Abstract
Study Objectives:
A primary focus of the National Institute of Mental Health's current strategic plan is “predicting” who is at risk for disease. As such, the current investigation examined the utility of premorbid sleep reactivity in identifying a specific and manageable population at elevated risk for future insomnia.
Methods:
A community-based sample of adults (n = 2,892; 59.3% female; 47.9 ± 13.3 y old) with no lifetime history of insomnia or depression completed web-based surveys across three annual assessments. Participants reported parental history of insomnia, demographic characteristics, sleep reactivity on the Ford Insomnia in Response to Stress Test (FIRST), and insomnia symptoms. DSM-IV diagnostic criteria were used to determine insomnia classification.
Results:
Baseline FIRST scores were used to predict incident insomnia at 1-y follow-up. Two clinically meaningful FIRST cutoff values were identified: FIRST ≥ 16 (sensitivity 77%; specificity 50%; odds ratio [OR] = 2.88, P < 0.001); and FIRST ≥ 18 (sensitivity 62%; specificity 67%; OR = 3.32, P < 0.001). Notably, both FIRST cut-points outperformed known maternal (OR = 1.49–1.59, P < 0.01) and paternal history (P = NS) in predicting insomnia onset, even after controlling for stress exposure and demographic characteristics. Of the incident cases, insomniacs with highly reactive sleep systems reported longer sleep onset latencies (FIRST ≥ 16: 65 min; FIRST ≥ 18: 68 min) than participants with nonreactive insomnia (FIRST < 16: 37 min; FIRST < 18: 44 min); these groups did not differ on any other sleep parameters.
Conclusions:
The current study established a cost- and time-effective strategy for identifying individuals at elevated risk for insomnia based on trait sleep reactivity. The FIRST accurately identifies a focused target population in which the psychobiological processes complicit in insomnia onset and progression can be better investigated, thus improving future preventive efforts.
Citation:
Kalmbach DA, Pillai V, Arnedt JT, Drake CL. Identifying at-risk individuals for insomnia using the ford insomnia response to stress test. SLEEP 2016;39(2):449–456.
Keywords: FIRST, insomnia, sleep onset, sleep reactivity, vulnerability
Significance.
A sleep system that is reactive to stress constitutes a robust vulnerability to incident insomnia. Individuals with such sleep reactivity can be easily identified using a brief self-report instrument: the Ford Insomnia Response to Stress Test. As improvements to patient care center on the ability of healthcare providers to detect patients at risk for insomnia prior to disease onset, this line of research has significant implications for preventative care. Further research on reactive sleepers, as identified by this study, will help discover other premorbid biomarkers of insomnia, offer insight into disease progression, and help examine the efficacy of prophylactic insomnia treatments.
INTRODUCTION
Insomnia is recognized as the most prevalent sleep disorder, afflicting approximately 9% to 20% of the adult population in the United States,1,2 with incidence rates estimated between 7% and 10%.2–4 Despite these staggering figures, the field has yet to develop promising preventive care interventions to curb incidence rates. Indeed, prevention efforts face many challenges, most notable of which is difficulty identifying individuals at elevated risk for insomnia. Importantly, given the established morbidity associated with insomnia—including a wide range of serious medical problems,5 depression,6,7 alcohol and substance use disorders, and suicide8—it is critical to identify at-risk individuals prior to the first onset of insomnia. Currently, there is no widely accepted empirical method for categorizing premorbid sleepers as high versus low risk for insomnia. Most of the research on insomnia risk factors has emerged from cross-sectional studies among already affected individuals,9 thus rendering it difficult to separate the underlying pathophysiology of the disorder from its morbidity. Though prospective investigations have demonstrated both poor physical and mental health conditions to increase risk for sleep disturbance and insomnia,3,4,10 no empirically validated method currently exists to classify sleepers accurately as high versus low risk for insomnia, irrespective of such morbidities. Accurately classifying high- and low-risk individuals would allow for the identification of premorbid biomarkers and causal pathways to incident insomnia among individuals without a lifetime history of the disorder. Thus, identifying at-risk sleepers carries great potential for the study of insomnia etiology and prevention. To address this gap in the literature, we sought to determine whether premorbid sleep reactivity—i.e., the tendency to exhibit sleep disturbance in response to a sleep challenge— can help identify individuals at risk for insomnia incidence and chronicity.
Owing to the robustness of the stress-diathesis model of insomnia,11,12 two widely supported risk factors have been demonstrated in the literature. First, family history of insomnia has long been viewed as a reliable indicator of risk, with recent findings showing maternal history as the strongest predictor.13,14 However, a number of methodological limitations diminish the clinical and research utility of evaluating risk based on this factor. Notably, as most laypeople are not trained in distinguishing between acute sleep disturbance and clinical insomnia, the validity of these reports—either positive or negative—cannot be established.14 Quite importantly, this method of risk assessment precludes classifying any individual who is unaware of his or her biological family's medical history.
