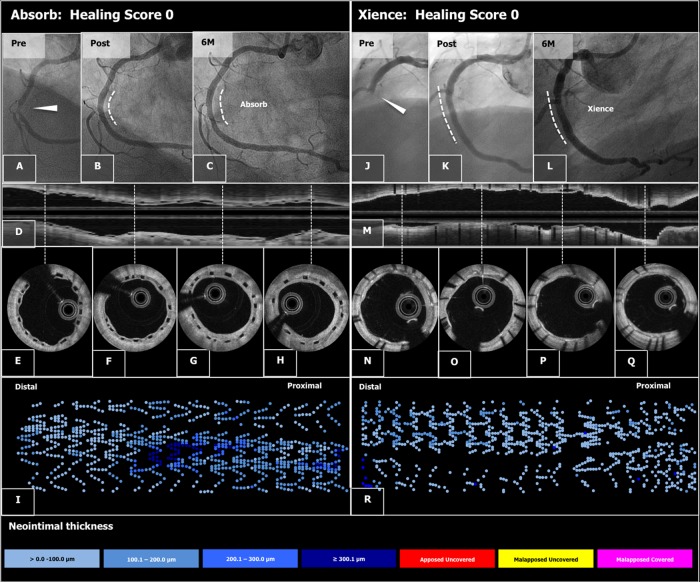
Figure 2.
Typical case examples of angiography and optical frequency domain imaging study in the Absorb arm (A–I) and the Xience arm (J–R). A patient presenting with ST-segment elevation myocardial infarction was successfully treated by implantation of an Absorb stent in the mid right coronary artery (A and B). At 6 months, the late loss was 0.13 mm (C). On optical frequency domain imaging (longitudinal view: D and cross sections: E–H), the polymeric struts were completely covered and apposed, resulting in a healing score of 0. The foldout representation of OFDI (I) illustrates graphically the distribution of neointimal thickness per strut throughout the stent. The extent of the thickness in micron is categorized and colour coded at the bottom of the figure. A patient presenting with ST-segment elevation myocardial infarction of RCA received a Xience stent (J and K). The angiography and optical frequency domain imaging at 6 months showed minimal neointimal hyperplasia (angiography: L, longitudinal and cross-sectional optical frequency domain imaging: M and N–Q, foldout view: R) with excellent neointimal coverage (HS = 0).
Figure 3.
Case examples of suboptimal neointimal healing in the Absorb arm (A–I) and the Xience arm (J–R). A patient received an Absorb stent in right coronary artery (A and B). The 6-month angiographic late loss was 0.16 mm (C), however, optical frequency domain imaging (longitudinal view: D, cross-sections: E–H) showed malapposed struts (E, F, and G) and uncovered struts (G and H). The foldout view of optical frequency domain imaging (I) depicted the distribution of malapposed and/or uncovered struts colour coded in red, pink, and yellow. The neointimal healing score was 9.8. A patient received a Xience stent in the infarct-related artery (J and K). At 6-month imaging follow-up (angiography: L), there was no restenosis but optical frequency domain imaging (M–Q) showed uncovered (N, P, and Q) and/or malapposed struts (O). The healing score of the case was 12.2 mainly due to the high frequency of malapposed struts (R).
Figure 4.
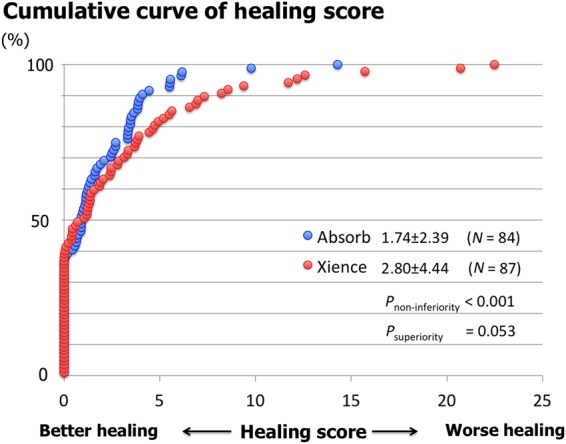
Cumulative distribution curves of the healing score between Absorb and everolimus-eluting stent arms. Healing score in the Absorb arm was non-inferior to that of the everolimus-eluting stent arm with a trend towards superiority.
