Abstract
Hypothesis
As the anterior and posterior semicircular canals are vital to the regulation of gaze stability, particularly during locomotion or vehicular travel, we tested whether the high velocity vestibulo-ocular reflex (VOR) of the three ipsilesional semicircular canals elicited by the modified Head Impulse Test would correlate with subjective dizziness or vertigo scores after vestibular neuritis (VN).
Background
Recovery following acute VN varies with around half reporting persistent symptoms long after the acute episode. However, an unanswered question is whether chronic symptoms are associated with impairment of the high velocity VOR of the anterior or posterior canals.
Methods
Twenty patients who had experienced an acute episode of VN at least three months earlier were included in this study. Participants were assessed with the video head impulse test (vHIT) of all six canals, bithermal caloric irrigation, the Dizziness Handicap Inventory (DHI) and the Vertigo Symptoms Scale short-form (VSS).
Results
Of these 20 patients, 12 felt that they had recovered from the initial episode whereas 8 did not and reported elevated DHI and VSS scores. However, we found no correlation between DHI or VSS scores and the ipsilesional single or combined vHIT gain, vHIT gain asymmetry or caloric paresis. The high velocity VOR was not different between patients who felt they had recovered and patients who felt they had not.
Conclusions
Our findings suggest that chronic symptoms of dizziness following VN are not associated with the high velocity VOR of the single or combined ipsilesional horizontal, anterior or posterior semicircular canals.
Keywords: Vestibular, vestibular neuritis, dizziness, vertigo, head-impulse test
Introduction
Vestibular neuritis (VN) is an acute disorder characterised by vertigo, nausea, vomiting and imbalance following sudden unilateral loss of peripheral vestibular function (1). Recovery is through peripheral and central vestibular compensation (2). Typically, symptoms last days or weeks but around 50% of patients experience chronic dizziness, unsteadiness and spatial disorientation (3,4).
It has been postulated that persistent peripheral vestibular loss could account for these chronic symptoms (5). The standard measure of peripheral vestibular loss is the gain of the vestibulo-ocular reflex (VOR) which is the ratio of the size of slow phase corrective eye movement to the size of head movement (peak slow phase eye velocity / peak head velocity). The VOR maintains gaze stability and preserves visual acuity during head movements. Impairment can cause visual blurring during head motion (6), which could be interpreted by the patient as dizziness, unsteadiness or spatial disorientation. Thus, a central question regarding the process of symptom recovery is whether this is related to a dysfunctional VOR.
Previous studies have shown that the low velocity VOR response from the caloric test does not predict chronic symptoms of dizziness or vertigo (3,7,8). However, recent advances have led to the development of a bedside clinical head thrust or impulse test (HIT) measuring the high velocity VOR of all six semicircular canals (9). The high velocity VOR elicited by the HIT recovers more slowly following acute VN compared to the low velocity VOR elicited by caloric irrigation (10-12), and may thus better reflect clinical outcome.
Interestingly, Palla and colleagues (13) have shown that there is no relationship between the high velocity horizontal canal VOR gain and chronic symptoms following VN. However, as the anterior and posterior semicircular canals are vital to the regulation of gaze stability (14), particularly during locomotion or vehicular travel, we posed the question of whether the high velocity VOR gain of the three ipsilesional semicircular canals (elicited by the modified HIT (9)) would predict subjective dizziness or vertigo scores after VN. The low velocity VOR of the horizontal canal (elicited by caloric irrigation) was measured for comparison.
Materials and Methods
Twenty patients (7 male, 31-87 years (mean 57.3 +/− 18) with clinical histories, physical examinations and function tests typical of acute VN were recruited i.e, horizontal nystagmus, clinically abnormal head-impulse test and a significant canal paresis. Of our patients, none had inferior vestibular neuritis. The exclusion criteria were patients with no current indications of overlapping vestibular migraine. For this study, all patients were tested in the chronic stage of VN (3-36 months after acute VN onset; mean 9.8 +/− 7.5), including a repeat caloric test. Informed consent was obtained from all subjects.
