Abstract
Trichuris suis, a nematode parasite of pigs, has attracted attention as its eggs have been administered to human patients as a potential therapy for inflammatory diseases. However, the immunomodulatory factors remain molecularly uncharacterised, but in vitro studies suggest that glycans on the parasite excretory/secretory proteins may have a role. Using an off-line LC-MS approach in combination with chemical and enzymatic treatments, we have examined the N-linked oligosaccharides of T. suis. In addition to the paucimannosidic and oligomannosidic N-glycans typical of many invertebrates, a number of glycans carry N,N′-diacetyllactosamine (LacdiNAc) modified by fucose and/or phosphorylcholine. Such antennal epitopes are similar to ones previously associated with immunomodulation by helminths, but here we can propose phosphorylcholine modifications predominantly of terminal N-acetylgalactosamine as well as of subterminal α1,3-fucosylated N-acetylglucosamine. Exact knowledge of the glycome of T. suis will facilitate more targeted studies on glycan receptors in the host as well enable engineering of cell lines to produce correctly-glycosylated recombinant forms of candidate proteins for future studies on immunomodulation.
Keywords: HPLC, mass spectrometry, fucose, N-glycans, nematode, phosphorylcholine
The relationship between mammals and helminths is both ancient and complex; thus, some have referred to parasitic worms as ‘old friends’ [1]. In developed countries, nematode parasites are no longer ubiquitous in the human population, but are of at least agricultural relevance. While mortality may not be high upon nematode infections, morbidity and poor response to vaccinations in the developing world are definite ‘negative’ effects; on the other hand, there is a debate as to whether increased levels of allergy and autoimmune diseases in more developed countries reflect that nematodes have a ‘positive’ side-effect on their hosts [2-4]. The co-evolution of mammalian immune systems and helminths may be an explanation for the disbalance when nematode infections are lacking. The difficulties in treatment of some autoimmune diseases have led to trials with nematodes in order to relieve inflammation and a few publications have appeared regarding the use of eggs of the porcine parasite, Trichuris suis, or the larvae of Necator americanus as potential therapies for, e.g., Crohn’s disease, coeliac disease or allergic rhinitis [5-7]; the therapeutic value of T. suis has also been disputed [8]. However, very often the molecular nature of the immunomodulatory substances from nematodes is unknown [9]; an exception is the phosphorylcholine-modified excretory/secretory protein ES-62 from Acanthocheilonema viteae [10]. In other cases, there are data to indicate that helminth glycans play a role and affect signalling pathways in cells of the immune system [11,12]; this has been reported also specifically for T. suis excretory-secretory products and their glycans, but without a thorough molecular characterisation [13,14].
The glycans of many helminth parasites have been characterised, especially the N-linked oligosaccharides attached to asparagine residues of proteins. Regardless of evolutionary distance (trematode, cestode or nematode), both recurring and unique glycosylation motifs have been found. However, it is probably correct to state that fucose and/or phosphorylcholine residues are found in most cases: examples include the antennal fucosylated epitopes of the trematode Schistosoma mansoni and the nematode Trichinella spiralis [15,16], the phosphorylcholine modifications of the nematodes Ascaris suum [17], Onchocerca volvulus [18] and Trichinella spiralis [19] or the core fucosylated motifs in Haemonchus contortus [20,21]. Occasionally individual glycoproteins such as ES-62 from the nematode Acanthocheilonema viteae [22] and Ag5 from the cestode Echinococcus granulosus [23] were specifically characterised. It is interesting to note that phosphorylcholine-modified N-glycans have been found in a cestode and in many nematodes, whereas the occurrence of fucosylated N,N’-diacetyllactosamine (fucosylated LacdiNAc) has been reported in only a few nematodes as well as in trematodes [15,16,20]. Nevertheless, the N-glycomic variation between species is very high and different biases in epitope abundance lead to a unique glycan population in each species, which may reflect also the patho-ecological niche, lifecycle or developmental stage.
Considering the evidence that glycans play a role in host-parasite interactions, including those of the porcine whipworm T. suis, we have performed the first glycomic analysis of this species using an off-line LC-MALDI-TOF MS strategy that we have applied to a number of other parasitic and non-parasitic nematode species [24,20,25-27]. Thus, we reveal that T. suis not only possesses a core fucosylation capacity, which is less complex than in some nematodes, but that it has fucosylated forms of phosphorylcholine-modified antennae which are, to date, unique to this species.
Experimental Procedures
N-glycan preparation and fractionation
Trichuris suis adults (kindly supplied by Dr. Stig Thamsborg of the Department of Veterinary Disease Biology, University of Copenhagen, Denmark) were homogenised and proteolysed with pepsin. N-glycans were then released from peptic peptides using peptide:N-glycosidase A (Roche) according to the procedures described previously [28]. Free glycans were labelled with 2-aminopyridine [29,28] prior to MALDI-TOF MS and fractionation by reversed-phase HPLC (RP-HPLC). Separation of PA-labeled glycans was performed on a Shimadzu HPLC system equipped with a fluorescence detector (RF 10 AXL; excitation at 320 nm and emission at 400 nm) and an Ascentis® Express 2.7 μ RP-Amide column (150 × 4.6 mm; Sigma-Aldrich). A gradient of 30% methanol (solvent B), with 100 mM ammonium acetate, pH 4.0, as buffer (solvent A), up to 35% over 34 minutes was applied at a flow rate of 0.8 ml/min as follows: 0-4 min, 0% B; 4-14 min, 0-5% B; 14-24 min, 5-15% B; 24-34 min, 15-35% B; 34-35 min, return to starting conditions [27]. The RP-HPLC analysis of the glycan preparation was performed twice together with MALDI-TOF MS of each fraction.
Mass spectrometry
Monoisotopic MALDI-TOF MS was performed in positive and negative reflectron modes with a Bruker Autoflex Speed instrument (equipped with a 1000 Hz Smartbeam™-II laser) and 6-aza-2-thiothymine (ATT) as matrix. MS/MS was performed by laser-induced dissociation. Spectra were processed with the manufacturer’s software (Bruker Flexanalysis 3.3.80) using the SNAP algorithm with a signal/noise threshold of 6 for MS (unsmoothed) and 3 for MS/MS (four-times smoothed). Interpretation of glycan spectra was performed manually on the basis of the masses of the predicted component monosaccharides, relative elution times as compared to previous studies, differences of mass in glycan series and fragmentation patterns. Glycans were primarily detected as [M+H]+, except for instances of [M+Na]+ in some digested fractions. As part of this study, some 600 MS and MS/MS spectra were recorded and approximately fifty selected mzXML files with the MS/MS spectra for all annotated glycans, prior to any chemical or enzymatic treatment, are available in a Supplementary ZIP file.
Enzymatic and chemical treatments
Selected fractions were subject to treatment overnight, prior to MALDI-TOF MS, with either α-mannosidases (jack bean [30] from Sigma or Xanthomonas α1,2/3- or α1,6-specific [31] from New England Biolabs), α-fucosidases (almond α1,3-fucosidase [32] from Prozyme or the more general bovine kidney α-fucosidase from Sigma-Aldrich) or β-N-acetylhexosaminidases (recombinant insect FDL prepared in-house, encoded by the fused lobes gene and specific for the N-acetylglucosamine attached to core α1,3-mannose [33], recombinant Caenorhabditis elegans HEX-4 prepared in-house, specific for terminal GalNAc residues [33], or recombinant Streptomyces β1,3/4/6-N-acetylhexosaminidase, also known as a chitinase [34], from New England Biolabs) in 25 mM ammonium acetate, pH 4.5 (or pH 6.5 in the case of HEX-4), at 37 °C. The substrate preferences of HEX-4 and the chitinase were confirmed by incubations with GlcNAcβ1,4GlcNAc-R and GalNAcβ1,4GlcNAc-R (where R is a methoxyamino-conjugate) in which HEX-4 was specific for the latter, while the chitinase cleaved both substrates. For removal of core or antennal α1,3-linked fucose or of phosphorylcholine, selected fractions were dried and incubated overnight at 0 °C with 3 μl 48% (v/v) hydrofluoric acid prior to evaporation [23]; the samples were diluted in water and re-evaporated, prior to redissolving once again prior to re-analysis by HPLC and/or MALDI-TOF MS.
