Abstract
Objectives
To investigate the relationship between serum uric acid (SUA) and left atrial spontaneous echo contrast (LA-SEC) in non-valvular atrial fibrillation (AF) patients.
Methods
We retrospectively screened 1,476 consecutive hospitalized patients with AF who underwent transesophageal echocardiography prior to radiofrequency catheter ablation, left atrial appendage closure and electric cardioversion at Guangdong General Hospital. Data on the clinical baseline characteristics of all patients were collected from electronic medical records and analyzed.
Results
After exclusion of patients with left atrial thrombus, 1,354 patients entered into present study and 57 were LA-SEC. The mean female SUA level (380.88 ± 94.35 µmol/L vs. 323.37 ± 72.19 µmol/L, P < 0.001) and male SUA level (416.97 ± 98.87 µmol/L vs. 367.88 ± 68.50 µmol/L, P = 0.008) were both significantly higher in patients with LA-SEC than in the controls. The mean left atrial dimension (41.32 ± 5.12 mm vs. 36.12 ± 5.66 mm, P < 0.001) was markedly larger in patients with LA-SEC. In multivariate regression analysis, SUA level was an independent risk factor for LA-SEC (OR: 1.008, P < 0.001). In receiver operating characteristic curve analysis, the corresponding area under the curve for SUA predicting LA-SEC in female and male were 0.670 and 0.657, respectively. SUA level is significantly higher in non-valvular AF patients with LA-SEC.
Conclusion
SUA level is an independent risk factor and has a moderate predictive value for LA-SEC among non-valvular AF patients in Southern China.
Keywords: Atrial fibrillation, Echo contrast, Left atrium, Serum uric acid, Thromboembolic events
1. Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia in clinical practice and represents an independent and important risk factor for thromboembolic events. Left atrial spontaneous echo contrast (LA-SEC) and left atrial appendage/chamber thrombus (LA-TH) on transesophageal echocardiography (TEE) account for at least one fifth of ischemic stroke events in non-valvular AF patients. Without anticoagulant therapy, LA-SEC would largely develop into LA-TH and sequentially progress to thromboembolic events, such as ischemic stroke or transient ischemic attack (TIA).
Recently, an increasing number of studies have focused on the relationship between serum uric acid (SUA) and thrombo-embolism risk.[1]–[3] Although SUA has been identified as an independent risk factor for LA-TH in non-valvular AF patients,[4] it remains unclear whether SUA can offer sufficient predictive value for LA-SEC in non-valvular AF patients, especially in the population of Southern China. The aims of the present study were to investigate the relationship between SUA and LA-SEC and to examine whether SUA plays a role in predicting LA-SEC in non-valvular AF patients.
2. Methods
2.1. Patient population
We retrospectively screened 1,476 consecutive hospitalized patients with AF who underwent transthoracic echocardiography (TTE) or TEE prior to radiofrequency catheter ablation (RFCA), left atrial appendage closure (LAAC) and electric cardioversion at Guangdong General Hospital between July 2007 and October 2014. The presence of AF was determined by patient medical history, serial electrocardiogram (ECG), and/or ambulatory ECG monitoring. The exclusion criteria were as follows: (1) definite medical history of structural heart disease and valvular abnormalities, such as rheumatic heart disease, infective endocarditis, and mitral valve prolapse syndrome; (2) history of cardiac surgery, such as valve replacement or valvuloplasty, coronary-artery bypass graft; (3) end-stage hepatic or renal dysfunction; and (4) drugs that affect SUA levels such as uric acid lowering agents.
Data on the clinical baseline characteristics of all patients including age, gender, type of AF, laboratory data, indices of echocardiography, and medical history were collected from electronic medical records and analyzed. Patients were categorized into an LA-SEC group and a normal group according to the TEE results. The Ethics Study Committee at Guangdong General Hospital approved the study protocols and agreed that informed consent was not necessary because of the observational nature of the study. Patient data were anonymized and de-identified prior to analysis.
2.2. Measurement by transthoracic echocardiography and transesophageal echocardiography
All patients consented to the TTE examination. Cardiac dimensions were measured by M-mode and two-dimensional echocardiography according to the recommendations of the American Society of Echocardiography.[5] Left atrial dimensions were obtained in the parasternal long axis view in M-mode at end systole. Left ventricular ejection fraction (LVEF) was calculated using the biplane method of discs (modified Simpson's rule) in the apical four- and two-chamber views at end diastole. Measurements were obtained as the mean value from the apical four- and two-chamber views. Data were acquired in three beats in patients with normal sinus rhythm and in five beats in atrial fibrillation and the mean was used for the analysis.
