Abstract
Background
Increased red blood cell distribution width (RDW) is associated with adverse outcomes in patients with heart failure (HF). The objective of this study was to compare the differences in the predictive value of RDW in patients with HF due to different causes.
Methods
We retrospectively investigated 1,021 HF patients from October 2009 to December 2011 at Fuwai Hospital (Beijing, China). HF in these patients was caused by three diseases; coronary heart disease (CHD), dilated cardiomyopathy (DCM) and valvular heart disease (VHD). Patients were followed-up for 21 ± 9 months.
Results
The RDW, mortality and survival duration were significantly different among the three groups. Kaplan–Meier analysis showed that the cumulative survival decreased significantly with increased RDW in patients with HF caused by CHD and DCM, but not in those with HF patients caused by VHD. In a multivariable model, RDW was identified as an independent predictor for the mortality of HF patients with CHD (P < 0.001, HR 1.315, 95% CI 1.122–1.543). The group with higher N-terminal pro-brain natriuretic peptide (NT-proBNP) and higher RDW than median had the lowest cumulative survival in patients with HF due to CHD, but not in patients with HF due to DCM.
Conclusions
RDW is a prognostic indicator for patients with HF caused by CHD and DCM; thus, RDW adds important information to NT-proBNP in CHD caused HF patients.
Keywords: Coronary heart disease, Dilated cardiomyopathy, Heart failure, Red blood cell distribution width, Valvular heart disease
1. Introduction
Heart failure (HF) is a chronic, progressive illness that carries a very poor prognosis and is highly prevalent worldwide.[1],[2] HF affects nearly four million Chinese people and is associated with elevated rates of mortality.[3] To target effective therapies for the most appropriate patients, there is a need for a simple but accurate prognostic indicator.
Red cell distribution width (RDW) is readily available from a standard full blood count and is a measure of variation in red blood cell (RBC) size. It is used clinically for morphological classification of anemia and the differential diagnosis of small cell anemia.[4] Furthermore, RDW has been shown to be a powerful predictor of short- and long-term outcomes in a patients with HF.[5]–[7] However, the etiology of heart failure is a complex and may be caused by myocardial systolic dysfunction, coronary artery disease, endocrine disease, heart valve disease, hypertension, acute pulmonary embolism, emphysema or other chronic lung diseases. Thus, in this study, our primary aim was to investigate differences in the prognostic value of RDW among patients with HF due to various heart diseases in order to expand the application of RDW in such patients.
2. Methods
2.1. Study samples
We identified 1,021 consecutive patients with HF hospitalized in the HF ward in Fuwai Hospital (Beijing, China), from October 2009 to December 2011. The diagnosis of HF was made by two clinicians with broad experience according to the European Society of Cardiology (ESC) guidelines.[8] To reduce the impact of other factors on RDW or the endpoint, the following patients were excluded: those with incomplete medical records, under 18 years old, with diseases such as infectious endocardial inflammation, aortic dissection, constrictive pericarditis, hydropericardium and pulmonary thromboembolism. In addition, patients with thyroid disease, acute cerebral vascular disease, cancer, chronic obstructive pulmonary diseases, end-stage renal disease and other diseases with the potential to change RDW were excluded. All the patients were given standard medication according to the guideline recommendations during the period of hospitalization and after discharge. Furthermore, all patients or their families were followed-up in telephone calls after discharge. The endpoint was defined as all-cause death. The study protocol was approved by the local ethics committee in accordance with the Declaration of Helsinki, and all study participants gave informed consent.
2.2. Clinical data collection
Clinical information on the patients, including age, sex, body mass index (BMI) and complicating diseases, was recorded on admission. Blood samples were taken from the participants for measurements at baseline, including routine blood tests, N-terminal pro-brain natriuretic peptide (NT-proBNP) and biochemical indexes. The blood samples were collected using a standard procedure after a 1-h fast, and were sent to the core laboratory of Fuwai Hospital for immediate testing using standard techniques. The RDW, RBC count, and hemoglobin (Hb) were tested using Sysmex XE-2100 blood cell analyzers and appropriate reagents; alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein (TP), albumin (ALB), total bilirubin (TBIL), direct bilirubin (DBIL), blood urea nitrogen (BUN), serum creatinine (CREA), uric acid (URIC) and high-sensitivity C-reactive protein (hs-CRP) were assayed using a Hitachi 7180 biochemistry autoanalyzer; plasma NT-proBNP was measured with a dedicated kit (NT-proBNP assays; Biomedica, Vienna, Austria). Chest X-radiography and echocardiography were performed and the left ventricular ejection fraction (LVEF) was measured according to the biplane Simpson rule. During the follow-up period, adverse events after discharge, such as rehospitalization due to HF or all-cause death, were recorded. If a patient was readmitted several times for HF exacerbation, we recorded the time of the first readmission. If a patient was readmitted to hospital and then died, death was regarded as an adverse event and the time of death was recorded. Following an all-cause death event, the follow-up period for this patient ended.
