Abstract
Objective
To investigate the safety and efficacy of a self-developed novel multi-electrode radiofrequency ablation catheter (Spark) for catheter-based renal denervation (RDN).
Methods
A total of 14 experimental miniature pigs were randomly divided into four groups (55°& 5-watt, 55°& 8-watt, 65°& 5-watt, and 65° & 8-watt groups). Spark was used for left and right renal artery radiofrequency ablation. Blood samples collected from renal arteries and veins as well as renal arteriography were performed on all animals before, immediately after, and three months after procedure to evaluate the effects of Spark on the levels of plasma renin, aldosterone, angiotensin I, and angiotensin II as well as the pathological changes of renal arteries.
Results
One pig died of an anesthetic accident, 13 pigs successfully underwent the bilateral renal artery ablation. Compared with basic measurements, pigs in all the four groups had significantly decreased mean arterial pressure after procedure. Histopathological analysis showed that this procedure could result in intimal hyperplasia, significant peripheral sympathetic nerve damage in the renal arteries such as inflammatory cell infiltration and fibrosis in perineurium, uneven distribution of nerve fibers, tissue necrosis, severe vacuolization, fragmented and unclear nucleoli myelin degeneration, sparse axons, and interruption of continuity. In addition, the renal artery radiofrequency ablation could significantly reduce the levels of plasma renin, aldosterone, angiotensin I, and angiotensin II in pigs.
Conclusions
The results suggest that this type of multi-electrode catheter-based radiofrequency ablation could effectively remove peripheral renal sympathetic nerves and reduce the activity of systemic renin-angiotensin system in pigs, thus facilitating the control of systemic blood pressure in pigs.
Keywords: Multi-electrode catheter, Radiofrequency ablation, Renal artery
1. Introduction
Systemic hypertension is a common disease that severely endangers the health of people in both developed and developing countries. It is also a major risk factor for the onset of the majority of cardiovascular and cerebrovascular diseases, such as coronary heart disease, heart failure, stroke, and chronic renal disease. Since the late 20th century, many antihypertensive drugs have been commercialized and have benefited numerous hypertensive patients. However, a certain percentage of hypertensive patients present with resistant hypertension. Even after the application of sufficient and reasonable combinations of three anti-hypertensive drugs (including diuretics) combined with improvement of life style, resistant hypertension patients' blood pressure remains above the target level; they require at least four anti-hypertensive drugs to have their blood pressure reach the target level.[1] It has been reported that resistant hypertension patients accounted for 5%−30% of all hypertensive patients;[2] these patients urgently require new replacement therapy strategies.
Enhancement of sympathetic nerve activity is always regarded as a basic link of the onset of hypertension.[3] In recent years, catheter-based renal denervation (RDN), as a new method for the inhibition of hyperactive sympathetic nerves has been confirmed (e.g., Symplicity HTN-1,[4]–[6] Symplicity HTN-2,[7],[8] DENERHTN[9]) to have the effect of reducing blood pressure in resistant hypertension in preliminary clinical trials. However, the single electrode catheter was required to be rotated spirally several times at the upper, lower, and side walls (anterior and posterior) of the renal artery to perform energy ablation. Technically, it was very difficult to achieve 360-degree comprehensive denervation therapy in one round of radiofrequency ablation treatment with this catheter; in addition, uneven ablation decreased the ablation effect, thus causing insufficient renal denervation. This might lead to the negative results of Symplicity HTN-3.[10]–[12] Therefore, to overcome this potential shortcoming, we first used a self-developed multi-electrode radiofrequency ablation catheter (Spark) of our own intellectual property. The effects of bilateral renal artery radiofrequency ablation in healthy experimental miniature pigs on heart rate, blood pressure, the systemic renin-angiotensin system (RAS), and the sympathetic nervous system were systematically evaluated. The aim of this study was to evaluate the efficacy and safety of a multi-electrode ablation catheter in RDN to provide an early experimental basis for future preliminary clinical trials.