TEE was performed after TTE on the same day in all cases. All patients received local pharyngeal anesthesia (1% lidocaine spray), or/and intravenous diazepam, 2–5 mg when necessary. The transesophageal probe was introduced with the patient lying supine in the left lateral position. LA-SEC was diagnosed by the presence of dynamic smog-like echoes in the left atrial cavity and left atrial appendage with a characteristic swirling motion distinct from a white noise artefact after properly adjusting the gain setting.[6]
2.3. Clinical assessment
The CHA2DS2-VASc score was recorded as a baseline characteristic. The CHA2DS2-VASc score was based on a point system,[7] in which two points were assigned for a history of stroke/TIA and age older than 75 years and one point was assigned for congestive heart failure (CHF), hypertension, age 65–75 years, diabetes mellitus (DM), vascular disease, and female gender. In the present study, coronary arterial disease, peripheral arterial disease and acute myocardial infarction were defined as “vascular disease”.
2.4. Statistical methods
All continuous variables were expressed as the mean ± SD. Categorical variables were expressed as percentages or ratios. The Student's t-test and the Mann-Whitney U-test were used to compare parametric and nonparametric continuous variables, respectively. The Chi-square test was used to compare categorical variables. Multivariate regression analysis was used to identify the potential independent predictors for LA-SEC and the predictive value of SUA for LA-SEC. Receiver operating characteristic (ROC) curve analysis was performed to estimate the diagnostic ability of SUA according to the area under the curve (AUC) statistic. AUC and the associated 95% CI were calculated. A two-tailed P value < 0.05 was considered to indicate statistical significance. All statistical analyses were performed using IBM SPSS Statistics 19.0 (IBM Inc., NY, USA).
3. Results
3.1. Baseline characteristics of the LA-SEC and normal groups
After applying the exclusion criteria, 1,419 patients with non-valvular AF were examined at the in-patient department of Guangdong General Hospital and underwent TEE. The results of TEE examination identified 122 (8.3%) patients with abnormalities, of which 65 were LA-TH and 57 were LA-SEC. After exclusion of patients with LA-TH, 1,354 patients were enrolled in the present study, including 910 men and 444 women with a mean age of 56.21 ± 12.24 years. There were 401 patients on warfarin or aspirin therapy. The baseline characteristics of patients in the LA-SEC and normal groups are summarized in Table 1.
Table 1. Baseline characteristics.
| Variables | LA-SEC group |
Normal group |
P Value | |
| n = 57 | n = 1297 | |||
| Male | 33 (57.9) | 877 (67.6) | 0.126 | |