2.3. Statistical analyses
The results are presented as percentages for dichotomous variables, mean ± SD for parametric continuous variables, and the median (interquartile range) for nonparametric continuous variables. Two groups were compared by t-test or Mann-Whitney U-test or the Kruskal-Wallis H-test; further comparisons were performed by the Bonferroni method. Univariate analysis was used to select clinical variables, which were related to an endpoint with a P-value of < 0.05. A multivariate Cox proportional hazards model was used to calculate risk ratios for independent predictors of mortality with incremental increases in continuous variables. The RDW was non-normally distributed and is represented as the median [first quartile, third quartile: M (Q1, Q3)]. The survival rate of patients, estimated by Kaplan–Meier and log-rank tests, was analyzed to investigate the difference between two groups. Receiver operating characteristic (ROC) curve analysis was used to estimate the predictive value of RDW for death risk in patients with HF; the areas under the curve (AUC) were compared by the Z-test. The data were analyzed statistically using SPSS version 17.0.
3. Results
3.1. Baseline characteristics
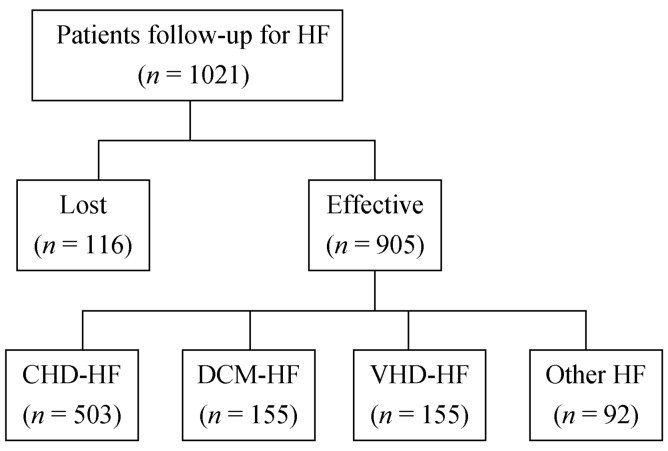
In total, 1,021 participants were enrolled consecutively in this study. During the follow-up period (21 ± 9 months), 116 (11.4%) participants were lost to follow-up, and 137 (15.1%) patients died. Effective follow-up was achieved for 905 cases (Figure 1). The mean age of the participants was 59.4 ± 13.6 years. The potential causes of HF were CHD (503, 55.6%), dilated cardiomyopathy (155, 17.1%), and valvular disease (155, 17.1%). Other characteristics of the patients are listed in Table 1.
Figure 1. Selection of follow-up patients.
CHD: coronary heart disease; DCM: dilated cardiomyopathy; HF: heart failure VHD: valvular heart disease.
Table 1. Patients' characteristics according to cardiac death events.
| All patients (n = 905) | Dead patients (n = 137) | Surviving patients (n = 768) | P-value | |
| Age, yrs | 59.4 ± 13.6 | 60.4 ± 14.4 | 59.3 ± 13.5 | 0.390 |
| Male, n (%) | 641 (70.5) | 93 (67.9) | 548 (71.4) | 0.410 |
| BMI, kg/m2 | 24.5 ± 4.1 | 22.6 ± 4.4 | 24.8 ± 3.9 | < 0.001 |
| SBP, mmHg | 121.8 ± 20.6 | 113.9 ± 20.5 | 123.2 ± 20.3 | < 0.001 |
| DBP, mmHg | 72.8 ± 12.6 | 69.7 ± 11.6 | 73.3 ± 12.7 | 0.002 |
| Heart rate, beats/min | 78.0 ± 16.7 | 81.4 ± 17.5 | 77.3 ± 16.5 | 0.009 |
| NYHA class II: III: IV, % | 18.1: 43.9: 38.0 | 11.1: 36.8: 52.1 | 25.8: 45.5: 28.7 | < 0.001 |
| LVEF, % | 47.0 ± 15.2 | 36.8 ± 13.8 | 48.7 ± 14.8 | < 0.001 |
| RDW, % (IQR) | 13.4 (12.8, 14.5) | 14.8 (13.6, 16.3) | 13.3 (12.8, 14.3) | < 0.001 |
| NT-proBNP, fmol/mL (IQR) | 1566.0 (766.0, 3003.2) | 4158.1 (2404.8, 6101.8) | 1347.8 (718.5, 2391.9) | < 0.001 |
| Hb, g/L (IQR) | 136 (124, 148) | 132 (119, 147) | 137 (125, 147) | 0.01 |