Vestibular assessment
Six-canal vHIT: Eye and head movements were simultaneously recorded using the ICS video Head Impulse system (vHIT, GN Otometrics, Denmark). The system consists of a pair of light-weight goggles containing 3-D gyroscopes to measure head velocity, and a small mounted video camera to record eye position. The video camera is mounted within the right eye-frame of the goggles, which were secured firmly to the subject’s head with an adjustable elastic strap.
The patient was instructed to fixate on a target positioned approximately 1.5 metres in front of them. The examiner, while holding the patient’s head from behind, then made a series of brisk head movements (10–20° amplitude) corresponding to the horizontal, left anterior-right posterior (LARP) and right anterior-left posterior (RALP) canal planes (15). In contrast to early papers measuring VOR responses along the LARP and RALP planes (16), with the vHIT technique the head must be turned in yaw by approximately 40-45° so that the head impulse delivered only (or mostly) elicits vertical VOR movements.
Eye and head velocities were sampled at 250 Hz and the ratio of eye-to-head peak velocity (VOR gain) was calculated for each semicircular canal from an average of 20 head impulses performed over a range of velocities (50–300°/s) (17). Asymmetry between the ipsilesional and contralesional canals was also calculated and expressed as a percentage (18).
In addition to the single canal gain values and asymmetry values generated automatically by the vHIT program, we calculated a total gain for each side: , and total right/left asymmetry (%). As previous studies have reported no correlation between horizontal canal vHIT gain or asymmetry with long-term recovery, we also focussed on the vertical canals and calculated a vertical canal gain , and vertical canal right/left asymmetry (%). These formulae provide overall values for the contributions from each canal.
Caloric test: Bithermal caloric irrigations (30 & 44°C) were performed (ICS CHARTR, GN Otometrics, Denmark)and the degree of canal paresis was calculated using Jongkees formula and expressed as a percentage as previous studies (18).
Symptoms questionnaires
In parallel, symptoms during the past month were scored with the Dizziness Handicap Inventory (DHI) (19) and the Vertigo Symptoms Scale short form (VSS) (20). We also asked each patient whether they felt they had recovered from the acute episode or not.
Pearson Correlation Coefficient analyses were employed between all measures. Independent samples t-tests were used as confirmation. Linear regression was used to test whether vHIT gains predict DHI or VSS scores. P-values were corrected for multiple comparisons.
Results
As shown in Table 1, eight patients felt that they had not fully recovered from the acute episode. These patients also had the highest DHI and VSS scores (paired t-test P<0.002). There was a strong significant correlation between DHI and VSS across the group of 20 VN patients (P<0.001, Pearson Correlation Coefficient=0.857). There was no correlation between caloric paresis and DHI score (Pearson correlation coefficient = −0.134, P=0.57) or between caloric paresis and VSS score (Pearson correlation coefficient = −0.076, P=0.572). There was also no correlation between caloric paresis and horizontal canal vHIT gain asymmetry (Pearson correlation coefficient = 0.176, P=0.458).
Table 1. Vestibular testing data and symptom scores from the patients who participated in this study (n=20).