| Age, yrs | 60.74 ± 10.76 | 56.01 ± 12.26 | 0.004* | |
| Serum uric acid, µmol/L | 401.77 ± 97.80 | 353.46 ± 72.74 | 0.001* | |
| Female | 380.88 ± 94.35 | 323.37 ± 72.19 | < 0.001* | |
| Male | 416.97 ± 98.87 | 367.88 ± 68.50 | 0.008* | |
| Plasma fibrinogen, g/L | 3.44 ± 0.90 | 3.08 ± 0.72 | 0.009* | |
| LDL-cholesterol, mmol/L | 2.94 ± 0.92 | 2.87 ± 0.80 | 0.617 | |
| Hemoglobin A1c, % | 6.00 ± 0.40 | 5.81 ± 0.49 | 0.036* | |
| Blood urea nitrogen, mmol/L | 5.62 ± 1.24 | 5.54 ± 1.18 | 0.617 | |
| Creatinine, µmol/L | 76.67 ± 20.30 | 84.26 ± 29.89 | 0.099 | |
| AF history, yrs | 4.86 ± 3.74 | 4.24 ± 3.18 | 0.226 | |
| Per-AF | 27 (47.4) | 210 (16.2) | < 0.001* | |
| Congestive heart failure | 10 (17.5) | 138 (10.6) | 0.102 | |
| Hypertension, | 27 (47.4) | 494 (38.1) | 0.159 | |
| Diabetes mellitus | 14 (24.6) | 178 (13.7) | 0.022* | |
| Previous Stroke/TIA | 4 (7.0) | 19 (1.5) | 0.014* | |
| Age ≥ 65, yrs | 24 (42.1) | 346 (26.7) | 0.011* | |
| Age ≥ 75, yrs | 3 (5.3) | 47 (3.6) | 0.464 | |
| Vascular disease | 8 (14.0) | 75 (5.8) | 0.020* | |
| CHA2DS2-VASc Score | ||||
| 0 | 3 (5.3) | 388 (29.9) | < 0.001* | |
| 1 | 18 (31.6) | 414 (31.9) | 0.957 | |
| ≥ 2 | 36 (63.2) | 496 (38.2) | < 0.001* | |
| LVDd, mm | 46.00 ± 5.71 | 45.83 ± 4.50 | 0.786 | |
| LAd, mm | 41.32 ± 5.12 | 36.12 ± 5.66 | < 0.001* | |
| LVEF, % | 64.51 ± 6.52 | 65.78 ± 6.90 | 0.174 | |
| Warfarin use | 16 (28.1) | 258 (19.9) | 0.133 | |
| Aspirin use, | 6 (10.5) | 122 (9.4) | 0.777 | |
Data are presented as mean ± SD or n (%). *With statistical significance. AF: Atrial fibrillation; LAd: Left atrial dimension; LA-SEC: left atrial spontaneous echo contrast; LDL: Low-density lipoprotein; LVDd: Left ventricular diastolic dimension; LVEF: Left ventricular ejection fraction; Per-AF: persistent/permanent atrial fibrillation; TIA: Transient ischemic attack.
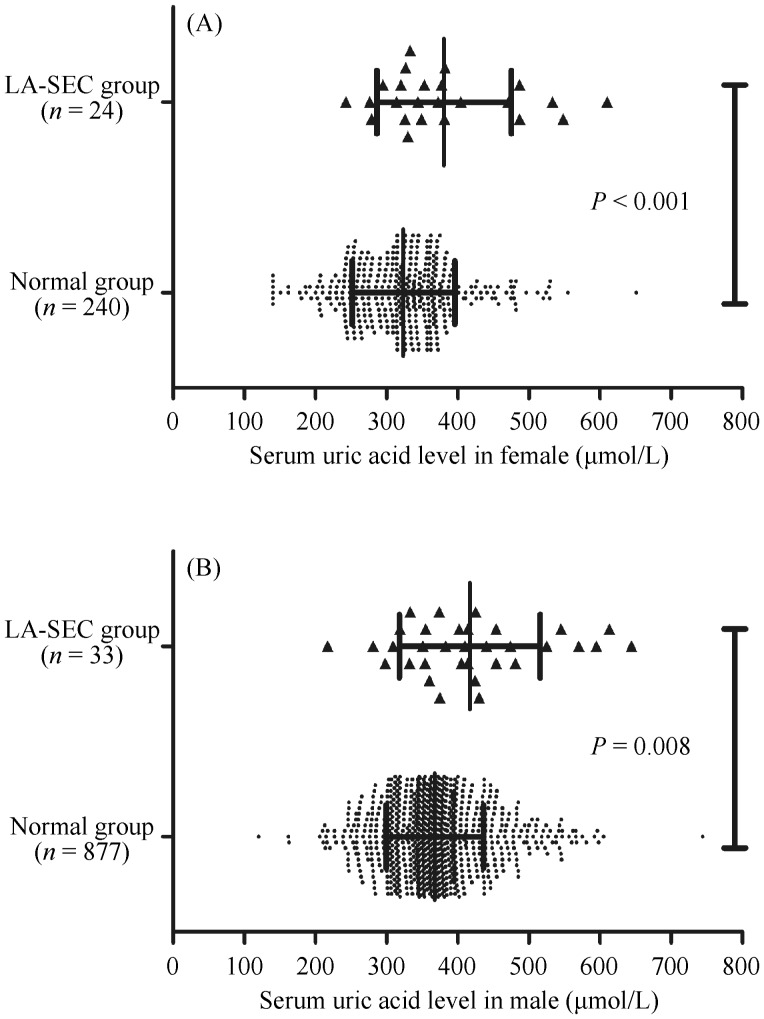
Patients with LA-SEC were markedly older (mean age: 60.74 ± 10.76 vs. 56.01 ± 12.26 years, P = 0.004). The mean female SUA level (380.88 ± 94.35 µmol/L vs. 323.37 ± 72.19 µmol/L, P < 0.001) and male SUA level (416.97 ± 98.87 µmol/L vs. 367.88 ± 68.50 µmol/L, P = 0.008) were both significantly higher in patients with LA-SEC than in the controls (Figure 1). And mean plasma fibrinogen (3.44 ± 0.90 g/L vs. 3.08 ± 0.72 g/L, P = 0.009) and mean Hemoglobin A1c (6.00% ± 0.40% vs. 5.81% ± 0.49%, P = 0.036) were significantly higher in patients with LA-SEC. The mean left atrial dimension (LAd) (41.32 ± 5.12 mm vs. 36.12 ± 5.66 mm, P < 0.001) was markedly larger in patients with LA-SEC. Patients with LA-SEC had a significantly higher proportion of CHA2DS2-VASc score ≥ 2 (63.2% vs. 38.2%, P < 0.001) and a lower proportion of CHA2DS2-VASc score = 0 (5.3% vs. 29.9%, P < 0.001).