2. Methods
2.1. Animals and equipments
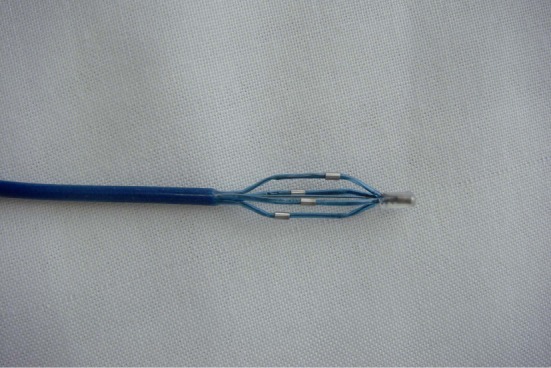
A total of 14 three-month-old miniature pigs (mean weight 20.7 ± 1.1 kg, regardless of sex) were provided by the Animal Center of the Shanghai Jiao Tong University. Animals were housed for three months and were fed with regular swine chow following Chinese animal feeding standard guidelines. This study program completely conformed to the relevant ethical standards of the National Institute of Health (NIH) and was approved by the Academic Ethics Committee of Shanghai Chest Hospital of Shanghai Jiaotong University. The four-electrode renal artery radiofrequency ablation catheter was developed in our group (Figure 1) using an 8F guiding catheter (Boston Scientific Corporation), a radiofrequency ablation generator (Spark, Endovascular Devices Corporation China), and a large-scale digital subtraction angiography (DSA) instrument (GE Innova 4100, USA).
Figure 1. A representative photo of the multi-electrode radio-frequency ablation catheter.
2.2. Percutaneous bilateral RDN
The protocol included the following steps: (1) pre-an-esthetic medication (diazepam 0.25 mg/kg and ketamine 15 mg/kg) was administered by a single intramuscular injection in the large muscle of the caudal thigh. The trachea was intubated with a 6.5−7.0 mm cuffed tracheal tube and ventilation was performed via a mechanical respirator. General anesthesia was maintained via 1%−2% isoflurane and oxygen. Continuous monitoring included: electrocardiogram, blood pressure and pulse oximetry. Heart rate and blood pressure were recorded. (2) Pigs were restrained in dorsal recumbency with cranial and caudal extension of the legs to adequately expose the inguinal areas. They were prepped and draped in a sterile fashion and prophylactic penicillium was administered. Femoral artery and femoral veins were isolated after lignocaine local injected, an arteriovenous sheath was placed, blood (20 mL) from both bilateral renal arteries and veins was collected. (3) An 8F guided catheter was inserted into the bilateral renal arteries using the standard percutaneous technique (modified Seldinger technique) after the sheaths were placed, and renal arteriography was performed to exclude renal vascular malformation. (4) Spark was inserted through the catheter, expanded in the renal arteries, and connected to an electrophysiological radiofrequency ablation instrument. The electrode impedance was recorded, the ablation energy and temperature were set, [The renal arteries of the miniature pigs were randomly divided into four groups: group A, 55°& 5 watts (n = 6); group B, 55°& 8 watts (n = 8); group C: 65°& 5 watts (n = 6); and group D, 65°& 8 watts (n = 8)] and the ablation time was 90 s. (5) After ablation, renal arteriography was performed again and blood from both bilateral renal arties and veins was collected again. (6) After the procedure, one miniature pig from each group was immediately euthanized, and the bilateral renal arteries were collected; (7) recovery and awakening from general anesthesia of other pigs, and (8) routine care of pigs for three months.
After three months, blood from both renal arteries and veins was collected using the same method, and final an-giography was performed again. Experimental animals were euthanized. Arteries and kidneys were then explanted and processed for histopathology and immunohistochemistry.
2.3. Detection of the systemic RAS and the sympathetic nervous system
Blood samples were collected from the renal artery and renal vein. After plasma separation, samples were stored at −20°C. The levels of angiotensin I (Ang I), angiotensin II (Ang II), renin, and aldosterone before, immediately after, and three months after procedure were measured using radioimmunoassays (reagent kits were purchased from Beijing Furui Biological Engineering Company; the sensitivities of the reagent kits were 0.033 ng/mL, 2.0 pmol/L, 0.033 ng/mL, and 1.4 pg/mL, respectively). The experimental me-thods were performed according to the instruction manuals in the reagent kits. Each sample was detected twice to obtain an average value.