| Patient | Duration (months) | Age | Caloric Paresis (%) | Ipsilesional horizontal canal vHIT gain | Horizontal vHIT gain Asymmetry (%) | Ipsilesional anterior canal vHIT gain | Anterior vHIT gain Asymmetry (%) | Ipsilesional posterior canal vHIT gain | Posterior vHIT gain Asymmetry (%) | DHI score (/100) | VSS score (/60) | Subjectively Recovered (YES/NO) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 | 44 | 62 | 0.44 | 52 | 0.44 | 53 | 0.59 | 40 | 24 | 18 | No |
| 2 | 4 | 75 | −12 | 0.79 | 15 | 0.77 | 12 | 0.9 | 17 | 22 | 10 | No |
| 3 | 12 | 61 | 75 | 0.69 | 31 | 0.74 | 11 | 0.62 | 23 | 42 | 18 | No |
| 4 | 6 | 47 | 10 | 0.81 | 2 | 0.93 | 16 | 0.33 | 56 | 0 | 4 | |
| 5 | 36 | 75 | 28 | 0.75 | 13 | 0.77 | −2 | 0.78 | 7 | 5 | 4 | |
| 6 | 6 | 54 | 0 | 1.25 | −7 | 1.11 | 0 | 1.2 | −26 | 12 | 5 | |
| 7 | 6 | 48 | 64 | 0.83 | 5 | 0.78 | 25 | 0.75 | 9 | 22 | 12 | No |
| 8 | 4 | 54 | 41 | 0.47 | 32 | 0.65 | −4 | 0.61 | −3 | 30 | 12 | No |
| 9 | 6 | 52 | 22 | 0.76 | −1 | 0.46 | 36 | 0.7 | 11 | 0 | 3 | |
| 10 | 8 | 32 | 17 | 0.93 | −4 | 0.91 | 20 | 0.94 | −5 | 32 | 10 | No |
| 11 | 12 | 33 | 5 | 0.94 | −4 | 0.73 | 28 | 0.96 | −26 | 28 | 13 | No |
| 12 | 8 | 71 | 100 | 0.64 | −17 | 1.03 | −17 | 0.6 | 41 | 8 | 1 | |
| 13 | 7 | 78 | 100 | 0.67 | 28 | 0.33 | 62 | 0.71 | −22 | 18 | 5 | |
| 14 | 3 | 70 | 62 | 0.88 | 7 | 0.75 | −6 | 0.71 | −9 | 12 | 3 | |
| 15 | 15 | 87 | 0 | 0.7 | 44 | 1.08 | −4 | 0.71 | 19 | 18 | 5 | |
| 16 | 5 | 31 | 51 | 0.18 | 87 | 1.1 | −4 | 0.92 | 2 | 8 | 1 | |
| 17 | 12 | 68 | 18 | 0.93 | 2 | 0.65 | 21 | 0.75 | 6 | 15 | 6 | |
| 18 | 18 | 78 | 26 | 1.08 | −10 | 1.18 | −23 | 0.93 | −5 | 0 | 0 | |
| 19 | 7 | 31 | 68 | 0.87 | 9 | 0.76 | 45 | 0.87 | 5 | 0 | 8 | |
| 20 | 24 | 62 | 3 | 0.88 | 9 | 0.78 | 17 | 0.84 | 24 | 70 | 22 | No |
With linear regression, the adjusted R-square was 0.02 for DHI scores and 0.084 for VSS scores. The regression was not significant for either DHI scores (F[0.47], P=0.82) or VSS scores (F[1.29], P=0.327). Similarly, stepwise linear regression identified no predicting independent variables in the analysis (no variables were entered into the analysis for either DHI or VSS).
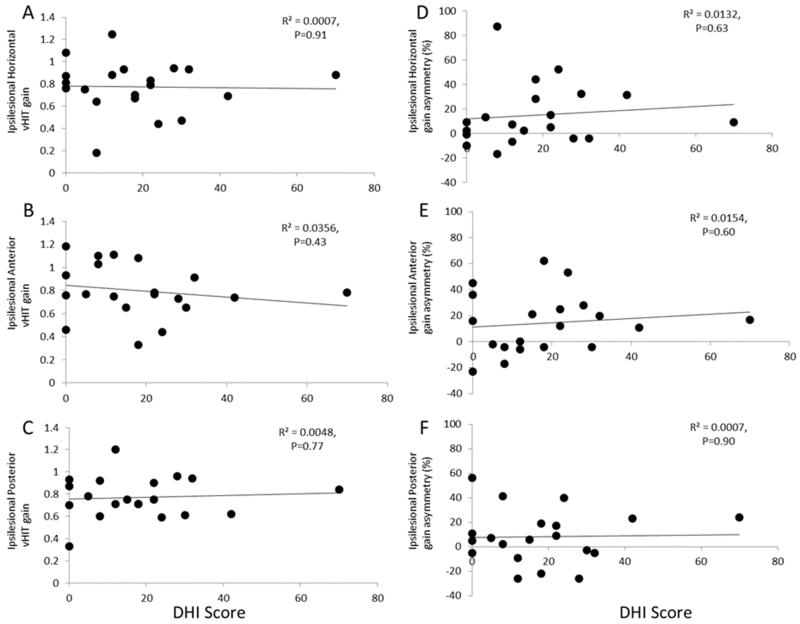
As shown in Figure 1 A-F, there was no correlation between the ipsilesional vHIT gains for the horizontal, anterior and posterior canals and vHIT gain asymmetry for the horizontal, anterior and posterior canals versus DHI score.