Figure 1. SUA levels between LA-SEC group and normal group.
(A): The mean female SUA level (380.88 ± 94.35 µmol/L vs. 323.37 ± 72.19 µmol/L, P < 0.001), and (B) male SUA level (416.97 ± 98.87 µmol/L vs. 367.88 ± 68.50 µmol/L, P = 0.008) were both significantly higher in patients with LA-SEC than in the controls. LA-SEC: left atrial spontaneous echo contrast; SUA: serum uric acid.
According to the medical history, the proportion of patients with persistent/permanent AF (47.4% vs. 16.2%, P < 0.001), DM (24.6% vs. 13.7%, P = 0.022), previous stroke/TIA (7.0% vs. 1.5%, P = 0.014) and vascular disease (14.0% vs. 5.8%, P = 0.020) was markedly higher among patients with LA-SEC than among the controls.
3.2. Predictive value of SUA for LA-SEC in non-valvular AF patients
Multivariate regression analysis was performed to identify the relationship between the potential risk factors for LA-SEC as screened by TEE in the study population. The odds ratios (OR) of these potential risk factors for LA-SEC are summarized in Table 2. In the multivariate regression analysis after adjustment for persistent/permanent AF, age, CHA2DS2-VASc score, LAd, LVEF, and warfarin and aspirin therapy, SUA level was an independent risk factor for LA-SEC (OR: 1.008, 95% CI: 1.004–1.011, P < 0.001) (Table 2). Excluding all patients who had received warfarin or aspirin therapy, after adjustment for persistent/permanent AF, age, CHA2DS2-VASc score, LAd, and LVEF, SUA remained significantly associated with LA-SEC (OR: 1.005, 95% CI: 1.001–1.009, P = 0.024).
Table 2. Multivariate regression analysis for LA-SEC.
| Multivariate analysis |
||
| OR (95% CI) | P Value | |
| Per-AF | 4.659 (2.713−7.998) | < 0.001* |
| Age | 1.036 (1.011−1.061) | 0.005* |
| LAd | 1.129 (1.086−1.173) | < 0.001* |
| LVEF | 0.977 (0.946−1.010) | 0.172 |
| Aspirin | 1.133 (0.477−2.694) | 0.777 |
| Warfarin | 1.572 (0.868−2.845) | 0.136 |
| Congestive heart failure | 1.787 (0.883−3.616) | 0.107 |
| Hypertension | 1.463 (0.859−2.490) | 0.161 |
| Diabetes mellitus | 2.047 (1.097−3.818) | 0.024* |
| Previous stroke/TIA | 5.076 (1.669−15.445) | 0.004* |
| Vascular disease | 2.660 (1.216−5.820) | 0.014* |
| Female | 1.519 (0.886−2.602) | 0.128 |
| Serum uric acid | 1.008 (1.004−1.011) | < 0.001* |
*With statistical significance. AF: atrial fibrillation; LAd: left atrial dimension; LA-SEC: left atrial spontaneous echo contrast; LVEF: left ventricular ejection fraction; Per-AF: persistent/permanent AF; TIA: transient ischemic attack.
