Abstract
Study Objectives:
To evaluate vitamin D (25(OH)D) levels in obstructive sleep apnea syndrome (OSAS) and possible relationships to OSAS severity, sleepiness, lung function, nocturnal heart rate (HR), and body composition. We also aimed to compare the 25(OH)D status of a subset of OSAS patients compared to controls matched for important determinants of both OSAS and vitamin D deficiency (VDD).
Methods:
This was a cross-sectional study conducted at an urban, clinical sleep medicine outpatient center. We recruited newly diagnosed, Caucasian adults who had recently undergone nocturnal polysomnography. We compared body mass index (BMI), body composition (bioelectrical impedance analysis), neck circumference, sleepiness (Epworth Sleepiness Scale), lung function, and vitamin D status (serum 25-hydrpoxyvitamin D (25(OH)D) across OSAS severity categories and non-OSAS subjects. Next, using a case-control design, we compared measures of serum 25(OH)D from OSAS cases to non-OSAS controls who were matched for age, gender, skin pigmentation, sleepiness, season, and BMI.
Results:
106 adults (77 male; median age = 54.5; median BMI = 34.3 kg/m2) resident in Dublin, Ireland (latitude 53°N) were recruited and categorized as non-OSAS or mild/moderate/severe OSAS. 98% of OSAS cases had insufficient 25(OH)D (< 75 nmol/L), including 72% with VDD (< 50 nmol/L). 25(OH)D levels decreased with OSAS severity (P = 0.003). 25(OH)D was inversely correlated with BMI, percent body fat, AHI, and nocturnal HR. Subsequent multivariate regression analysis revealed that 25(OH)D was independently associated with both AHI (P = 0.016) and nocturnal HR (P = 0.0419). Our separate case-control study revealed that 25(OH)D was significantly lower in OSAS cases than matched, non-OSAS subjects (P = 0.001).
Conclusions:
We observed widespread vitamin D deficiency and insufficiency in a Caucasian, OSAS population. There were significant, independent, inverse relationships between 25(OH)D and AHI as well as nocturnal HR, a known cardiovascular risk factor. Further, 25(OH)D was significantly lower in OSAS cases compared to matched, non-OSAS subjects. We provide evidence that 25(OH)D and OSAS are related, but the role, if any, of replenishment has not been investigated.
Citation:
Kerley CP, Hutchinson K, Bolger K, McGowan A, Faul J, Comican L. Serum vitamin D is significantly inversely associated with disease severity in Caucasian adults with obstructive sleep apnea syndrome. SLEEP 2016;39(2):293–300.
Keywords: vitamin D, obstructive sleep apnea, obesity, apnea-hypopnea index, nutrition, diet, sunshine
Significance.
We demonstrate that vitamin D deficiency/insufficiency is almost universal in a cohort with obstructive sleep apnea syndrome (OSAS). Further, we observed significant, independent, inverse relationships between vitamin D levels and both OSAS severity and nocturnal heart rate, a known cardiovascular risk factor. Although previous studies have found a link between vitamin D levels and OSAS, this could be due to confounding. We compared relationships between vitamin D levels and OSAS severity. We also compared the difference between vitamin D levels in OSAS cases and controls matched for important determinants of OSAS and vitamin D, including BMI, age, gender and sleepiness. Prospective and/or randomized trials are warranted to fully assess the effect, if any, of vitamin D in OSAS.
INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) represents a major public health problem.1 One major risk factor for OSAS is obesity, which is reported in up to 70% of cases. The incidence and/ or severity of OSAS also appears related to ethnicity, winter season, and lack of physical activity.2–4 Additionally, OSAS has been associated with multiple metabolic disturbances including excess systemic inflammation, hyperglycemia, hyper-lipidemia, cardiovascular disease, and increased bone loss.5,6
Vitamin D receptors and enzymes have been discovered in most cell types and tissues leading to the realization that vitamin D exerts non-skeletal, pleiotropic effects in multiple organs.7 However, vitamin D deficiency (VDD) remains highly prevalent worldwide.7 Similar to OSAS, VDD is associated with adiposity, dark skin pigmentation, winter season, and physical inactivity. The non-skeletal consequences of VDD are not well understood; however, VDD has been associated with similar metabolic disturbances as OSAS, including elevated systemic inflammation, impaired glucose metabolism, dyslipidemia, and bone deformities as well as many of the comorbidi-ties associated with OSAS, including cardiovascular disease.7
Heart rate (HR) parameters provide important information regarding cardiovascular regulatory mechanisms and are mainly affected by the sympathetic nervous system (SNS). One of the most important effects of OSAS is SNS activation,8 and numerous studies have noted HR perturbations in OSAS.9 Elevated HR has been associated with low 25-hydroxyvitamin D (25(OH)D) in both cross-sectional10 and prospective11 studies. However, there is a lack of studies regarding HR and 25(OH) D in OSAS.
