Abstract
Study Objectives:
Narcolepsy is caused by loss of the orexin (also known as hypocretin) neurons. In addition to the orexin peptides, these neurons release additional neurotransmitters, which may produce complex effects on sleep/wake behavior. Currently, it remains unknown whether the orexin neurons promote the initiation as well as the maintenance of wakefulness, and whether the orexin neurons influence initiation or maintenance of sleep. To determine the effects of the orexin neurons on the dynamics of sleep/wake behavior, we analyzed sleep/wake architecture in a novel mouse model of acute orexin neuron loss.
Methods:
We used survival analysis and other statistical methods to analyze sleep/wake architecture in orexin-tTA ; TetO diphtheria toxin A mice at different stages of orexin neuron degeneration.
Results:
Progressive loss of the orexin neurons dramatically reduced survival of long wake bouts, but it also improved survival of brief wake bouts. In addition, with loss of the orexin neurons, mice were more likely to wake during the first 30 sec of nonrapid eye movement sleep and then less likely to return to sleep during the first 60 sec of wakefulness.
Conclusions:
These findings help explain the sleepiness and fragmented sleep that are characteristic of narcolepsy. Orexin neuron loss impairs survival of long wake bouts resulting in poor maintenance of wakefulness, but this neuronal loss also fragments sleep by increasing the risk of awakening at the beginning of sleep and then reducing the likelihood of quickly returning to sleep.
Citation:
Branch AF, Navidi W, Tabuchi S, Terao A, Yamanaka A, Scammell TE, Diniz Behn C. Progressive loss of the orexin neurons reveals dual effects on wakefulness. SLEEP 2016;39(2):369–377.
Keywords: hypocretin, narcolepsy, orexin, sleep, survival analysis
Significance.
The loss of orexin neurons is associated with the sleep disorder narcolepsy. This study examined sleep/wake behavior in a novel rodent model of narcolepsy that mimics the progressive orexin cell loss that is thought to occur with this disease. With loss of orexin neurons, mice showed progressively worse maintenance of wakefulness. However, the effect varied for wake periods of different durations: maintenance of wake less than a few minutes was increased, and there was a higher probability of waking just after sleep onset. These findings suggest that the modulatory effect of orexin may be more complex than previously thought, and they may help to explain the fragmented sleep of patients with narcolepsy.
INTRODUCTION
The orexin neuropeptides (orexin-A and -B, also known as hypocretin-1 and -2) are involved in the promotion and consolidation of wakefulness, and selective loss of the orexin-producing neurons causes the chronic sleep disorder narcolepsy.1–3 Narcolepsy is characterized by fragmented sleep/wake behavior with excessive daytime sleepiness and disturbed nocturnal sleep; in addition, dysregulation of rapid eye movement (REM) sleep results in unusual symptoms such as cataplexy, a state in which the loss of muscle tone typically associated with REM sleep occurs during wakefulness.4 Similar disruption of both wake and sleep also occurs in animal models of narcolepsy, including dogs with a mutation in the orexin 2 receptor gene5 and mouse models in which the orexin neurons, receptors, or ligands are disrupted.6–10 Because disruption of the orexin system affects both wake and sleep states, a better understanding of the role of orexins in sleep/wake dynamics may lead to novel approaches for clinical interventions.
We previously identified key differences in the sleep/wake architecture of orexin knockout (OXKO) mice, a model of narcolepsy in which the orexin neurons are intact but the orexins are constitutively absent.11 Although the number of bouts of wake, nonrapid eye movement (NREM) sleep, and REM sleep was increased in OXKO mice compared to wild-type (WT) mice,12 only wake bouts displayed a clear change in the distribution of bout lengths.11 The altered structure of this distribution reflected a bout duration-dependence in the action of the orexins; for example, during the light period, OXKO mice had normal survival of wake bouts < 60 sec but poor maintenance of wake bouts of durations between 60–1000 sec or longer than > 1000 sec.
Although OXKO mice lack orexin signaling, the orexin neurons also release glutamate, dynorphin, and possibly other neurotransmitters.13–15 To more accurately model the loss of orexin neurons in human narcolepsy, Tabuchi and colleagues recently produced orexin-tTA; TetO diphtheria toxin A mice (referred to here simply as DTA mice) in which acute orexin neuron degeneration leads to signs of sleepiness and cataplexy.16 In these mice, discontinuation of doxycycline (DOX) induces expression of diphtheria toxin A selectively in the orexin neurons, thereby killing the orexin neurons over 3–4 w: 1 w after DOX removal, the number of orexin neurons was reduced to 14% of baseline, and by week 4, the number of orexin neurons was only 3% of baseline.16 In parallel with this loss of the orexin neurons, these mice show increasingly frequent transitions into sleep, less time awake in the dark period, and frequent episodes of cataplexy.16
These behaviors are consistent with a wake-promoting role for the orexin neurons, but it remains unclear just how the orexin neurons affect the dynamics of wakefulness and sleep. For example, optogenetic stimulation of locus coeruleus neurons produces immediate awakenings17; by contrast, opto-genetic stimulation of the orexin neurons increases the propensity of transitions to wakefulness but does not elicit immediate awakening,18 suggesting that the orexin neurons do not directly trigger awakenings from sleep. In fact, many important questions remain unanswered: Do the orexin neurons promote the initiation as well as the maintenance of wake bouts? Do the orexin neurons influence initiation or maintenance of NREM sleep?17,18 To determine the effects of orexin neurons on the dynamics of sleep/wake behavior, we analyzed sleep/wake architecture in DTA mice at different stages of orexin neuron degeneration.
