Abstract
Study Objectives:
Restless legs syndrome (RLS) prevalence estimates range from 0% to 52% in Parkinson disease (PD), but the causal relationship between the two disorders is still debated. The present study aims to evaluate RLS prevalence in de novo PD subjects, its incidence during the first 4 years from diagnosis, and possible relationships with clinical, laboratory, and neuroradiological data.
Methods:
One hundred nine newly diagnosed, drug-naïve PD subjects were evaluated at the time of PD diagnosis, and after 2- and 4-years. RLS diagnosis was performed with the RLS Diagnostic Index at each visit. Motor features, additional non-motor symptoms (NMS), and concomitant dopaminergic and nondopaminergic treatments were also gathered. Moreover, at baseline, 65 subjects were randomly selected to undergo a FP-CIT SPECT to study dopamine transporter availability.
Results:
RLS prevalence rose from 4.6% at baseline evaluation to 6.5% after 2 years and to 16.3% after 4 years (P = 0.007). A multinomial logistic stepwise regression model selected NMS Questionnaire items more likely to be associated with RLS at diagnosis (insomnia, OR = 15.555; P = 0.040) and with occurrence of RLS during follow-up (dizziness, OR = 1.153; P = 0.022; and daytime sleepiness; OR = 9.557; P = 0.001), as compared to patients without RLS. Older age was more likely associated to increased RLS occurrence during follow-up in a random effect logistic regression model (OR = 1.187; P = 0.036). A multinomial logistic stepwise model found increased dopaminergic transporter availability of affected caudate and putamen to be more likely associated with RLS presence at diagnosis (n = 5; OR = 75.711; P = 0.077), and RLS occurrence during follow-up (n = 16; OR = 12.004; P = 0.059), respectively, as compared to patients without RLS (n = 88).
Conclusions:
RLS is present since PD diagnosis, and increases in prevalence during the course of PD. PD subjects with RLS have higher age at PD onset, more preserved dopaminergic pathways, and worse sleep and cardiovascular disturbances.
Citation:
Moccia M, Erro R, Picillo M, Santangelo G, Spina E, Allocca R, Longo K, Amboni M, Palladino R, Assante R, Pappatà S, Pellecchia MT, Barone P, Vitale C. A four-year longitudinal study on restless legs syndrome in Parkinson disease. SLEEP 2016;39(2):405–412.
Keywords: Parkinson, restless, RLS, sleep, progression, DAT, SPECT
Significance.
Prevalence of RLS at the time of PD diagnosis is similar to estimates in the general population. However, it significantly increases during the first 4 years from diagnosis, being likely secondary to PD progression. PD patients with RLS have a better dopaminergic transmission, as measured by FP-CIT SPECT, than patients without RLS, suggesting that other neurotransmitters might be implicated. In line with this proposition, there is the significant co-occurrence of RLS and other non-motor symptoms such as sleep disturbances and autonomic dysfunction, which are mostly claimed to be nondopaminergic. This would also explain the partial response of RLS to dopaminergic treatments.
INTRODUCTION
Restless legs syndrome (RLS) is characterized by an urge to move the legs and unpleasant sensations, occurring at rest, with a circadian pattern, and diminishing during motor activity.1 RLS can be idiopathic, or associated to different conditions, such as iron deficiency, neuropathy, renal failure, or Parkinson disease (PD).1,2 The latter is of great interest since RLS and PD have different pathogenesis and evolution, but both are treated with dopaminergic drugs.3
In particular, RLS has a prevalence ranging from 0% to 52% in PD,2,4–11 and has been reported to occur either before or after the onset of PD. Although RLS has been rarely reported before the diagnosis of PD,7,9,10 it has been suggested to be part of the premotor phenotype in individuals at risk for developing PD,12 possibly predicting an older age at PD onset and a benign motor progression.13
On the other hand, over 75% of PD subjects with RLS develop RLS symptoms after PD diagnosis and treatment,14–16 particularly within the first 5 years of PD onset.7 PD subjects with RLS differ clinically from idiopathic RLS patients, in the way that they are older at onset, have lower rates of positive family history, and milder symptoms.8,17
Overall, it remains unclear whether RLS in PD is one of its early symptoms, a late complication, or just reflects an association by chance. This knowledge gap partly relies that evidence come from cross-sectional studies. There are in fact no longitudinal studies on RLS prevalence during the course of PD. Therefore, the present study aims to investigate: (1) the prevalence of RLS in a population of newly diagnosed, drug-naïve PD subjects; (2) the incidence of RLS in the same population during a prospective 4-year follow-up; and (3) laboratory, clinical, and neuroradiological correlates of RLS in PD.
METHODS
Study Design and Population
This is an observational longitudinal study evaluating RLS prevalence in a population of newly diagnosed, drug-naïve PD subjects, and RLS incidence during 4-year disease course. PD subjects have been consecutively enrolled in the Movement Disorder Unit, at the Federico II University Hospital (Naples, Italy), between January 2008 and June 2009 (with the last 4-year follow-up visit occurring in June 2013). The local Ethics Committee approved the study and all subjects gave informed consent. The study was performed in accordance with good clinical practices and the Declaration of Helsinki.
Inclusion criteria were: presence of Parkinson disease according to the United Kingdom Parkinson's Disease Society Brain Bank Diagnostic Criteria18,19; reported symptom duration < 24 months; no previous or current treatment with dopaminergic drugs; and lack of significant cerebral lesions on MRI or CT. Exclusion criteria were: diagnosis of secondary, familial, or atypical parkinsonism, according to current diagnostic criteria.19–23 Patients with clinical signs suggestive of an alternative diagnosis at 2- and 4-year follow-up evaluations were excluded.