The abluminal mean stent areas amounted to 8.73 and 8.19 mm2 in Absorb and EES, respectively (P = 0.07). The neointima area was significantly larger in the Absorb compared with EES (1.52 and 1.35 mm2P = 0.018). As a result, the mean luminal area was similar in both groups 7.06 and 7.02 mm2 in Absorb and EES, respectively (P = 0.89). In addition, the minimum stent area (7.30 and 7.04 mm2 in Absorb and EES, respectively; P = 0.34) and the minimum luminal area (5.40 and 5.53 mm2 in Absorb and EES, respectively; P = 0.65) were similar in both groups (Supplementary material online, Figure SB and appendix). The percentage of volume obstruction was 17.9 and 16.9% in Absorb and EES, respectively (P = 0.27).
Quantitative coronary angiography data at 6-month follow-up are summarized in Table 3. The mean in-device late lumen loss was 0.17 ± 0.24 mm in the Absorb group vs. 0.08 ± 0.28 mm in the EES arm (P = 0.024). Cumulative curves of late loss are presented in Supplementary material online, Figure SC. In-device, binary restenosis rate was similarly low in both groups (0% in Absorb vs. 1.1% in EES; difference −1.1% [−3.3%, 1.1%]; P = 1.0).
The DOCE rates were low (Absorb: 1.1% vs. Xience: 0%) at 6 months. There was no death in both groups. There was only one patient in the Absorb group suffering a subacute definite stent thrombosis leading to MI and clinically driven TLR. In this patient, an inadequate matching of the vessel and device size was observed (vessel size 1.92 mm; stent size 2.5 mm). There were in each group one non-clinically driven TLR at 178 and 198 days. One patient in the Xience arm underwent a non-clinically driven target vessel revascularization outside of the target lesion at 198 days. In addition, there were five and four non-target vessel revascularization (mostly staged) in the Absorb arm and the Xience arm, respectively.
Most of the patients were on dual antiplatelet regimen (aspirin + ticagrelor: 46.2% in Absorb vs. 46.9% in EES arm; P = 0.92; aspirin + prasugrel: 31.9% in Absorb vs. 37.9% in EES arm; P = 0.39). At follow-up, angina-free patients were 91.4 vs. 91.7% in the Absorb and EES group, respectively (P = 0.94).
Discussion
This is the first randomized clinical trial which investigated stenting of culprit lesion in the setting of STEMI comparing Absorb stent with the conventional EES. The principal findings can be summarized as follows: These findings are notable for several reasons. First, rupture plaques in patients with STEMI have been shown to be prone to delay arterial healing. Specifically, mean rate of uncovered stents appeared to be as high as 49% in culprit lesions from STEMI when compared with 9% in stable plaques after first-generation DES implantation.7 The advent of second-generation DES has improved the arterial healing response. In an in vivo animal model, the use of EES when compared with first-generation sirolimus-eluting stent was associated with a lower incidence of uncovered struts (0.4 ± 0.8 vs. 41.7 ± 27.0%; P = 0.004) and a minimal degree of inflammatory reaction (inflammation score 0.5 ± 0.4 vs. 2.9 ± 1.4; P = 0.001).24 These findings have been corroborated in humans in whom EES evidenced lower frequencies of uncovered struts and malapposed struts compared with paclitaxel-eluting stent (2.3 vs. 5.2% and 2.1 vs. 5.7%, respectively; P < 0.001) as assessed by optical coherence tomography (OCT).25 Histopathology and OCT studies have also confirmed the superiority of EES in vascular healing process when compared with first-generation DES.26 In a multi-imaging study, rates of uncovered struts (1.2 ± 2.3% vs. 1.0 ± 2.3%) and percentage of stents fully covered with neointima (60.9 vs. 61.1%) appeared to be similar in STEMI vs. stable coronary plaques at 12 months with the use of EES.27
Stenting of culprit lesions with Absorb in the setting of STEMI resulted in nearly complete arterial healing, which was comparable with that of metallic EES at 6 months.