Results
Overall glycomic characteristics
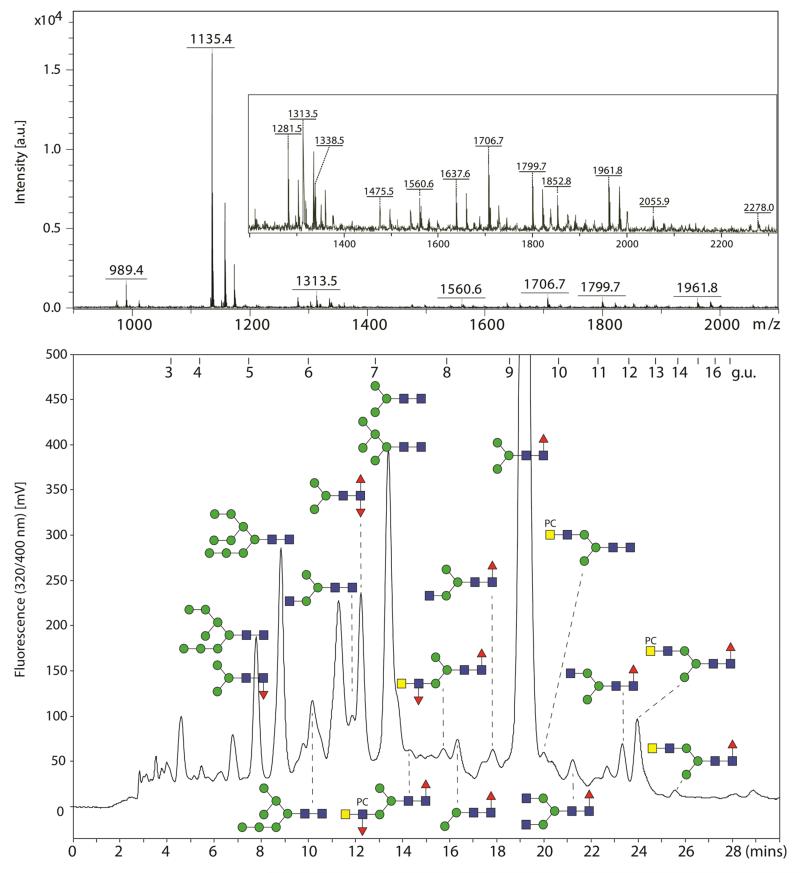
N-glycans from Trichuris suis adults were released using PNGase A from almonds as this enzyme can cleave N-glycans, regardless of the type of core fucosylation, from glycopeptides; bacterial PNGase F was not used as this enzyme cannot cleave core α1,3-fucosylated N-glycans [35], which are commonly found in plants, insects and nematodes [36]. The N-glycans were then fluorescently labelled using 2-aminopyridine, which is a well-established method and suited for reversed-phase chromatography in order to obtain also isomeric information [37,38]. In this study, we used a fused core RP-amide column calibrated with a partial dextran hydrolysate in terms of glucose units (g.u.) in conjunction with MALDI-TOF MS (see Figure 1) as previously employed for separation and analysis of insect and nematode N-glycans [27,39]. Approximately 40 RP-HPLC peaks were collected and individually analysed with the focus on those glycans whose MS/MS spectra indicated the presence of fucose and/or phosphorylcholine. A detailed analysis of glycans predicted to be oligomannosidic was not performed, but they were assigned (see Figure 2) due to being comparable in terms of retention time and MS/MS fragmentation pattern with either commercial standards or structures from another nematode (Pristionchus pacificus) analysed in parallel using the same RP-amide column [27].
Figure 1. MALDI-TOF MS and RP-HPLC analysis of Trichuris suis N-glycans.
Pyridylaminated N-glycans derived from a PNGase A digest of T. suis glycopeptides were analysed by MALDI-TOF MS in positive ion mode and by RP-HPLC on an fused core Ascentis RP-amide column calibrated in terms of glucose units (g.u.). The MALDI-TOF MS spectrum is dominated by a Man3GlcNAc2Fuc1-PA glycan (m/z 1135 as the protonated quasimolecular ion); the region m/z 1200-2300 is also shown magnified with further protonated ions annotated. Selected HPLC fractions are annotated according to the nomenclature of the Consortium for Functional Glycomics (circles, hexose; squares, N-acetylhexosamine; triangles, fucose; PC, phosphorylcholine).
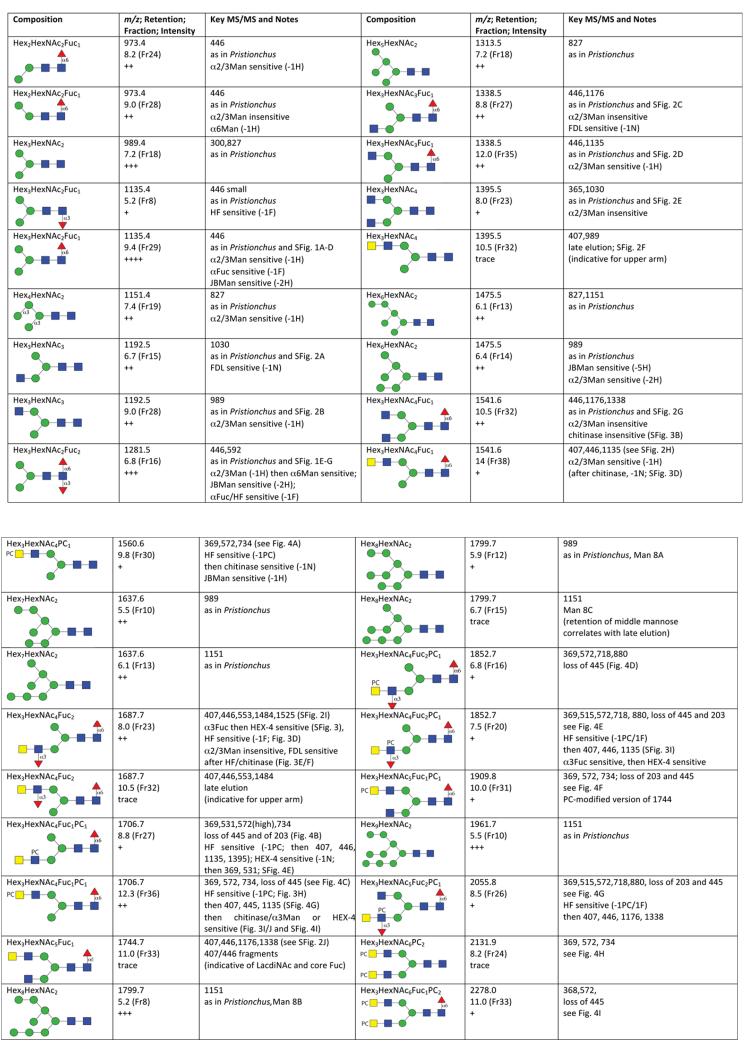
Figure 2. Summary of N-glycan assignments.