Recently there has been interest in the idea that vitamin D could be important for sleep disorders.12–14 Several cross-sectional studies have reported a high prevalence of VDD in OSAS,15–18 which is more pronounced in severe OSAS16,17 and associated with abnormal glucose metabolism.15,16 These studies suggest a possible link between OSAS and VDD but are limited by the lack of controls15 and the inclusion of controls who had not undergone polysomnography18 or were not matched for important determinants of OSAS and VDD such as BMI, age, gender, and degree of sleepiness.16,18
In this cross-sectional study, we examined the prevalence of vitamin D insufficiency and deficiency among urban adults with newly diagnosed, untreated OSAS. We also wanted to assess relationships between 25(OH)D and OSAS severity, sleepiness, lung function, nocturnal HR, and body composition. Finally, we wanted to compare vitamin D levels from selected OSAS cases with non-OSAS subjects matched for important determinants of OSAS and VDD.
METHODS
Subjects/Study Population
We conducted a cross-sectional study among consecutive Caucasian, urban adults who had been referred to the Sleep Clinics of Connolly Hospital, Blanchardstown, Dublin, Ireland. All subjects underwent overnight polysomnography (PSG) and were recruited after signing an institutional ethics committee approved consent form. We excluded participants currently using multivitamin or vitamin D supplements or medications that modulate vitamin D metabolism, and those with disorders known to influence vitamin D metabolism/absorption.
Sleep Indices
The presence or absence of OSAS was based on the results of full, in-house, nocturnal PSG (SomnoScreen Plus), which were conducted and analyzed at the Sleep Laboratory of Connolly Hospital. PSG data were analyzed manually by a respiratory physiologist using computer software (Somno-Medics Domino Software) according to Irish Sleep Society/ AASM guidelines. An obstructive apnea was defined as a drop in airflow to ≤ 90% of baseline for ≥ 10s as recorded with the oronasal sensor with continued respiratory effort. A hypopnea was defined as a drop in airflow ≥ 30% from baseline as recorded with the nasal cannula for ≥ 10s accompanied by ≥ 3% oxygen desaturation. A decrease in SpO2 ≥ 3% was considered to represent an oxygen desaturation. The average number of apneas and hypopneas per hour of sleep was defined as the apnea-hypopnea index (AHI). The diagnosis and severity of OSAS was based on the definitions recommended by the American Academy of Sleep Medicine as follows: non-OSAS (AHI < 5), mild OSAS (5 ≤ AHI < 15), moderate OSAS (15 ≥ AHI < 30) and severe OSAS (AHI ≥ 30). The non-OSAS group comprised subjects with snoring or insomnia but without OSAS as confirmed by PSG. Consenting and eligible, non-OSAS subjects for the case-control study were matched in a paired fashion to individual OSAS cases in terms of important determinants of both OSAS and VDD, including age, gender, skin pigmentation, sleepiness, season, and BMI. We evaluated daytime sleepiness with the Epworth Sleepiness Scale (ESS), which is a widely used, validated tool.
Pulmonary Function Testing
Values for percentage of predicted forced expiratory volume in 1 second (FEV1%) and percentage of predicted forced expiratory volume (FVC%) were obtained according to ATS/ ERS recommended techniques by an experienced respiratory physiologist. The results were compared with predicted normal values from the European Community for Coal and Steel/European Respiratory Society.
Anthropometry
Body mass index (BMI) was calculated from measured height and weight. Neck circumference was measured by a trained dietitian (CPK). We estimated total fat mass (FM), fat free mass (FFM), and trunk fat with bioelectrical impedance analysis (BIA) using the Tanita Segmental Body Composition Analyzer, Model BC-418 (Tanita Corporation, Tokyo, Japan), which correlates highly with DEXA measurements (r = 0.82–0.87).19,20
Vitamin D Analysis
Blood samples were collected, centrifuged, aliquoted, and frozen to −80°C, which does not affect 25(OH)D. Circulating levels of total 25(OH)D are considered the most reliable measure of overall vitamin D status since they reflect vitamin D2 + D3 contributions from all sources.21 25(OH)D was measured using competitive chemiluminescence immunoassays (Dia-Sorin, Dietzenbach, Germany) with inter-assay coefficient of variation of 6.8%. Internal quality control was determined using kit controls of 2 different concentrations.