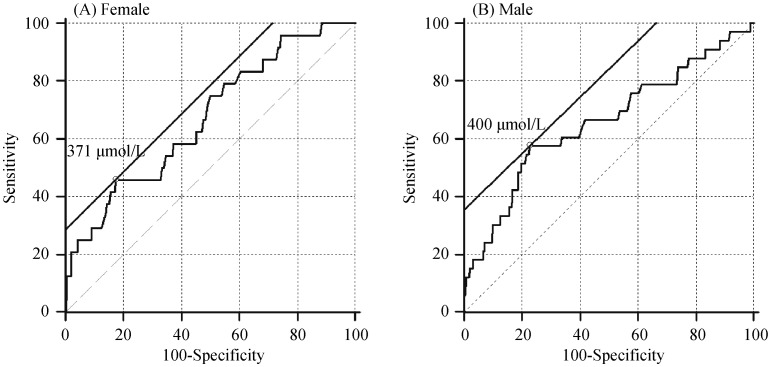
The ROC curve and AUC statistical method were used to examine the diagnostic value of SUA levels for the detection of LA-SEC. The corresponding AUC for SUA predicting LA-SEC in female was 0.670 (95% CI: 0.624–0.714) and the best cut-off point for SUA predicting LA-SEC was 371 µmol/L; AUC in male was 0.657 (95% CI: 0.625–0.688) and the best cut-off point for SUA predicting LA-SEC was 400 µmol/L, as shown in Figure 2.
Figure 2. ROC curve for SUA level in predicting LA-SEC.
(A): The corresponding AUC for SUA predicting LA-SEC in female was 0.670 (95% CI 0.624–0.714) and the best cut-off point for SUA predicting LA-SEC was 371 µmol/L; and (B): AUC in male was 0.657 (95% CI 0.625–0.688) and the best cut-off point for SUA predicting LA-SEC was 400 µmol/L. AUC: area under the curve; LA-SEC: left atrial spontaneous echo contrast; ROC curve: receiver operator characteristic curve; SUA: serum uric acid.
4. Discussion
4.1. Main findings
In the present study, we explored the relationship between SUA and LA-SEC screened by TEE in a total of 1,345 non-valvular AF patients using a single-center database. The main findings were as follows: (1) Patients in the LA-SEC group had significantly higher SUA levels than those in the TEE normal group; (2) SUA was an independent risk factor for LA-SEC in non-valvular AF patients; and (3) SUA might have a moderate predictive value for LA-SEC in non-valvular AF patients.
4.2. Pathophysiological mechanisms between SUA and LA-SEC in non-valvular AF patients
Stasis of blood in the left atrium plays an important role in the occurrence of thromboembolic events. LA-SEC is a dynamic smog-like echo with a characteristic swirling motion of the fluid that is detected by TEE. LA-SEC is commonly screened in the left atrium/appendage and in patients with AF.[8],[9]
Several pathophysiological mechanisms of LA-SEC are widely accepted. Manjunath, et al.[10] suggested that LA-SEC is mainly attributed to low velocity of the blood stream, erythrocyte aggregation in the blood plasma, or a combination of both. Sigel, et al.[11] revealed that erythrocyte aggregation in the blood plasma predominantly results in echogenicity of LA-SEC. Similarly, a relationship between inflammatory status and prothrombotic status has been described in previous studies.[1],[12]–[14] Conway, et al.[15] demonstrated that inflammatory markers including C-reactive protein (CRP) and interleukin-6 are associated with indices of LA-SEC/LA-TH in patients with AF. Akpek, et al.[16] confirmed that high sensitive-CRP, which is indicative of inflammatory status, is independently associated with LA-SEC in patients with valvular AF.
Uric acid is a by-product of normal purine catabolism that is excreted mostly in the urine, but also through the gastrointestinal tract. Xanthine oxidase catalyzes the conversion of hypoxanthine to xanthine and xanthine to uric acid. SUA has been shown to be associated with left ventricular diastolic (LVD) dysfunction,[17] essential hypertension,[14] insulin resistance,[18] arterial atherosclerosis,[19] and ischemic stroke in previous studies.[1],[3] Hypertension can cause left ventricular hypertrophy and impairment of the left ventricular diastolic function, which can lead to CHF, insulin resistance leading to DM, and arterial stiffness and carotid atherosclerosis leading to vascular disease. These disorders are components of the CHA2DS2-VASc scheme and directly or indirectly induce prothrombotic status and thromboembolic events. AF patients with abnormally elevated SUA and concomitant disorders such as hypertension and LVD dysfunction frequently show elevated LVD pressure and intra-atrial pressure. Elevated LVD and intra-atrial pressure results in low velocity of the blood stream, blood cell aggregation in the plasma, left atrial stretch impulses, and maintenance of AF, which might exacerbate blood stasis and the impairment of the left atrial endocardium. The relationship between SUA and endothelial or endocardial dysfunction, oxidant stress, and excessive inflammation has been widely examined in in vitro studies, animal models, and human studies. SUA is considered as a trigger or a danger signal that results in the activation of inflammatory markers and the interleukin-1 pathway, such as C-reactive protein and interleukin-6, which may promote left atrial cell apoptosis and fibrosis. Therefore, elevated SUA might induce cardiac structural remodeling to directly increase the left atrial dimensions and indirectly increase LVD and intra-atrial pressure. This could result in the generation of an atrial arrhythmogenic substrate, reduce the antithrombotic ability, exacerbate blood stasis, and promote the LA-SEC status. Consequently, elevated SUA could be an indicator of the risk of LA-SEC in non-valvular patients.