A second risk factor in the literature to receive much attention is sleep reactivity.15–19 Sleep reactivity is a heritable15,20 trait predisposition to insomnia that manifests as a sleep system that is sensitive or “reactive” to stress.17 Prior investigations have shown that individuals with high sleep reactivity but no current/ prior insomnia report greater disruption in sleep in response to caffeine intake,18,19 circadian misalignment,19,21 and interpersonal stressors22 than do non-reactive sleepers. The Ford Insomnia Response to Stress Test (FIRST)18 is a psychometrically validated nine-item self-report instrument commonly used to measure sleep reactivity. As a self-report measure, it is not constrained by many of the limitations of patient-reported biological family history of sleep disorders. Furthermore, it presents a viable risk evaluation that is expeditious and cost effective, minimizing burden in both research and clinical settings.
The goal of the current investigation was to identify individuals at elevated risk for the development of insomnia using the FIRST, and to identify clinically relevant cut-points for the measure in predicting initial onset of insomnia. We sought to evaluate the predictive qualities of the FIRST in comparison to participant-reported parental history of insomnia while controlling for stress exposure (i.e., number of major life events). To accomplish these goals, we analyzed data from a large community-based adult sample recruited in the Evolutions of Pathways to Insomnia Cohort (EPIC)22 study. Using a prospective design, we collected three waves of annual data from an adult sample with no current or lifetime history of insomnia or depression. We hypothesized that the risk for insomnia as indicated by high sleep reactivity would accurately distinguish between acute insomniacs, chronic insomniacs, and individuals without the disorder.
METHODS
Participants
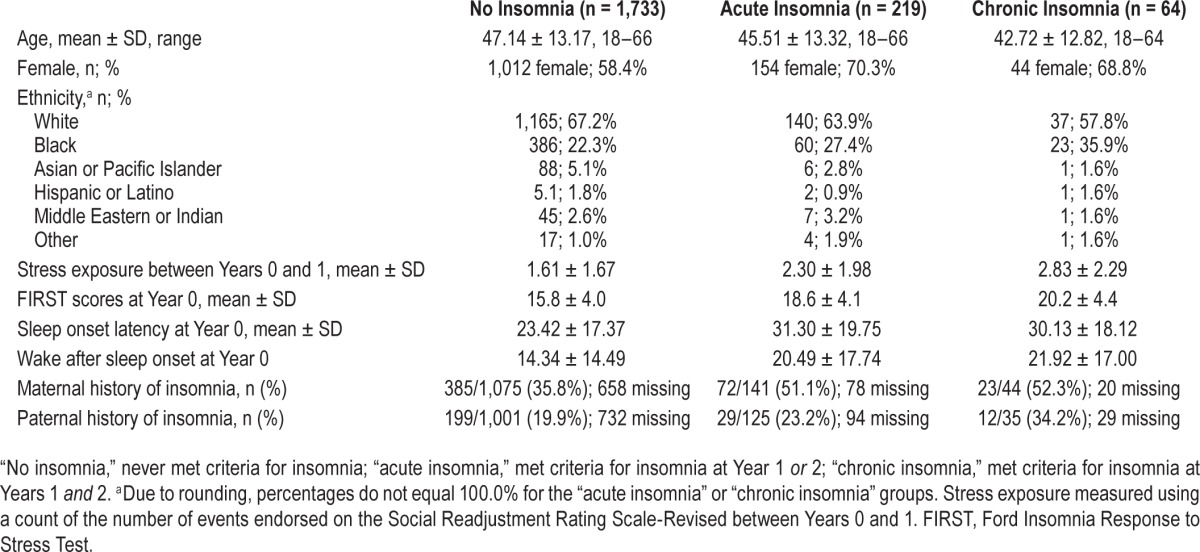
Data were collected from a large community sample in southeastern Michigan as part of a 3-y National Institute of Mental Health-funded investigation. Detailed recruitment, eligibility, and demographic information has been reported elsewhere.11 For initial assessment (Year 0), study invitations were mailed to a randomly generated list of individuals (n = 36,002) from a major Health Maintenance Organization database. Of these individuals, 7,608 completed an online eligibility survey, which screened for current/lifetime history of insomnia and depression based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition23 (DSM-IV) criteria, and inconsistent or indiscriminate responding. A total of 2,590 individuals screened positive for current/lifetime insomnia and/or depression, whereas 149 individuals were identified as inconsistent or indiscriminate responders, resulting in the exclusion of 2,739 respondents. In addition, 1,339 individuals declined to participate in the study. A total of 3,530 individuals participated in the Year 0 assessment. One year later, 2,892 completed Year 1 follow-up (attrition rate = 18.1%). Finally, 2,016 participants completed the third and final wave of data collection at Year 2 (attrition rate = 30.3% from Year 1). Baseline demographic characteristics revealed the sample to be largely middle-aged, female, and white, though some ethnic diversity was observed (see Table 1 for sample characteristics).