METHODS
Animals
DTA mice were generated by breeding orexin-tTA mice, which express tTA exclusively in the orexin neurons under the control of the human prepro-orexin promoter19 with TetO diphtheria toxin A fragment mice (B6.Cg-Tg(tetO-DTA)1Gfi/J, The Jackson Laboratory, Sacramento, CA). Both orexin-tTA mice and TetO diphtheria toxin A mice were on a C57BL/6J genetic background. In double transgenic DTA mice, diphtheria toxin A expression occurs in the absence of DOX, thereby causing rapid orexin neuron degeneration, but in the presence of DOX, there is no loss of the orexin neurons.16
Mating pairs of orexin-tTA mice and TetO diphtheria toxin A mice were fed DOX-containing chow (DOX(+) chow) at a concentration of 100 mg/kg from the day of mating. DOX was supplied to pups via the maternal circulation or lactation during the prenatal and early postnatal periods, respectively. After weaning, DTA mice were fed DOX(+) chow until experiments were begun at approximately 12 w of age.16 The eight mice analyzed in this cohort were recorded in the laboratory of Dr. Akihiro Yamanaka and represent a subset of those presented by Tabuchi and colleagues with complete electroencephalography/electromyography (EEG/EMG) recordings at baseline (week 0) and over weeks 1–4 after switching from DOX(+) to DOX(−) chow.16 Four weeks after DOX removal, these mice had 27 ± 4 orexin neurons, representing only 3% of the baseline number of orexin neurons (891 ± 54, n = 5).16 These experiments were approved by the Institutional Animal Care and Use Committee of the Research Institute of Environmental Medicine at Nagoya University and were in accordance with National Institutes of Health guidelines.
To establish whether DOX removal was the primary factor in the changes in bout length after removal of DOX, we analyzed an additional control group of DTA mice that were maintained on DOX(+) chow throughout the 4-w recording period (n = 5) (Figure S3). This separate cohort was recorded in the laboratory of Dr. Akira Terao. These experiments were approved by the Animal Care and Use Committee of Hokkaido University and were in accordance with National Institutes of Health guidelines.
The surgery, recording, and scoring techniques were the same for both cohorts of mice. All efforts were made to minimize animal discomfort and the number of animals used.
Surgery and EEG/EMG Recordings
Surgeries and EEG/EMG recordings were performed as described previously.16 Briefly, male DTA mice (n = 8) were anesthetized with pentobarbital and implanted with EEG and EMG electrodes for polysomnogram recordings at age 10 w. A slip ring (Air Precision, Le Plessis-Robinson, France) was used to allow unrestricted movement of the mouse during EEG and EMG recordings. Mice were anesthetized with isoflurane, tethered to the recording cables, and acclimated to the recording cables for at least 7 d. Mice were housed individually in chambers with a 12 h/12 h light/dark (LD) cycle (light onset 08:00). They had unrestricted access to food and water, and they remained tethered throughout the weeks of the experiment. At age 12 w, DOX(+) chow was replaced with DOX(−) chow for the cohort of mice recorded at Nagoya University. Mice in the cohort at Hokkaido University were maintained on DOX(+) chow throughout the experiment. EEG and EMG recordings were obtained approximately weekly from age 12 w to 16 w (0–4 w after DOX removal in the Nagoya University cohort). Therefore, our analysis focuses on the changes in sleep/wake architecture in the Nagoya University cohort during weeks 1, 2, 3, and 4 DOX(−) compared to baseline data collected in the 3 d prior to DOX withdrawal. This corresponds to the period during which most orexin neurons are lost and the major features of the narcolepsy phenotype emerge.
EEG and EMG signals were amplified (AB-610J, Nihon Koden, Tokyo, Japan), filtered (EEG 1.5–30 Hz; EMG 15–300 Hz), and digitally recorded at a sampling rate of 128 Hz using SleepSign version 3 (Kissei Comtec, Matsumoto, Japan). In addition, we monitored behavior via infrared video recordings synchronized with the EEG and EMG recordings using the SleepSign video option system (Kissei Comtec, Matsumoto, Japan).
Scoring of Behavioral States
EEG/EMG recordings were semiautomatically scored in 4-sec epochs and classified as wakefulness, NREM sleep, or rapid eye movement (REM) sleep by SleepSign software according to standard criteria.20,21 Vigilance state classifications assigned by SleepSign were examined and visually corrected by a trained scorer who was blinded to genotype and experimental condition. For wake bouts, an additional scoring criterion requiring five consecutive epochs (20 sec) was imposed to avoid improperly scoring brief movements or artifact as wakefulness. We refer to wake bouts ≤ 60 sec as “brief.” Cataplexy was scored according to a consensus definition of cataplexy with four key criteria including an abrupt episode of EMG atonia lasting ≥ 10 sec.22 Therefore, the minimum duration for an episode of cataplexy was 12 sec. Each continuous string of epochs of wake, NREM sleep, REM sleep, or cataplexy were considered to be a bout. We analyzed the percentage of time spent in each behavioral state, the duration of bouts, and the number of bouts (means ± standard error of the mean). Because the distributions were highly skewed, we used the sign test to compare summary data reporting the number and mean durations of bouts. All data analysis was performed using built-in functions and custom scripts written for MATLAB (MathWorks, Natick, MA) and the statistical software R (http://www/R-project.org).
To compare the distributions of wake bouts at different stages of orexin neuron degeneration, we compared the proportions of subsets of wake bouts of various durations at baseline and week 4 DOX(−). We selected interval endpoints to approximately balance the proportions of bouts in the intervals considering baseline and week 4 DOX(−) bouts together. This choice enabled comparisons of bouts of different durations without a priori assumptions regarding relevant intervals. We tested for statistical significance in the number of bouts in each interval with the z-test for the difference between two proportions (means ± standard error of the mean).
Survival Analysis of Sleep/Wake Behavior
For wake, NREM sleep, and REM sleep, we calculated Kaplan-Meier survival curves for bout duration data at baseline and each week after discontinuation of DOX. To assess differences between survival curves, we used both weighted and unweighted versions of the log-rank test applied to all data (see Methods in supplemental material). In addition, to investigate in detail the possibility of a differential orexin neuron action on “short” and “long” time scales in more detail, we calculated separate survival curves for specific subsets of wake bout data. By considering subsets of the full distribution, we focused on survival of specific subsets and eliminated the possibility that differences in survival curves were biased by differences in maximal bout durations. To avoid imposing bias from the post hoc choice of a threshold dividing “short” and “long” wake bouts, we performed this analysis with thresholds ranging from 60 sec to 1000 sec.11 We also analyzed bouts between 64 and 1000 sec in order to compare to bouts of intermediate length as defined in previous work.11 We compared the survival of these subsets across weeks DOX(−) with the log-rank test. To further investigate differences in the survival of brief wake bouts at different stages of orexin neuron degeneration, we computed the proportion of bouts that failed of all bouts at risk for each scored interval less than 60 sec. We tested for statistical significance with the z-test for the difference between two proportions (means ± standard error of the mean). We also computed the empirical hazards for the survival curves for wake, NREM sleep, and REM sleep (see Methods in supplemental material).