2.4. Pathological detection methods
Renal artery samples were conventionally embedded into paraffin blocks and sectioned. A renal artery-kidney tissue block, which included arterial surrounding tissue and posterior underlying psoas and sublumbar muscles, was extracted as a unit. Considering a preclinical research, which demonstrated that the depth of renal denervation therapies can reach beyond 9 mm, we decided to analyze up to a threshold of 10 mm in depth.[13] Sections were cut at approximately 5 μm serially and immunostained for neurofilament protein (NF200 antibody purchased from Wuhan Saiweisi Biological Science Technologies Co.). Sections were rehydrated and heat treated in EDTA buffer for epitope retrieval before exposing them to the staining procedure. Immunostained sections were directly visualized under × 200 magnification and assessed for staining intensity or changes in staining pattern on a descriptive basis.
2.5. Statistical methods
Experimental data were analyzed using the SPSS13.0 software package. Measurement data that conformed to the normal distribution were presented as the means ± SD and analyzed using the paired t test and analysis of variance (ANOVA). Data that did not conform to the normal distribution were analyzed using the rank sum test. In either case, P < 0.05 indicated that the difference was statistically significant.
3. Results
During the experimental process, one miniature pig died of an anesthetic accident. The remaining 13 miniature pigs successfully received bilateral renal artery ablation. After procedure, four miniature pigs were sacrificed immediately, and the remaining nine miniature pigs survived three months after procedure.
3.1. Comparison of renal arteries before and after RDN procedure
Bilateral renal arteriography was performed on all miniature pigs before, immediately after, and three months after procedure. Renal artery spasm results were defined as follows: severe spasm with vascular diameter stenosis ≥ 50%, moderate spasm with vascular diameter stenosis 25%−50%, mild spasm with vascular diameter stenosis < 25%, and no spasm. The results suggested that immediately after procedure, all renal arteries were free of dissections and significant stenosis (stenosis > 75%). Group A did not have significant renal artery spasm; moderate renal artery spasm occurred in 25% of group B, in 60% of group C, and in 25% of group D (Table 1). Three months after procedure, no spasm or stenosis was observed in all renal arteries.
Table 1. Incidence of vascular spasm in renal arteriography immediately after RDN procedure.
| Groups | Spasm |
|||
| Control | Mild (stenosis < 25%) | Moderate (stenosis 25%−50%) | Severe (stenosis ≥ 50%) | |
| A | 1 (20%) | 4 (80%) | 0 (0%) | 0 (0%) |
| B | 2 (50%) | 1 (25%) | 1 (25%) | 0 (0%) |
| C | 0 (0%) | 2 (40%) | 3 (60%) | 0 (0%) |
| D | 0 (0%) | 3 (75%) | 1 (25%) | 0 (0%) |
Data are presented as n (%). RDN: renal denervation.
3.2. Comparison of intimal hyperplasia of renal arteries before and after RDN
Immediately after procedure, the intimal thickness of the renal arteries was measured. The vascular intimal thickness was 0.06 mm (n = 6). Three months after intervention, 28 renal artery samples from 14 different miniature pigs were all collected based on proper harvesting procedure and were sent for histologic analysis. NF-200 immunohistochemical staining was performed on sections. The average arterial diameter was 4.3 ± 0.5 mm (respectively in the Group A to D was 4.2 ± 0.5 mm, 4.3 ± 0.5 mm, 4.3 ± 0.6 mm, 4.2 ± 0.4 mm, P = 0.98). Three random sections (in approximate segments proximal, middle, and distal to the renal aortic ostium) from each artery samples were selected, and the locations of vascular intimal hyperplasia were photographed at × 200 field. The vascular intimal thickness was measured using the Image Pro-plus 6.0 software. The results suggested that the intimal thickness after three months of intervention increased in all cases compared with those in the arteries after immediately intervention (Figure 2). However, the intimal thicknesses of arteries in the Group A to D were 0.06 ± 0.01 mm, 0.21 ± 0.15 mm, 0.10 ± 0.04 mm and 0.11 ± 0.04 mm, respectively, and did not exhibit significant differences among the four groups.