| RBC, 1012/L (IQR) | 4.5 (4.1, 4.9) | 4.4 (3.9, 5.0) | 4.5 (4.1, 4.9) | 0.033 |
| TP, g/L(IQR) | 70.1 (65.7, 74.9) | 68.0 (62.8, 73.6) | 70.4 (66.0, 75.5) | 0.008 |
| ALB, g/L(IQR) | 40.7 (37.7, 43.4) | 37.7 (33.9, 41.1) | 41.1 (38.3, 43.9) | < 0.001 |
| ALT, IU/L (IQR) | 24.0 (16.0, 39.0) | 23 (15.5, 44.5) | 24.0 (16.0, 38.0) | 0.916 |
| AST, IU/L (IQR) | 26.5 (20.0, 37.8) | 31 (22, 41) | 26 (20, 37) | 0.023 |
| TBIL, µmol/L (IQR) | 19.0 (13.5, 29.3) | 27.3 (17.0, 41.4) | 18.6 (13.1, 27.7) | < 0.001 |
| DBIL, mol/L (IQR) | 3.2 (2.1, 5.3) | 5.2 (3.1, 10.8) | 3.0 (2.0, 4.8) | < 0.001 |
| BUN, mmol/L (IQR) | 7.35 (5.74, 9.67) | 9.4 (7.6, 12.7) | 7.3 (5.7, 9.3) | < 0.001 |
| URIC, µmol/L (IQR) | 377.5 (303.0, 485.2) | 458.9 (354.5, 548.6) | 368.5 (307.0, 467.5) | < 0.001 |
| CREA, µmol/L (IQR) | 91.4 (76.4, 113.5) | 103.0 (80.7, 129.7) | 91.4 (76.9, 111.9) | < 0.001 |
| hs-CRP, mg/L (IQR) | 3.62 (1.64, 9.66) | 8.51 (3.20, 11.2) | 3.17 (1.54, 9.13) | < 0.001 |
ALB: albumin; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BMI: body mass index; BUN: blood urea nitrogen; CERA: creatinine; DBIL: direct bilirubin; DBP: diastolic blood pressure; Hb: hemoglobin; hs-CRP: highly sensitive C-reactive protein; IQR: interquartile range; LVEF: left ventricular ejection fraction; NT-proBNP: amino terminal B-type natriuretic peptide; NYHA class: New York Heart Association functional class; RBC: red blood cell; RDW: red blood cell distribution width; SBP: systolic blood pressure; TBIL: total bilirubin; TP: total protein; URIC; uric acid.
3.2. RDW is an independent risk factor for death in patients with HF
In the univariate analysis, the significant clinical variables were BMI, NYHA heart function classification, blood pressure, heart rate, LVEF, RDW, NT-proBNP, hs-CRP, Hb, TP, ALB, ALT, AST, TBIL, DBIL, BUN, CREA, and URIC. In the multivariate Cox proportional hazard analysis, RDW was identified as a significant variable in predicting mortality (Table 2).
Table 2. Cox multivariate risk ratio for mortality.
| Variable | Increment | P-value | Multivariate Risk Ratio (95% CI) |
| RDW, % | + 1 | < 0.001 | 1.102 (1.038−1.171) |
| NT-proBNP, fmol/mL | + 100 | < 0.001 | 1.020 (1.014−1.027) |
| Hb, g/L | + 10 | 0.487 | - |
| LVEF, % | + 10 | < 0.05 | 0.915 (0.840−0.999) |
| CREA, µmol/L | + 10 | 0.392 | - |
| Age, yrs | + 10 | 0.06 | - |
CERA: creatinine; Hb: hemoglobin; LVEF: left ventricular ejection fraction; NT-proBNP: amino terminal B-type natriuretic peptide; RDW: red blood cell distribution width.
3.3. Differences in the prognostic value of RDW in patients with HF due to various heart diseases
CHD, DCM, and VHD are the main causes of HF. Therefore, we selected patients with these three main causes for this study and stratified the population into three groups accordingly. In the univariate analysis, RDW remained a predictor of mortality in patients with CHD and DCM, but not in those with VHD (Table 3).