Clinical Evaluation
At baseline evaluation, demographic features, concomitant diseases and treatments, and disease duration (expressed in months from the reported motor onset) were recorded. Motor disability was evaluated with the Unified Parkinson's Disease Rating Scale (UPDRS) part III. All subjects completed the non-motor symptom (NMS) Questionnaire (NMSQ), a validated tool for detection of NMS. The NMSQ consists of 30 questions with dichotomous (yes/no) answers, and of a total score, which ranges between 0 and 30, with higher scores reflecting more NMS.24,25
Due to the observational nature of the study, after enrollment and baseline evaluation, dopaminergic and non-dopaminergic treatments (i.e., antidepressants) were started depending on the supervising physician. Generally, patients were initially treated with either a dopamine agonist (DA) or a monoamine oxidase-B inhibitor (MAOB-I), while L-dopa was proposed for subjects developing a more severe motor disability. Visits were performed according to clinical practice with 3–5 months intervals.
Study visits were performed after 24 ± 3 and 48 ± 3 months. At each visit, concomitant diseases and treatments were investigated. Dopaminergic treatment was recorded and levodopa-equivalent daily dose (LEDD) was calculated for each drug class (i.e., DA, MAOB-I). A total LEDD was calculated from the sum of drug class LEDDs and eventually adjusted for the treatment with catechol-O-methyltransferase inhibitor (COMT-I).26 Time occurring from PD diagnosis to L-dopa treatment was recorded (time to L-dopa). UPDRS part III was evaluated during practically defined off-state (12 hours off drugs) and also during the on-state, and NMS were checked by means of the NMSQ, as at baseline. The presence of motor complications was investigated with the UPDRS part IV.
RLS Diagnosis
All subjects were evaluated for RLS at baseline visit, and at 2-and 4-year follow-ups. RLS diagnosis was performed with the RLS Diagnostic Index (RLS-DI), a recommended diagnostic instrument for RLS diagnosis in a clinical setting.27 In particular, the RLS-DI consists of 10 items, including essential criteria from the International RLS Study Group (IRLSSG),1 together with supportive features (sleep disturbances, family history, and treatment response).28 The severity of RLS in the previous week was measured with the IRLSSG rating scale (IRLSSG-RS),29 consisting of 10 questions, graded into 5 severity categories from 0 to 4 (maximum total score of 40). Generally, an IRLSSG-RS between 1 and 10 is considered mild, between 11 and 20 moderate, between 21 and 30 severe, and between 31 and 40 very severe RLS.29
In order to exclude possible causes of secondary RLS, all subjects underwent complete neurological examination. In particular, the presence of peripheral neuropathy was excluded according to consensus recommendations for case definition in epidemiological studies.30,31 All subjects were screened for neuropathic symptoms (numbness, altered sensation, or pain in the feet), ankle reflexes, distal sensation (pin sensation and vibration), distal muscle weakness, and atrophy, were accordingly categorized for the likelihood of having peripheral neuropathy, and eventually addressed to electrodiagnostic studies to further confirm the diagnosis.30,31 Moreover, PD subjects were investigated for all possible RLS “mimics” (i.e., hypnic jerks, habitual foot tapping, leg shaking, general nervous movements, arthritis).32
Serum iron, ferritin, hemoglobin, and creatinine were determined with COBAS c501 Analyzer (Roche Diagnostic, Mannheim, Germany) at baseline visit for the entire population, and at 2- and 4-year follow-up evaluations for PD subjects developing RLS.
At baseline visit, concomitant diseases and treatments were recorded in order to calculate the comorbidity index, as suggested in different studies on general and RLS populations.33,34 In particular, conditions previously associated with RLS or with severe impact on health (diabetes, hypertension, myocardial infarction, obesity, stroke, cancer, renal disease, anemia, depression, thyroid disease, and migraine) were considered and weighted equally, determining the following categories of the comorbidity index: none, 1, 2, and 3 or more conditions.34
For statistical purposes the PD population was categorized in relation to presence or absence of RLS during the study period. Furthermore, PD subjects with RLS were also categorized in prevalent (prRLS) if RLS was diagnosed at baseline evaluation simultaneously with PD, or incident (inRLS) if RLS occurred during the follow-up visits.
SPECT Imaging
At baseline visit, all subjects were asked to undergo an iodine-123 fluoropropyl-carbomethoxy-3 β-(4-iodophenyltropane) single-photon emission computed tomography (FP-CIT SPECT) (DaTSCAN, GE Healthcare, Little Chalfont, Buckingham-shire, UK) to study dopamine transporter (DAT) availability. Full methods have been reported elsewhere.35,36 The outcome measure was the specific-to-non-displaceable binding ratio, V3” (ROIstriatum − ROIoccipital / ROIoccipital). V3” values were calculated for the caudate and the putamen contralateral (affected) and ipsilateral (unaffected) to the most clinically affected body side, and were used for statistical analysis.
Statistical Analyses
Means and proportions of demographics (age, gender), laboratory findings (iron, ferritin, hemoglobin, creatinine), comorbidities (categories of the comorbidity index), clinical features (UPDRS part III and IV, NMSQ), treatments (LEDD, dopaminergic and non-dopaminergic drug use), and SPECT imaging (V3” values for affected and unaffected caudate and putamen) were calculated for PD subjects without RLS, with prRLS and with inRLS. Then, different subgroups were compared for study measures with χ2 test, Fisher exact test, or analysis of variance (ANOVA), as appropriate. Incidence rates at the second and fourth year were calculated; the cumulative incidence was calculated as the percentage of all new cases occurred in our study population during the follow-up time.
Multinomial logistic regression analyses were performed to evaluate the likelihood of having PD subjects with prRLS or with inRLS in relation to baseline demographics, comorbidities, clinical features, and SPECT imaging, as compared to PD subjects without RLS. Models were subsequently adjusted for age and gender. Odds ratio (OR) and 95% confidence intervals (95% CI) were calculated. Considering the noticeable amount of variables in both NMSQ and SPECT, a parsimonious approach to identify possible correlates of RLS was applied using 2 different multinomial logistic regression stepwise models, with backward selection for P = 0.20 as the critical value for entering variables in the model, while RLS status was the dependent variable.