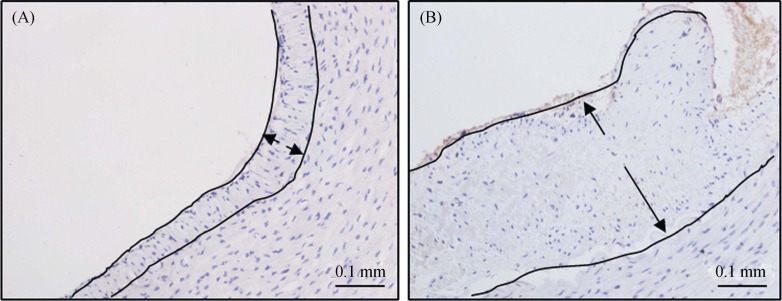
Figure 2. Effect of ablation on the intima of renal arteries.
A representative normal renal artery intima in the acute phase after surgery (A) and renal artery intimal hyperplasia three months after RDN surgery (B) (× 200 light microscope; NF-200 staining). RDN: renal denervation.
3.3. Changes in nerve staining in renal artery after RDN procedure
After NF-200 immunohistochemical staining, peripheral sympathetic nerve fiber cells were positive in sections of renal artery samples from pigs. The positive standard was dark brown yellow granules in the cytoplasm (Figure 3A). Three months after the radiofrequency ablation treatment, the presentations in histological sections showed inflammatory cell infiltration and fibrosis in perineurium and complete degeneration and necrosis of nerve cells, digestion cambers, cell degeneration at the edge, nuclear atrophy, severe vacuolization, fragmented and unclear nucleoli, uneven distribution of nerve fibers, and edema in adjacent tissues. In addition, degeneration, swelling, segmental demyelination of myelin as well as sparseness, disruption of continuity, fracture and disappearance, and loss of normal structure of axons were also observed (Figure 3B-D).
Figure 3. Histological analysis of renal sympathetic nerve after ablation.
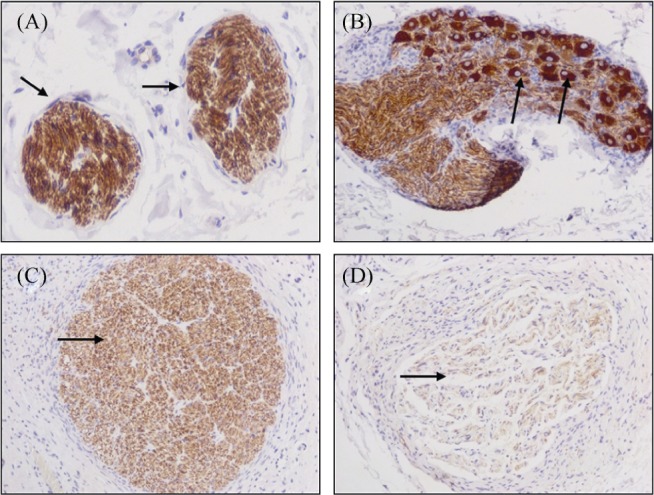
(A): Normal renal artery peripheral sympathetic nerve was dark brown yellow color (indicated by arrows); (B): mild inflammation and fibrosis, vacuolization, fragmented and unclear nucleoli; (C): moderate inflammation and fibrosis, cell degeneration at the edge, nuclear atrophy, digestion cambers; and (D): severe inflammation and fibrosis, complete degeneration and necrosis of nerve cells as well as sparseness, disruption of continuity, fracture and disappearance, and loss of normal structure of axons) (× 200; NF-200 staining).