Table 3. Univariate analysis of predictors for major causes of heart failure.
| CHD (n = 503) |
DCM (n = 155) |
VHD (n = 155) |
||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age, yrs | 1.020 (0.995−1.045) | 0.121 | 1.003 (0.980−1.027) | 0.788 | 1.027 (0.995−1.060) | 0.100 |
| Male vs. female | 1.641 (0.957−2.813) | 0.072 | 0.942 (0.429−2.066) | 0.881 | 0.672 (0.314−1.438) | 0.306 |
| BMI, kg/m2 | 0.835 (0.769−0.906) | 0.000 | 0.909 (0.837−0.987) | 0.024 | 0.902 (0.814−1.001) | 0.052 |
| SBP, mmHg | 0.981 (0.967−0.995) | 0.007 | 0.971 (0.949−0.994) | 0.015 | 0.988 (0.968−1.009) | 0.254 |
| DBP, mmHg | 0.988 (0.966−1.011) | 0.300 | 0.954 (0.924−0.985) | 0.004 | 0.986 (0.959−1.014) | 0.312 |
| Heart rate, beats/min | 1.036 (1.020−1.052) | 0.000 | 0.983 (0.965−1.001) | 0.069 | 1.009 (0.990−1.029) | 0.341 |
| NYHA class | 1.894 (1.308−2.743) | 0.001 | 1.751 (1.016−3.020) | 0.044 | 2.530 (1.357−4.718) | 0.003 |
| LVEF, % | 0.932 (0.913−0.952) | 0.000 | 0.958 (0.915−1.003) | 0.067 | 0.966 (0.942−0.991) | 0.009 |
| RDW, % (IQR) | 1.464 (1.327−1.616) | 0.000 | 1.355 (1.225−1.499) | 0.000 | 1.086 (0.915−1.289) | 0.344 |
| NT-proBNP, fmol/mL(IQR) | 1.000 (1.000−1.000) | 0.000 | 1.001 (1.000−1.001) | 0.000 | 1.000 (1.000−1.001) | 0.000 |
| Hb, g/L (IQR) | 0.976 (0.964−0.988) | 0.000 | 0.993 (0.975−1.011) | 0.456 | 0.989 (0.973−1.005) | 0.193 |
| RBC, 1012/L (IQR) | 0.673 (0.443−1.023) | 0.064 | 0.849 (0.521−1.386) | 0.513 | 0.640 (0.385−1.062) | 0.084 |
| TP, g/L (IQR) | 0.965 (0.930−1.001) | 0.059 | 0.953 (0.911−0.998) | 0.039 | 0.960 (0.925−0.996) | 0.031 |
| ALB, g/L (IQR) | 0.844 (0.802−0.887) | 0.000 | 0.884 (0.832−0.939) | 0.000 | 0.915 (0.864−0.970) | 0.003 |
| ALT, IU/L (IQR) | 1.003 (1.002−1.004) | 0.000 | 1.000 (0.993−1.007) | 0.929 | 1.003 (1.001−1.005) | 0.001 |
| AST, IU/L (IQR) | 1.001 (1.001−1.002) | 0.000 | 1.000 (0.992−1.008) | 0.959 | 1.004 (1.002−1.006) | 0.000 |
| TBIL, µmol/L (IQR) | 1.011 (1.003−1.020) | 0.006 | 1.017 (1.008−1.026) | 0.000 | 1.005 (0.995−1.016) | 0.330 |
| DBIL, µmol/L (IQR) | 1.113 (1.075−1.151) | 0.000 | 1.045 (1.030−1.061) | 0.000 | 1.012 (0.990−1.035) | 0.294 |
| BUN, mmol/L (IQR) | 1.119 (1.087−1.152) | 0.000 | 1.197 (1.094−1.309) | 0.000 | 1.067 (1.008−1.131) | 0.027 |
| URIC, µmol/L (IQR) | 1.005 (1.003−1.006) | 0.000 | 1.003 (1.001−1.006) | 0.001 | 1.002 (1.000−1.005) | 0.025 |
| CREA, µmol/L (IQR) | 1.006 (1.003−1.000) | 0.000 | 1.002 (0.990−1.014) | 0.729 | 1.011 (1.005−1.018) | 0.000 |
| hs-CRP, mg/L (IQR) | 1.093 (1.034−1.154) | 0.002 | 1.104 (1.023−1.192) | 0.011 | 1.158 (1.066−1.258) | 0.001 |
ALB: albumin; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BMI: body mass index; BUN: blood urea nitrogen; CERA: creatinine; CHD: coronary heart disease; DBIL: direct bilirubin; DBP: diastolic blood pressure; DCM: dilated cardiomyopathy; Hb: hemoglobin; hs-CRP: highly sensitive C-reactive protein; HR: hazard ratio; IQR: interquartile range; LVEF: left ventricular ejection fraction; NT-proBNP: amino terminal B-type natriuretic peptide; NYHA class: New York Heart Association functional class; RBC: red blood cell; RDW: red blood cell distribution width; SBP: systolic blood pressure; TBIL: total bilirubin; TP: total protein; URIC; uric acid; VHD: valvular heart disease.