RESULTS
Basic Sleep/Wake Architecture in DTA Mice
In DTA mice, progressive loss of the orexin neurons over 4 w altered sleep architecture in a pattern consistent with our previous report.16 Specifically, in the weeks after discontinuation of DOX, these mice spent less time in wake during the dark period, less time in REM sleep during the light period, and more time in cataplexy in both the dark and light periods. Mice gained 1–2 g across the recording period (data not shown). The reduction in wake during the dark period was due to a large decrease in the mean duration of wake bouts that was not fully offset by the increased number of wake bouts (Figures 1 and 2). In the light period, the mean duration of wake bouts did not change significantly. There was a trend toward an increase in the number of wake bouts during the light period, though this only reached significance at week 3. NREM sleep bouts were generally more frequent but shorter during the dark period, resulting in no overall change in the amount of NREM sleep. The reduction in REM sleep during the light period was due to a large decrease in the number of REM sleep bouts without any change in REM sleep bout duration. In the dark period, small increases in mean REM sleep bout duration did not significantly affect the total time in REM sleep. In addition, the number of cataplexy bouts increased, but the duration did not vary with loss of the orexin neurons (data not shown).
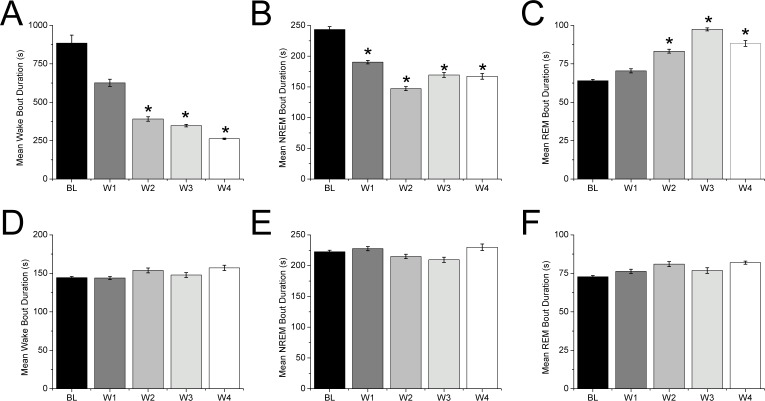
Figure 1.
Progressive loss of the orexin neurons in DTA mice after discontinuation of DOX disrupts wake and sleep bouts. (A) In the dark period, the mean duration of wake bouts gradually and dramatically shortens in the weeks after DOX is stopped (B,C) During this period, NREM sleep bouts shorten while the mean durations of REM sleep bouts increase. (D–F) In the light period, the mean durations of wake, NREM sleep, and REM sleep bouts are unchanged. (sign test, *P < 0.05 compared to baseline day). BL, baseline; W1, W2, W3, W4, weeks 1, 2, 3, 4 respectively following discontinuation of DOX.
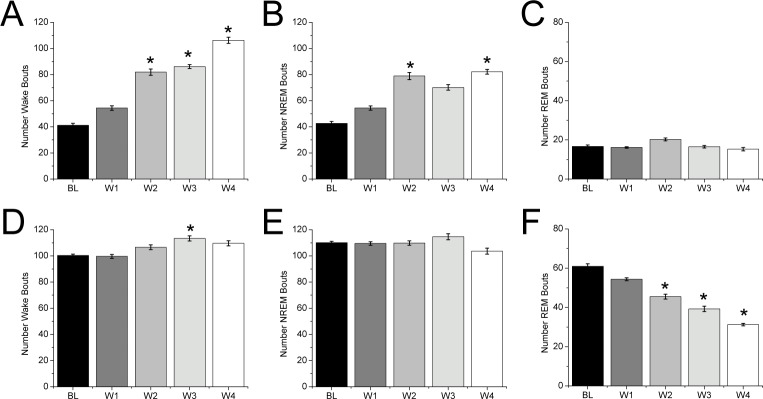
Figure 2.
Removal of DOX alters the number of wake and sleep bouts in the dark and light periods. (A) In the dark period, the number of wake bouts progressively increases in the weeks after discontinuation of DOX. (B,C) NREM sleep bouts become more numerous, but the number of REM sleep bouts is unchanged. (D) During the light period, the number of wake bouts increases, (E) the number of NREM bouts is unchanged, and (F) the number of REM sleep bouts decreases (sign test, *P < 0.05 compared to the baseline day). BL, baseline; W1, W2, W3, W4, weeks 1, 2, 3, 4 respectively following discontinuation of DOX.
Altered Distribution of Wake Bouts with Orexin Neuron Degeneration
To investigate how loss of the orexin neurons affects the maintenance of wake, we examined the distributions of wake bouts of specific durations. To control for the variable number of bouts in each condition, we normalized by the total number of wake bouts. The distribution of bout lengths in the dark period was clearly altered: brief wake bouts were reduced (difference significant for bouts < 36 sec and between 48 and 60 sec; z-test, P < 0.05); midlength bouts were increased (bouts between 64 and 1000 sec; z-test, P < 0.01); and very long wake bouts were nearly eliminated (> 2000 sec; z-test, P < 0.01) (Figure 3). During the light period, this general pattern persisted: mice had relatively fewer very short wake bouts, more intermediate bouts, and fewer very long bouts (all differences significant, z-test, P < 0.01).
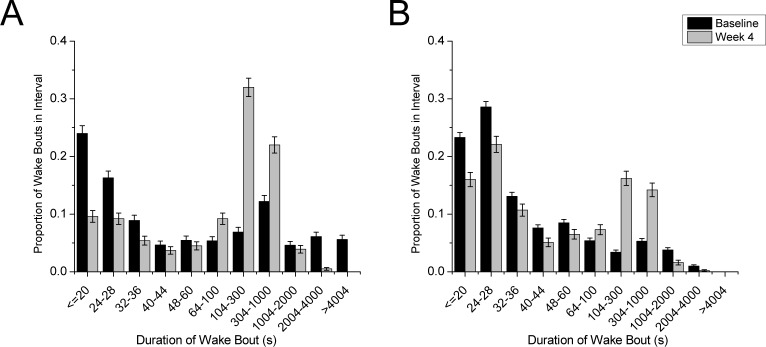
Figure 3.