3.4. Comparison of blood pressure and heart rate before and after RDN procedure
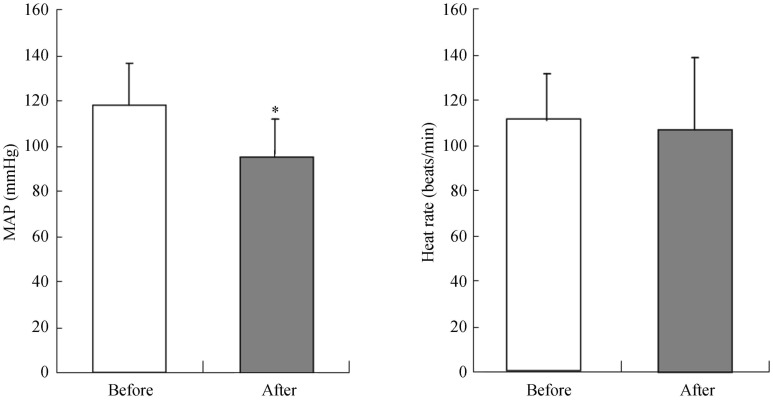
Three months after RDN ablation procedure, all miniature pigs showed significantly decreased mean arterial pressure (−22 ± 18 mmHg, P = 0.002) and there was no difference among these groups in baseline blood pressure and follow-up blood pressure (baseline: for group A, 133/107 mmHg; group B, 145/106 mmHg; group C, 137/105 mmHg; group D, 145/110 mmHg. Follow-up: group A 112/88 mmHg, group B 117/85 mmHg, group C 116/95 mmHg, group D 114/83 mmHg), while the heart rate did not exhibit significant changes (−4 ± 33 beats/min, P = 0.667) (Figure 4).
Figure 4. MAP and heart rates before and after ablation surgery.
The mean arterial pressure and heart rates were measured before and after surgery. The results were expressed as mean ± SE, *P < 0.05. MAP: Mean arterial pressure, n = 13.
3.5. Changes in blood indicators before and after RDN procedure
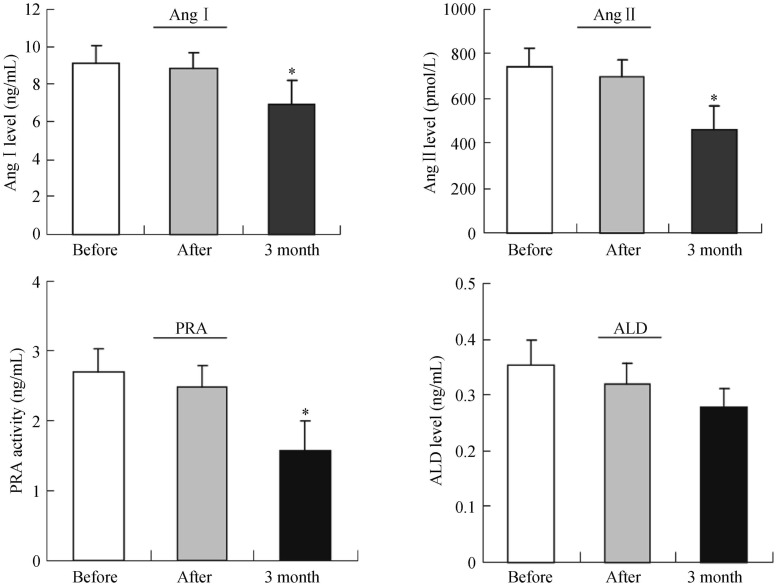
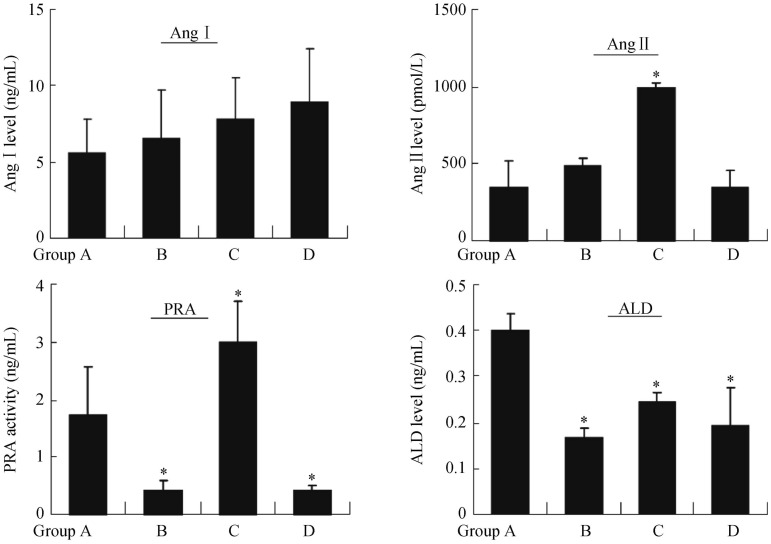
Renal arterial and venous blood was collected from all miniature pigs before, immediately after, and three months after RDN. The results showed that, compared with levels before surgery, the levels of plasma renin, Ang I, Ang II, and aldosterone all significantly decreased three months after surgery, and the differences were statistically significant (Figure 5). Further analysis of differences among these groups showed that three months after surgery, the levels of blood rennin (PRA) and aldosterone in group B and D were lower than those in group A (P < 0.05). However, the levels of Ang II and PRA in group C were higher than those in group A (P < 0.05). The level of Ang I remained to be similar among the four groups (Figure 6).