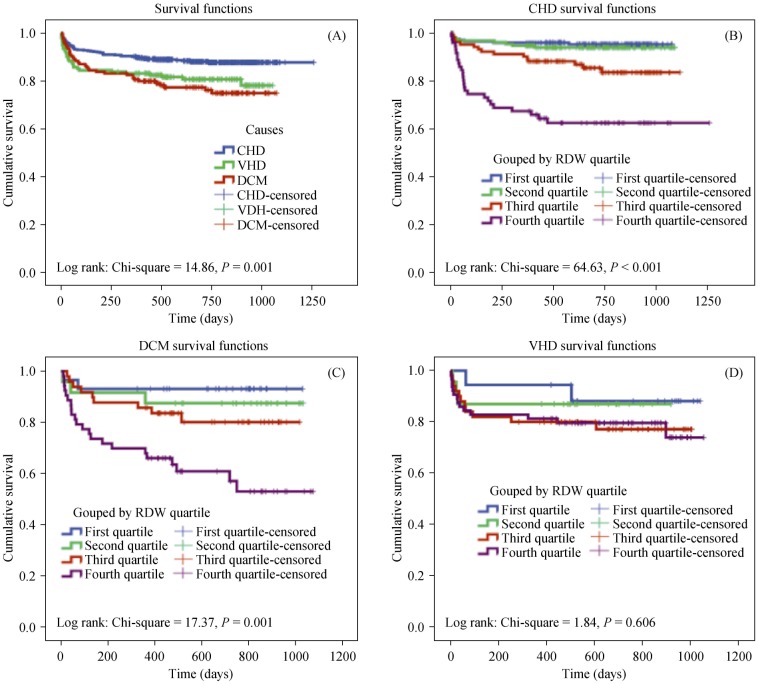
Kaplan–Meier analysis was used to evaluate the predictive ability of RDW on cumulative survival by stratification of the patients into four groups according to the quartiles of RDW. It was found that cumulative survival was significantly lower in patients with CHD and DCM in the higher quartiles of RDW, but cumulative survival was not significantly different for those with VHD (Figure 2).
Figure 2. Survival curves for patients with heart failure of different etiologies.
Kaplan–Meier survival curves showing the total HF patients with heart failure (A); HF patients due to coronary heart disease (B); dilated cardiomyopathy (C); and valvular heart disease (D) grouped by RDW quartile. CHD: coronary heart disease; DCM: dilated cardiomyopathy; HF: heart failure; RDW: red cell distribution width; VHD: valvular heart disease.
As shown in Table 4, after adjustment for potential confounding factors (NT-proBNP, Hb, LVEF, and age) in a multivariable Cox proportional hazards model, RDW remained an independent predictor for CHD mortality; however, RDW was no longer a predictor for DCM mortality.
Table 4. Cox multivariate risk ratio for mortality from CHD and DCM.
| Variable | Increment | CHD |
DCM |
||
| P-value | Multivariate Risk Ratio (95% CI) | P-value | Multivariate Risk Ratio (95% CI) | ||
| RDW, % | + 1 | < 0.001 | 1.347 (1.150, 1.578) | 0.264 | |
| NT-proBNP, fmol/mL | + 100 | 0.029 | 1.016 (1.002, 1.031) | < 0.001 | 1.057 (1.026, 1.088) |
| Hb, g/L | + 10 | 0.104 | - | ||
| LVEF, % | + 10 | 0.003 | 0.636 (0.474, 0854) | 0.843 | |
| Age, yrs | + 10 | 0.395 | 0.767 | ||
CHD: coronary heart disease; DCM: dilated cardiomyopathy; Hb: hemoglobin; LVEF: left ventricular ejection fraction; NT-proBNP: amino terminal B-type natriuretic peptide; RDW: red blood cell distribution width.
We stratified the population into three groups according to the cause of HF and found that the median RDW, mortality during the follow-up period and median survival time were significantly different in the three groups of patients. Median RDW and mortality were significantly higher in patients with VHD and DCM than in patients with CHD and the survival time in these patients was shorter than that of patients with CHD, with no differences between the patients with VHD and DCM (Table 5).