The distribution of wake bout durations changes between baseline and week 4 after discontinuation of DOX. In the dark (A) and light (B) periods, the proportion of both short and long wake bouts decreases with DOX removal while the proportion of intermediate length bouts increases. In the dark period, differences are significant for all bins except 40–44 sec and 1004–2000 sec (z-test, P < 0.05). In the light period, all differences are significant (z-test, P < 0.01). At baseline, DTA mice averaged 40 wake bouts in the dark period and 100 wake bouts in the light period. At week 4, DTA mice averaged 106 wake bouts in the dark period and 110 wake bouts in the light period.
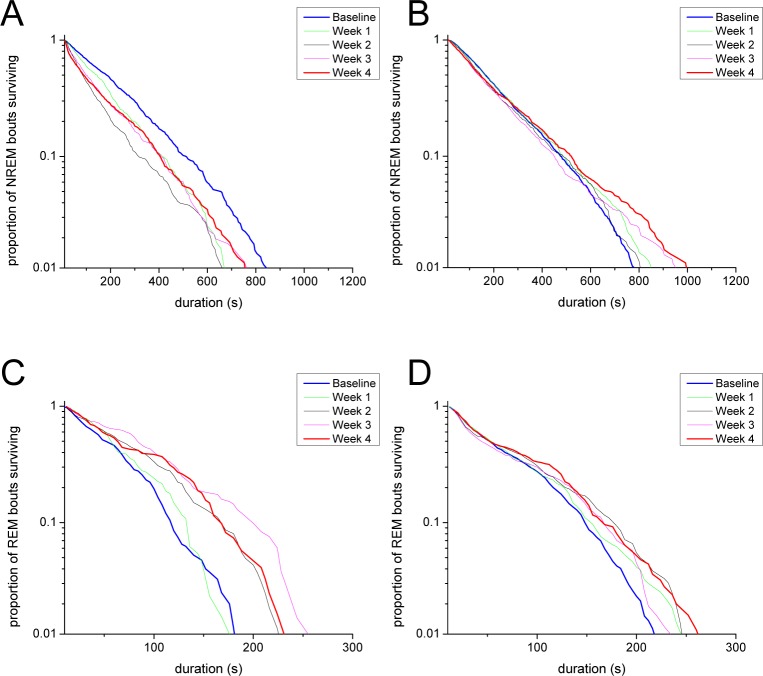
Survival Analysis of Wake Bouts
Because orexin neuron loss differentially affected long and short bouts of wake, we used Kaplan-Meier survival curves to analyze the survival of wake bouts after discontinuation of DOX. As orexin neuron loss progressed, wake bouts became shorter during the dark and light periods (Figure 4). By week 4, the maximum duration of wake bouts in the dark period decreased nearly an order of magnitude from ∼10,000 sec to less than 2,000 sec. To compare survival curves, we applied weighted log-rank tests that emphasize differences in survival of short or long bouts (weights W(t) = S(t) and W(t) = 1 − S(t), respectively, where S(t) is the survival function, see Methods in supplemental material). When weights were chosen to emphasize long durations, we found significant, duration-dependent changes in the distributions for all dark period conditions except week 1 (W(t) = 1 − S(t), P < 0.05). In the light period, changes were apparent for weeks 1–4 when shorter durations were emphasized (W(t) = S(t), P < 0.05) and for weeks 2 and 4 when longer durations were emphasized (W(t) = 1 − S(t), P < 0.05).
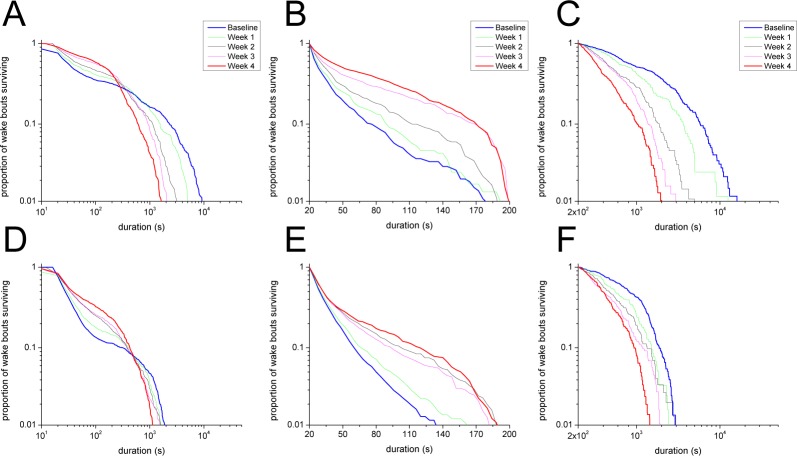
Figure 4.
Kaplan-Meier survival curves for wake bouts of different durations reveal decreased maintenance of wake in the weeks after DOX discontinuation. (A) In the dark period, DTA mice show a progressive inability to produce very long wake bouts, but survival of short wake bouts increases (weighted log-rank test, P < 0.01). (B) The dependence of these changes on bout duration is highlighted when the survival of subsets of dark period wake bouts are considered separately: the survival of wake bouts ≤ 200 sec increases from baseline to week 4 (log-rank test, P < 0.05), whereas (C) the survival of wake bouts > 200 sec decreases from baseline to week 4 (log-rank test, P < 0.01). (D) In the light period, the overall survival curve undergoes similar changes across time though the loss of very long wake bouts is less dramatic (weighted log-rank test, P < 0.01). As in the dark period, the survival of light period wake bouts ≤ 200 sec (E) and > 200 s (F) increases and decreases, respectively after DOX discontinuation (log-rank test, P < 0.05).