Finally, a random effect logistic regression model was used to estimate possible relationships of clinical features and treatments, with inRLS occurrence over time.
Statistical and graphical methods have been applied to test normal distribution of variables and residuals, when appropriate. Stata 12.0 and Microsoft Excel have been used for data processing and analysis. Results are considered statistically significant if P < 0.05.
RESULTS
Study Population and RLS Prevalence
The present study included 109 newly diagnosed, drug-naïve PD subjects, whose demographics are reported in Table 1. During the study period, 10 subjects were lost to follow-up (9.1%): 1 subject after 2 years, and an additional 9 subjects after 4 years (5 subjects moved to a different Clinic, 4 withdrew their consent to the study, 1 died from colon cancer). Their demographics and clinical features at baseline were not different from the remaining population (data not shown).
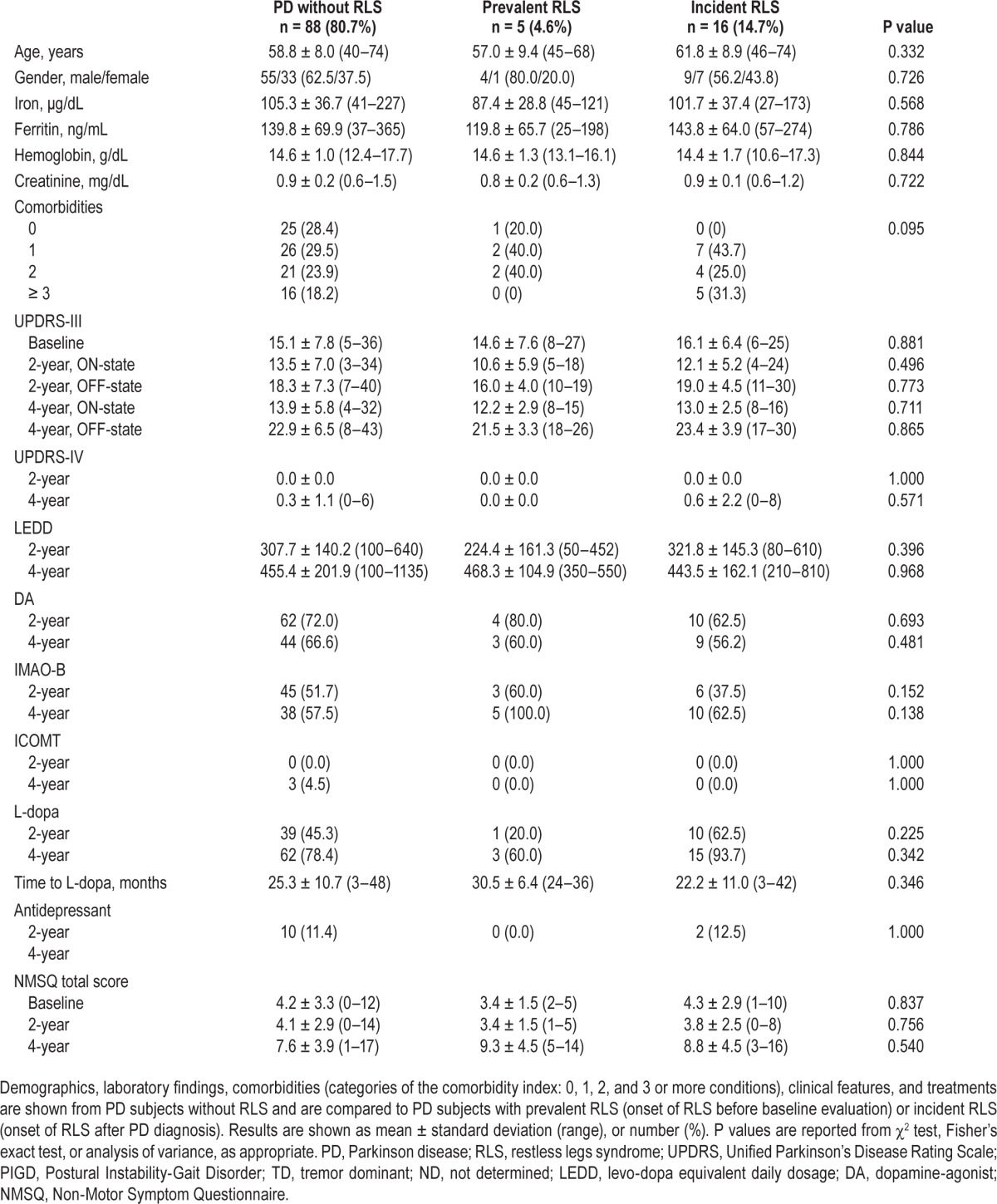
Table 1.
Demographics and clinical features of PD population.
RLS prevalence rose from 4.6% (n = 5) at baseline evaluation to 6.5% after 2 years (n = 7), and to 16.3% after 4 years (n = 16) (P = 0.007) (Figure 1). Incidence rate at the second year was 5.7% (95% CI = 2.6–12.8), while the incidence rate at the fourth year was 10.2% (95% CI = 5.5–19.0); cumulative incidence was 6.8% (95% CI = 4.4–10.4). RLS severity was not different among the baseline evaluation, 2-year and 4-year follow-up visits (Figure 1). In the whole RLS population, only 2 subjects (9.5%) with prRLS reported family history for RLS (first-degree relatives) (supplemental material).
Figure 1.