Predicted compositions in terms of hexose, N-acetylhexosamine, fucose and phosphorylcholine residues and CFG-type structures are presented together with (i) calculated m/z as [M+H]+ for the pyridylaminated N-glycans, (ii) retention time in terms of glucose units and fraction number (RP-amide column), (iii) a qualitative indication of abundance (trace through to +++), (iv) important MS/MS fragments and (v) notes regarding identical properties as glycans from Pristionchus, sensitivity to chemical and enzymatic treatments and relevant figure (Fig) and supplementary figure (SFig) numbers. For reasons of space, two variants of Man2GlcNAc2 (m/z 827) as well as a Glc1Man9GlcNAc2 (m/z 2123) are not included. The MS/MS of the untreated glycans are also available as supplementary mzXML files.
Fucosylated paucimannosidic N-glycans
Initial perusal of the Trichuris full pyridylaminylated N-glycome by RP-HPLC and MALDI-TOF MS indicated the presence of a major fucosylated peak (Hex3HexNAc2Fuc1-PA; 9.4 g.u.; m/z 1135) as well as a range of glycans of lower abundance with predicted compositions Hex3HexNAc2Fuc1-PA (5.2 g.u.; m/z 1135), Hex2HexNAc2Fuc1-PA (8.2 and 9.0 g.u.; m/z 973) or Hex3HexNAc2Fuc2-PA (6.8 g.u.; m/z 1281). According to the positive ion mode MS/MS spectra yielding fragments of m/z 446 and 592 (HexNAc1Fuc1-PA and HexNAc1Fuc2-PA; Supplementary Figure 1 A and E), the fucose residues were linked to the reducing terminal GlcNAc. Based also on the sensitivity to α-mannosidases, bovine α-fucosidase and hydrofluoric acid (see Supplementary Figure 1 B, C, F and G), these glycans are Man2-3GlcNAc2 paucimannosidic structures modified with either core α1,3- or α1,6-fucose or both in the case of Hex3HexNAc2Fuc2 with the most abundant being the α1,6-fucosylated form of Man3GlcNAc2. Due to the high abundance of this glycan, MS/MS was also performed in the negative ion mode (m/z 1133; Supplementary Figure 1D), resulting in typical N-glycan fragmentation ions at m/z 323, 545 and 688 respectively corresponding to an unprocessed antenna (Hex2), cross-ring cleavage of penultimate GlcNAc (2,4A3) and glycosidic cleavage within the chitobiose unit (B3, loss of GlcNAc1Fuc1-PA from the parent ion) [40]; these fragments as well as the Y1α and Z1α ions at m/z 444 (GlcNAc1Fuc1-PA) and 426 are also compatible with core fucosylation.
Hybrid and pseudohybrid N-glycans
A range of Trichuris oligosaccharides had putative compositions of Hex3HexNAc3-5Fuc0-2-PA, very often in two separate HPLC fractions. Thus, there were typical isomeric pairs of Hex3HexNAc3-PA (m/z 1192; 6.7 and 9.0 g.u.) and Hex3HexNAc3Fuc1-PA (m/z 1338; 8.8 and 12.0 g.u.) with retention times, MS/MS fragmentation patterns (Supplementary Figure 2) and enzymatic digestion patterns compatible with the presence of antennal non-reducing GlcNAc on either the α1,3- or α1,6-arm (summarised in Figure 2); the pattern of respective early or later retention for the so-called MGn and GnM isomers (m/z 1192) has been reported previously [41]. Other pairs of glycans, possessing an additional N-acetylhexosamine residue, were Hex3HexNAc4-PA (m/z 1395; 8.0 and 10.5 g.u.) and Hex3HexNAc4Fuc1-PA (m/z 1541; 10.5 and 14.0 g.u.); the earlier eluting isobaric structure in each case had a retention time and MS/MS spectrum compatible with that of an ‘uncapped’ biantennary glycan, whereas MS/MS of the later eluting structures in both cases yielded m/z 407 fragments (i.e., HexNAc2) suggestive for a HexdiNAc antennal modification. While the 10.5 g.u. form of m/z 1541 was resistant, the 14.0 g.u. species lost one HexNAc when incubated with a chitinase displaying β1,3/4/6-, but no β1,2-, hexosaminidase specificity (Supplementary Figure 3 A-D). The late retention time of the latter is suggestive for an upper arm HexdiNAc modification, a presumption confirmed by the loss of one hexose upon subsequent incubation with α1,2/3-specific mannosidase. The same m/z 407 fragment was also observed in the case of the single form of Hex3HexNAc5Fuc1-PA (m/z 1744; 11.0 g.u.; Supplementary Figure 2J); as also judged by the relative retention times, this glycan is probably identical to one isolated from human urinary kallikrein [42]. Furthermore, GalNAcβ1,4GlcNAc is a known motif for N-glycans and glycolipids from other nematodes, it is probable that the m/z 407 fragment derives from a LacdiNAc modification, as described below for fucosylated or phosphorylcholinylated antennae.
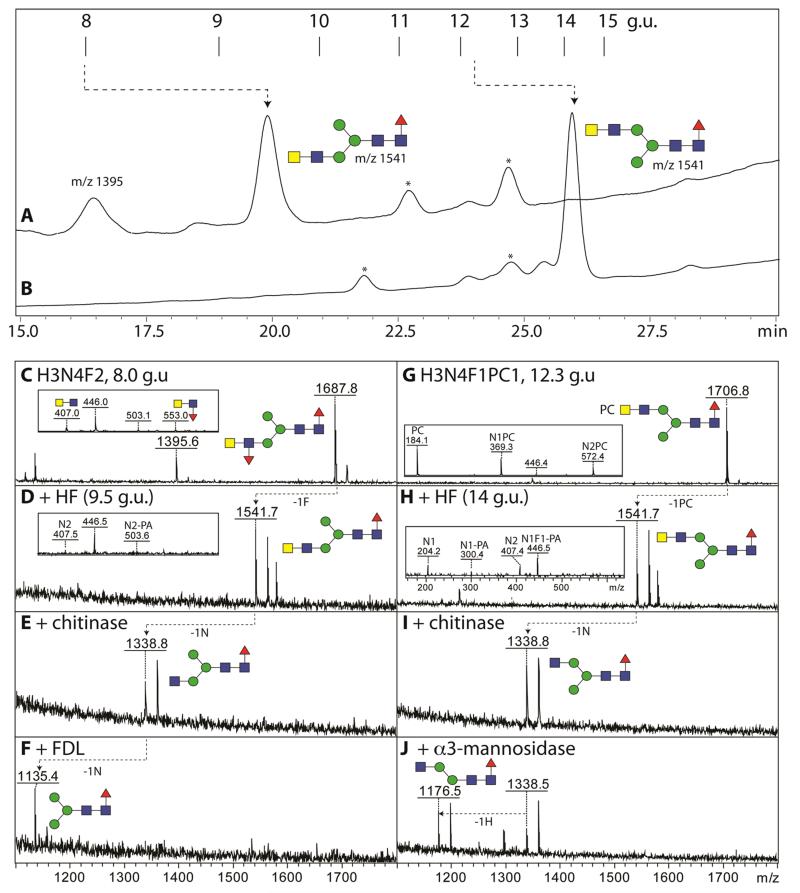
A further glycan variant had the composition Hex3HexNAc4Fuc2-PA (m/z 1687; 8.0 g.u.). Considering the lack of an m/z 592 fragment consistent with core difucosylation (compare with Supplementary Figure 1E), but the presence of ones of m/z 407 and 553 (HexNAc2Fuc0-1; Supplementary Figure 2I and 3E), it was probable that this structure carries an α1,3-fucosylated LacdiNAc motif. Therefore, the glycan was treated with hydrofluoric acid or with almond α1,3-fucosidase; both treatments resulted in the loss of one fucose residue (Figure 3D and Supplementary Figure 3F). Thereby, the fucosylated LacdiNAc fragment (m/z 553) was no longer apparent, while the core fucose-related one at m/z 446 was retained in both cases. When reinjected onto the RP-amide column (Figure 3A), the hydrofluoric acid treated form of the glycan eluted later (9.4 g.u.:, m/z 1541), but still earlier than the aforementioned forms of m/z 1541 present in the original glycome, which is consistent with a lower arm modification; furthermore, the defucosylated glycan was sensitive to subsequent bacterial chitinase and insect FDL-hexosaminidase (Figure 3 E and F). Due to the FDL-sensitivity of the HF/chitinase-treated form, the fucosylated LacdiNAc motif is concluded to be on the α1,3-arm, whereby the definition of the terminal HexNAc as GalNAc is verified by its removal by C. elegans HEX-4 (Supplementary Figure 3G), an enzyme specific for GalNAc residues. Furthermore, based on the shared elution properties, the glycan has the same structure as ones proposed in Haemonchus [20], mosquitoes [39] and honeybee venom phospholipase A2 [43].