Because changes in survival appeared to be bout-duration dependent, we separately analyzed brief (< 60 sec), intermediate (between 60 and 1000 sec) and long (> 1000 sec) duration wake bouts using Kaplan-Meier survival curves (Figure S1, supplemental material). Surprisingly, the survival of brief wake bouts in the dark period increased 4 w after DOX removal, suggesting that with loss of the orexin neurons, mice are less likely to return to sleep soon after awakening. In addition, the survival of intermediate and long bouts was impaired, consistent with a role of the orexin neurons in sustaining longer periods of wake (log-rank test, P < 0.05). In the light period, differences in the survival of brief and intermediate wake bouts did not reach significance, but the survival of long bouts was clearly impaired (log-rank test, P < 0.05). This differential effect on short and long bouts persisted over a range of short and long subsets where the dividing threshold for each subset ranged from 60 sec to 1000 sec (Table S1, supplemental material). The opposite evolutions of survival curves for wake bouts ≤ 200 sec and wake bouts > 200 sec from baseline to week 4 DOX(−) provide one example of this differential effect (Figures 4B, 4C, 4E, 4F).
To better understand the increased survival of brief wake bouts with orexin neuron loss, we examined the proportion of wake bouts that failed of those at risk for each scored bin less than 60 sec (Figure 5). We found that this proportion was significantly reduced at week 4 after DOX compared to baseline for bouts ≤ 40 sec in the dark period and bouts ≤ 60 sec in the light period, providing further evidence that mice with few orexin neurons are less likely to return to sleep quickly (z-test, P < 0.05). We also computed the hazards associated with each survival curve over all bout durations. In both the dark and light periods, hazards for brief wake bouts (≤ 40 sec in the dark period; ≤ 60 sec in the light period) were lower at week 4 after DOX compared to baseline (z-test, P < 0.05), and there was a trend for higher hazards for long wake bouts at week 4 after DOX compared to baseline (Figure S2, supplemental material). Although the differences in hazards for wake bouts > 100 sec did not reach statistical significance, they are consistent with the hypothesis that the orexin neurons help sustain long bouts of wakefulness. The absence of a proportional relationship between the baseline and week 4 hazard curves indicates that the role of orexins in sustaining wakefulness depends on wake bout duration.
Figure 5.
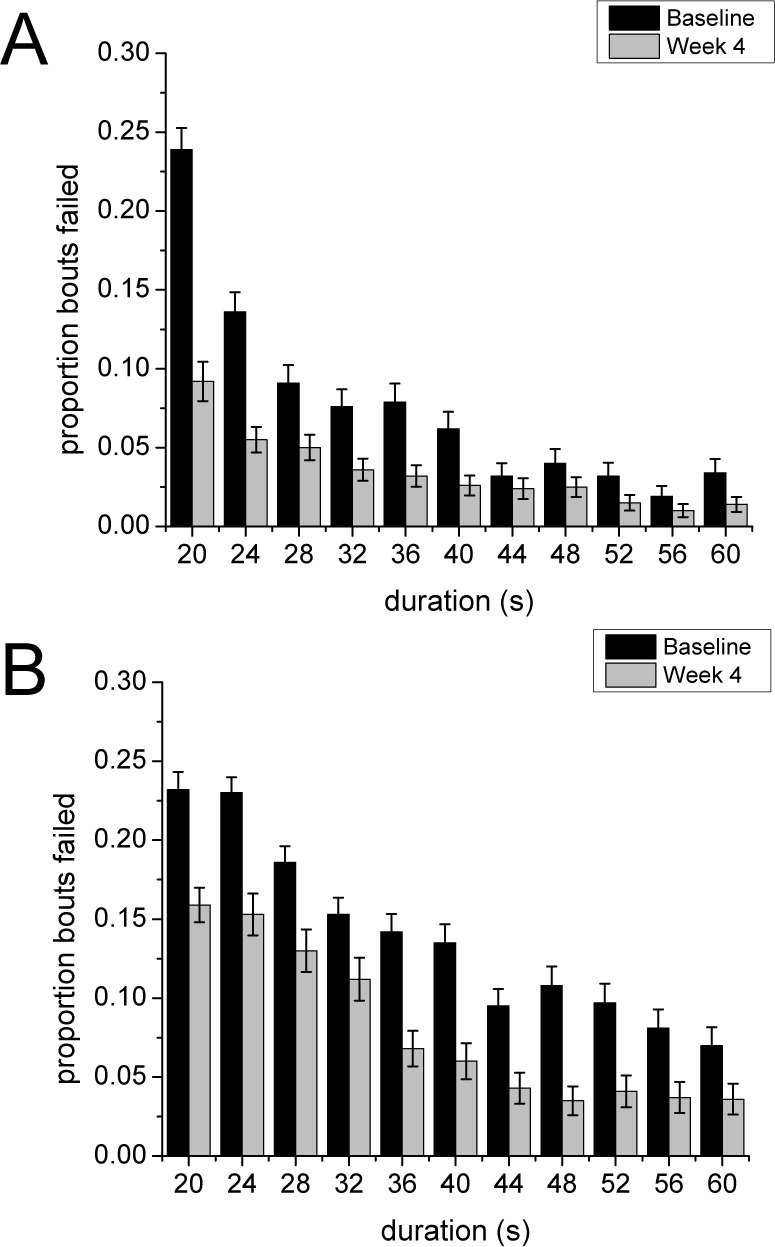
Of bouts at risk, the proportion of brief wake bouts that fail is lower 4 w after discontinuation of DOX compared to baseline. (A) In the dark period, the decrease in the failure proportion of brief bouts at week 4 is limited to bouts ≤ 40 sec (z-test, P < 0.01). (B) In the light period, all bouts ≤ 60 sec have a lower failure proportion at week 4 (z-test, P < 0.05).
Survival Analysis of Bouts of NREM and REM Sleep
To determine whether the distributions of sleep bouts underwent similar changes with loss of the orexin neurons, we computed Kaplan-Meier survival curves for the durations of NREM and REM sleep bouts. The survival of NREM sleep bouts in the dark period gradually decreased in the weeks after discontinuation of DOX (log-rank test, P < 0.01) (Figure 6). No difference in survival of NREM sleep bouts was detected in the light period. In the dark period, the hazard rate was consistently higher for short NREM sleep bouts at week 4 DOX(−) compared to baseline (bout durations between 12 and 32 sec; z-test, P < 0.05). This trend was also observed for light period NREM sleep bouts of duration between 16 and 28 sec, suggesting that orexin neuron loss destabilizes NREM sleep in the first 30 sec of sleep (Figures S2C and S2D).
Figure 6.