Frequency of malapposed, and both malapposed and uncovered struts were lower in the Absorb arm, while there was no difference in intraluminal mass between groups.
QCA revealed similar acute gain and MLD post-procedure. At 6 months, late lumen loss was lower in the EES arm, but binary restenosis rate was comparably low between groups.
Secondly, EES is currently the best-in-class DES and has become the reference in the stent comparative trials.21,28 A comprehensive network meta-analysis involving 51 trials that included a total of 52 158 randomized patients with follow-up duration ≥3 years recently demonstrated that EES was associated with lower rates of mortality, definite stent thrombosis, and myocardial infarction than bare-metal stents and first-generation DES.29 In the setting of STEMI, EES also evidenced the safest and the most efficacious profile among their counterparts.6,30 Its safe vascular healing process following implantation could be the basis of this clinical advantage.
Third, OFDI was used at follow-up for the primary endpoint analysis. This intracoronary diagnostic technique is able to detect to a nearly microscopic resolution any potential finding that could be different in the two groups. Up-to-date, there was only one OFDI study that reported the arterial response head-to-head between Absorb and EES, in stable coronary artery disease.31 At 1-year after implantation, neointimal thickness and percentage in-device area obstruction were comparable between groups. In the same way, uncovered strut rate was also similar between devices (5.3 vs. 4.5%; P = 0.11). Our results are promising as they have been obtained at a shorter follow-up period (6 months) and in the context of high thrombogenic milieu.
Fourth, the implantation technique was guided by angiography alone (maximum diameter assessed by on-line QCA), which corresponds to routine clinical practice.
In this context, the acute performance of both devices was comparable (Table 3). This is reassuring with respect to the initial findings of a previous randomized trial performed in stable coronary artery lesions where Absorb did not achieve the same acute lumen gain as EES.32
Finally, late loss was significantly higher in the Absorb arm (0.17 vs. 0.08 mm in the EES arm; P = 0.024) although this was not translated into any increase in binary restenosis rate (Absorb: 0% vs. Xience: 1%, Table 3). The observed late lumen loss is comparable with that of the EVERBIO II (comparison of everolimus- and biolimus-eluting stents with everolimus-eluting bioresorbable vascular scaffold stents II) randomized trial that compared the angiographic performance of Absorb vs. EES vs. biolimus-eluting stent (BES).33 The primary endpoint (late loss at 9 months) was comparable between groups (0.28 mm in Absorb, 0.25 mm in EES/BES group). Of note, only 10% of patients included in EVERBIO II trial presented with STEMI. The thicker struts of the Absorb, when compared with those of EES (150 vs. 81 µm) may induce a more intense neointimal response that leads to higher late lumen loss.34
The results of this study may have relevant clinical implications. We have demonstrated that Absorb can deliver the same acute and mid-term results as second-generation DES when using an appropriate implantation technique.12,20 Patients presenting with STEMI may represent the ideal scenario for the use of Absorb.35 Culprit lesions are frequently localized in the proximal segments of the coronary artery tree.36 Therefore, restoration of physiological vasomotion may have a greater effect in patients with STEMI when compared with patients with stable coronary artery disease.
Recent study suggests that Absorb may eventually decrease the incidence of angina during follow-up, by reducing fixed and dynamic restenoses and by improving vasomotor responses.32 In our trial, >90% of the patients were found to be free from angina at 6-month follow-up. Finally, the potential advantages of implanting Absorb (vs. other DES) in STEMI may be mostly related to the young age of these patients. Indeed, they usually have less extensive coronary artery disease (compared with other forms of coronary artery disease), may have a long life expectancy and thus may benefit more from not having a permanent caging of the coronary artery. Despite all the above, current evidence by the use of Absorb in STEMI is still scarce and limited to few registries involving a low number of selected patients.35 Recently, a propensity score matching between Absorb (n = 290), bare-metal stent (n = 290), and EES (n = 290) in the context of STEMI showed a comparable incidence of device-oriented endpoint either at 30 days or at 1 year. Of interest, definite/probable thrombosis rate was found to be numerically higher with Absorb either at 30 days (2.1% vs. 0.3%, vs. 1.0%) or 1 year (2.4% vs. 1.4%, vs. 1.7%), when compared with EES or bare-metal stent, respectively.37 Conversely, in our trial a very low event rate has been observed, which may be related to refinement and standardization of the implantation technique.12,20 As mentioned above, the only episode of stent thrombosis was presumably due to an inappropriate matching of the stent to the vessel size. Finally, another potential clinical implication regards to the observed nearly complete healing at 6 months. This is reassuring for an eventual need of antiplatelet treatment disruption or cessation before the 1-year prescription time.