Values were reported as nmol/L. Here we use the 2011 Endocrine Society guidelines to define serum 25(OH)D status,22 whereby 25(OH)D < 50 nmol/L equates to VDD, while < 75 nmol/L equates to vitamin D insufficiency (VDI) and > 75 nmol/L denotes vitamin D sufficiency (VDS).
Skin pigmentation, which is a major influencer of vitamin D photosynthesis,7 was assessed using the 6 point Fitzpatrick scale.23
Statistical Methods
The Shapiro-Wilk test was performed to check the data for normality. The majority of variables were not normally distributed. Therefore we utilized Kruskal-Wallis, one-way, nonparametric, ANOVA tests to detect differences between non-OSAS subjects and OSAS severities and if differences were detected, the Mann-Whitney test was performed for pairwise comparisons. We used Spearman rho derived from bivariate correlation analysis to assess relevant correlations. Associations between variables on univariate regression analysis with P values < 0.05 were entered into multivariate models to determine the independent correlations to AHI and HR. Paired t-tests were used to compare differences between OSAS cases and matched, non-OSAS subjects. All analyses were performed using SPSS statistical software (version 20.0. Armonk, NY: IBM Corp). Results were expressed as median ± interquartile range (IQR). All P values reported are 2-tailed with statistical significance set at < 0.05.
RESULTS
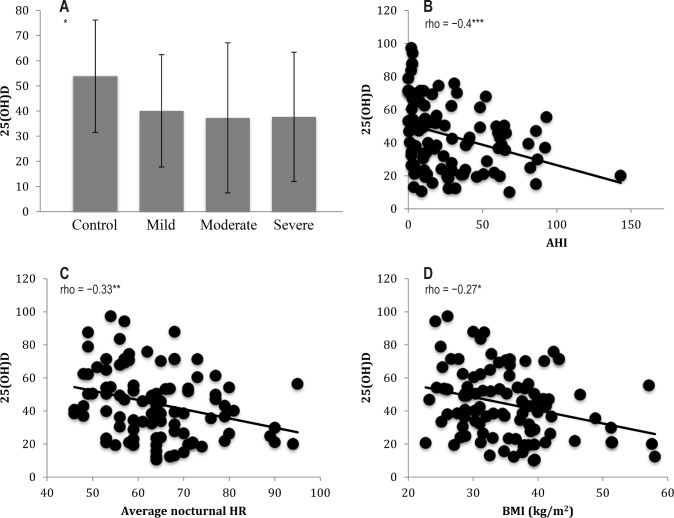
We recruited 106 untreated Caucasian adults who had recently undergone nocturnal PSG. Table 1 displays all parameters and results of the non-OSAS, control group, and the OSAS subjects grouped by severity. Severe OSAS was overrepresented compared to mild and moderate OSAS, reflecting the typical demographics of our clinic population. Similarly, males were overrepresented compared to females, reflecting OSAS epidemiology. The age profile of the 4 groups was similar. Patients with severe OSAS group had significantly lower FEV1% and FVC% than other groups, as well as a more adverse anthropo-metric profile (higher BMI, percentage body fat, FM, trunk fat, neck circumference, but significantly lower FFM) and higher nocturnal mean HR. Median ESS scores were only slightly elevated in severe OSAS compared to other groups but the difference was statistically significant. As expected PSG variables increased across OSAS severity classes (AHI, total apnea number, total hypopnea number, desaturation index). Although the severe OSAS group had less total sleep time and less deep sleep than other groups, there was no difference in measures of sleep efficiency. Finally, 25(OH)D values were highest in non-OSAS subjects and decreased across OSAS severities (Figure 1A, P = 0.003), while the proportion of subjects with VDD was lowest in non-OSAS subjects and increased across OSAS severity classes.
Table 1.
Demographic and physiologic characteristics of subjects by OSAS severity.
Figure 1.
Vitamin D levels and relationships to demographics. (A) 25(OH)D (median, interquartile range) by group. (B–D) Correlations between 25(OH)D and AHI, nocturnal HR and BMI. *P = 0.01; **P = 0.001; ***P = 0.0001; 25(OH)D, 25-hydroxyvitamin D; AHI, apnea-hypopnea index; BMI, body mass index; HR, heart rate.