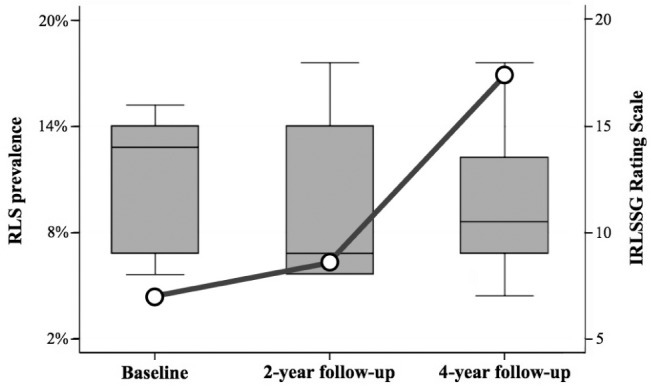
RLS prevalence and severity. Profile plot shows increasing prevalence of Restless Legs Syndrome (RLS) from the time of Parkinson's disease diagnosis (baseline evaluation), to 2-year and 4-year follow-up visits (P = 0.007). Box and Whisker plot shows similar RLS severity evaluated by the International RLS Study Group (IRLSSG) Rating Scale, at baseline evaluation (12.4 ± 3.6/40, ranging from 8 to 16), after 2-year (11.1 ± 3.8/40, ranging from 8 to 18), and 4-year follow-up visits (11.3 ± 3.2/40, ranging from 7 to 18) (F[2,20] = 1.88; P = 0.245).
Demographics, clinical features, laboratory findings, and treatments of RLS subjects are reported in the supplemental material.
RLS and PD Clinical Features
PD subjects without RLS, with prRLS and with inRLS were not different in terms of any demographics, laboratory findings, motor symptoms, treatments, and NMS (Table 1). The likelihood of PD patients having prRLS or inRLS, as compared to patients without RLS, was not associated with baseline demographics, laboratory findings, motor symptoms, and NMS (Table 2). The likelihood of PD patients having prRLS was not associated with comorbidities, whereas a positive but not significant OR was found for inRLS, as compared to patients without RLS (Table 2).
Table 2.
Baseline clinical features and likelihood of RLS.
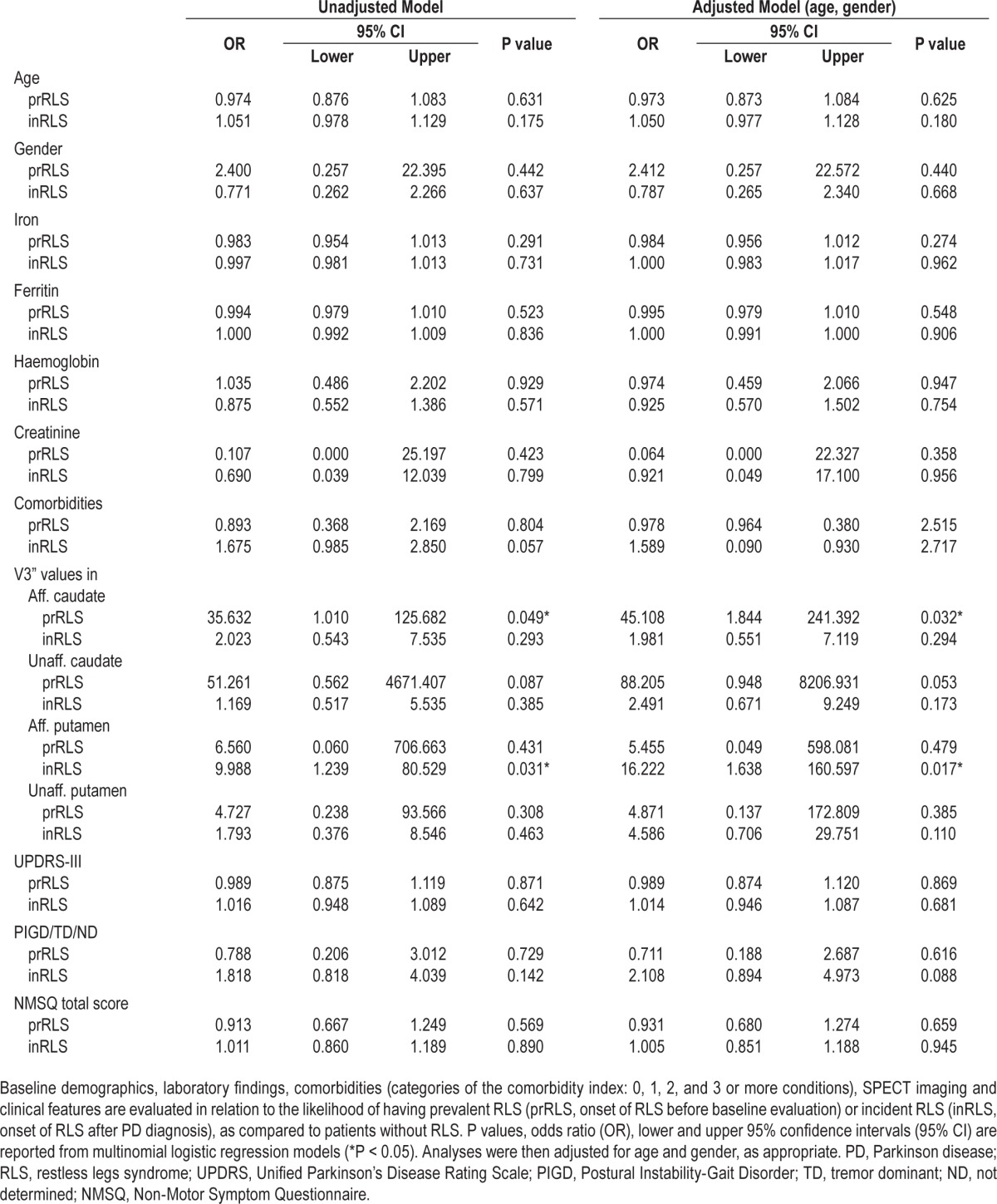
A multinomial logistic stepwise regression model selected NMSQ items more likely to be associated with prRLS: anxiety (OR = 0.056; 95% CI = 0.002–1.079; P = 0.056), dizziness (OR = 9.256; 95% CI = 0.731–117.131; P = 0.086), and insomnia (OR = 15.555; 95% CI = 1.132–213.619; P = 0.040); and with inRLS: dizziness (OR = 1.153; 95% CI = 1.296–29.211; P = 0.022), and daytime sleepiness (OR = 9.557; 95% CI = 2.505–36.457; P = 0.001), as compared to patients without RLS.