Several limitations have to be acknowledged. The observed event rate was exceedingly low. This may be related to the highly selected population (only 10% of the STEMI patients admitted during the recruitment period were included), due to inclusion and exclusion criteria, need to consent in the acute phase of STEMI, need to randomize after successful lesion preparation, and the requirement of an angiographic follow-up. Thus, results cannot be representative of more complex population suffering from STEMI. Secondly, our study assess arterial healing at 6 months which is an intermediate time point in which the resorption process is not complete and the process of neointima formation in EES has not picked yet. Six-month follow-up represents, however, the time when the mechanical support of the Absorb stent starts to decrease and thus the healing should be completed at this crucial time. A longer-term follow-up is needed to further characterize the healing process. Third, results in HS refer to current Absorb technology with relatively thick struts (150 µm). Therefore, these findings cannot be extrapolated to other bioresorbable devices with different materials or strut thickness. Fourthly, the observed HS was lower than the assumed score in the sample size calculation (actual HS 1.74 vs. assumed HS 9.0). The original non-inferiority margin of 4.5 was determined as 50% of the observed HS of 9.0 in the Absorb stent in a stable angina population. If we apply this relative non-inferiority margin to the current observation, the (post hoc) non-inferiority margin would be 1.4 (50% of the HS in the control group: 2.80). Non-inferiority is met with this post hoc NI margin. In addition, the distribution of observed HS was not normal. When the log-transformation was performed, the non-inferiority was still achieved with a P-value of <0.001 (log-transformed HS: 0.73 ± 0.72 vs. Xience: 0.86 ± 0.92, difference: −0.13). Finally, sample size does not allow us to draw any meaningful conclusion regarding clinical outcomes.
In conclusion, this randomized trial demonstrated a nearly complete arterial healing at 6 months after both Absorb and EES implantation in the setting of STEMI. This trial provides the basis for further exploration in clinical outcomes trials.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
Sponsor: European Cardiovascular Research Institute (ECRI); grant givers: Abbott Vascular and Terumo Europe N.V. Funding to pay the Open Access publication charges for this article was provided by ECRI.
Conflict of interest: L.O.J. has received institutional research grants from Terumo, Biosensors and St Jude Medical. S.W. has received institutional research contracts from Abbott, Boston Scientific, Biosensors, Biotronik, Edward Lifesciences, Medtronic and St Jude. He has received speaker fees from Astra Zeneca, Bayer, Eli Lilly, Abbott, Boston Scientific, Biosensors, Biotronik, Edward Lifesciences, Medtronic and St Jude. M.S. received a consultant/speaker fee from the Abbott Vascular. A.C. received research grants from Abbott Vascular, Medtronic, Biomenco and Spanish Society and he also received a consulting or lectures fees from Abbott Vascular, Medtronic and Boston Scientific. Y.O. and P.W.S. are a member of advisory board of Abbott Vascular.