Figure 5. Effect of ablation on the plasma rennin-angiotensin-aldosterone activities.
The levels of plasma Ang I, Ang II, PRA and aldosterone were measured using radioimmunoassays methods. The results were expressed as mean ± SE, *P < 0.05 vs. before surgery, n = 9. ALD: aldosterone; Ang I: angiotensin I; Ang II: angiotensin II; PRA: rennin.
Figure 6. Comparison of plasma rennin-angiotensin-aldosterone activities three months after surgery among different groups.
The levels of plasma Ang I, Ang II, PRA and aldosterone were measured using radioimmunoassays methods. The results were expressed as mean ± SE, *P < 0.05 vs. before surgery. ALD: aldosterone; Ang I: angiotensin I; Ang II: angiotensin II; PRA: rennin.
4. Discussion
In this study, we demonstrated that Spark catheter could effectively ablate the bilateral renal peripheral sympathetic nerves in a pig model. The denervation treatment resulted in a significant inhibition of RAS activation and thus contributed to lower systemic blood pressure.
The swine model is the most frequently used because of its similarity to the renovascular anatomy and size of humans.[14] New histological study of porcine model clarified normal renal arteries from pigs to elucidate renovascular anatomy.[15]–[17] Maximal ablation zones varied in orientation and shape, and often included or bordered anatomic structures, such as lymph nodes, other large blood vessels, and the hypaxial skeletal muscle. Quantitative morphometry of maximal ablation zones estimated depth (4.5 ± 3.1 mm), width (8.0 ± 4.2 mm) and area (26.6 ± 31.0 mm2).[13] These anatomical features made it warranted to use healthy experimental miniature pigs for bilateral renal artery radiofrequency ablation. The energy released by the radiofrequency catheter inserted into renal arteries could penetrate the intima and media of renal arteries to selectively destroy some afferent and efferent nerve fibers in adventitia, which could reduce the discharge of nerve impulses by the renal afferent nerves into the sympathetic nervous center under various conditions and also block the effect of renal efferent nerves on the kidneys during the activation of the sympathetic nervous system, thus achieving the goals of anti-hyper-ten-sion and inhibition of the sympathetic nerve activity in the whole body. The high heat during the treatment process would also inevitably cause some damage to surrounding nerves and tissues.[18] NF200 can be used for specific staining of the neuronal perikarya, nerve dendrites, and axons.[19] Our histological results showed that after radiofrequency ablation, all sections showed inflammatory cell infiltration and fibrosis in perineurium, uneven distribution of nerve fibers, tissue necrosis, severe vacuolization, fragmented and unclear nucleoli myelin degeneration, sparse axons, and interruption of continuity. Therefore, these pathological data again provided an important experimental basis for the application of this catheter to renal artery denervation therapy.