Table 5. RDW, mortality and survival time in the three groups, and ROC curve analysis.
| Cause of heart failure (n) | Mortality (%) | RDW (%) |
Survival time (day) |
ROC curve analysis |
||
| M (Q1, Q3) | M (Q1, Q3) | AUC | P-value | AUC 95% CI | ||
| CHD (503) | 11.7 | 13.1 (12.6, 13.8) | 726.0 (495.0, 882) | 0.704 | < 0.001 | 0.609−0.799 |
| VHD (155) | 19.4 | 14.0 (13.4, 15.3)** | 657.0 (444.0, 864.0)* | 0.593 | 0.168 | 0.462−0.724 |
| DCM (155) | 23.2 | 13.9 (13.0, 14.9)** | 628.0 (412.0, 852.0)** | 0.753 | < 0.001 | 0.647−0.860 |
| P-value | 0.001 | < 0.001 | 0.005 | > 0.05a | — | — |
*P < 0.05, **P < 0.01; A P-values of AUC are calculated by comparison between CHD and DCM. AUC: area under the curve; CHD: coronary heart disease; DCM: dilated cardiomyopathy; ROC: receiver operator characteristic; VHD: valvular heart disease.
Parameters of the ROC curves examining the power of RDW to predict mortality in patients with HF are shown in Table 5. The prognostic values of the RDW in various heart diseases were different. The AUC of RDW for predicting mortality due to CHD and DCM was 0.704 (P < 0.001, 95% CI: 0.609–0.799) and 0.753 (P < 0.001, 95% CI: 0.647–0.860), respectively, with no significant difference between the two values (P > 0.05). However, the AUC of the RDW for predicting mortality from VHD was 0.593 (P = 0.168).
3.4. RDW adds important prognostic information to NT-proBNP in HF due to CHD
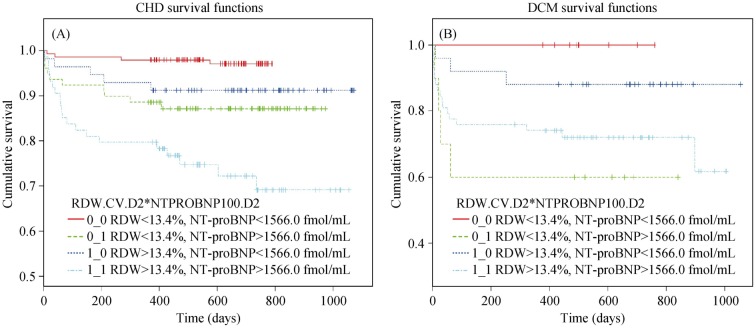
We then stratified the population into four groups according to the median of RDW (13.4%) and NT-proBNP (1566.0 fmol/mL) with the purpose of exploring the power of RDW to adding prognostic information to NT-proBNP. We found that the group of patients with RDW > 13.4% and NT-proBNP > 1566.0 fmol/mL had the lowest cumulative survival in patients with HF due to CHD (Figure 3A). However, for those patients with the NT-proBNP phase at the same level, the cumulative survival was significantly lower in patients with increased RDW. In patients with HF due to DCM, it was found that cumulative survival of patients with RDW < 13.4% and NT-proBNP > 1566.0 fmol/mL was significantly lower than the group of patients with RDW > 13.4% and NT-proBNP > 1566.0 fmol/mL (Figure 3B).
Figure 3. Survival curves for patients with heart failure of different etiologies according to median concentrations of RDW and NT-proBNP.
(A): Survival curves of patients with coronary heart disease grouped by median concentrations of RDW and NT-proBNP; (B): survival curves of patients with dilated cardiomyopathy grouped by median concentrations of RDW and NT-proBNP. CHD: coronary heart disease; DCM: dilated cardiomyopathy; NT-proBNP: amino terminal B-type natriuretic peptide; RDW: red cell distribution width.
4. Discussion
RDW reflects the variability in size of circulating RBCs and, when elevated, defines the state of anisocytosis. Felker et al.[9] first reported the increase in mortality and morbidity in patients with CHF and elevated RDW. Subsequent studies showed that increased RDW is associated with increased mortality in patients with chronic and acute HF,[10]–[12] acute myocardial infarction,[13],[14] coronary artery disease,[15]–[17] acute coronary syndromes,[18] and stroke.[19] However, HF is a complex disease, with a variety of causes.[20] Therefore, in this study our primary aim was to investigate differences in the prognostic value of RDW among patients with HF due to various heart diseases and to apply it more effectively in predicting outcomes of HF patients.