Supplementary Material
References
- 1. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003;361:13–20. [DOI] [PubMed] [Google Scholar]
- 2. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 3. Sabate M, Cequier A, Iniguez A, Serra A, Hernandez-Antolin R, Mainar V, Valgimigli M, Tespili M, den Heijer P, Bethencourt A, Vazquez N, Gomez-Hospital JA, Baz JA, Martin-Yuste V, van Geuns RJ, Alfonso F, Bordes P, Tebaldi M, Masotti M, Silvestro A, Backx B, Brugaletta S, van Es GA, Serruys PW. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet 2012;380:1482–1490. [DOI] [PubMed] [Google Scholar]
- 4. Raber L, Kelbaek H, Ostojic M, Baumbach A, Heg D, Tuller D, von Birgelen C, Roffi M, Moschovitis A, Khattab AA, Wenaweser P, Bonvini R, Pedrazzini G, Kornowski R, Weber K, Trelle S, Luscher TF, Taniwaki M, Matter CM, Meier B, Juni P, Windecker S, Investigators CAT. Effect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA 2012;308:777–787. [DOI] [PubMed] [Google Scholar]
- 5. Sabate M, Raber L, Heg D, Brugaletta S, Kelbaek H, Cequier A, Ostojic M, Iniguez A, Tuller D, Serra A, Baumbach A, von Birgelen C, Hernandez-Antolin R, Roffi M, Mainar V, Valgimigli M, Serruys PW, Juni P, Windecker S. Comparison of newer-generation drug-eluting with bare-metal stents in patients with acute ST-segment elevation myocardial infarction: a pooled analysis of the EXAMINATION (clinical Evaluation of the Xience-V stent in Acute Myocardial INfArcTION) and COMFORTABLE-AMI (Comparison of Biolimus Eluted From an Erodible Stent Coating With Bare Metal Stents in Acute ST-Elevation Myocardial Infarction) trials. JACC Cardiovasc Intervent 2014;7:55–63. [DOI] [PubMed] [Google Scholar]
- 6. Palmerini T, Biondi-Zoccai G, Della Riva D, Mariani A, Sabate M, Valgimigli M, Frati G, Kedhi E, Smits PC, Kaiser C, Genereux P, Galatius S, Kirtane AJ, Stone GW. Clinical outcomes with drug-eluting and bare-metal stents in patients with ST-segment elevation myocardial infarction: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol 2013;62:496–504. [DOI] [PubMed] [Google Scholar]
- 7. Nakazawa G, Finn AV, Joner M, Ladich E, Kutys R, Mont EK, Gold HK, Burke AP, Kolodgie FD, Virmani R. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients: an autopsy study. Circulation 2008;118:1138–1145. [DOI] [PubMed] [Google Scholar]
- 8. Gonzalo N, Barlis P, Serruys PW, Garcia-Garcia HM, Onuma Y, Ligthart J, Regar E. Incomplete stent apposition and delayed tissue coverage are more frequent in drug-eluting stents implanted during primary percutaneous coronary intervention for ST-segment elevation myocardial infarction than in drug-eluting stents implanted for stable/unstable angina: insights from optical coherence tomography. JACC Cardiovasc Intervent 2009;2:445–452. [DOI] [PubMed] [Google Scholar]
- 9. Raber L, Zanchin T, Baumgartner S, Taniwaki M, Kalesan B, Moschovitis A, Garcia-Garcia HM, Justiz J, Pilgrim T, Wenaweser P, Meier B, Juni P, Windecker S. Differential healing response attributed to culprit lesions of patients with acute coronary syndromes and stable coronary artery after implantation of drug-eluting stents: an optical coherence tomography study. Int J Cardiol 2014;173:259–267. [DOI] [PubMed] [Google Scholar]
- 10. Brugaletta S, Heo JH, Garcia-Garcia HM, Farooq V, van Geuns RJ, de Bruyne B, Dudek D, Smits PC, Koolen J, McClean D, Dorange C, Veldhof S, Rapoza R, Onuma Y, Bruining N, Ormiston JA, Serruys PW. Endothelial-dependent vasomotion in a coronary segment treated by ABSORB everolimus-eluting bioresorbable vascular scaffold system is related to plaque composition at the time of bioresorption of the polymer: indirect finding of vascular reparative therapy? Eur Heart J 2012;33:1325–1333. [DOI] [PubMed] [Google Scholar]