In order to estimate potential associations between 25(OH)D levels, PSG measures and anthropometric variables, we initially performed bivariate Spearman correlation analysis. Table 2 displays correlations between 25(OH)D and relevant variables. There were statistically significant, inverse correlations between 25(OH)D and anthropometric variables including BMI, total percentage body fat, and total FM (Figure 1D). Further, there were statistically significant, inverse correlations between 25(OH)D and OSAS variables including AHI (Figure 1B), desaturation index, time spent below 90% oxygen saturation, and average nocturnal HR (Figure 1C). Conversely, there was a positive correlation between 25(OH)D and baseline oxygen saturation. There was no association between 25(OH)D and age, FFM, neck circumference, lung function, ESS score, or PSG variables not listed in Table 2.
Table 2.
Correlations between 25(OH)D and anthropometric, demographic, and polysomnographic measures.
Many factors influencing 25(OH)D status, AHI, and HR are related, and correlations between variables may be due to confounding. Therefore, we conducted regression analysis to determine if there were independent association between variables of interest.
ESS score (β = 0.22; P = 0.028), 25(OH)D (β = 0.34; P = 0.001), and several measures of adiposity were significantly associated with AHI in univariate regression analysis. Therefore we selected the most significant adiposity association (BMI) and entered BMI, ESS score, and 25(OH)D level into a multivariate model. These variables explained 56.7% of the variance in AHI in this cohort. However, only BMI (β = 0.42; P < 0.0001) and 25(OH)D (β = −0.22; P = 0.016) were significant independent predictors of AHI, while ESS score was of borderline significance (β = −0.19; P = 0.065).
Similarly, 25(OH)D level (β = 0.35; P < 0.0001) as well as several measures of both adiposity and sleep were significantly associated with HR in univariate regression analysis. We selected the most significant associations—BMI for adiposity and AHI for sleep variables – and entered these values into a multivariate model which revealed that these variables explained 46.4% of the variance in mean nocturnal HR in this cohort. However, only 25(OH)D level could be considered a significant independent predictor of HR (β = −0.24; P = 0.019), while BMI was of borderline significance (β = 0.21; P = 0.057). AHI was not associated with HR after adjusting for 25(OH)D level and BMI (β = 0.17; P = 0.14).
Case-Control Study
To further assess if there was any relationship between OSAS and 25(OH)D, we wanted to examine 25(OH)D levels between a separate cohort of OSAS cases and non-OSAS subjects after matching for potential confounders: age, gender, skin pigmentation, sleepiness, season, and BMI (Table 3).
Table 3.
Characteristics of OSAS subjects vs. matched, non-OSAS subjects.
Nocturnal HR was significantly higher in OSAS vs. matched, non-OSAS subjects, while FVC% was significantly lower. Additionally, 25(OH)D levels were significantly lower in OSAS cases vs. non-OSAS subjects and the proportion of subjects with VDD was markedly higher in severe OSAS compared to non-OSAS subjects.
DISCUSSION
In the present study, we investigated the relation of 25(OH)D levels to OSAS indices, lung function, mean nocturnal HR, and body composition. 25(OH)D levels were highest in non-OSAS subjects and decreased with increasing OSAS severity. Of the 75 OSAS cases, there was only one subject who had sufficient vitamin D (1.5%), while 6 of 31 non-OSAS subjects (18%) were VDS. Further, the proportion of subjects with VDD was markedly higher in severe OSAS (82%) compared to mild OSAS, moderate OSAS, and non-OSAS subjects (61%, 61%, and 32%, respectively). Our results agree with previous cross-sectional reports of widespread VDD in OSAS, whereby levels were lowest in severe OSAS.15–18
We conducted this study at high latitude in a country with limited sun exposure (Dublin, Ireland, 53°N). It is perhaps not surprising that a mostly obese cohort with OSAS living in such an environment exhibited decreased vitamin D levels. However, in a recent cohort study carried out in a representative Irish population, it was observed that 40.1% were VDD and 75.6% were VDS.24 In this context, we report a very high prevalence of VDD (71%) and VDI (99%) in OSAS cases, which is particularly pronounced in severe OSAS cases (82% VDD).