Figure 3. Analysis of antennal fucose and phosphorylcholine modifications on N-glycans from T. suis.
(A, B) Two RP-HPLC fractions (8.0 and 12.3 g.u., respectively containing glycans of m/z 1687 and 1706) were treated with hydrofluoric acid prior to re-injection onto the HPLC column (collected fractions are annotated with the relevant m/z; those marked with an asterisk contained no detectable glycans). (C, D, G and H) The product fractions were analysed by MALDI-TOF MS showing the loss of either α1,3-fucose or phosphorylcholine as compared to the untreated glycans. (E, I) Both product fractions (i.e., those of 9.5 and 14 g.u.) were treated with β1,3/4/6-specific hexosaminidase (chitinase) prior to diagnostic digests with either (F, J) FDL-hexosaminidase or α1,2/3-specific mannosidase (respectively specific for ‘lower arm’ GlcNAc or mannose residues). The effect of the hydrofluoric acid treatment is also obvious by the alteration of the fragment ions (insets in panels C, D, G and H); the loss of antennal fucose from the m/z 1687 glycan was also realized with almond α1,3-fucosidase (Supplementary Figure 3F). The contrasting HPLC retention times for the HF-treated glycans (m/z 1541) correlate with different arms being modified with the LacdiNAc motif. Partial shifts to sodiated adducts were observed; for full MS/MS of the two glycans, see Supplementary Figure 2I and 4F or the Supplementary mzXML files.
Phosphorylcholine-modified N-glycans
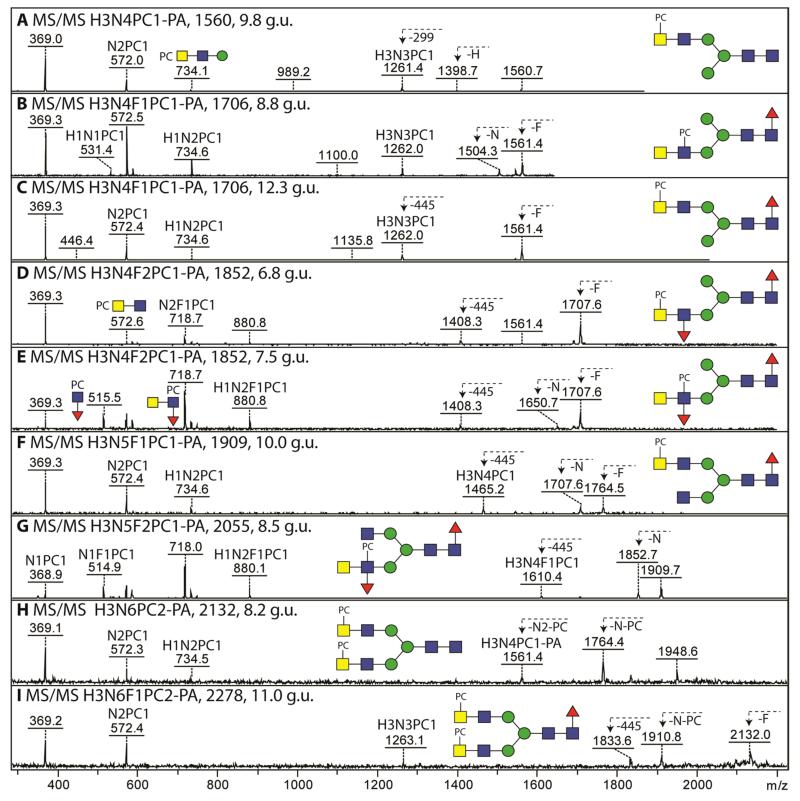
MALDI-TOF MS analysis of various fractions of the Trichuris N-glycome contained glycans whose masses were 165 Da greater than some of the hybrid and pseudohybrid N-glycans discussed above which indicated modification by phosphorylcholine. MS/MS of these oligosaccharides, which all contained at least four N-acetylhexosamine residues, revealed fragment ions at m/z 369, 572 and 734 compatible with HexNAc1-2PC1 and Hex1HexNAc2PC1 motifs (Figure 4). The general lack of m/z 531 fragment ions (Hex1HexNAc1PC1, showing close vicinity of the PC-HexNAc to a hexose) contrasts with glycans of the same mass in, e.g., Pristionchus (see Supplementary Figure 4A-C) and was indicative of a different location for the phosphorylcholine moiety as compared to many other nematodes. Together with the relatively higher m/z 369 fragment (as compared to that at m/z 572) for most phosphorylcholine-modified glycans of Trichuris, the occurrence of [M+H-HexNAc1PC1] fragment ions in the case of structures carrying two phosphorylcholine residues (Figure 4 H and I) and the general lack of [M+H-HexNAc1] fragments, we conclude that it is the terminal HexNAc of the HexdiNAc motif which is most often modified by phosphorylcholine. We therefore postulate that the phosphorylcholine moieties on Hex3HexNAc4-6Fuc0-1PC1-2 glycans (m/z 1560, 1706, 1909, 2131 and 2278; Figure 4 A, C, F, H and I) are generally separated from a core α-mannose by two N-acetylhexosamine residues.
Figure 4. MS/MS analysis of phosphorylcholine-modified N-glycans from T. suis.
(A-I) Positive ion mode MALDI-TOF MS/MS of phosphorylcholine-modified N-glycans in various RP-amide fractions (with the relevant glucose units given). Key fragments are annotated with abbreviated compositions: F, fucose; H, hexose; N, N-acetylhexosamine; PA, 2-aminopyridine; PC, phosphorylcholine. The B-fragment ions at m/z 369 (HexNAc1PC1) are typical for phosphorylcholine-modified N-glycans from this and other nematode species. Examples of ions resulting from loss of fucose (F), hexose (H), HexNAc (N), phosphorylcholine-modified HexNAc (N-PC) or of the reducing terminus (299 or 445) are also indicated. Further analyses of the 7.5 g.u. (m/z 1852) and 12.3 g.u. (m/z 1706) glycans are shown in Figure 3 and in Supplementary Figures 3 and 4. The terminal position for the phosphorylcholine in the case of m/z 1706 (12.3 g.u.; panel C) correlates with the resistance of this glycan (prior to hydrofluoric acid treatment) to the GalNAc-specific C. elegans HEX-4 (see Supplementary Figure 4 F-I), whereas the 8.8 g.u. isoform (panel B) is sensitive to this enzyme without removal of the zwitterionic moiety (see Supplementary Figure 4 D and E).