Survival of NREM and REM sleep bouts changes after discontinuation of DOX. After DOX removal, survival of NREM sleep bouts decreases in the dark period (A) (log-rank test, P < 0.01) but not in the light period (B). In contrast, in the weeks after DOX discontinuation, survival of REM sleep bouts clearly increases in the dark period (log-rank test, P < 0.01) (C) and increases to a lesser extent in the light period (log-rank test, P < 0.05) (D).
Kaplan-Meier survival curves for REM sleep bout durations across the weeks after DOX showed moderately improved survival in both the dark and light periods (log-rank test, P < 0.01), though the time-dependent nature of these changes was more apparent in the dark period (Figure 6). In both the dark and light periods, the hazard rate was significantly lower at week 4 DOX(−) compared to baseline for longer bouts (dark period bouts > 96 sec; light period bouts > 144 s; z-test, P < 0.05), suggesting that loss of the orexin neurons may allow longer REM sleep bouts (Figures S2E and S2F).
To determine whether age or duration of sleep recordings might affect sleep architecture, we also analyzed dark period data from DTA mice that were maintained on DOX(+) chow throughout the 4-w recording period. The survival of wake and sleep bouts did not change when DOX was maintained (Figure S3, supplemental material).
DISCUSSION
We analyzed sleep/wake architecture in DTA mice at different stages of orexin neuron degeneration to understand how loss of these key neurons affects sleep/wake behavior. Consistent with previous work, we found that progressive loss of the orexin neurons dramatically altered wakefulness, with poor maintenance of long wake bouts, especially in the dark period. Unexpectedly, orexin neuron loss increased survival of brief wake bouts. These findings suggest that the effects of the orexin neurons on wakefulness are dependent on the duration of the wake bout, with apparent promotion of long wake bouts and inhibition of short wake bouts.
In addition, we found that with loss of the orexin neurons, mice are more likely to wake during the first 30 sec of NREM sleep, and they take longer to return to sleep. Considered together, these findings shed light on the fragmented sleep that often troubles patients with narcolepsy.
Implications for the Influence of Orexins on Wake
Across the weeks after discontinuation of DOX, DTA mice had progressively worse maintenance of long bouts of wakefulness and better maintenance of short bouts of wakefulness. The necessity of the orexin neurons for the maintenance of long periods of wake has been observed in other animal models of narcolepsy,5–12 but the differential effect on short wake bouts is a novel finding.
These seemingly contradictory effects on wake bouts of different durations may be due to loss of the multiple signaling molecules in the orexin neurons. Recent optogenetic studies suggest that both glutamate and orexins contribute to the wake-promoting effects of orexin neurons.23 Release of glutamate by the orexin neurons may briefly promote wake, whereas release of orexins probably drives long wake bouts because the effects of orexins last much longer than those of glutamate.15,24,25 Importantly, these excitatory effects of orexin neuron activation may be initially masked by the inhibitory effects of dynorphin, a neuropeptide likely coreleased with orexins.13,14 Li and colleagues26 showed that the postsynaptic inhibitory effects of dynorphin can block the excitatory effects of orexin A, but this dynorphin response desensitizes in a few minutes.11,26,27 Perhaps, when the orexin neurons fire, the initial net response is inhibition of target wake-promoting neurons, and thus, loss of the orexin neurons improves survival of short wake bouts.11 These changes in orexin signaling may be exacerbated by the disruption of local network interactions involving orexin neurons.28–31
Alternatively, increased survival of short wake bouts may be a consequence of decreased survival of long wake bouts and lower homeostatic sleep pressure. Long periods of wakefulness increase sleep pressure and are usually followed by deeper NREM sleep. In previous work, we showed that OXKO mice spend less time in deep NREM sleep and more time in light NREM sleep.32 Although brief wake bouts may be less likely to occur when an animal is in deep NREM sleep, it is probable that these wake bouts would have a high hazard; in other words, an animal would likely return to sleep quickly after awakening from deep NREM sleep. In contrast, the probability of awakening from light NREM sleep is likely higher, but the relatively low sleep pressure should reduce the hazard of falling back to sleep. Therefore, changes in the propensity to be in deep or light NREM sleep could alter the hazards for brief wake bouts. Our data regarding this point are limited because our scoring considered only wake bouts ≥ 20 sec, and spectral analysis of DTA mice shows increases in multiple bands.16 Future experiments using sleep deprivation to increase sleep pressure in orexin neuron-deficient animals could shed light on the relationship between sleep pressure and the hazards associated with brief wake bouts.
Implications for the Influence of Orexins on Sleep
Although the orexin neurons are primarily wake active,33,34 disrupted sleep is a common clinical feature of narcolepsy, and its cause is a major, outstanding mystery. In this study, we found that in the weeks after discontinuation of DOX, DTA mice developed increasingly fragmented NREM sleep in the dark period. In contrast to the results for wake bouts, the survival curve for NREM sleep in the dark period at 4 w DOX(−) was approximately proportional to the baseline survival curve. The mostly constant hazard associated with NREM sleep is consistent with previous work suggesting that NREM sleep bouts follow an exponential distribution.11,35,36
In DTA mice, the fragmentation of NREM sleep was caused by both a higher probability of awakening during the first 30 sec of NREM sleep and a lower probability of returning to sleep after a brief awakening. Older human subjects also have an increased hazard for awakening compared to younger adults, but their probability of falling back asleep is similar to young subjects.37 With aging, poor maintenance of sleep may be due to loss of sleep-promoting preoptic neurons,38 but a similar mechanism seems unlikely in DTA mice as the orexin neurons are not very active during sleep.33,34 Instead, the fragmented sleep with loss of the orexin neurons may be caused by compensatory changes in histaminergic and perhaps other wake-promoting populations that may improve wakefulness yet disrupt sleep.39,40 Alternatively, unstable NREM sleep could result from lower homeostatic pressure for sleep as previously discussed.12
In contrast with the effects on NREM sleep, survival analysis revealed a lengthening of REM sleep bouts after DOX removal in both the light and dark periods. This change was evident in the increased mean duration of REM bouts in the dark period, although the change in light period REM bout durations did not reach significance. This discrepancy highlights the limitation of a metric such as mean bout duration for identifying changes that occur primarily in the tail of a distribution. By accounting for the entire distribution, survival analysis provides a more sensitive tool for detecting key differences. Mathematical modeling has suggested that decreased activity in wake-promoting neuronal populations can lengthen REM sleep bouts via network effects.41,42 In addition, disruption to local interactions among orexin neurons and melanin-concentrating hormone neurons in the lateral hypothalamus may contribute to the altered REM sleep-related behaviors observed after DOX removal.28–30 A decrease in orexin or glutamate signaling associated with orexin neuron loss is consistent with such a mechanism, but OXKO mice do not have longer REM sleep bout durations12 nor improved survival of REM sleep.11 Additional work is needed to determine the robustness of these REM sleep effects and their relationship to other signaling molecules in orexin neurons.