Figure 1. vHIT VOR gains for the ipsilesional A). Horizontal, B). Anterior and C). Posterior canals and vHIT gain asymmetry for the A). Horizontal, B). Anterior and C). Posterior canals versus DHI score. vHIT assessment did not correlate with DHI score.
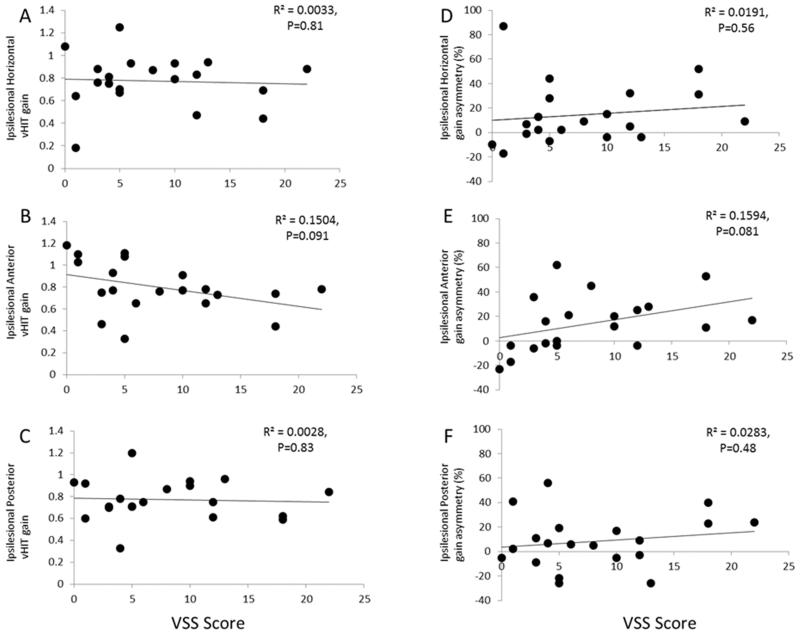
As shown in Figure 2 A-F, there was also no correlation between the ipsilesional vHIT gains for the horizontal, anterior and posterior canals and vHIT gain asymmetry for the horizontal, anterior and posterior canals versus VSS score.
Figure 2. vHIT VOR gains for the ipsilesional A). Horizontal, B). Anterior and C). Posterior canals and vHIT gain asymmetry for the A). Horizontal, B). Anterior and C). Posterior canals versus VSS score. vHIT assessment did not correlate with VSS score.
We also compared vHIT gains and vHIT gain asymmetries for the horizontal, anterior and posterior canals between the 8 patients who felt they had not recovered and the 12 patients who felt they had recovered. Independent samples t-tests showed no difference between these groups (P=0.26-0.92).
We also investigated the relationship between vHIT response and recovery by grouping the vHIT single canal gains into the mean sum of the canal vectors to give a single gain value for the ipsilesional and contralesional sides. We also grouped the ipsilesional semicircular canals into a single value for the anterior and posterior (vertical) canals gain and asymmetry, as described in Methods.