Table 1.
Sample descriptive characteristics at baseline (n = 2,016 with full data).
Procedure
All study protocols were approved by the Henry Ford Hospital institutional review board. Individuals were required to provide informed consent prior to participating. The setting of this study was a web-based epidemiological survey. Data were collected in three annual waves. Year 0 data were collected after participants met eligibility requirements using web-delivered questionnaires. One month prior to each annual follow-up (at Years 1 and 2), participants received Email reminders. Each assessment took approximately 30 min to complete.
Measures
Insomnia
Individuals were classified with insomnia at Years 0, 1, and 2. At Year 0, those who met criteria for insomnia were excluded from the study, whereas insomnia status was the target outcome at Years 1 and 2. DSM-IV based insomnia classifications were established using the following questions: “In the past year, have you experienced difficulty falling asleep for at least 1 mo?” “In the past year, have you experienced difficulty staying asleep for at least 1 mo?” and “In the past year, have you experienced difficulty with nonrefreshing sleep for at least 1 mo?” Responses ranged from 0 (“not at all”) to 4 (“very much”), and participants who reported a score of 2 (“somewhat”) or higher met that nocturnal symptom criterion. In addition, participants were asked to estimate the number of days per week these nocturnal insomnia symptoms occurred. Further, participants had to endorse daytime impairment secondary to sleep difficulties in response to the following question: “To what extent do you consider your sleep problems to interfere with your daily functioning?” Responses again ranged from 0 to 4, and participants who reported a score of 2 (“somewhat”) or higher met the daytime impairment criterion. To meet criteria, participants had to report experiencing one or more of these nocturnal insomnia symptoms three or more nights per week for at least a 1-mo period, and meet the daytime impairment criterion for a duration of 1 mo or longer.
Insomnia Symptom Presentation
Participants were asked to respond to the following items to offer a more detailed depiction of baseline sleep and insomnia complaints at Years 1 and 2. Sleep onset latency (SOL): “On average (including weekdays and weekends), how long does it take you to fall asleep (in the past month)?” with responses in minutes. Wake after sleep onset (WASO): “On average, how long does it take you to fall back asleep after waking up (during the past month)?” with responses in minutes. Subjective insomnia severity: “How severe were your sleep problems?” with responses ranging from 0 “low” to 10 “high.” Daytime impairment: “To what extent do you consider your sleep problem to interfere with your daily functioning (e.g. daytime fatigue, ability to function at work/daily chores, concentration, memory, mood, etc.)?” with response options ranging from 0 “not at all” to 4 “very much.” Frequency of nighttime symptoms: “During your insomnia, on average, how many days per week did you experience the sleep problem?” with responses in number of days.
Parental History of Insomnia
To assess parental history of insomnia, participants were asked to respond “yes” or “no” to the two following questions: “Has your biological mother had difficulty falling asleep, staying asleep, or nonrefreshing sleep three or more times per week for at least 1 mo?” and “Has your biological father had difficulty falling asleep, staying asleep, or nonrefreshing sleep three or more times per week for at least 1 mo?”
Sleep Reactivity
The current study examined trait sleep reactivity at Year 0, prior to any lifetime history of insomnia among our eligible participants. The FIRST18 is a self-report measure of trait sleep reactivity. Items on the FIRST ask respondents to rate the likelihood (not, somewhat, moderately, and very likely) that they would experience sleep difficulties in reaction to nine hypothetical stressful situations (e.g., “after a stressful experience during the day,” “before an important meeting the next day”). Higher scores on the FIRST indicate a more highly reactive sleep system.
Stress Exposure: Number of Events
Stress exposure was based on the Social Readjustment Rating Scale (SRRS-R),24,25 an inventory of 52 stressful life events commonly reported by US adults (e.g., divorce, change in residence, loss of employment). In the current study, we examined the total number of endorsed events for each participant reported at Year 1.
Analysis Plan
Logistic regression with maximum likelihood estimation was used to evaluate risk for insomnia incidence and chronicity as predicted by sleep reactivity and stress exposure, controlling for demographic characteristics. To determine FIRST cut-points, a receiver operating characteristic (ROC) curve was employed, and evaluation of Youden J index associated with potential cut-point values informed balance of sensitivity and specificity in the selection of optimal clinical cutoffs. Next, the positive and negative predictive values of these cutoffs were examined and compared to the sample base rate. We then compared low-risk versus at-risk individuals for their risk for insomnia onset and chronicity. Finally, we compared insomnia symptom presentation between insomniacs with and without highly reactive sleep systems.