Limitations of the Study
The progressive and thorough loss of orexin neurons in DTA mice provides an excellent model of narcolepsy in humans. However, additional changes in phenotype, such as further increases in cataplexy, occur after the 4-w period that we analyzed.16 In addition, the time frame of our study may be too short to reveal changes in sleep/wake architecture due to compensatory physiological mechanisms. For example, in addition to a large reduction in orexin neurons, people with narcolepsy also have a large increase in histaminergic neurons that may help compensate for the loss of the orexin neurons.39,40 Six weeks after discontinuation of DOX, DTA mice do not show an increase in histamine neurons,40 but changes in the hista-mine neurons might develop later with consequent effects on sleep architecture. Furthermore, although the dramatic loss of orexin neurons is likely a major factor in the observed changes in sleep/wake architecture, other factors, such as age, altered locomotor activity, the presence of DOX, and compensatory physiological mechanisms, may also contribute. Our recordings began in 12-w-old mice, so the effect of age on sleep is probably small as previous work has demonstrated that mouse sleep/wake behavior is relatively stable by this time, although that study did not consider behavior beyond 14 w of age.43 Similarly, although changes in locomotor activity are known to affect mouse sleep/wake architecture in both normal44,45 and orexin-deficient animals,46 there was no evidence for significant changes in locomotor activity over the duration of our experiments. Additional work is needed to determine the relative contributions of these factors.
CONCLUSIONS
Interestingly, we observed a relatively uniform progression in changes to sleep/wake architecture across weeks despite the nonuniform changes in orexin cell loss across this time period: behavior at weeks 1, 2, and 4 DOX(−) corresponded to ∼85%, 95%, and 97% cell loss, respectively.16 These results suggest that small numbers of orexin neurons may be able to maintain normal function, and they are consistent with the markedly low orexin levels that are typically found in cerebrospinal fluid at the time of diagnosis in narcolepsy patients. Future work in DTA mice and other mouse models of narcolepsy such as orexin/ataxin-3 mice10 will be needed to better understand the relationship between moderate orexin neuron loss and sleep/ wake architecture.
Our results support the current paradigm that the orexin neurons promote long bouts of wakefulness, but they also suggest that the orexin neurons may inhibit short wake bouts. Most likely, these opposite effects arise from network-level interactions among the neuronal populations that regulate sleep/wake behavior, but they may also result from the complex signaling capabilities of the orexin neurons. The different postsynaptic effects of orexins, dynorphin, and glutamate provide a level of flexibility in signaling that we are just beginning to understand.15 Clinically, this suggests that optimal therapies for narcolepsy may require a multi-faceted approach that addresses several aspects of altered sleep/wake behavior in addition to the current focus on wake consolidation to improve excessive daytime sleepiness.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by National Science Foundation grant DMS 1121361 (CDB); National Science Foundation grant DMS 1412571 (CDB); Grant-in-Aid for Scientific Research on Innovative Areas “Mesoscopic Neurocircuitry” (23115103) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (AY); Akiyama Foundation (AT); and a Colorado School of Mines Undergraduate Research Fellowship (AFB). Dr. Scammell has received research support from Eisai and has consulted for Merck, Symphony Pharma Life Sciences, Prexa, Jazz and Cereve. The other authors have indicated no financial conflicts of interest. The study did not include any off-label/investigational use. The work was performed at the Colorado School of Mines, Golden, CO and at Hokkaido University, Hokkaido, Japan.
REFERENCES
- 1.Sakurai T. Orexin deficiency and narcolepsy. Curr Opin Neurobiol. 2013;23:760–6. doi: 10.1016/j.conb.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet. 2007;369:499–511. doi: 10.1016/S0140-6736(07)60237-2. [DOI] [PubMed] [Google Scholar]
- 3.Scammell TE. The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol. 2003;53:154–66. doi: 10.1002/ana.10444. [DOI] [PubMed] [Google Scholar]
- 4.Burgess CR, Scammell TE. Narcolepsy: neural mechanisms of sleepiness and cataplexy. J Neurosci. 2012;32:12305–11. doi: 10.1523/JNEUROSCI.2630-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin L, Faraco J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–76. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 6.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 7.Willie JT, Chemelli RM, Sinton CM, et al. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–30. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 8.Kisanuki Y, Chemelli R, Tokita S, Willie J, Sinton C, Yanagisawa M. Behavioral and polysomnographic characterization of orexin-1 receptor and orexin-2 receptor double knockout mice. Sleep. 2001;24:A22. (Abstract Suppl) [Google Scholar]
- 9.Kalogiannis M, Hsu E, Willie JT, et al. Cholinergic modulation of narcoleptic attacks in double orexin receptor knockout mice. PLoS One. 2011;6:e18697. doi: 10.1371/journal.pone.0018697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hara J, Beuckmann CT, Nambu T, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–54. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 11.Diniz Behn CG, Kopell N, Brown EN, Mochizuki T, Scammell TE. Delayed orexin signaling consolidates wakefulness and sleep: physiology and modeling. J Neurophysiol. 2008;99:3090–103. doi: 10.1152/jn.01243.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mochizuki T, Crocker A, McCormack S, Yanagisawa M, Sakurai T, Scammell TE. Behavioral state instability in orexin knock-out mice. J Neurosci. 2004;24:6291–300. doi: 10.1523/JNEUROSCI.0586-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou TC, Lee CE, Lu J, et al. Orexin (hypocretin) neurons contain dynorphin. J Neurosci. 2001;21:RC168. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muschamp JW, Hollander JA, Thompson JL, et al. Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci U S A. 2014;111:E1648–55. doi: 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schone C, Apergis-Schoute J, Sakurai T, Adamantidis A, Burdakov D. Coreleased orexin and glutamate evoke nonredundant spike outputs and computations in histamine neurons. Cell Rep. 2014;7:697–704. doi: 10.1016/j.celrep.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tabuchi S, Tsunematsu T, Black SW, et al. Conditional ablation of orexin/hypocretin neurons: a new mouse model for the study of narcolepsy and orexin system function. J Neurosci. 2014;34:6495–509. doi: 10.1523/JNEUROSCI.0073-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter ME, Yizhar O, Chikahisa S, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–33. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–4. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakurai T, Moriguchi T, Furuya K, et al. Structure and function of human prepro-orexin gene. J Biol Chem. 1999;274:17771–6. doi: 10.1074/jbc.274.25.17771. [DOI] [PubMed] [Google Scholar]
- 20.Tobler I, Deboer T, Fischer M. Sleep and sleep regulation in normal and prion protein-deficient mice. J Neurosci. 1997;17:1869–79. doi: 10.1523/JNEUROSCI.17-05-01869.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamanaka A, Tsujino N, Funahashi H, et al. Orexins activate histaminergic neurons via the orexin 2 receptor. Biochem Biophys Res Commun. 2002;290:1237–45. doi: 10.1006/bbrc.2001.6318. [DOI] [PubMed] [Google Scholar]
- 22.Scammell TE, Willie JT, Guilleminault C, Siegel JM. A consensus definition of cataplexy in mouse models of narcolepsy. Sleep. 2009;32:111–6. [PMC free article] [PubMed] [Google Scholar]
- 23.Kostin A, Siegel JM, Alam MN. Lack of hypocretin attenuates behavioral changes produced by glutamatergic activation of the perifornical-lateral hypothalamic area. Sleep. 2014;37:1011–20. doi: 10.5665/sleep.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hagan J, Leslie R, Patel S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96:10911–6. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, van den Pol AN. Differential target-dependent actions of coexpressed inhibitory dynorphin and excitatory hypocretin/orexin neuropeptides. J Neurosci. 2006;26:13037–47. doi: 10.1523/JNEUROSCI.3380-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams K, Diniz Behn CG. A Hodgkin-Huxley-type model orexin neuron. Sleep. 2009;32:A25. (Abstract Suppl) [Google Scholar]
- 28.Verret L, Goutagny R, Fort P, et al. A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. BMC Neurosci. 2003;4:19. doi: 10.1186/1471-2202-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jego S, Glasgow SD, Herrera CG, et al. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat Neurosci. 2013;16:1637–43. doi: 10.1038/nn.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassani OK, Lee MG, Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc Natl Acad Sci U S A. 2009;106:2418–22. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosqueiro T, de Lecea L, Huerta R. Control of sleep-to-wake transitions via fast amino acid and slow neuropeptide transmission. New J Phys. 2014;16:115010. doi: 10.1088/1367-2630/16/11/115010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diniz Behn CG, Klerman EB, Mochizuki T, Lin SC, Scammell TE. Abnormal sleep/wake dynamics in orexin knockout mice. Sleep. 2010;33:297–306. doi: 10.1093/sleep/33.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–20. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–98. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lo CC, Chou T, Penzel T, et al. Common scale-invariant patterns of sleep-wake transitions across mammalian species. Proc Natl Acad Sci U S A. 2004;101:17545–8. doi: 10.1073/pnas.0408242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blumberg MS, Seelke AM, Lowen SB, Karlsson KA. Dynamics of sleep-wake cyclicity in developing rats. Proc Natl Acad Sci U S A. 2005;102:14860–4. doi: 10.1073/pnas.0506340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klerman EB, Wang W, Duffy JF, Dijk DJ, Czeisler CA, Kronauer RE. Survival analysis indicates that age-related decline in sleep continuity occurs exclusively during NREM sleep. Neurobiol Aging. 2013;34:309–18. doi: 10.1016/j.neurobiolaging.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim AS, Ellison BA, Wang JL, et al. Sleep is related to neuron numbers in the ventrolateral preoptic/intermediate nucleus in older adults with and without Alzheimer's disease. Brain. 2014;137:2847–61. doi: 10.1093/brain/awu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valko PO, Gavrilov YV, Yamamoto M, et al. Increase of histaminergic tuberomammillary neurons in narcolepsy. Ann Neurol. 2013;74:794–804. doi: 10.1002/ana.24019. [DOI] [PubMed] [Google Scholar]
- 40.John J, Thannickal TC, McGregor R, et al. Greatly increased numbers of histamine cells in human narcolepsy with cataplexy. Ann Neurol. 2013;74:786–93. doi: 10.1002/ana.23968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diniz Behn CG, Booth V. A fast-slow analysis of the dynamics of REM sleep. Siam J Appl Dyn Syst. 2012;11:212–42. [Google Scholar]
- 42.Diniz Behn C, Booth V. Simulating microinjection experiments in a novel model of the rat sleep-wake regulatory network. J Neurophysiol. 2010;103:1937–53. doi: 10.1152/jn.00795.2009. [DOI] [PubMed] [Google Scholar]
- 43.Daszuta A, Gambarelli F, Ternaux JP. Sleep variations in C57BL and BALBc mice from 3 weeks to 14 weeks of age. Brain Res. 1983;283:87–96. doi: 10.1016/0165-3806(83)90084-6. [DOI] [PubMed] [Google Scholar]
- 44.Edgar DM, Kilduff TS, Martin CE, Dement WC. Influence of running wheel activity on free-running sleep/wake and drinking circadian rhythms in mice. Physiol Behav. 1991;50:373–8. doi: 10.1016/0031-9384(91)90080-8. [DOI] [PubMed] [Google Scholar]
- 45.Tang X, Orchard SM, Liu X, Sanford LD. Effect of varying recording cable weight and flexibility on activity and sleep in mice. Sleep. 2004;27:803–10. [PubMed] [Google Scholar]
- 46.Espana RA, McCormack SL, Mochizuki T, Scammell TE. Running promotes wakefulness and increases cataplexy in orexin knockout mice. Sleep. 2007;30:1417–25. doi: 10.1093/sleep/30.11.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.