4.1. Prognostic value of RDW in patients with HF due to various heart diseases
Kaplan–Meier analysis was used to evaluate the effect of RDW on cumulative survival by stratification of the patients into four groups according to the quartile of RDW.
4.1.1. RDW and CHD-HF
Cumulative survival was significantly lower in patients with CHD in the higher quartiles of RDW. The AUC of RDW for predicting the mortality of HF patients due to CHD was 0.704, showing that that RDW is an effective predictor of mortality in this group.
After adjustment for potential confounding factors (NT-proBNP, Hb, LVEF, and age) in a multivariable Cox proportional hazards model, RDW remained an independent predictor for CHD mortality. Consequently, we considered combining RDW with another biomarker to testify whether RDW could provide additional information for prognosis. On this occasion, NT-proBNP is a good choice as it has relatively authoritative power in the prognosis of HF.[21],[22] The lowest cumulative survival for the patients with CHD was observed in the group with both RDW and NT-proBNP above the median values. In addition, with NT-proBNP values of the same level, the cumulative survival of patients with RDW above the median was lower than those with RDW below the median (dark blue line vs. red line and light blue line vs. green line in Figure 3A). These data indicate that the combination of NT-proBNP and RDW provide more information on the prognosis of patients with HF due to CHD than that provided by NT-proBNP alone.
4.1.2. RDW and DCM-HF
Cumulative survival was significantly lower in patients with DCM in the higher quartiles of RDW. The AUC of RDW for predicting the mortality of HF patients due to DCM was 0.753, showing that that RDW is an effective predictor of mortality in this group.
However, after adjustment for potential confounding factors (NT-proBNP, Hb, LVEF, and age) in a multivariable Cox proportional hazards model, RDW was no longer a predictor for DCM mortality. The combination of NT-proBNP and RDW failed to provide additional information for prognosis of patients with HF due to DCM than that provided by NT-proBNP alone. This may be related to the small number of patients in this group.
4.1.3. RDW and VHD-HF
Cumulative survival in every RDW quartile group was similar in patients with VHD. The AUC of RDW for predicting the mortality of HF patients due to VHD was 0.593, indicating that RDW is not an effective predictor of mortality in this group.
These published data and the novel observations of this study provide a further understanding of why measurement of RDW is more appropriate in HF caused by coronary artery disease and DCM, than in VHD.
4.2. Study limitations
The current study has several limitations. Although it was a single-center study with consecutive patient enrolment from both in- and outpatient departments, patient heterogeneity may represent some background bias. HF is associated with many diseases. Due to the small number of cases of HF due to some of these diseases, in this study, we investigated only three main causes: CHD, DCM, and VHD. CHD (55.7%) is first leading cause of heart failures in China, followed by other caused including DCM, VHD, and hypertension. In the present study, the heart failure patients were continuously included, and thus the number of the patients with CHD was the highest (n = 503), while the number of the ones with the other two diseases, namely DCM (n = 155) and VHD (n = 155), was lower. Further large-scale studies are required to obtain more comprehensive data.
4.3. Conclusions
Accumulating evidence shows that different strategies are required to treat HF caused by different diseases. We identified differences in the prognostic value of RDW in patients with HF due to CHD, DCM and VHD. In conclusion, our study indicates that RDW is predicative for the mortality of HF patients due to CHD and DCM, but not VHD. In a multivariable Cox proportional hazards model, RDW was no longer found to be an independent predictor of DCM mortality. The combination of NT-proBNP and RDW provide more information on the prognosis of patients with HF due to CHD than that provided by NT-proBNP alone. This study should prompt further evaluation of the association between RDW and outcome in HF due to different to improve our understanding of the pathophysiology and to improve risk-stratification.
Acknowledgments
This study received Beijing Capital Special Foundation (Jinsuo Kang, Z121107005112014) and Plan of Excellent Talents in Beijing City (Yang Zhang, 2011B009008000003) support. We would like to thank Prof. Zhang Jian for his invaluable help in preparing the manuscript.