The loss of a terminal HexNAc upon MS/MS was only observed for a minority of phosphorylcholine-modified glycans. For example, MS/MS of the 8.8 g.u. isoform of m/z 1706 yielded an [M+H-HexNAc1] fragment (m/z 1503); furthermore, the MS/MS shows a relatively high intensity of the m/z 572 fragment as well as an ion at m/z 531, contrasting to the case for the 12.3 g.u. isoform (compare Figures 4 B and C). This suggests that the former has a subterminal phosphorylcholine modification (i.e., on a GlcNAc directly substituting a mannose), a presumption in keeping with the ability to remove one HexNAc with the C. elegans HEX-4 hexosaminidase (Supplementary Figure 4E). The terminal loss of a HexNAc is also observed for m/z 1909 (Figure 4F), which anyway possesses one non-modified GlcNAc on one arm.
Other cases of a subterminal position for the phosphorylcholine modification are two glycans also carrying a fucose on the antennae (Hex3HexNAc4-5Fuc2PC1; m/z 1852 and m/z 2055; 7.5 and 8.5 g.u.). For these glycans, fragment ions at m/z 515, 718 and 880 were observed (HexNAc1Fuc1PC1, HexNAc2Fuc1PC1 and Hex1HexNAc2Fuc1PC1; Figure 4 E and G) suggestive of trisubstitution of the subterminal GlcNAc. Consistent with the non-substitution of the terminal GalNAc, the MS/MS spectra of m/z 1852 and 2055 displayed fragments ion compatible with the loss of a single HexNAc (respectively, m/z 1650 and 1852). The antenna was also sensitive to almond α1,3-fucosidase and subsequently to HEX-4 (as summarised in Figure 2). There was nevertheless a second form of Hex3HexNAc4Fuc2PC1 (m/z 1852; 6.8 g.u.) for which a terminal phosphorylcholine modification is presumed on the basis of the lack of the m/z 515 fragment (Figure 4 D).
Other than fragmentation, another proof for the modification by phosphorylcholine was sensitivity towards hydrofluoric acid, which not only cleaves α1,3-fucose linkages (as described above), but also effectively removes phosphodiesters [44]. For example, hydrofluoric acid treatment of the 12.3 g.u. form of Hex3HexNAc4Fuc1PC1 (m/z 1706) yielded a product with m/z 1541 (Hex3HexNAc4Fuc1-PA), which eluted at 14.0 g.u. when reinjected onto the RP-amide column (thus co-eluting with the aforementioned ‘upper’ arm HexdiNAc-modified isomer occurring in the original glycome; Figure 3A and B) and which displayed a definite m/z 407 fragment ion (Figure 3H). This product lost, as for the HexdiNAc glycans discussed above, one HexNAc residue when incubated with chitinase (Figure 3I) and then a hexose when subsequently treated with α1,2/3-mannosidase (Figure 3J). Furthermore, recombinant C. elegans HEX-4 could remove one HexNAc after, but not before, hydrofluoric acid treatment (Supplementary Figure 4I); as HEX-4 only removes GalNAc residues from standard N-glycan substrates [33], the overall conclusion is that the phosphorylcholine modification of this isomer is on the terminal GalNAc of a LacdiNAc motif on the α1,6-arm. Another example of the effect of hydrofluoric acid is exemplified by the analysis of the m/z 1852 glycan eluting at 8.5 g.u., for which the loss of antennal fucose and phosphorylcholine is accompanied by the appearance of the otherwise suppressed core fragments (e.g., HexNAc1Fuc1-PA, m/z 446, rather than the HexNAc2Fuc1PC1 fragment ion at m/z 718; compare Supplementary Figure 3 H and I).
Discussion
In this study, we reveal the variety of N-glycans present in adults of the porcine whipworm, T. suis, whose eggs have been used as a potential therapy for autoimmune and allergic disorders. Using the ability of a fused core RP-amide HPLC column to fractionate isomeric forms of pyridylaminated N-glycans prior to MALDI-TOF MS and MS/MS, we could distinguish core α1,3- from core α1,6-fucosylation or the antennae carrying GlcNAc or HexdiNAc modifications (summarised in Figure 2). Based on the HEX-4 hexosaminidase sensitivity and analogies to glycans from insects or from other nematodes (as discussed below), we consider it most probable that the HexdiNAc is indeed LacdiNAc. Targetted digests with enzymes or hydrofluoric acid, as well as comparisons to the N-glycans of other nematodes, enable us to propose some forty oligosaccharide structures, including some modified by antennal fucose or phosphorylcholine; a number of these have not been previously described by any method or only now were analysed by ‘off-line’ LC-MALDI-TOF MS for the first time. As many invertebrates, the N-glycome of T. suis contains a large percentage of paucimannosidic and oligomannosidic glycans [45]; however, it is remarkable for its range of antennal fucose and phosphorylcholine modifications based on HexdiNAc motifs. Indeed, we did not detect phosphorylcholine on a single terminal HexNAc, as common in Caenorhabditis elegans, Pristionchus pacificus, Acanthocheilonema viteae, Ascaris suum or Haemonchus contortus [46,27,22,17,20]. We propose that, in contrast to these organisms, which are members of nematode clades III and V [47], T. suis tends to add phosphorylcholine to terminal GalNAc of the putative LacdiNAc motif unless the subterminal GlcNAc is fucosylated.
The association of phosphorylcholine solely with HexdiNAc may be shared with Trichinella spiralis, as the smallest phosphorylcholine-modified glycan in this species may be Hex3HexNAc4Fuc1PC1 as judged by analysis of perdeuteroacetylated forms by FAB-MS; although it was not determined in T. spiralis as to whether the modification was on the terminal or subterminal HexNAc, a relatively high amount of terminal GalNAc was detected by GC-MS of the hydrofluoric acid-treated T. spiralis N-glycan pool suggesting the presence of LacdiNAc [19]. Thus, as T. suis and T. spiralis are both members of the Dorylaimia (clade I nematodes, rather distant phylogenetically from C. elegans), it could be that they share some phosphorylcholine-modified N-glycan structures. On the other hand, no longer HexNAc repeats were observed as described for some filarial nematodes [18] which are members of clade III and no disubstitution of HexdiNAc by phosphorylcholine was obvious.
Antennal fucosylation in T. suis also occurred in the absence of a phosphorylcholine modification and is probably in the form of fucosylated LacdiNAc as present in insects and trematodes [43,48]. As judged by retention times and example digests, antennal fucose is predominantly on the lower arm and we recently found co-eluting N-glycans in both H. contortus and Anopheles gambiae [39,20]; both these species express compatible fucosyltransferase activities [49,50]. However, an antibody recognising fucosylated LacdiNAc only poorly reacted with T. suis soluble products [13], which could be due to the localisation of this epitope only in certain tissues or proteins of the parasite or steric hindrance when this motif also carries phosphorylcholine. Although fucosylated LacdiNAc is also known from T. spiralis, all glycans in that organism with this motif were apparently capped with tyvelose [16], a sugar modification not found during any other N-glycan analysis of a eukaryote. Thus, our off-line LC-MALDI-TOF MS study is the first to demonstrate uncapped and phosphorylcholine-modified fucosylated LacdiNAc in any clade I nematode.