Finally, demographics (age, gender), motor symptoms (UPDRS-III during on and off state, UPDRS-IV), treatments (DA, IMAO-B, ICOMT, L-dopa, time to L-dopa, antidepressant), and NMS (NMSQ total score and items) were included in a random effect logistic regression model in order to evaluate factors associated with the presence of RLS over time (inRLS). Among different data included in the model, only age was more likely associated to occurrence of inRLS during the study period (OR = 1.187; 95% CI = 1.011–1.393; P = 0.036).
RLS and SPECT Imaging
At baseline evaluation, 65 subjects agreed to perform the [123I] FP-CIT SPECT (4 PD subjects with prRLS, 14 with inRLS, and 47 without RLS).
Increased V3” values of affected caudate were more likely associated with prRLS (P = 0.049; and P = 0.032 after adjusting for age and gender), but not with inRLS occurrence, as compared to patients without RLS (Figure 2). No association was found between increased V3” values of unaffected caudate and prRLS, or inRLS, as compared to not having RLS (Figure 2). Increased V3” values of affected putamen were more likely associated with inRLS occurrence (P = 0.031; and P = 0.017 after adjusting for age and gender), but not with prRLS, as compared to patients without RLS (Figure 2). No association was found between increased V3” values of unaffected putamen and prRLS, or inRLS, as compared to patients without RLS (Figure 2).
Figure 2.
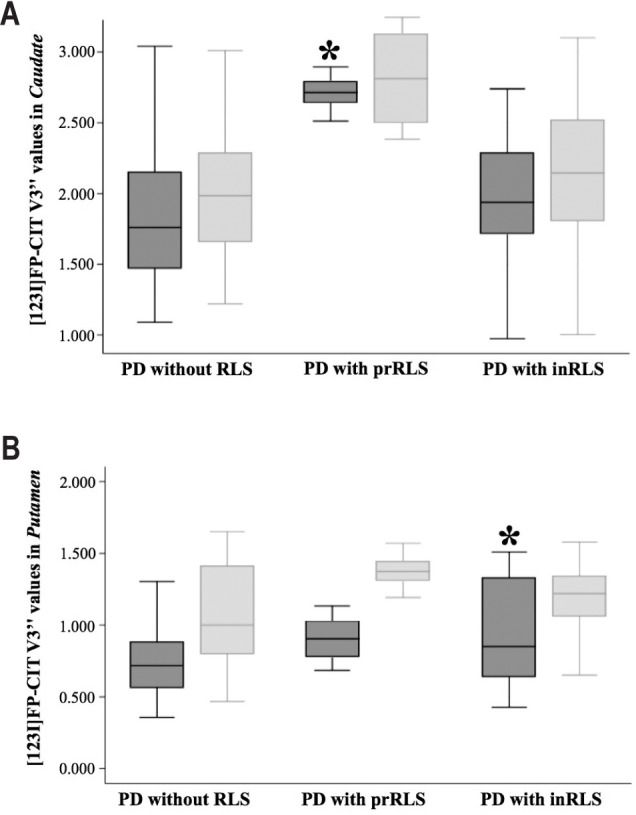
DAT availability and RLS in PD. Box and whisker plots show differences in dopamine transporter (DAT) availability between Parkinson disease (PD) subjects without restless legs syndrome (RLS), and developing RLS before baseline evaluation (prevalent RLS, prRLS), or after PD diagnosis (incident RLS, inRLS). A multinomial logistic stepwise model selected increased V3” values of affected caudate to be more likely associated with prRLS (OR = 75.711; 95% CI = 0.493– 1162.232; P = 0.077) (A), and increased V3” values of affected putamen with inRLS occurrence (OR = 12.004; 95% CI = 0.135–4.398; P = 0.059) (B), as compared to patients without RLS (affected caudate and putamen are shown in dark gray, unaffected caudate and putamen are shown in light gray).
Finally, a multinomial logistic stepwise model selected increased V3” values of affected caudate to be more likely associated with prRLS (P = 0.077), and increased V3” values of affected putamen with inRLS occurrence (P = 0.059), as compared to not having RLS (Figure 2).
DISCUSSION
The current study investigated RLS prevalence at the time of PD diagnosis, and RLS incidence during the course of PD with a longitudinal design. In the present de novo PD population, RLS prevalence at PD diagnosis seems not different from that previously described for idiopathic RLS in the general population,31,37 as already suggested in different studies on de novo PD patients.15,17 However, our longitudinal design showed that RLS prevalence progressively increased during the course of PD (from 4.6% to 16.3% at 4-year follow-up), reaching prevalence rates in line with previous cross-sectional investigations on PD populations.9,14 Interestingly, the incidence rate for RLS is apparently higher in the present de novo PD population (6.8%), as compared to previous studies on representative general populations where it accounts for 0.2% to 2.2% within similar age ranges, although a formal comparison is not possible due to differences in the study populations.38–40 It is also worth discussing that comorbidities previously associated with RLS occurrence in the general population34,38 only had a partial effect on RLS incidence in the current PD population. Therefore, RLS occurring during the course of PD can be considered secondary to PD evolution, and possibly presents pathophysiological correlates.