In the present study, we showed that SUA is independently associated with LA-SEC in non-valvular AF patients. Several recent studies showed that allopurinol and colchicine,[20]–[22] which are urate lowering agents, effectively suppress the onset and development of AF and prevent atrial endocardium impairment. Moreover, maintenance of AF and atrial endocardial dysfunction are regarded as critical factors for LA-SEC formation. Accordingly, AF patients with elevated SUA who are not under anticoagulant therapy have an increased susceptibility to LA-SEC, and even thromboembolic events.
If the relationship between SUA and LA-SEC in AF patients is confirmed, SUA could be regarded as a therapeutic target for the prevention of thromboembolic events and as an efficient indicator for antithrombotic decision-making. Our results suggest that SUA plays an essential role in LA-SEC formation and has independent predictive value for LA-SEC in non-valvular AF patients.
4.3. Limitations
The present study had three main limitations. First, the number of patients was relatively insufficient to determine the real predictive value of SUA for LA-SEC. Furthermore, the study population was mainly selected from the population of Southern China. Second, owing to most of the patients were eligible for RFCA, there were few non-valvular AF patients in poor conditions, such as few patients aged ≥ 75 years (3.7%) or ≥ 65 years (27.3%), with a history of stroke/TIA (1.7%) and CHF (10.9%). Therefore, selection bias may have limited the present statistical analysis, and the results may not be representative of all non-valvular AF patients. Finally, the diagnostic ability of intracardiac echocardiography might be better than that of TEE for LA-SEC or LA-TH. Therefore, rather than a gold standard, TEE just is a good standard. In consideration of the retrospective design of the present study, further prospective clinical trials are warranted to validate the predictive value of SUA for LA-SEC or/and thromboembolic events in different populations and for making anticoagulant decisions.
4.4. Conclusion
SUA level is significantly higher in non-valvular AF patients with LA-SEC. SUA level is an independent risk factor and has a moderate predictive value for LA-SEC among non-valvular AF patients in Southern China.
Acknowledgments
This work was supported by National Nature Science Foundation of China [No.81370295], Science and Technology Planning Project of Guangdong Province [No. 2010B031600166, 2012B031800316, 2012B 061800047, 2012B031800317], Science and Technology Planning Project of Guangzhou [No. 158100073]. We appreciated the great help of Xin LI, Li REN, and Xuan JIANG. The authors declare no conflict of interest.