25(OH)D was inversely associated with numerous OSAS parameters as documented with PSG, including AHI, total apnea number, total hypopnea number, baseline oxygen concentration as well as time spent below 90% oxygen saturation, minimal oxygen concentration, and desaturation index. The inverse relationship between 25(OH)D and AHI was still present after multivariate analysis suggesting an independent association. An inverse correlation between 25(OH)D and various OSAS measures on PSG has been reported previously by some authors16 but not others.15,18 However, in one of these studies,18 25(OH)D was associated with OSAS according to multivariate logistic regression analysis. It is noteworthy that the studies that did not report an association between 25(OH)D and AHI reported higher mean 25(OH)D levels compared to the 25(OH) D levels we and others16 observed among OSAS cohorts. In this context, it is possible that VDD is more strongly associated with OSAS compared to VDI.
An interesting and novel finding of this study is the independent, inverse relationship between 25(OH)D and mean nocturnal HR. This is consistent with previous cross-sectional10 and prospective11 studies in non-OSAS adults. Further, low 25(OH)D levels are associated with cardiac autonomic dys-function.25 In this context, it is noteworthy that previous trials have demonstrated that vitamin D can significantly reduce HR.26,27 Elevated HR has been associated with metabolic syndrome, type 2 diabetes, as well as CVD and mortality.28 The possibility that vitamin D repletion offers opportunity to reduce HR and morbidity in OSAS cannot be discounted. However, our preliminary findings require confirmation.
Here, 25(OH)D was inversely associated with BMI and percentage body fat, and there was a trend to an inverse relationship with FM. These observations are consistent with previous reports in OSAS.1,5,16,18 It is well established that there is an inverse association between 25(OH)D and BMI. There was a suggestion that low 25(OH)D could lead to weight gain,29 potentially through elevated parathyroid hormone (PTH) promoting fat accumulation through increased calcium influx into adipocytes, which would theoretically enhance lipogenesis and inhibit lipolysis.29 Support for this theory comes from murine work showing that vitamin D regulates energy expenditure and enhances fatty acid oxidation.30 However, most vitamin D supplementation trials have demonstrated little impact on FM,31 and a recent bi-directional Mendelian randomization analysis of multiple cohorts concluded that higher BMI leads to lower 25(OH)D and any effects of lower 25(OH)D increasing BMI are likely minor.32 Indeed, there is consensus that the bioavailability of vitamin D (either from dietary sources or ultraviolet irradiation) is truly decreased in obesity. Although it has traditionally been assumed that sequestration of 25(OH)D by adipocytes explained this association, it has recently been demonstrated that volumetric dilution offers a superior explanation.33
We failed to demonstrate an association between sleepiness and 25(OH)D. This is in contrast to cross-sectional reports among varied ethnicities.34,35 The discrepancy regarding associations between sleepiness and 25(OH)D may be race dependent.34 Nevertheless, our results do not support a relationship between vitamin D levels and sleepiness, at least not in Caucasians with untreated OSAS. This raises the intriguing possibility that race modifies the relationship between vitamin D level and excessive daytime sleepiness. Similarly, although epidemiologic data demonstrate a positive association between 25(OH)D level and pulmonary function in healthy adults and those with asthma or COPD,36 we did not observe an association here. The lack of association might be explained by the presence of OSAS and/or excess adiposity.
Our subsequent analysis of OSAS cases and non-OSAS subjects matched for important determinants of 25(OH)D and OSAS revealed that 25(OH)D levels were significantly decreased in OSAS. This observation is consistent with most previous reports,16,18,38 but not others.17
In the present study, all patients and non-OSAS subjects were Caucasian and resided in Dublin, Ireland. All of the 25(OH)D assays were conducted using the same batch of commercial assays and were performed concomitantly by the same biochemist in the same laboratory, thereby reducing biochemical variability. The strength of the case-control analysis was in comparing vitamin D levels in patients with OSAS to subjects without OSAS confirmed by PSG who were matched for important determinants of both VDD and OSAS.