More in detail, the pathogenesis of PD and RLS seems to be intriguingly conflicting.41 In fact, previous pathological and neuroradiological studies associated idiopathic RLS with enhanced dopaminergic activity, whereas, in PD, nigrostriatal dopaminergic cells are noticeably reduced.3,41–46 However, few studies investigated neuroradiological correlates of RLS in PD, and generally found a trend of reduced hyperechogenicity in the substantia nigra at transcranial brain sonography in PD subjects with RLS, as compared to PD subjects without RLS, conversely presenting more impaired sonographic images.47,48 Thus, current findings of PD subjects with RLS presenting more preserved nigrostriatal dopaminergic pathways at FPCIT SPECT as compared to PD subjects without RLS, are in line with previous investigations, and possibly suggest the involvement of neurotransmitters different from dopamine.49
With a view to iron status, PD has been associated with iron accumulation in the brain, where it would exert different toxic effects and drive one of the main pathophysiological mechanisms in PD, whereas opposite findings were found in idiopathic RLS.3,42–44,50 In the attempt to reconcile such different views, we might speculate that presence of RLS in PD would identify a subset of patients with relatively less iron pathology and, in turn, with a better phenotype.13,48 Accordingly, in our population, PD patients with RLS had more preserved nigrostriatal dopaminergic pathways than those without RLS. The latter finding would also support a nonlinear relationship between dopaminergic dysfunction and presence of RLS, possibly suggesting a RLS generator outside of the nigrostriatal region. In particular, several studies have explored the diencephalospinal pathway, responsible for a variety of afferent and efferent mechanisms leading to RLS.51 Therefore, since this structure is rostral to basal ganglia, it is possibly involved only after the onset of typical motor symptoms of PD.52
Furthermore, there are additional results of the present study that need to be discussed and compared to previous investigations. In particular, no significant relationships were found between RLS and dopaminergic treatments or motor complications of PD, not supporting previous hypotheses of PD-related treatments and motor complications possibly determining restlessness.17 Moreover, RLS incidence appeared to be more likely associated with higher age of PD subjects, as previously suggested.13 In addition, RLS in PD seems to be mild to moderately severe, as previously reported,8 possibly also because of concomitant dopaminergic medications. It has to be discussed that due to the observational design of the present study, the treating physician decided on dopaminergic treatments and, thus, their potential benefit on RLS is difficult to evaluate. However, considering that the overall dopaminergic dose progressively increased during the course of PD, together with RLS prevalence, it is possible to hypothesize that RLS occurring in PD only partially responds to dopaminergic treatments.
With regard to the non-motor phenotype, the PD population was screened with the NMSQ, an effective tool for screening sleep disorders and, more in general, non-motor problems in PD in a clinical setting.24,25,53,54 PD subjects with RLS complained more frequently of autonomic disturbances with particular regard to cardiovascular ones (such as dizziness), and of sleep disturbances (in particular insomnia and daytime sleepiness), in accordance with previous studies.6,7,55–58 It is worth noting that the current non-motor profile might also suggest the presence of non-dopaminergic factors involved in the relationships between RLS and PD.6 In line with this, the possible involvement of different neurotransmitters in RLS occurring in PD might be responsible, at least in part, for the partial response to dopaminergic treatments.
Finally, there are some limitations of the present study to be acknowledged, such as the specificity of the diagnostic criteria of RLS in patients with PD, and similarly, the difficulty of rating RLS severity in patients with PD, which may interfere and confound RLS symptoms.32,59 Nonetheless, noticeable efforts have been made to avoid the risk of secondary forms of RLS, and of confounding factors, such as motor restlessness in PD, a motor phenomenon that increases with the progression of PD.8,14 An underestimation of RLS prevalence during follow-up visits is also possible because of concomitant treatments. In this regard, it is interesting to note that dopaminergic drugs were not found to be effective in patients with inRLS, possibly suggesting that were only identified more severe forms, thus leading to an underestimation of RLS in PD. Finally, the family history for RLS should have been investigated not only among RLS subjects, but in the whole population in order to have an estimate of the overall RLS predisposition in PD.
In conclusion, motor, non-motor and neuroimaging correlates of RLS in PD have been presented but need to be considered preliminary, whereas future larger studies also including a control group and additional investigations, such as polysomnography or sleep specific scales, are warranted to specifically investigate these issues.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. The work for this study was performed at the department of Neurosciences, Reproductive Sciences and Odontostomatology, Federico II University, Naples, Italy.
REFERENCES
- 1.Allen R, Picchietti D, Hening W, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 2.Gómez-Esteban JC, Zarranz JJ, Tijero B, et al. Restless legs syndrome in Parkinson's disease. Mov Disord. 2007;22:1912–6. doi: 10.1002/mds.21624. [DOI] [PubMed] [Google Scholar]
- 3.Connor JR, Wang XS, Allen RP, et al. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain. 2009;132:2403–12. doi: 10.1093/brain/awp125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peralta CM, Frauscher B, Seppi K, et al. Restless legs syndrome in Parkinson's disease. Mov Disord. 2009;24:2076–80. doi: 10.1002/mds.22694. [DOI] [PubMed] [Google Scholar]