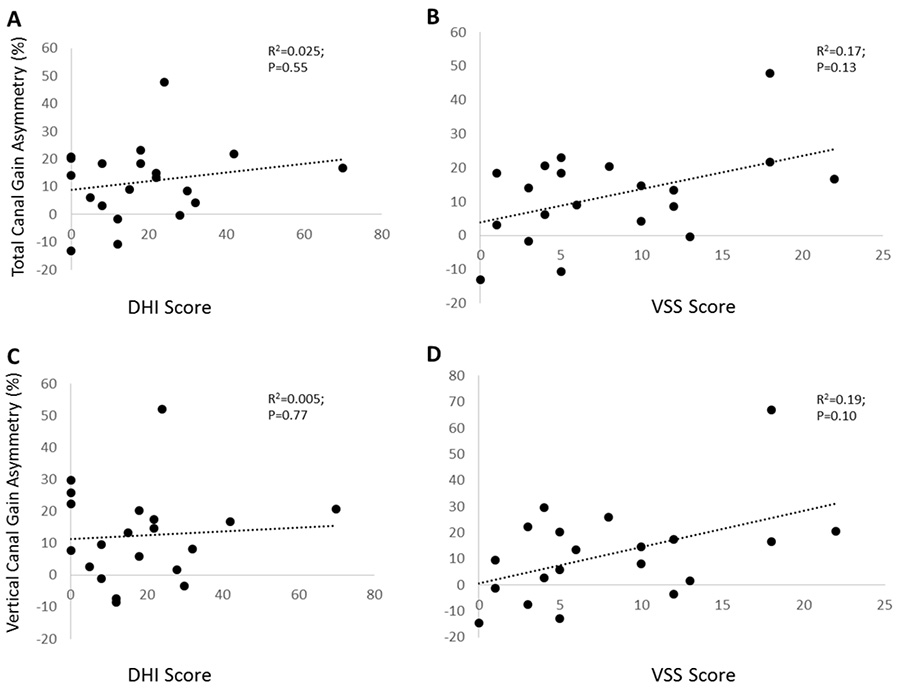
We found no significant correlation between the vector sum of the three ipsilesional canal gains (horizontal + anterior + posterior) and DHI scores (Pearson correlation coefficient =−0.124, P=0.60) or VSS scores (Pearson correlation coefficient =−0.302, P=0.196). Asymmetry did not correlate to DHI (P=0.55) or VSS scores (P=0.13) as shown in Figures 3A and 3B.
Figure 3. vHIT VOR gain for the total response versus DHI score (A) and VSS score (B) & vHIT VOR gain for the vertical canal (anterior + posterior canals) response versus DHI score (C) and VSS score (D).
In addition, there was no significant correlation between the vector sum of the vertical canals (anterior + posterior) and DHI scores (Pearson correlation coefficient = −0.125, P=0.60) or VSS scores (Pearson correlation coefficient=−0.152, P=0.15). Asymmetry did not correlate with DHI (P=0.77) or VSS scores (P=0.10) as shown in Figures 3C and 3D.
Neither total nor vertical canal gain and asymmetry values were significant predictors of DHI or VSS scores with multiple regression analysis, i.e., no values were entered into the analysis during stepwise regression.
Discussion
Here, we find no evidence to support the hypothesis that chronic symptoms of dizziness or vertigo following acute VN are associated with the high velocity VOR of the three ipsilesional semicircular canals. There was no correlation between ipsilesional high velocity VOR gain or gain asymmetry of the single or combined horizontal, anterior and posterior canals measured with the vHIT and DHI or VSS scores. Patient 4 is a representative example: this individual was asymptomatic (DHI=0) but had an ipsilesional posterior canal gain of 0.33. In contrast, patient 20 who was the most symptomatic individual (DHI=70) had normal vHIT gains for each of the canals (above 0.78).
Also, as in previous studies, there was no correlation between caloric paresis and chronic symptoms after VN (21,22) or between caloric paresis and horizontal canal HIT asymmetry (11) probably reflecting the different frequency ranges of these tests.
There is little doubt that acute VN triggered the patients’ chronic symptoms, however residual semicircular canal deficits might not be a crucial factor. As the otoliths are involved in the translational VOR (tVOR) (23), it is possible that impaired otolith function could explain chronic symptoms in some patients. Utricular function is typically affected in VN as measured with ocular VEMP (oVEMP) (24). In a one-year follow-up study in VN patients, Magliulo and colleagues (25) found that four out of five patients with chronic symptoms, had absent ipsilesional oVEMP responses. Saccular function is impaired when the inferior branch of the vestibular nerve is affected. However, it is unlikely that otolith damage would be the critical variable predicting long term outcome in VN given that even patients with vestibular neurectomy recover well (26).
Another explanation is that the relative weightings of vestibular, visual and somatosensory signals change following unilateral vestibular loss. Indeed, we have found that chronic symptoms after VN may relate to increased visual dependence (3). Psychological (22,27) and spatial orientation factors (28), also have a strong influence on long term outcome.