Our glycomic analyses suggest that there are at least three N-glycan modifying fucosyltransferase activities in T. suis (core α1,3-, core α1,6- and Lewis-like α1,3-fucosyltransferases). Indeed, homology searching of the genome [51] indicates perhaps fifteen possibly relevant predicted gene products, although key protein motifs are in some cases not conserved (unpublished data). Missing from this repertoire are almost certainly an α1,3-fucosyltransferase modifying the distal core GlcNAc or any galactose-modifying α1,2-fucosyltransferase. Whereas distal core fucosylation and galactosylation of core α1,6-fucose is known from C. elegans, A. suum, Oesophagostomum dentatum or H. contortus [25,20,52] and bisecting galactose is seemingly a unique feature of C. elegans [26], there is no sign of such modifications in T. suis. Indeed, as there is a complete lack of tri- and tetrafucosylated N-glycans, it can be argued that the N-glycomic potential of T. suis is rather simple as compared to some other nematodes, but this may be related to its phylogeny and its pathoecology.
The identification of the N-glycome of T. suis should aid studies regarding interactions of the proteins secreted by this and related parasites with components of the immune system. Indeed, there are reports regarding binding of T. muris and T. suis proteins to various C-type lectins such as the mannose receptor, macrophage galactose receptor and DC-SIGN [53,13]; although the nature of the T. suis structures recognised is not known, in other studies fucose-containing glycans are proven ligands for all three lectins. Also potentially relevant to the fucose-containing glycans, it has been shown by antibody cross-reactivity that fucosylated LacdiNAc is present on a host-protective protein fraction from H. contortus [54], whereas core α1,3-fucose is an epitope for IgE from sheep infected with H. contortus [55]. Furthermore, nematode-derived phosphorylcholine is a ligand for C-reactive protein as well as from antibodies raised against nematodes [56-59], whereas core fucose modifications of nematode parasite N-glycans are also targets of nematotoxic lectins derived from fungi [60]. While some of the relevant glycan ligands can be synthesised chemoenzymatically, synthetic approaches are required for those containing phosphorylcholine. Thus, knowledge of the actual N-glycans of T. suis is the starting point for targeted studies (e.g., with glycan arrays) to understand the molecular basis of carbohydrate-mediated effects of this parasite in potential therapeutic settings.
Supplementary Material
Acknowledgments
This work was funded in part by the Austrian Fonds zur Förderung der wissenschaftlichen Forschung (FWF) [grants P21946 to K.P. and P23922 to I.B.H.W.]. The authors thank Dr. Martin Dragosits for preparing the recombinant FDL and HEX-4 hexosaminidases, Dr. Chunsheng Jin for his comments on negative ion mode spectra and Dr. Stig Thamsborg for the parasite material.
Footnotes
Conflict of Interest:
The authors declare that they have no conflict of interest.
References
- 1.Rook GA. Review series on helminths, immune modulation and the hygiene hypothesis: the broader implications of the hygiene hypothesis. Immunology. 2009;126:3–11. doi: 10.1111/j.1365-2567.2008.03007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoerauf A, Satoguina J, Saeftel M, Specht S. Immunomodulation by filarial nematodes. Parasite Immunol. 2005;27:417–429. doi: 10.1111/j.1365-3024.2005.00792.x. doi:10.1111/j.1365-3024.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- 3.Wammes LJ, Mpairwe H, Elliott AM, Yazdanbakhsh M. Helminth therapy or elimination: epidemiological, immunological, and clinical considerations. Lancet Infect Dis. 2014;14:1150–1162. doi: 10.1016/S1473-3099(14)70771-6. doi:10.1016/S1473-3099(14)70771-6. [DOI] [PubMed] [Google Scholar]
- 4.McSorley HJ, Hewitson JP, Maizels RM. Immunomodulation by helminth parasites: defining mechanisms and mediators. Int J Parasitol. 2013;43:301–310. doi: 10.1016/j.ijpara.2012.11.011. doi:10.1016/j.ijpara.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Weinstock JV, Elliott DE. Translatability of helminth therapy in inflammatory bowel diseases. Int J Parasitol. 2013;43:245–251. doi: 10.1016/j.ijpara.2012.10.016. doi:10.1016/j.ijpara.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebner F, Hepworth MR, Rausch S, Janek K, Niewienda A, Kuhl A, Henklein P, Lucius R, Hamelmann E, Hartmann S. Therapeutic potential of larval excretory/secretory proteins of the pig whipworm Trichuris suis in allergic disease. Allergy. 2014;69:1489–1497. doi: 10.1111/all.12496. doi:10.1111/all.12496. [DOI] [PubMed] [Google Scholar]
- 7.Croese J, Giacomin P, Navarro S, Clouston A, McCann L, Dougall A, Ferreira I, Susianto A, O’Rourke P, Howlett M, McCarthy J, Engwerda C, Jones D, Loukas A. Experimental hookworm infection and gluten microchallenge promote tolerance in celiac disease. J Allergy Clin Immunol. 2015;135:508–516. doi: 10.1016/j.jaci.2014.07.022. doi:10.1016/j.jaci.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Bager P, Arnved J, Ronborg S, Wohlfahrt J, Poulsen LK, Westergaard T, Petersen HW, Kristensen B, Thamsborg S, Roepstorff A, Kapel C, Melbye M. Trichuris suis ova therapy for allergic rhinitis: a randomized, double-blind, placebo-controlled clinical trial. J Allergy Clin Immunol. 2010;125:123–130. e121–123. doi: 10.1016/j.jaci.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Bian K, Zhong M, Harari Y, Lai M, Weisbrodt N, Murad F. Helminth regulation of host IL-4Ra/Stat6 signaling: mechanism underlying NOS-2 inhibition by Trichinella spiralis. Proc Natl Acad Sci U S A. 2005;102:3936–3941. doi: 10.1073/pnas.0409461102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harnett W. Secretory products of helminth parasites as immunomodulators. Mol Biochem Parasitol. 2014;195:130–136. doi: 10.1016/j.molbiopara.2014.03.007. doi:10.1016/j.molbiopara.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava L, Tundup S, Choi BS, Norberg T, Harn D. Immunomodulatory glycan lacto-N-fucopentaose III requires clathrin-mediated endocytosis to induce alternative activation of antigen-presenting cells. Infect Immun. 2014;82:1891–1903. doi: 10.1128/IAI.01293-13. doi:10.1128/IAI.01293-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas PG, Carter MR, Da’dara AA, DeSimone TM, Harn DA. A helminth glycan induces APC maturation via alternative NF-κ B activation independent of I κ B α degradation. J Immunol. 2005;175:2082–2090. doi: 10.4049/jimmunol.175.4.2082. [DOI] [PubMed] [Google Scholar]