- 11. Brugaletta S, Radu MD, Garcia-Garcia HM, Heo JH, Farooq V, Girasis C, van Geuns RJ, Thuesen L, McClean D, Chevalier B, Windecker S, Koolen J, Rapoza R, Miquel-Hebert K, Ormiston J, Serruys PW. Circumferential evaluation of the neointima by optical coherence tomography after ABSORB bioresorbable vascular scaffold implantation: can the scaffold cap the plaque? Atherosclerosis 2012;221:106–112. [DOI] [PubMed] [Google Scholar]
- 12. Bourantas CV, Serruys PW, Nakatani S, Zhang YJ, Farooq V, Diletti R, Ligthart J, Sheehy A, van Geuns RJ, McClean D, Chevalier B, Windecker S, Koolen J, Ormiston J, Whitbourn R, Rapoza R, Veldhof S, Onuma Y, Garcia-Garcia HM. Bioresorbable vascular scaffold treatment induces the formation of neointimal cap that seals the underlying plaque without compromising the luminal dimensions: a concept based on serial optical coherence tomography data. EuroIntervention 2014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13. Raber L, Onuma Y, Brugaletta S, García-García H, Backx B, Iñiguez A, Okkels-Jensen L, Cequier A, Christiansen E, Suttorp M, Serruys P, Sabate M, Windecker S. Arterial healing following primary PCI using the ABSORB everolimus eluting bioresorbable vascular scaffold (ABSORB BVS) versus the durable polymer everolimus-eluting metallic stent (Xience) in patients with acute ST-elevation myocardial infarction: rationale and design of the randomized TROFI II TRIAL. EuroIntervention 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14. Onuma Y, Thuesen L, van Geuns RJ, van der Ent M, Desch S, Fajadet J, Christiansen E, Smits P, Holm NR, Regar E, van Mieghem N, Borovicanin V, Paunovic D, Senshu K, van Es GA, Muramatsu T, Lee IS, Schuler G, Zijlstra F, Garcia-Garcia HM, Serruys PW, Investigators T. Randomized study to assess the effect of thrombus aspiration on flow area in patients with ST-elevation myocardial infarction: an optical frequency domain imaging study – TROFI trial. Eur Heart J 2013;34:1050–1060. [DOI] [PubMed] [Google Scholar]
- 15. Garcia-Garcia HM, Muramatsu T, Nakatani S, Lee IS, Holm NR, Thuesen L, van Geuns RJ, van der Ent M, Borovicanin V, Paunovic D, Onuma Y, Serruys PW. Serial optical frequency domain imaging in STEMI patients: the follow-up report of TROFI study. Eur Heart J Cardiovasc Imaging 2014;15:987–995. [DOI] [PubMed] [Google Scholar]
- 16. Nakatani S, Sotomi Y, Ishibashi Y, Grundeken M, Tateishi H, Tenekecioglu E, Zeng Y, Suwannasom P, Regar E, Radu M, Räber L, Bezerra H, Costa M, Prati F, Costa R, Dijkstra J, Kimura T, Kozuma K, Tanabe K, Akasaka T, Mario C, Serruys P, Onuma Y. Comparative analysis method of permanent metallic stents (Xience) and bioresorbable poly-L-lactide (PLLA) scaffolds (Absorb) on optical coherence tomography at baseline and follow-up. EuroIntervention 2015;in press. [DOI] [PubMed] [Google Scholar]
- 17. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW, Academic Research C. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344–2351. [DOI] [PubMed] [Google Scholar]
- 18. Vranckx P, Cutlip DE, Mehran R, Kint PP, Silber S, Windecker S, Serruys PW. Myocardial infarction adjudication in contemporary all-comer stent trials: balancing sensitivity and specificity. Addendum to the Historical MI definitions used in stent studies . EuroIntervention 2010;5:871–874. [DOI] [PubMed] [Google Scholar]