This study has some limitations. We included a relatively small sample size and did not assess sun exposure, dietary habits or metabolic biomarkers, such as PTH, lipids, or glycemic indices. Further, we did not assess polymorphisms in the vitamin D metabolism pathway (vitamin D receptor, 25-hydroxylase and 1-α-hydroxylase). It is well known that variants in this pathway are associated with perturbed vitamin D metabolism and clinical outcomes. The cross-sectional nature of our study and other studies relating vitamin D to OSAS makes it impossible to infer that VDD predisposes to OSAS or vice versa
In our cohort, BMI did not fully explain the association between AHI and 25(OH)D. This is supported by the largest report of 25(OH)D levels in OSAS to date, which reported no association with obesity.15 Therefore, an alternative explanation regarding low 25(OH)D in OSAS is needed. It is possible that 25(OH)D level reflects healthy behaviors such as outdoor exercise or fish consumption. However, there is an idea that OSAS is an inflammatory disorder.5 Therefore, it is possible the VDD either predisposes to or exacerbates OSAS through an upregulation of inflammatory pathways. It is also plausible that VDD may predispose to OSAS through mediation by myopathy, inflammatory rhinitis, and/or tonsillar hypertrophy as first suggested by McCarty and colleagues.13 On the other hand, it is possible that the chronic low-grade inflammation accompanying untreated OSAS degrades vitamin D stores. 25(OH)D may be rapidly depleted by acute stress leading to the suggestion that 25(OH)D acts as an acute phase reagent.37 This hypothesis is further supported by a recent report demonstrating markedly increased 25(OH)D levels after only 7 days of CPAP use in male OSAS subjects.38
VDD has been associated with similar metabolic disturbances as OSAS, including elevated systemic inflammation, impaired glucose metabolism, dyslipidemia, and bone deformities, as well as many of the comorbidities associated with OSAS, including cardiovascular disease.7 Specific to OSAS, previous studies have demonstrated that 25(OH)D levels were inversely correlated with multiple metabolic parameters including HbA1c, HOMA-IR, fasting insulin, fasting glucose, total cholesterol, and triglyceride levels, as well as incidence of insulin resistance, type 2 diabetes, and metabolic syndrome.15,16 Further there is interventional data suggesting that vitamin D supplementation may have benefits regarding inflammation,39 glycemic indices, and lipids.40 Although, effects of vitamin D supplementation are inconsistent and controversial, these observations raise the intriguing possibility that reduced bio-availability and activity of 25(OH)D in clinical settings such as obesity or OSAS may facilitate the emergence of insulin resistance, systematic inflammation, dyslipidemia, and other OSAS-related morbidities.
CONCLUSION
We report a high prevalence of VDD in OSAS and that 25(OH) D is significantly and independently associated with AHI and mean nocturnal HR. We further demonstrate significantly lower 25(OH)D in a group of OSAS cases compared to non-OSAS subjects matched for important determinants of both OSAS and VDD. Our results are consistent with several recent reports of 25(OH)D levels in OSAS. To our knowledge, there are no reports of vitamin D supplementation in OSAS. Therefore, existing cross-sectional evidence must be interpreted with caution. Considering the cheap nature of vitamin D repletion as well as the non-skeletal effects of vitamin D in many disorders of relevance to OSAS, prospective studies and interventional trials of vitamin D supplementation are warranted to determine the effect, if any, on core symptoms of OSAS and related metabolic disturbances (e.g. inflammation, hyperglycemia, hyperlipidemia).
DISCLOSURE STATEMENT
This was not an industry supported study. Mr. Kerley is supported by funding from the Irish Thoracic Society and the Irish Lung Foundation. Dr. Hutchinson is supported by funding from the Irish Research Council. The funding bodies had no involvement in study design, data collection, analysis or interpretation. The authors have indicated no financial conflicts of interest. The work for this study was completed at the Respiratory and Sleep Diagnostics Department, Connolly Hospital, Blanchardstown, Dublin, Ireland.
ACKNOWLEDGMENTS
The authors express our thanks to the respiratory physiologists of the Respiratory and Sleep Diagnostics Department, Connolly Hospital, Blanchardstown and also the subjects who agreed to take part in this study.