- 13.Klaver EJ, Kuijk LM, Laan LC, Kringel H, van Vliet SJ, Bouma G, Cummings RD, Kraal G, van Die I. Trichuris suis-induced modulation of human dendritic cell function is glycan-mediated. Int J Parasitol. 2013;43:191–200. doi: 10.1016/j.ijpara.2012.10.021. doi:10.1016/j.ijpara.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 14.Hiemstra IH, Klaver EJ, Vrijland K, Kringel H, Andreasen A, Bouma G, Kraal G, van Die I, den Haan JM. Excreted/secreted Trichuris suis products reduce barrier function and suppress inflammatory cytokine production of intestinal epithelial cells. Mol Immunol. 2014;60:1–7. doi: 10.1016/j.molimm.2014.03.003. doi:10.1016/j.molimm.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Smit CH, van Diepen A, Nguyen DL, Wuhrer M, Hoffmann KF, Deelder AM, Hokke CH. Glycomic Analysis of Life Stages of the Human Parasite Schistosoma mansoni Reveals Developmental Expression Profiles of Functional and Antigenic Glycan Motifs. Mol Cell Proteomics. 2015;14:1750–1769. doi: 10.1074/mcp.M115.048280. doi:10.1074/mcp.M115.048280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reason AJ, Ellis LA, Appleton JA, Wisnewski N, Grieve RB, McNeil M, Wassom DL, Morris HR, Dell A. Novel tyvelose-containing tri- and tetra-antennary N-glycans in the immunodominant antigens of the intracellular parasite Trichinella spiralis. Glycobiology. 1994;4:593–604. doi: 10.1093/glycob/4.5.593. [DOI] [PubMed] [Google Scholar]
- 17.Pöltl G, Kerner D, Paschinger K, Wilson IBH. N-Glycans of the porcine nematode parasite Ascaris suum are modified with phosphorylcholine and core fucose residues. FEBS J. 2007;274:714–726. doi: 10.1111/j.1742-4658.2006.05615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haslam SM, Houston KM, Harnett W, Reason AJ, Morris HR, Dell A. Structural studies of N-glycans of filarial parasites. Conservation of phosphorylcholine-substituted glycans among species and discovery of novel chito-oligomers. J Biol Chem. 1999;274:20953–20960. doi: 10.1074/jbc.274.30.20953. [DOI] [PubMed] [Google Scholar]
- 19.Morelle W, Haslam SM, Olivier V, Appleton JA, Morris HR, Dell A. Phosphorylcholine-containing N-glycans of Trichinella spiralis: identification of multiantennary lacdiNAc structures. Glycobiology. 2000;10:941–950. doi: 10.1093/glycob/10.9.941. [DOI] [PubMed] [Google Scholar]
- 20.Paschinger K, Wilson IBH. Two types of galactosylated fucose motifs are present on N-glycans of Haemonchus contortus. Glycobiology. 2015;25:585–590. doi: 10.1093/glycob/cwv015. doi:10.1093/glycob/cwv015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haslam SM, Coles GC, Munn EA, Smith TS, Smith HF, Morris HR, Dell A. Haemonchus contortus glycoproteins contain N-linked oligosaccharides with novel highly fucosylated core structures. J Biol Chem. 1996;271:30561–30570. doi: 10.1074/jbc.271.48.30561. [DOI] [PubMed] [Google Scholar]
- 22.Haslam SM, Khoo KH, Houston KM, Harnett W, Morris HR, Dell A. Characterisation of the phosphorylcholine-containing N-linked oligosaccharides in the excretory-secretory 62 kDa glycoprotein of Acanthocheilonema viteae. Mol Biochem Parasitol. 1997;85:53–66. doi: 10.1016/s0166-6851(96)02807-1. [DOI] [PubMed] [Google Scholar]
- 23.Paschinger K, Gonzalez-Sapienza GG, Wilson IBH. Mass spectrometric analysis of the immunodominant glycan epitope of Echinococcus granulosus antigen Ag5. Int J Parasitol. 2012;42:279–285. doi: 10.1016/j.ijpara.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paschinger K, Razzazi-Fazeli E, Furukawa K, Wilson IBH. Presence of galactosylated core fucose on N-glycans in the planaria Dugesia japonica. J Mass Spectrom. 2011;46:561–567. doi: 10.1002/jms.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan S, Bleuler-Martinez S, Plaza Gutierrez DF, Künzler M, Aebi M, Joachim A, Razzazi-Fazeli E, Jantsch V, Geyer R, Wilson IBH, Paschinger K. Galactosylated fucose epitopes in nematodes: increased expression in a Caenorhabditis mutant associated with altered lectin sensitivity and occurrence in parasitic species. J Biol Chem. 2012;287:28276–28290. doi: 10.1074/jbc.M112.353128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan S, Brecker L, Jin C, Titz A, Dragosits M, Karlsson N, Jantsch V, Wilson IBH, Paschinger K. Bisecting galactose as a feature of N-glycans of wild-type and mutant Caenorhabditis elegans. Mol Cell Proteomics. 2015;14:2111–2125. doi: 10.1074/mcp.M115.049817. doi:10.1074/mcp.M115.049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan S, Wilson IBH, Paschinger K. Comparison of RP-HPLC modes to analyse the N-glycome of the free-living nematode Pristionchus pacificus. Electrophoresis. 2015;36:1314–1329. doi: 10.1002/elps.201400528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paschinger K, Hykollari A, Razzazi-Fazeli E, Greenwell P, Leitsch D, Walochnik J, Wilson IBH. The N-glycans of Trichomonas vaginalis contain variable core and antennal modifications. Glycobiology. 2012;22:300–313. doi: 10.1093/glycob/cwr149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hase S, Ibuki T, Ikenaka T. Reexamination of the pyridylamination used for fluorescence labelling of oligosaccharides and its application to glycoproteins. J Biochem (Tokyo) 1984;95:197–203. doi: 10.1093/oxfordjournals.jbchem.a134585. [DOI] [PubMed] [Google Scholar]
- 30.Li YT. Presence of α-D-mannosidic linkage in glycoproteins. Liberation of D-mannose from various glycoproteins by α-mannosidase isolated from jack bean meal. J Biol Chem. 1966;241:1010–1012. [PubMed] [Google Scholar]
- 31.Wong-Madden ST, Landry D. Purification and characterization of novel glycosidases from the bacterial genus Xanthomonas. Glycobiology. 1995;5:19–28. doi: 10.1093/glycob/5.1.19. [DOI] [PubMed] [Google Scholar]
- 32.Scudder P, Neville DC, Butters TD, Fleet GW, Dwek RA, Rademacher TW, Jacob GS. The isolation by ligand affinity chromatography of a novel form of α-L-fucosidase from almond. J Biol Chem. 1990;265:16472–16477. [PubMed] [Google Scholar]
- 33.Dragosits M, Yan S, Razzazi-Fazeli E, Wilson IBH, Rendić D. Enzymatic properties and subtle differences in the substrate specificity of phylogenetically distinct invertebrate N-glycan processing hexosaminidases. Glycobiology. 2015;25:448–464. doi: 10.1093/glycob/cwu132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robbins PW, Overbye K, Albright C, Benfield B, Pero J. Cloning and high-level expression of chitinase-encoding gene of Streptomyces plicatus. Gene. 1992;111:69–76. doi: 10.1016/0378-1119(92)90604-n. [DOI] [PubMed] [Google Scholar]
- 35.Tretter V, Altmann F, März L. Peptide-N4-(N-acetyl-β-glucosaminyl)asparagine amidase F cannot release glycans with fucose attached α1→3 to the asparagine-linked N-acetylglucosamine residue. Eur J Biochem. 1991;199:647–652. doi: 10.1111/j.1432-1033.1991.tb16166.x. [DOI] [PubMed] [Google Scholar]
- 36.Paschinger K, Rendić D, Wilson IBH. Revealing the anti-HRP epitope in Drosophila and Caenorhabditis. Glycoconj J. 2009;26:385–395. doi: 10.1007/s10719-008-9155-3. [DOI] [PubMed] [Google Scholar]