- 19. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Thygesen K, Alpert JS, White HD, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Chaitman BA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S, Guidelines, Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction, ESCCfP. Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551–2567.22922414 [Google Scholar]
- 20. Tamburino C, Latib A, van Geuns RJ, Sabate M, Mehilli J, Gori T, Achenbach S, Alvarez MP, Nef H, Lesiak M, Di Mario C, Colombo A, Naber CK, Caramanno G, Capranzano P, Brugaletta S, Geraci S, Araszkiewicz A, Mattesini A, Pyxaras SA, Rzeszutko L, Depukat R, Diletti R, Boone E, Capodanno D, Dudek D. Contemporary practice and technical aspects in coronary intervention with bioresorbable scaffolds: a European perspective. EuroIntervention 2015;11:45–52. [DOI] [PubMed] [Google Scholar]
- 21. Serruys PW, Silber S, Garg S, van Geuns RJ, Richardt G, Buszman PE, Kelbaek H, van Boven AJ, Hofma SH, Linke A, Klauss V, Wijns W, Macaya C, Garot P, DiMario C, Manoharan G, Kornowski R, Ischinger T, Bartorelli A, Ronden J, Bressers M, Gobbens P, Negoita M, van Leeuwen F, Windecker S. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med 2010;363:136–146. [DOI] [PubMed] [Google Scholar]
- 22. Serruys PW, Onuma Y, Garcia-Garcia HM, Muramatsu T, van Geuns RJ, de Bruyne B, Dudek D, Thuesen L, Smits PC, Chevalier B, McClean D, Koolen J, Windecker S, Whitbourn R, Meredith I, Dorange C, Veldhof S, Hebert KM, Rapoza R, Ormiston JA. Dynamics of vessel wall changes following the implantation of the absorb everolimus-eluting bioresorbable vascular scaffold: a multi-imaging modality study at 6, 12, 24 and 36 months. EuroIntervention 2014;9:1271–1284. [DOI] [PubMed] [Google Scholar]
- 23. Gutierrez-Chico JL, van Geuns RJ, Regar E, van der Giessen WJ, Kelbaek H, Saunamaki K, Escaned J, Gonzalo N, di Mario C, Borgia F, Nuesch E, Garcia-Garcia HM, Silber S, Windecker S, Serruys PW. Tissue coverage of a hydrophilic polymer-coated zotarolimus-eluting stent vs. a fluoropolymer-coated everolimus-eluting stent at 13-month follow-up: an optical coherence tomography substudy from the RESOLUTE All Comers trial. Eur Heart J 2011;32:2454–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakazawa G, Shinke T, Ijichi T, Matsumoto D, Otake H, Torii S, Hiranuma N, Ohsue T, Otsuka F, Shite J, Hirata K, Ikari Y. Comparison of vascular response between durable and biodegradable polymer-based drug-eluting stents in a porcine coronary artery model. EuroIntervention 2014;10:717–723. [DOI] [PubMed] [Google Scholar]
- 25. Takano M, Murakami D, Yamamoto M, Kurihara O, Murai K, Inami T, Kimata N, Ohba T, Seino Y, Mizuno K. Six-month follow-up evaluation for everolimus-eluting stents by intracoronary optical coherence tomography: comparison with paclitaxel-eluting stents. Int J Cardiol 2013;166:181–186. [DOI] [PubMed] [Google Scholar]
- 26. Hiranuma N, Shinke T, Nakazawa G, Otake H, Matsumoto D, Ijichi T, Kawamori H, Nagoshi R, Osue T, Shite J, Hirata K. Optical coherence tomography and histopathology assessment after implantation of first- and second-generation drug-eluting stents in a porcine coronary model. Circ J 2014;78:2665–2673. [DOI] [PubMed] [Google Scholar]
- 27. Mizoguchi T, Sawada T, Shinke T, Yamada S, Okamoto H, Kim SS, Takarada A, Yasaka Y. Detailed comparison of intra-stent conditions 12 months after implantation of everolimus-eluting stents in patients with ST-segment elevation myocardial infarction or stable angina pectoris. Int J Cardiol 2014;171:224–230. [DOI] [PubMed] [Google Scholar]