- 5.Ondo WG, Vuong KD, Jankovic J. Exploring the relationship between Parkinson disease and restless legs syndrome. Arch Neurol. 2002;59:421–4. doi: 10.1001/archneur.59.3.421. [DOI] [PubMed] [Google Scholar]
- 6.Verbaan D, van Rooden SM, van Hilten JJ, Rijsman RM. Prevalence and clinical profile of restless legs syndrome in Parkinson's disease. Mov Disord. 2010;25:2142–7. doi: 10.1002/mds.23241. [DOI] [PubMed] [Google Scholar]
- 7.Nomura T, Inoue Y, Nakashima K. Clinical characteristics of Restless legs syndrome in patients with Parkinson's disease. J Neurol Sci. 2006;250:39–44. doi: 10.1016/j.jns.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 8.Rijsman RM, Schoolderman LF, Rundervoort RS, Louter M. Restless legs syndrome in Parkinson's disease. Parkinsonism Relat Disord. 2014;20:S5–9. doi: 10.1016/S1353-8020(13)70004-X. [DOI] [PubMed] [Google Scholar]
- 9.Calzetti S, Negrotti A, Bonavina G, Angelini M, Marchesi E. Absence of comorbidity of Parkinson disease and restless legs syndrome: a case-control study in patients attending a movement disorders clinic. Neurol Sci. 2009;30:119–22. doi: 10.1007/s10072-009-0037-7. [DOI] [PubMed] [Google Scholar]
- 10.Loo H-V, Tan E-K. Case-control study of restless legs syndrome and quality of sleep in Parkinson's disease. J Neurol Sci. 2008;266:145–9. doi: 10.1016/j.jns.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 11.Tan E, Lum S, Wong M. Restless legs syndrome in Parkinson's disease. J Neurol Sci. 2002;196:33–6. doi: 10.1016/s0022-510x(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 12.Wong JC, Li Y, Schwarzschild M a, Ascherio a, Gao X. Restless legs syndrome: an early clinical feature of Parkinson disease in men. Sleep. 2014;37:369–72. doi: 10.5665/sleep.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dragan EM, Chen Z, Ondo WG. Does idiopathic restless legs syndrome delay onset and reduce severity of Parkinson's disease: a pilot study. Int J Neurosci. 2015;125:526–30. doi: 10.3109/00207454.2014.987771. [DOI] [PubMed] [Google Scholar]
- 14.Gjerstad MD, Tysnes OB, Larsen JP. Increased risk of leg motor restlessness but not RLS in early Parkinson disease. Neurology. 2011;77:1941–6. doi: 10.1212/WNL.0b013e31823a0cc8. [DOI] [PubMed] [Google Scholar]
- 15.Angelini M, Negrotti A, Marchesi E, Bonavina G, Calzetti S. A study of the prevalence of restless legs syndrome in previously untreated Parkinson's disease patients: absence of co-morbid association. J Neurol Sci. 2011;310:286–8. doi: 10.1016/j.jns.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Bhalsing K, Suresh K, Muthane UB, Pal PK. Prevalence and profile of Restless Legs Syndrome in Parkinson's disease and other neurodegenerative disorders: a case-control study. Parkinsonism Relat Disord. 2013;19:426–30. doi: 10.1016/j.parkreldis.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Lee JE, Shin H-W, Kim KS, Sohn YH. Factors contributing to the development of restless legs syndrome in patients with Parkinson disease. Mov Disord. 2009;24:579–82. doi: 10.1002/mds.22410. [DOI] [PubMed] [Google Scholar]
- 18.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berardelli A, Wenning GK, Antonini A, et al. EFNS/MDS-ES recommendations for the diagnosis of Parkinson's disease. Eur J Neurol. 2013;20:16–34. doi: 10.1111/ene.12022. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Reich SG. Criteria for the diagnosis of corticobasal degeneration criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80:496–503. doi: 10.1212/WNL.0b013e31827f0fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilman S, Wenning G, Low P, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–6. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson's disease dementia: recommendations from the Movement Disorder Society Task Force. Mov Disord. 2007;22:2314–24. doi: 10.1002/mds.21844. [DOI] [PubMed] [Google Scholar]
- 23.McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–24. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 24.Chaudhuri KR, Martinez-Martin P. Quantitation of non-motor symptoms in Parkinson's disease. Eur J Neurol. 2008;15:2–7. doi: 10.1111/j.1468-1331.2008.02212.x. [DOI] [PubMed] [Google Scholar]
- 25.Chaudhuri KR, Martinez-Martin P, Schapira AH, et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson's disease: the NMSQuest study. Mov Disord. 2006;21:916–23. doi: 10.1002/mds.20844. [DOI] [PubMed] [Google Scholar]
- 26.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649–53. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 27.Walters A, Frauscher B, Allen R, et al. Review of Diagnostic Instruments for the Restless Legs Syndrome/Willis-Ekbom Disease (RLS/WED): critique and recommendations. J Clin Sleep Med. 2014;10:1343–9. doi: 10.5664/jcsm.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beneš H, Kohnen R. Validation of an algorithm for the diagnosis of restless legs syndrome: the Restless Legs Syndrome-Diagnostic Index (RLS-DI) Sleep Med. 2009;10:515–23. doi: 10.1016/j.sleep.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–32. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 30.England JD, Gronseth GS, Franklin G, et al. Distal symmetrical polyneuropathy: definition for clinical research. Muscle Nerve. 2005;31:113–23. doi: 10.1002/mus.20233. [DOI] [PubMed] [Google Scholar]
- 31.Moccia M, Pellecchia MT, Erro R, et al. Restless legs syndrome is a common feature of adult celiac disease. Mov Disord. 2010;25:877–81. doi: 10.1002/mds.22903. [DOI] [PubMed] [Google Scholar]
- 32.Hening WA, Allen RP, Washburn M, Lesage SR, Earley CJ. The four diagnostic criteria for restless legs syndrome are unable to exclude confounding conditions (“mimics”) Sleep Med. 2009;10:976–81. doi: 10.1016/j.sleep.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diederichs CP, Wellmann J, Bartels DB, Ellert U, Hoffmann W, Berger K. How to weight chronic diseases in multimorbidity indices? Development of a new method on the basis of individual data from five population-based studies. J Clin Epidemiol. 2012;65:679–85. doi: 10.1016/j.jclinepi.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Szentkirályi A, Völzke H, Hoffmann W. Multimorbidity and the risk of restless legs syndrome in 2 prospective cohort studies. Neurology. 2014;82:2026–33. doi: 10.1212/WNL.0000000000000470. [DOI] [PubMed] [Google Scholar]