Compared to SymplicityTM catheter, Spark catheter could use the same energy and temperature for simultaneous multi-point ablation. Based on the limitations in the single electrode design, many new types of novel renal artery ablation catheters have entered clinical trials or are being developed including the EnligHTN simultaneous multi-elec-trode catheter used in the EnligHTN study (St. Jude Medical),[20]–[22] the Vessix V2 balloon-mounted multi-electrode catheter used in the REDUCE-HTN study (Boston Scientific), the OneShot single-electrode balloon-mounted spiral ablation catheter used in the RAPID study (Covidien), and the Paradise ultrasound catheter used in the REALISE study (ReCor Medical). Compared to these simultaneous multi-electrode ablation catheters, the four ablation electrodes of Spark catheter were not at the same planes, which could reduce the occurrence of intimal spasm and other complications. Furthermore, the ablation electrodes expanded according to the renal artery diameter and sticked to the artery wall by drawing and rotating the catheter basket. So it fitted for various diameters of renal artery and reduced the ablation impedance. The impedance more than 300 Ω always resulted in failed ablation because of not reaching the preset ablation energy, or resulted in the intimal thickening. After ablation and three months of follow-up, we found that there was no significant renal artery dissection and stenosis or renal infarction. The degrees of intimal thickening among all groups did not exhibit significant differences, indicating that the multi-electrode radiofrequency ablation catheter could be safely used in the RDN procedure. Of course, the incidence of intraoperative renal artery spasm in each group was not quite the same, and increasing temperature or energy might increase the risk for spasm. So we could conclude that when the ablation temperature and energy were lower during procedure, the occurrence of renal artery spasm was also lower Overall, as there was no renal artery spasm in the 55°& 5-watt group (vasoconstriction stenosis > 25%), we considered that the 55°& 5-watt group was safer than the other three groups.
This study observed that after bilateral RDN treatment, the mean arterial pressure in miniature pigs significantly decreased. Consistently, we also observed that RDN could effectively inhibit the RAS activity in the body, thus significantly decreasing the levels of plasma Ang II, Ang I, and aldosterone after procedure.[23] This anti-RAS activation effect could be maintained until three months after procedure, especially in the 65°& 8-watt group, which had even lower plasma aldosterone and PRA levels than the other treatment groups. These results suggested that this type of multi-electrode radiofrequency ablation catheter could not only effectively perform renal denervation, but also effectively inhibit RAS activity in the body, and thus be an excellent choice for the RDN procedure.
4.1. Study limitations
The sample size observed in this study was relatively small. In addition, the experimental models were normal miniature pigs, which could not completely reflect the situation of sympathetic hyperactivity. Nevertheless, this study was devoted to investigating the safety and efficacy of this multi-electrode catheter, and the mechanism underlying its effect on the sympathetic nerves will be further investigated in future studies.
4.2. Conclusion
In summary, this multi-electrode radiofrequency ablation catheter can be used as a safe and effective ablation catheter in the RDN procedure.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81370361), Science and Technology Commission of Shanghai Municipality (12140902800, 13140903700), Shanghai Shen-kang Hospital Development Center ( SHDC12012101).