- 28. Windecker S, Haude M, Neumann FJ, Stangl K, Witzenbichler B, Slagboom T, Sabate M, Goicolea J, Barragan P, Cook S, Piot C, Richardt G, Merkely B, Schneider H, Bilger J, Erne P, Waksman R, Zaugg S, Juni P, Lefevre T. Comparison of a novel biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent: results of the randomized BIOFLOW-II trial. Circ Cardiovasc Intervent 2015;8:e001441. [DOI] [PubMed] [Google Scholar]
- 29. Palmerini T, Benedetto U, Biondi-Zoccai G, Della Riva D, Bacchi-Reggiani L, Smits PC, Vlachojannis GJ, Jensen LO, Christiansen EH, Berencsi K, Valgimigli M, Orlandi C, Petrou M, Rapezzi C, Stone GW. Long-term safety of drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol 2015;65:2496–2507. [DOI] [PubMed] [Google Scholar]
- 30. Sabate M, Brugaletta S, Cequier A, Iniguez A, Serra A, Hernadez-Antolin R, Mainar V, Valgimigli M, Tespili M, den Heijer P, Bethencourt A, Vazquez N, Backx B, Serruys PW. The EXAMINATION trial (everolimus-eluting stents versus bare-metal stents in ST-segment elevation myocardial infarction): 2-year results from a multicenter randomized controlled trial. JACC Cardiovasc Intervent 2014;7:64–71. [DOI] [PubMed] [Google Scholar]
- 31. Gomez-Lara J, Brugaletta S, Farooq V, Onuma Y, Diletti R, Windecker S, Thuesen L, McClean D, Koolen J, Whitbourn R, Dudek D, Smits PC, Chevalier B, Regar E, Veldhof S, Rapoza R, Ormiston JA, Garcia-Garcia HM, Serruys PW. Head-to-head comparison of the neointimal response between metallic and bioresorbable everolimus-eluting scaffolds using optical coherence tomography. JACC Cardiovasc Intervent 2011;4:1271–1280. [DOI] [PubMed] [Google Scholar]
- 32. Serruys PW, Chevalier B, Dudek D, Cequier A, Carrie D, Iniguez A, Dominici M, van der Schaaf RJ, Haude M, Wasungu L, Veldhof S, Peng L, Staehr P, Grundeken MJ, Ishibashi Y, Garcia-Garcia HM, Onuma Y. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial. Lancet 2015;385:43–54. [DOI] [PubMed] [Google Scholar]
- 33. Puricel S, Arroyo D, Corpataux N, Baeriswyl G, Lehmann S, Kallinikou Z, Muller O, Allard L, Stauffer JC, Togni M, Goy JJ, Cook S. Comparison of everolimus- and biolimus-eluting coronary stents with everolimus-eluting bioresorbable vascular scaffolds. J Am Coll Cardiol 2015;65:791–801. [DOI] [PubMed] [Google Scholar]
- 34. Kastrati A, Mehilli J, Dirschinger J, Dotzer F, Schuhlen H, Neumann FJ, Fleckenstein M, Pfafferott C, Seyfarth M, Schomig A. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation 2001;103:2816–2821. [DOI] [PubMed] [Google Scholar]
- 35. Scalone G, Brugaletta S, Gomez-Monterrosas O, Otsuki S, Sabate M. ST-segment elevation myocardial infarction - ideal scenario for bioresorbable vascular scaffold implantation? Circ J 2015;79:263–270. [DOI] [PubMed] [Google Scholar]
- 36. Cheruvu PK, Finn AV, Gardner C, Caplan J, Goldstein J, Stone GW, Virmani R, Muller JE. Frequency and distribution of thin-cap fibroatheroma and ruptured plaques in human coronary arteries: a pathologic study. J Am Coll Cardiol 2007;50:940–949. [DOI] [PubMed] [Google Scholar]
- 37. Brugaletta S, Gori T, Low AF, Tousek P, Pinar E, Gomez-Lara J, Scalone G, Schulz E, Chan MY, Kocka V, Hurtado J, Gomez-Hospital JA, Munzel T, Lee CH, Cequier A, Valdes M, Widimsky P, Serruys PW, Sabate M. Absorb bioresorbable vascular scaffold versus everolimus-eluting metallic stent in ST-segment elevation myocardial infarction: 1-year results of a propensity score matching comparison: the BVS-EXAMINATION Study (bioresorbable vascular scaffold-a clinical evaluation of everolimus eluting coronary stents in the treatment of patients with ST-segment elevation myocardial infarction). JACC Cardiovasc Intervent 2015;8(1 Pt B):189–197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.