- 37.Hase S. Pyridylamination as a means of analyzing complex sugar chains. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:378–390. doi: 10.2183/pjab.86.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomiya N, Kurono M, Ishihara H, Tejima S, Endo S, Arata Y, Takahashi N. Structural analysis of N-linked oligosaccharides by a combination of glycopeptidase, exoglycosidases, and high-performance liquid chromatography. Anal Biochem. 1987;163:489–499. doi: 10.1016/0003-2697(87)90253-3. [DOI] [PubMed] [Google Scholar]
- 39.Kurz S, Aoki K, Jin C, Karlsson NG, Tiemeyer M, Wilson IB, Paschinger K. Targetted release and fractionation reveal glucuronylated and sulphated N- and O-glycans in larvae of dipteran insects. J Proteomics. 2015;126:172–188. doi: 10.1016/j.jprot.2015.05.030. doi:10.1016/j.jprot.2015.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harvey DJ. Fragmentation of negative ions from carbohydrates: part 2. Fragmentation of high-mannose N-linked glycans. J Am Soc Mass Spectrom. 2005;16:631–646. doi: 10.1016/j.jasms.2005.01.005. doi:10.1016/j.jasms.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Gutternigg M, Kretschmer-Lubich D, Paschinger K, Rendić D, Hader J, Geier P, Ranftl R, Jantsch V, Lochnit G, Wilson IBH. Biosynthesis of truncated N-linked oligosaccharides results from non-orthologous hexosaminidase-mediated mechanisms in nematodes, plants and insects. J Biol Chem. 2007;282:27825–27840. doi: 10.1074/jbc.M704235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomiya N, Awaya J, Kurono M, Hanzawa H, Shimada I, Arata Y, Yoshida T, Takahashi N. Structural elucidation of a variety of GalNAc-containing N-linked oligosaccharides from human urinary kallidinogenase. Journal of Biological Chemistry. 1993;268:113–126. [PubMed] [Google Scholar]
- 43.Kubelka V, Altmann F, Staudacher E, Tretter V, März L, Hård K, Kamerling JP, Vliegenthart JFG. Primary structures of the N-linked carbohydrate chains from honeybee venom phospholipase A2. Eur J Biochem. 1993;213:1193–1204. doi: 10.1111/j.1432-1033.1993.tb17870.x. [DOI] [PubMed] [Google Scholar]
- 44.Schneider P, Ferguson MAJ. Microscale analysis of glycosylphosphatidylinositol structures. Methods Enzymol. 1995;250:614–630. doi: 10.1016/0076-6879(95)50100-2. [DOI] [PubMed] [Google Scholar]
- 45.Schachter H. Paucimannose N-glycans in Caenorhabditis elegans and Drosophila melanogaster. Carbohydr Res. 2009;344:1391–1396. doi: 10.1016/j.carres.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 46.Paschinger K, Hackl M, Gutternigg M, Kretschmer-Lubich D, Stemmer U, Jantsch V, Lochnit G, Wilson IBH. A deletion in the Golgi α-mannosidase II gene of Caenorhabditis elegans results in unexpected non-wild type N-glycan structures. J Biol Chem. 2006;281:28265–28277. doi: 10.1074/jbc.M602878200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sommer RJ, Streit A. Comparative genetics and genomics of nematodes: genome structure, development, and lifestyle. Annu Rev Genet. 2011;45:1–20. doi: 10.1146/annurev-genet-110410-132417. doi:10.1146/annurev-genet-110410-132417. [DOI] [PubMed] [Google Scholar]
- 48.Wuhrer M, Koeleman CA, Deelder AM, Hokke CH. Repeats of LacdiNAc and fucosylated LacdiNAc on N-glycans of the human parasite Schistosoma mansoni. FEBS J. 2006;273:347–361. doi: 10.1111/j.1742-4658.2005.05068.x. [DOI] [PubMed] [Google Scholar]
- 49.DeBose-Boyd RA, Nyame AK, Jasmer DP, Cummings RD. The ruminant parasite Haemonchus contortus expresses an α1,3-fucosyltransferase capable of synthesizing the Lewis x and sialyl Lewis x antigens. Glycoconjugate J. 1998;15:789–798. doi: 10.1023/a:1006912032273. [DOI] [PubMed] [Google Scholar]
- 50.Kurz S, King JG, Dinglasan RR, Paschinger K, Wilson IBH. The fucomic potential of mosquitoes: Fucosylated N-glycan epitopes and their cognate fucosyltransferases. 2015 doi: 10.1016/j.ibmb.2015.11.001. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jex AR, Nejsum P, Schwarz EM, Hu L, Young ND, Hall RS, Korhonen PK, Liao S, Thamsborg S, Xia J, Xu P, Wang S, Scheerlinck JP, Hofmann A, Sternberg PW, Wang J, Gasser RB. Genome and transcriptome of the porcine whipworm Trichuris suis. Nat Genet. 2014;46:701–706. doi: 10.1038/ng.3012. doi:10.1038/ng.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanneman AJ, Rosa JC, Ashline D, Reinhold V. Isomer and glycomer complexities of core GlcNAcs in Caenorhabditis elegans. Glycobiology. 2006;16:874–890. doi: 10.1093/glycob/cwl011. [DOI] [PubMed] [Google Scholar]
- 53.deSchoolmeester ML, Martinez-Pomares L, Gordon S, Else KJ. The mannose receptor binds Trichuris muris excretory/secretory proteins but is not essential for protective immunity. Immunology. 2009;126:246–255. doi: 10.1111/j.1365-2567.2008.02893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Geldhof P, Newlands GF, Nyame K, Cummings R, Smith WD, Knox DP. Presence of the LDNF glycan on the host-protective H-gal-GP fraction from Haemonchus contortus. Parasite Immunol. 2005;27:55–60. doi: 10.1111/j.1365-3024.2005.00744.x. [DOI] [PubMed] [Google Scholar]
- 55.van Die I, Gomord V, Kooyman FNJ, van der Berg TK, Cummings RD, Vervelde L. Core α1→3-fucose is a common modifcation of N-glycans in parasitic helminths and constitutes an important epitope for IgE from Haemonchus contortus infected sheep. FEBS Lett. 1999;463:189–193. doi: 10.1016/s0014-5793(99)01508-2. [DOI] [PubMed] [Google Scholar]
- 56.Hewitson JP, Filbey KJ, Grainger JR, Dowle AA, Pearson M, Murray J, Harcus Y, Maizels RM. Heligmosomoides polygyrus elicits a dominant nonprotective antibody response directed against restricted glycan and peptide epitopes. J Immunol. 2011;187:4764–4777. doi: 10.4049/jimmunol.1004140. doi:10.4049/jimmunol.1004140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maizels RM, Burke J, Denham DA. Phosphorylcholine-bearing antigens in filarial nematode parasites: analysis of somatic extracts, in-vitro secretions and infection sera from Brugia malayi and B. pahangi. Parasite Immunol. 1987;9:49–66. doi: 10.1111/j.1365-3024.1987.tb00488.x. [DOI] [PubMed] [Google Scholar]
- 58.Sugane K, Oshima T. Activation of complement in C-reactive protein positive sera by phosphorylcholine-bearing component isolated from parasite extract. Parasite Immunol. 1983;5:385–395. doi: 10.1111/j.1365-3024.1983.tb00754.x. [DOI] [PubMed] [Google Scholar]
- 59.Kooyman FN, de Vries E, Ploeger HW, van Putten JP. Antibodies elicited by the bovine lungworm, Dictyocaulus viviparus, cross-react with platelet-activating factor. Infect Immun. 2007;75:4456–4462. doi: 10.1128/IAI.00633-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heim C, Hertzberg H, Butschi A, Bleuler-Martinez S, Aebi M, Deplazes P, Kunzler M, Stefanic S. Inhibition of Haemonchus contortus larval development by fungal lectins. Parasit Vectors. 2015;8:425. doi: 10.1186/s13071-015-1032-x. doi:10.1186/s13071-015-1032-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.