The sample size used in this study (n=20) is also a potential limitation, but if this were the case it would imply that the relationship between clinical outcome and vHIT gains is very weak and therefore unlikely to be sensitive enough to be of practical use in a clinical environment. Using mean and standard deviation data from our strongest correlation coefficient (Figure 2E, anterior canal gain vs VSS) we calculated that subjects recruited would need to equal n=58 to achieve P<0.05 (Power= 0.8) before correction for multiple comparisons.
To conclude, chronic symptoms of dizziness or vertigo following acute VN were not related to the high velocity VOR of the horizontal, anterior or posterior semicircular canals. It is likely that clinical recovery and outcome depends mostly on central compensation, including higher level processing in the brain.
Acknowledgments
Sources of Funding
The research was supported by the UK Medical Research Council (MR/J004685/1).
Footnotes
Conflicts of Interest
The authors report no conflicts of interest.
References
- 1.Dix MR, Hallpike CS. The pathology, symptomatology and diagnosis of certain common disorders of the vestibular system. The Annals of otology, rhinology, and laryngology. 1952;61:987–1016. doi: 10.1177/000348945206100403. [DOI] [PubMed] [Google Scholar]
- 2.Jeong SH, Kim HJ, Kim JS. Vestibular neuritis. Seminars in neurology. 2013;33:185–94. doi: 10.1055/s-0033-1354598. [DOI] [PubMed] [Google Scholar]
- 3.Cousins S, Cutfield NJ, Kaski D, et al. Visual dependency and dizziness after vestibular neuritis. PLoS One. 2014;9:e105426. doi: 10.1371/journal.pone.0105426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kammerlind AS, Ledin TE, Skargren EI, et al. Long-term follow-up after acute unilateral vestibular loss and comparison between subjects with and without remaining symptoms. Acta oto-laryngologica. 2005;125:946–53. doi: 10.1080/00016480510043477. [DOI] [PubMed] [Google Scholar]
- 5.Curthoys IS, Halmagyi GM. Vestibular compensation: a review of the oculomotor, neural, and clinical consequences of unilateral vestibular loss. Journal of vestibular research : equilibrium & orientation. 1995;5:67–107. [PubMed] [Google Scholar]
- 6.Macdougall HG, Curthoys IS. Plasticity during Vestibular Compensation: The Role of Saccades. Frontiers in neurology. 2012;3:21. doi: 10.3389/fneur.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shupak A, Issa A, Golz A, et al. Prednisone treatment for vestibular neuritis. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2008;29:368–74. doi: 10.1097/MAO.0b013e3181692804. [DOI] [PubMed] [Google Scholar]
- 8.Okinaka Y, Sekitani T, Okazaki H, et al. Progress of caloric response of vestibular neuronitis. Acta oto-laryngologica. Supplementum. 1993;503:18–22. doi: 10.3109/00016489309128064. [DOI] [PubMed] [Google Scholar]
- 9.Migliaccio AA, Cremer PD. The 2D modified head impulse test: a 2D technique for measuring function in all six semi-circular canals. Journal of vestibular research : equilibrium & orientation. 2011;21:227–34. doi: 10.3233/VES-2011-0421. [DOI] [PubMed] [Google Scholar]
- 10.Schmid-Priscoveanu A, Bohmer A, Obzina H, et al. Caloric and search-coil head-impulse testing in patients after vestibular neuritis. Journal of the Association for Research in Otolaryngology : JARO. 2001;2:72–8. doi: 10.1007/s101620010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zellhuber S, Mahringer A, Rambold HA. Relation of video-head-impulse test and caloric irrigation: a study on the recovery in unilateral vestibular neuritis. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies. 2014;271:2375–83. doi: 10.1007/s00405-013-2723-6. [DOI] [PubMed] [Google Scholar]
- 12.Bartolomeo M, Biboulet R, Pierre G, et al. Value of the video head impulse test in assessing vestibular deficits following vestibular neuritis. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies. 2014;271:681–8. doi: 10.1007/s00405-013-2451-y. [DOI] [PubMed] [Google Scholar]