References
- 1.Vongpatanasin W. Resistant hypertension: a review of diagnosis and management. JAMA. 2014;311:2216–2224. doi: 10.1001/jama.2014.5180. [DOI] [PubMed] [Google Scholar]
- 2.Persell S. Prevalence of resistant hypertension in the United States, 2003-2008. Hypertension. 2011;57:1076–1080. doi: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- 3.Vink EE, Blankestijn PJ. Evidence and consequences of the central role of the kidneys in the pathophysiology of sympathetic hyperactivity. Front Physiol. 2012;3:29. doi: 10.3389/fphys.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 5.Krum H, Schlaich M, Whitboum R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011;57:911–917. doi: 10.1161/HYPERTENSIONAHA.110.163014. [DOI] [PubMed] [Google Scholar]
- 6.Krum H, Schlaich MP, Bohm M, et al. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet. 2014;383:622–629. doi: 10.1016/S0140-6736(13)62192-3. [DOI] [PubMed] [Google Scholar]
- 7.Symplicity HTN-2 Investigators. Esler MD, Krum H. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomized controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 8.Esler MD, Krum H, Schlaich M, et al. Renal sympathetic denervation for treatment of drug-resistant hypertension. One-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126:2976–2982. doi: 10.1161/CIRCULATIONAHA.112.130880. [DOI] [PubMed] [Google Scholar]
- 9.Azizi M, Sapoval M, Gosse P, et al. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet. 2015;385:1957–1965. doi: 10.1016/S0140-6736(14)61942-5. [DOI] [PubMed] [Google Scholar]
- 10.Kandzari DE, Bhatt DL, Sobotka PA, et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the Symplicity HTN-3 Trial. Clin Cardiol. 2012;35:528–535. doi: 10.1002/clc.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel HC, Hayward C, Di Mario C. SYMPLICITY HTN 3: the death knell for renal denervation in hypertension? Glob Cardiol Sci Pract. 2014;2014:94–98. doi: 10.5339/gcsp.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hering D. Renal denervation superior to drug therapy in hypertension. Lancet. 2015;385:1922–1924. doi: 10.1016/S0140-6736(14)62050-X. [DOI] [PubMed] [Google Scholar]
- 13.Tzafriri AR, Mahfoud F, Keating JH, et al. Innervation patterns may limit response to endovascular renal denervation. J Am Coll Cardiol. 2014;64:1079–1087. doi: 10.1016/j.jacc.2014.07.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsioufis C, Papademetriou V, Dimitriadis K, et al. Catheter-based renal sympathetic denervation exerts acute and chronic effects on renal hemodynamics in swine. Int J Cardiol. 2013;168:987–992. doi: 10.1016/j.ijcard.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 15.Sakakura K, Ladich E, Edelman ER, et al. Methodological standardization for the pre-clinical evaluation of renal sympathetic denervation. JACC Cardiovasc Interv. 2014;7:1184–1193. doi: 10.1016/j.jcin.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steigerwald K, Titova A, Kennerknecht E, et al. Morphological assessment of renal arteries after radiofrequency catheter-based sympathetic denervation in a porcine model. J Hypertens. 2012;30:2230–2239. doi: 10.1097/HJH.0b013e32835821e5. [DOI] [PubMed] [Google Scholar]
- 17.Tellez A, Rousselle S, Palmieri T, et al. Renal artery nerve distribution and density in the porcine model: biologic implications for the development of radiofrequency ablation therapies. Transl Res. 2013;162:381–389. doi: 10.1016/j.trsl.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Kornick C, Kramarich SS, Lamer TJ, et al. Complications of lumbar facet radiofrequency denervation. Spine. 2004;29:1352–1354. doi: 10.1097/01.brs.0000128263.67291.a0. [DOI] [PubMed] [Google Scholar]
- 19.Uchida K, Baba H, Maezawa Y, et al. Progressive changes in neurofilament proteins and growth-associated protein-43 immunoreactivities at the site of cervical spinal cord compression in spinal hyperostotic mice. Spine. 2002;27:480–486. doi: 10.1097/00007632-200203010-00008. [DOI] [PubMed] [Google Scholar]
- 20.Froeschl M, Hadziomerovic A, Ruzicka M. Percutaneous renal sympathetic denervation: 2013 and beyond. Can J Cardiol. 2014;30:64–74. doi: 10.1016/j.cjca.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Worthley SG, Tsioufis CP, Worthley MI, et al. Safety and efficacy of a multi-electrode renal sympathetic denervation system in resistant hypertension: the EnligHTN I trial. Eur Heart J. 2013;34:2132–2140. doi: 10.1093/eurheartj/eht197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papademetriou V, Tsioufis CP, Sinhal A, et al. Catheter-based renal denervation for resistant hypertension: 12-month results of the EnligHTN I first-in-human study using a multielectrode ablation system. Hypertension. 2014;64:565–572. doi: 10.1161/HYPERTENSIONAHA.114.03605. [DOI] [PubMed] [Google Scholar]
- 23.Bertog SC, Sobotka PA, Sievert H. Renal denervation for hypertension. J Am Coll Cardiol Intv. 2012;5:249–258. doi: 10.1016/j.jcin.2011.12.011. [DOI] [PubMed] [Google Scholar]