ABBREVIATIONS
- 25(OH)D
25-hydroxyvitamin D
- AHI
apnea-hypopnea index
- BM
body mass index
- EDS
excessive daytime sleepiness
- ESS
Epworth sleepiness scale
- FEV1%
percentage of predicted forced expiratory volume in 1 second
- FVC%
percentage of predicted
- FFM
fat free mass
- FM
fat mass
- FVC
forced vital capacity
- HR
heart rate
- LLNS
lifelong non-smoker
- NC
neck circumference
- O2
oxygen
- OSAS
obstructive sleep apnea syndrome
- PLM
periodic leg movements
- PSG
polysomnography
- PTH
parathyroid hormone
- REM
rapid eye movement
- TST
total sleep time
- VDD
vitamin D deficiency
- VDI
vitamin D insufficient
- VDS
vitamin D sufficient
REFERENCES
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Ruiter ME, DeCoster J, Jacobs L, Lichstein KL. Sleep disorders in African Americans and Caucasian Americans: a meta-analysis. Behav Sleep Med. 2010;8:246–59. doi: 10.1080/15402002.2010.509251. [DOI] [PubMed] [Google Scholar]
- 3.Cassol CM, Martinez D, da Silva FA, Fischer MK, Lenz Mdo C, Bós ÂJ. Is sleep apnea a winter disease? meteorologic and sleep laboratory evidence collected over 1 decade. Chest. 2012;142:1499–507. doi: 10.1378/chest.11-0493. [DOI] [PubMed] [Google Scholar]
- 4.Peppard PE, Young T. Exercise and sleep-disordered breathing: an association independent of body habitus. Sleep. 2004;1:480–4. doi: 10.1093/sleep/27.3.480. 27. [DOI] [PubMed] [Google Scholar]
- 5.Lin QC, Chen LD, Yu YH, Liu KX, Gao SY. Obstructive sleep apnea syndrome is associated with metabolic syndrome and inflammation. Eur Arch Otorhinolaryngol. 2014;271:825–31. doi: 10.1007/s00405-013-2669-8. [DOI] [PubMed] [Google Scholar]
- 6.Chakhtoura M, Nasrallah M, Chami H. Bone loss in obesity and obstructive sleep apnea: a review of literature. J Clin Sleep Med. 2015;11:575–80. doi: 10.5664/jcsm.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 8.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guilleminault C, Poyares D, Rosa A, Huang YS. Heart rate variability, sympathetic and vagal balance and EEG arousals in upper airway resistance and mild obstructive sleep apnea syndromes. Sleep Med. 2005;6:451–7. doi: 10.1016/j.sleep.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Scragg RK, Camargo CA, Jr, Simpson RU. Relation of serum 25-hydroxyvitamin D to heart rate and cardiac work (from the National Health and Nutrition Examination Surveys) Am J Cardiol. 2010;105:122–8. doi: 10.1016/j.amjcard.2009.08.661. [DOI] [PubMed] [Google Scholar]
- 11.Ke L, Graubard BI, Albanes D, et al. Hypertension, pulse, and other cardiovascular risk factors and vitamin D status in Finnish men. Am J Hypertens. 2013;26:951–6. doi: 10.1093/ajh/hpt051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gominak SC, Stumpf WE. The world epidemic of sleep disorders is linked to vitamin D deficiency. Med Hypotheses. 2012;79:132–5. doi: 10.1016/j.mehy.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 13.McCarty DE, Chesson AL, Jr, Jain SK, Marino AA. The link between vitamin D metabolism and sleep medicine. Sleep Med Rev. 2014;18:311–9. doi: 10.1016/j.smrv.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Evatt ML. Vitamin D associations and sleep physiology-promising rays of information. Sleep. 2015;38:171–2. doi: 10.5665/sleep.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barceló A, Esquinas C, Piérola J, et al. Vitamin D status and parathyroid hormone levels in patients with obstructive sleep apnea. Respiration. 2013;86:295–301. doi: 10.1159/000342748. [DOI] [PubMed] [Google Scholar]
- 16.Bozkurt NC, Cakal E, Sahin M, Ozkaya EC, Firat H, Delibasi T. The relation of serum 25-hydroxyvitamin-D levels with severity of obstructive sleep apnea and glucose metabolism abnormalities. Endocrine. 2012;41:518–25. doi: 10.1007/s12020-012-9595-1. [DOI] [PubMed] [Google Scholar]
- 17.Mete T, Yalcın Y, Berker D, et al. Obstructive sleep apnea syndrome and its association with vitamin D deficiency. J Endocrinol Invest. 2013;36:681–5. doi: 10.3275/8923. [DOI] [PubMed] [Google Scholar]
- 18.Erden ES, Genc S, Motor S, et al. Investigation of serum bisphenol A, vitamin D, and parathyroid hormone levels in patients with obstructive sleep apnea syndrome. Endocrine. 2014;45:311–8. doi: 10.1007/s12020-013-0022-z. [DOI] [PubMed] [Google Scholar]