References
- 1.Fang J, Mensah GA, Croft JB, et al. Heart failure-related hospitalization in the U.S. 1979 to 2004. J Am Coll Cardiol. 2008;52:428–434. doi: 10.1016/j.jacc.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 2.Loehr LR, Rosamond WD, Chang PP, et al. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 3.Gu DF, Huang GY, He J. Investigation of prevalence and distributing feature of chronic heart failure in Chinese adult population. Chin J Cardiol. 2003;31:3–6. [Google Scholar]
- 4.Simel DL, DeLong ER, Feussner JR, et al. Erythrocyte anisocytosis. Visual inspection of blood films vs. automated analysis of red blood cell distribution width. Arch Intern Med. 1988;148:822–824. doi: 10.1001/archinte.148.4.822. [DOI] [PubMed] [Google Scholar]
- 5.Allen L A, Felker GM, Mehra MR, et al. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Cardiac Fail. 2010;16:230–238. doi: 10.1016/j.cardfail.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Najjar YA, Goode KM, Zhang J, et al. Red cell distribution width: an inexpensive and powerful prognostic marker in heart failure. Eur J Heart Fail. 2009;11:1155–1162. doi: 10.1093/eurjhf/hfp147. [DOI] [PubMed] [Google Scholar]
- 7.Forhécz Z, Combos T, Borgulya G, et al. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. 2009;158:659–666. doi: 10.1016/j.ahj.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the task force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur J Heart Fail. 2008;10:933–989. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Felker GM, Allen LA, Pocuck SJ, et al. Red cell distribution width as a novel prognostic market in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol. 2007;50:40–47. doi: 10.1016/j.jacc.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 10.Pascual-Figal DA, Bonaque JC, Redondo B, et al. Red blood cell distribution width predicts long-term outcome regardless of anaemia status in acute heart failure patients. Eur J Heart Fail. 2009;11:840–846. doi: 10.1093/eurjhf/hfp109. [DOI] [PubMed] [Google Scholar]
- 11.Jaewon Oh, Kang SM, Hong Nk, et al. Relation between red cell distribution width with echocardiographic parameters in patients with acute heart failure. J Cardiac Fail. 2009;15:517–522. doi: 10.1016/j.cardfail.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Van Kimmenade RR, Mohammed AA, Uthamalingam S, et al. Red blood cell distribution width and 1-year mortality in acute heart failure. Eur J Heart Fail. 2010;12:129–136. doi: 10.1093/eurjhf/hfp179. [DOI] [PubMed] [Google Scholar]
- 13.Dabbah S, Hammerman H, Markiewicz W, et al. Relation between red cell distribution width and clinical outcomes after acute myocardial infarction. Am J Cardiol. 2010;105:312–317. doi: 10.1016/j.amjcard.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 14.Azab B, Torbey E, Hatoum H, et al. Usefulness of red cell distribution width in predicting all-cause long-term mortality after non-ST-elevation myocardial infarction. Cardiology. 2011;119:72–80. doi: 10.1159/000329920. [DOI] [PubMed] [Google Scholar]
- 15.Lappé JM, Horne BD, Shah SH, et al. Red cell distribution width, C-reactive protein, the complete blood count, and mortality in patients with coronary disease and a normal comparison population. Clin Chim Acta. 2011;412:2094–2099. doi: 10.1016/j.cca.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Tonelli M, Sacks F, Arnold M, et al. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation. 2008;117:163–168. doi: 10.1161/CIRCULATIONAHA.107.727545. [DOI] [PubMed] [Google Scholar]
- 17.Nabais S, Losa N, Gaspar A, et al. Association between red blood cell distribution width and outcomes at six months in patients with acute coronary syndromes. Rev Port Cardiol. 2009;28:905–924. [PubMed] [Google Scholar]
- 18.Lippi G, Giuseppe L, Filippozzi M, et al. Clinical usefulness of measuring red blood cell distribution width on admission in patients with acute coronary syndromes. Clin Chem Lab Med. 2009;47:353–357. doi: 10.1515/cclm.2009.066. [DOI] [PubMed] [Google Scholar]
- 19.Ani C, Ovbiagele B. Elevated red blood cell distribution width predicts mortality in persons with known stroke. J Neurol Sci. 2009;277:103–108. doi: 10.1016/j.jns.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 20.Biermann J, Neumann T, Angermann CE, et al. Economic burden of patients with various etiologies of chronic systolic heart failure analyzed by resource use and costs. Int J Cardiol. 2012;156:323–325. doi: 10.1016/j.ijcard.2012.01.099. [DOI] [PubMed] [Google Scholar]
- 21.Tang WH, Francis GS, Morrow DA, et al. National Academy of Clinical Biochemistry Laboratory Medicine practice guidelines: clinical utilization of cardiac biomarker testing in heart failure. Circulation. 2007;116:e99–e109. doi: 10.1161/CIRCULATIONAHA.107.185267. [DOI] [PubMed] [Google Scholar]
- 22.Heart Failure Society Of America Evaluation and management of patients with acute decompensated heart failure. J Card Fail. 2006;12:e86–e103. doi: 10.1016/j.cardfail.2005.11.017. [DOI] [PubMed] [Google Scholar]