- 35.Moccia M, Pappatà S, Erro R, et al. Uric acid relates to dopamine transporter availability in Parkinson's disease. Acta Neurol Scand. 2015;123:127–31. doi: 10.1111/ane.12295. [DOI] [PubMed] [Google Scholar]
- 36.Moccia M, Pappatà S, Picillo M, et al. Dopamine transporter availability in motor subtypes of de novo drug-naïve Parkinson's disease. J Neurol. 2014;261:2112–8. doi: 10.1007/s00415-014-7459-8. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Borreguero D, Egatz R, Winkelmann J, Berger K. Epidemiology of restless legs syndrome: the current status. Sleep Med Rev. 2006;10:153–67. doi: 10.1016/j.smrv.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 38.De Vito K, Li Y, Batool-Anwar S, Ning Y, Han J, Gao X. Prospective study of obesity, hypertension, high cholesterol, and risk of restless legs syndrome. Mov Disord. 2014;29:1044–52. doi: 10.1002/mds.25860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Budhiraja P, Budhiraja R, Goodwin JL, et al. Incidence of restless legs syndrome and its correlates. J Clin Sleep Med. 2012;8:119–24. doi: 10.5664/jcsm.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szentkiralyi A, Fendrich K, Hoffmann W, Happe S, Berger K. Incidence of restless legs syndrome in two population-based cohort studies in Germany. Sleep Med. 2011;12:815–20. doi: 10.1016/j.sleep.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 41.Slow EJ, Postuma RB, Lang AE. Implications of nocturnal symptoms towards the early diagnosis of Parkinson's disease. J Neural Transm. 2014;121:49–57. doi: 10.1007/s00702-014-1168-4. [DOI] [PubMed] [Google Scholar]
- 42.Connor JR, Boyer PJ, Menzies SL, et al. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology. 2003;61:304–9. doi: 10.1212/01.wnl.0000078887.16593.12. [DOI] [PubMed] [Google Scholar]
- 43.Earley CJ, Barker PB, Horská A, Allen RP. MRI-determined regional brain iron concentrations in early- and late-onset restless legs syndrome. Sleep Med. 2006;7:458–61. doi: 10.1016/j.sleep.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 44.Friedman A, Galazka-Friedman J, Koziorowski D. Iron as a cause of Parkinson disease - a myth or a well established hypothesis? Parkinsonism Relat Disord. 2009;15:S212–4. doi: 10.1016/S1353-8020(09)70817-X. [DOI] [PubMed] [Google Scholar]
- 45.Godau J, Sojer M. Transcranial sonography in restless legs syndrome. Int Rev Neurobiol. 2010;90:199–215. doi: 10.1016/S0074-7742(10)90015-9. [DOI] [PubMed] [Google Scholar]
- 46.Schmidauer C, Sojer M, Seppi K, et al. Transcranial ultrasound shows nigral hypoechogenicity in restless legs syndrome. Ann Neurol. 2005;58:630–4. doi: 10.1002/ana.20572. [DOI] [PubMed] [Google Scholar]
- 47.Kwon DY, Seo WK, Yoon HK, Park MH, Koh SB, Park KW. Transcranial brain sonography in Parkinson's disease with restless legs syndrome. Mov Disord. 2010;25:1373–8. doi: 10.1002/mds.23066. [DOI] [PubMed] [Google Scholar]
- 48.Pedroso JL, Bor-Seng-Shu E, Felicio AC, et al. Severity of restless legs syndrome is inversely correlated with echogenicity of the substantia nigra in different neurodegenerative movement disorders. A preliminary observation. J Neurol Sci. 2012;319:59–62. doi: 10.1016/j.jns.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 49.Rye DB. Parkinson's disease and RLS: the dopaminergic bridge. Sleep Med. 2004;5:317–28. doi: 10.1016/j.sleep.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 50.Allen RP, Barker PB, Wehrl F, Song HK, Earley CJ. MRI measurement of brain iron in patients with restless legs syndrome. Neurology. 2001;56:263–5. doi: 10.1212/wnl.56.2.263. [DOI] [PubMed] [Google Scholar]
- 51.Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009;32:589–97. doi: 10.1093/sleep/32.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hawkes CH, Del Tredici K, Braak H. A timeline for Parkinson's disease. Parkinsonism Relat Disord. 2010;16:79–84. doi: 10.1016/j.parkreldis.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Rios Romenets S, Wolfson C, Galatas C, et al. Validation of the non-motor symptoms questionnaire (NMS-Quest) Parkinsonism Relat Disord. 2012;18:54–8. doi: 10.1016/j.parkreldis.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 54.Perez Lloret S, Rossi M, Cardinali DP, Merello M. Validation of the sleep related items of the Non-motor Symptoms Questionnaire for Parkinson's disease (NMSQuest) Parkinsonism Relat Disord. 2008;14:641–5. doi: 10.1016/j.parkreldis.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 55.Shneyder N, Adler CH, Hentz JG, et al. Autonomic complaints in patients with restless legs syndrome. Sleep Med. 2013;14:1413–6. doi: 10.1016/j.sleep.2013.08.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fereshtehnejad S, Shafieesabet M, Shahidi G, Delbari A, Lökk J. Restless legs syndrome in patients with Parkinson's disease: a comparative study on prevalence, clinical characteristics, quality of life and nutritional status. Acta Neurol Scand. 2015;131:211–8. doi: 10.1111/ane.12307. [DOI] [PubMed] [Google Scholar]
- 57.Oh YS, Kim JS, Park IS, et al. Association between nocturnal/supine hypertension and restless legs syndrome in patients with Parkinson's disease. J Neurol Sci. 2014;344:186–9. doi: 10.1016/j.jns.2014.06.056. [DOI] [PubMed] [Google Scholar]
- 58.Neikrug AB, Maglione JE, Liu L, et al. Effects of sleep disorders on the non-motor symptoms of Parkinson disease. J Clin Sleep Med. 2013;9:1119–29. doi: 10.5664/jcsm.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Högl B, Gschliesser V. RLS assessment and sleep questionnaires in practice - lessons learned from Parkinson's disease. Sleep Med. 2007;8:7–12. doi: 10.1016/j.sleep.2007.04.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