- 19.Pietrobelli A, Rubiano F, St-Onge MP, Heymsfield SB. New bioimpedance analysis system: improved phenotyping with whole-body analysis. Eur J Clin Nutr. 2004;58:1479–84. doi: 10.1038/sj.ejcn.1601993. [DOI] [PubMed] [Google Scholar]
- 20.Sung RY, So HK, Choi KC, Li AM, Yin J, Nelson EA. Body fat measured by bioelectrical impedance in Hong Kong Chinese children. Hong Kong Med J. 2009;15:110–7. [PubMed] [Google Scholar]
- 21.Hollis BW. Assessment of vitamin D status and definition of a normal circulating range of 25-hydroxyvitamin D. Curr Opin Endocrinol Diabetes Obes. 2008;15:489–94. doi: 10.1097/MED.0b013e328317ca6c. [DOI] [PubMed] [Google Scholar]
- 22.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 23.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–71. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 24.Cashman KD, Muldowney S, McNulty B, et al. Vitamin D status of Irish adults: findings from the National Adult Nutrition Survey. Br J Nutr. 2013;14:1248–56. doi: 10.1017/S0007114512003212. 109. [DOI] [PubMed] [Google Scholar]
- 25.Canpolat U, Ozcan F, Ozeke O, et al. Impaired cardiac autonomic functions in apparently healthy subjects with Vitamin D deficiency. Ann Noninvasive Electrocardiol. 2015;20:378–85. doi: 10.1111/anec.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86:1633–37. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 27.Scragg R, Khaw KT, Murphy S. Effect of winter oral vitamin D3 supplementation on cardiovascular risk factors in elderly adults. Eur J Clin Nutr. 1995;49:640–6. [PubMed] [Google Scholar]
- 28.Tverdal A, Hjellvik V, Selmer R. Heart rate and mortality from cardiovascular causes: a 12 year follow-up study of 379,843 men and women aged 40-45 years. Eur Heart J. 2008;29:2772–81. doi: 10.1093/eurheartj/ehn435. [DOI] [PubMed] [Google Scholar]
- 29.McCarty MF, Thomas CA. PTH excess may promote weight gain by impeding catecholamine-induced lipolysis-implications for the impact of calcium, vitamin D, and alcohol on body weight. Med Hypotheses. 2003;61:535–42. doi: 10.1016/s0306-9877(03)00227-5. [DOI] [PubMed] [Google Scholar]
- 30.Marcotorchino J, Tourniaire F, Astier J, et al. Vitamin D protects against diet-induced obesity by enhancing fatty acid oxidation. J Nutr Biochem. 2014;25:1077–83. doi: 10.1016/j.jnutbio.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Bouillon R, Carmeliet G, Lieben L, et al. Vitamin D and energy homeostasis: of mice and men. Nat Rev Endocrinol. 2014;10:79–87. doi: 10.1038/nrendo.2013.226. [DOI] [PubMed] [Google Scholar]
- 32.Vimaleswaran KS, Berry DJ, Lu C, et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013;10:e1001383. doi: 10.1371/journal.pmed.1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drincic AT, Armas LA, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring) 2012;20:1444–8. doi: 10.1038/oby.2011.404. [DOI] [PubMed] [Google Scholar]
- 34.McCarty DE, Reddy A, Keigley Q, Kim PY, Marino AA. Vitamin D, race, and excessive daytime sleepiness. J Clin Sleep Med. 2012;8:693–7. doi: 10.5664/jcsm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertisch SM, Sillau S, de Boer IH, Szklo M, Redline S. 25-hydroxyvitamin D concentration and sleep duration and continuity: multi-ethnic study of atherosclerosis. Sleep. 2015;38:1305–11. doi: 10.5665/sleep.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerley CP, Elnazir B, Faul J, Cormican L. Vitamin D as an adjunctive therapy in asthma. Part 2: a review of human studies. Pulm Pharmacol Ther. 2015;32:75–92. doi: 10.1016/j.pupt.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Silva MC, Furlanetto TW. Does serum 25-hydroxyvitamin D decrease during acute-phase response? A systematic review. Nutr Res. 2015;35:91–6. doi: 10.1016/j.nutres.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Liguori C, Romigi A, Izzi F, et al. continuous positive airway pressure treatment increases serum vitamin d levels in male patients with obstructive sleep apnea. J Clin Sleep Med. 2015;11:603–7. doi: 10.5664/jcsm.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–9. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 40.Schnatz PF, Jiang X, Vila-Wright S, et al. Calcium/vitamin D supplementation, serum 25-hydroxyvitamin D concentrations, and cholesterol profiles in the Women's Health Initiative calcium/vitamin D randomized trial. Menopause. 2014;21:823–33. doi: 10.1097/GME.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]