- 13.Palla A, Straumann D, Bronstein AM. Vestibular neuritis: vertigo and the high-acceleration vestibulo-ocular reflex. Journal of neurology. 2008;255:1479–82. doi: 10.1007/s00415-008-0935-2. [DOI] [PubMed] [Google Scholar]
- 14.Angelaki DE, Hess BJ. Adaptation of primate vestibuloocular reflex to altered peripheral vestibular inputs. II Spatiotemporal properties of the adapted slow-phase eye velocity. Journal of neurophysiology. 1996;76:2954–71. doi: 10.1152/jn.1996.76.5.2954. [DOI] [PubMed] [Google Scholar]
- 15.Bronstein AM, Patel M, Arshad Q. A brief review of the clinical anatomy of the vestibular-ocular connections-how much do we know? Eye. 2015;29:163–70. doi: 10.1038/eye.2014.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanayama R, Bronstein AM, Gresty MA, et al. Vertical and torsional VOR in posterior canal occlusion. Acta oto-laryngologica. Supplementum. 1995;520(Pt 2):362–5. doi: 10.3109/00016489509125271. [DOI] [PubMed] [Google Scholar]
- 17.Taylor RL, Kong J, Flanagan S, et al. Prevalence of vestibular dysfunction in patients with vestibular schwannoma using video head-impulses and vestibular-evoked potentials. Journal of neurology. 2015;262:1228–37. doi: 10.1007/s00415-015-7697-4. [DOI] [PubMed] [Google Scholar]
- 18.Cousins S, Kaski D, Cutfield N, et al. Vestibular perception following acute unilateral vestibular lesions. PLoS One. 2013;8:e61862. doi: 10.1371/journal.pone.0061862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobson GP, Newman CW, Hunter L, et al. Balance function test correlates of the Dizziness Handicap Inventory. Journal of the American Academy of Audiology. 1991;2:253–60. [PubMed] [Google Scholar]
- 20.Yardley L, Masson E, Verschuur C, et al. Symptoms, anxiety and handicap in dizzy patients: development of the vertigo symptom scale. Journal of psychosomatic research. 1992;36:731–41. doi: 10.1016/0022-3999(92)90131-k. [DOI] [PubMed] [Google Scholar]
- 21.Bergenius J, Perols O. Vestibular neuritis: a follow-up study. Acta oto-laryngologica. 1999;119:895–9. doi: 10.1080/00016489950180243. [DOI] [PubMed] [Google Scholar]
- 22.Godemann F, Siefert K, Hantschke-Bruggemann M, et al. What accounts for vertigo one year after neuritis vestibularis - anxiety or a dysfunctional vestibular organ? Journal of psychiatric research. 2005;39:529–34. doi: 10.1016/j.jpsychires.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Walker MF, Tian J, Shan X, et al. The cerebellar nodulus/uvula integrates otolith signals for the translational vestibulo-ocular reflex. PloS one. 2010;5:e13981. doi: 10.1371/journal.pone.0013981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwasaki S, Chihara Y, Smulders YE, et al. The role of the superior vestibular nerve in generating ocular vestibular-evoked myogenic potentials to bone conducted vibration at Fz. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2009;120:588–93. doi: 10.1016/j.clinph.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 25.Magliulo G, Gagliardi S, Ciniglio Appiani M, et al. Vestibular neurolabyrinthitis: a follow-up study with cervical and ocular vestibular evoked myogenic potentials and the video head impulse test. The Annals of otology, rhinology, and laryngology. 2014;123:162–73. doi: 10.1177/0003489414522974. [DOI] [PubMed] [Google Scholar]
- 26.Lempert T, Gianna C, Brookes G, et al. Horizontal otolith-ocular responses in humans after unilateral vestibular deafferentation. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1998;118:533–40. doi: 10.1007/s002210050309. [DOI] [PubMed] [Google Scholar]
- 27.Staab JP. Chronic subjective dizziness. Continuum. 2012;18:1118–41. doi: 10.1212/01.CON.0000421622.56525.58. [DOI] [PubMed] [Google Scholar]
- 28.Jauregui-Renaud K, Sang FY, Gresty MA, et al. Depersonalisation/derealisation symptoms and updating orientation in patients with vestibular disease. Journal of neurology, neurosurgery, and psychiatry. 2008;79:276–83. doi: 10.1136/jnnp.2007.122119. [DOI] [PubMed] [Google Scholar]