References
- 1.Bos MJ, Koudstaal PJ, Hofman A, et al. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 2006;37:1503–1507. doi: 10.1161/01.STR.0000221716.55088.d4. [DOI] [PubMed] [Google Scholar]
- 2.Heo SH, Lee SH. High levels of serum uric acid are associated with silent brain infarction. J Neurol Sci. 2010;297:6–10. doi: 10.1016/j.jns.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Hozawa A, Folsom AR, Ibrahim H, et al. Serum uric acid and risk of ischemic stroke: the aric study. Atherosclerosis. 2006;187:401–407. doi: 10.1016/j.atherosclerosis.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Tang RB, Dong JZ, Yan XL, et al. Serum uric acid and risk of left atrial thrombus in patients with nonvalvular atrial fibrillation. Can J Cardiol. 2014;30:1415–1421. doi: 10.1016/j.cjca.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Romero J, Cao JJ, Garcia MJ, et al. Cardiac imaging for assessment of left atrial appendage stasis and thrombosis. Nat Rev Cardiol. 2014;11:470–480. doi: 10.1038/nrcardio.2014.77. [DOI] [PubMed] [Google Scholar]
- 7.Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 8.Castello R, Pearson AC, Labovitz AJ. Prevalence and clinical implications of atrial spontaneous contrast in patients undergoing transesophageal echocardiography. Am J Cardiol. 1990;65:1149–1153. doi: 10.1016/0002-9149(90)90330-4. [DOI] [PubMed] [Google Scholar]
- 9.Sahin T, Ural D, Kilic T, et al. Right atrial appendage function in different etiologies of permanent atrial fibrillation: a transesophageal echocardiography and tissue Doppler imaging study. Echocardiography. 2010;27:384–393. doi: 10.1111/j.1540-8175.2009.01027.x. [DOI] [PubMed] [Google Scholar]
- 10.Manjunath CN, Srinivasa KH, Panneerselvam A, et al. Incidence and predictors of left atrial thrombus in patients with rheumatic mitral stenosis and sinus rhythm: a transesophageal echocardiographic study. Echocardiography. 2011;28:457–460. doi: 10.1111/j.1540-8175.2010.01361.x. [DOI] [PubMed] [Google Scholar]
- 11.Sigel B, Coelho JC, Spigos DG, et al. Ultrasonography of blood during stasis and coagulation. Invest Radiol. 1981;16:71–76. doi: 10.1097/00004424-198101000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Hess K, Grant PJ. Inflammation and thrombosis in diabetes. Thromb Haemost. 2011;105(Suppl. 1):S43–S54. doi: 10.1160/THS10-11-0739. [DOI] [PubMed] [Google Scholar]
- 13.Kaya MG, Yarlioglues M, Gunebakmaz O, et al. Platelet activation and inflammatory response in patients with non-dipper hypertension. Atherosclerosis. 2010;209:278–282. doi: 10.1016/j.atherosclerosis.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Krishnan E. Interaction of inflammation, hyperuricemia, and the prevalence of hypertension among adults free of metabolic syndrome: NHANES 2009-2010. J Am Heart Assoc. 2014;3:e 000157. doi: 10.1161/JAHA.113.000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conway DS, Buggins P, Hughes E, et al. Relationship of interleukin-6 and c-reactive protein to the prothrombotic state in chronic atrial fibrillation. J Am Coll Cardiol. 2004;43:2075–2082. doi: 10.1016/j.jacc.2003.11.062. [DOI] [PubMed] [Google Scholar]
- 16.Akpek M, Sahin O, Sarli B, et al. Association of left atrial spontaneous echo contrast to uric acid in patients with mitral stenosis. Echocardiography. 2015;32:1477–1482. doi: 10.1111/echo.12897. [DOI] [PubMed] [Google Scholar]
- 17.Bergamini C, Cicoira M, Rossi A, et al. Oxidative stress and hyperuricaemia: pathophysiology, clinical relevance, and therapeutic implications in chronic heart failure. Eur J Heart Fail. 2009;11:444–452. doi: 10.1093/eurjhf/hfp042. [DOI] [PubMed] [Google Scholar]
- 18.Krishnan E, Pandya BJ, Chung L, et al. Hyperuricemia in young adults and risk of insulin resistance, prediabetes, and diabetes: a 15-year follow-up study. Am J Epidemiol. 2012;176:108–116. doi: 10.1093/aje/kws002. [DOI] [PubMed] [Google Scholar]
- 19.Ishizaka N, Ishizaka Y, Toda E, et al. Association between serum uric acid, metabolic syndrome, and carotid atherosclerosis in Japanese individuals. Arterioscler Thromb Vasc Biol. 2005;25:1038–1044. doi: 10.1161/01.ATV.0000161274.87407.26. [DOI] [PubMed] [Google Scholar]
- 20.Kim SC, Schneeweiss S, Choudhry N, et al. Effects of xanthine oxidase inhibitors on cardiovascular disease in patients with gout: a cohort study. Am J Med. 2015;128:653.e7–653.e16. doi: 10.1016/j.amjmed.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakabe M, Fujiki A, Sakamoto T, et al. Xanthine oxidase inhibition prevents atrial fibrillation in a canine model of atrial pacing-induced left ventricular dysfunction. J Cardiovasc Electrophysiol. 2012;23:1130–1135. doi: 10.1111/j.1540-8167.2012.02356.x. [DOI] [PubMed] [Google Scholar]
- 22.Nidorf SM, Eikelboom JW, Budgeon CA, et al. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404–410. doi: 10.1016/j.jacc.2012.10.027. [DOI] [PubMed] [Google Scholar]