RESULTS
Preliminary Analyses
We observed 262 new cases of insomnia at 1-y follow-up (of n = 2,892 at Year 1), representing an incidence rate of 9.1%., which is consistent with other population-based incidence estimates2,3 (see Table 1 for sample descriptive characteristics). Regarding parental history of insomnia, more than one-third of the sample reported that they “did not know” their sleep-related parental history. Of those who were cognizant of their parental history, 37.9% of participants indicated maternal history of insomnia, as compared to 20.9% who indicated a paternal history. Additionally, the average number of major life stressors leading up to Year 1 was 1.7 ± 1.7. Next, using oneway analysis of variance, we compared FIRST scores among never-insomniacs, acute insomniacs (met criteria at Year 1 or 2), and chronic insomniacs (met criteria at Years 1 and 2). Analyses revealed that the groups significantly differed in baseline FIRST scores (F(2,2013) = 78.42, P < 0.001), corresponding to a medium-large effect (η2 = 0.07). Post hoc Bonferroni tests showed that never-insomniacs rated lower sleep reactivity as compared to both acute (MΔ = 2.8, P < 0.001) and chronic (MΔ = 4.4, P < 0.001) insomniacs. Moreover, individuals with chronic insomnia indicated greater sleep reactivity than those with acute insomnia (MΔ = 1.6, P = 0.02).
To determine relevant covariates for testing our substantive hypotheses, we fit a logistic regression model estimating risk for insomnia as predicted by sex, age, stress exposure between baseline and 1-y follow-up, and known parental history of insomnia. The overall model reliably distinguished between insomniacs and those in whom the disorder did not develop (χ2(5) = 50.62, P < 0.001), and the Hosmer-Lemeshow test revealed that the model fit the data well (χ2(8) = 6.17, P = 0.63). Evaluation of the individual predictors revealed that maternal history of sleep disturbance (b = 0.60, odds ratio [OR] = 1.82, 95% confidence interval [CI] = 1.22–2.71, P < 0.01) and stress exposure (b = 0.27, OR = 1.32, 95% CI = 1.21–1.43, P < 0.001) increased likelihood for the development of insomnia. Specifically, the odds of the development of insomnia among individuals with a maternal history of insomnia were nearly twice than for those without, and each major life stressor increased odds for insomnia by 32%. Age (P = 0.97), sex (P = 0.24), and paternal history of insomnia (P = 0.78) were not significantly related to insomnia incidence in the overall model. Consequently, maternal history and stress exposure were used as covariates in models predicting insomnia onset.
Determining FIRST Cut-points
Prior to determining FIRST cut-points, we began by examining its predictive qualities for insomnia incidence, independent of maternal history and stress exposure. We fit a logistic regression model estimating likelihood of insomnia incidence as predicted by total FIRST scores and controlling for maternal history and stress exposure. The model reliably distinguished between individuals with insomnia and individuals without the disorder (χ2(3) = 108.773, P < 0.001), and fit the data well (χ2(8) = 3.79, P = 0.88). As predicted, baseline FIRST scores predicted insomnia onset at 1-y follow-up (b = 0.13, OR = 1.14, 95% CI = 1.10–1.18, P < 0.01) such that each one-point increase on the FIRST was associated with a 14% increase in the odds for the development of insomnia while controlling for maternal history (OR = 1.53, P = 0.01) and stress exposure (OR = 1.28, P < 0.001).
Next, we constructed a ROC curve: sleep reactivity predicting incident insomnia. Based on Youden J index, a cutoff of 18 was identified as providing optimal balance of sensitivity and specificity (Youden J index: 0.62 (sensitivity) + 0.67 (specificity) – 1 = 0.29). That is, of the 262 individuals in whom insomnia developed at 1-y follow-up, 163 scored 18 or above on the FIRST at baseline, whereas 67% of individuals without insomnia scored below threshold at baseline. As such, individuals who scored 18 or higher on the FIRST were considered at elevated risk for insomnia. However, because preventive efforts may benefit from a more sensitive test, we examined additional potential cut-points indicating risk for insomnia with greater sensitivity. Specifically, we examined potential cut-points of 16 (the sample median) and 17. The Youden J for a cutoff of 16 (sensitivity 0.77 and specificity 0.50; Youden J = 0.27) outperformed that of a 17-point cutoff (Youden J = 0.26). Of the 262 Year 1 individuals with insomnia, 202 scored ≥ 16 on the FIRST at baseline, whereas 1,301 of the 2,630 individuals in whom the disorder did not develop scored below 16. Next, we further explored the predictive values and risk associated with the 16- and 18-point FIRST cutoffs.
Comparing Sleep Reactive and Nonreactive Individuals
FIRST ≥ 16
When using FIRST ≥ 16 as the cut-point, 1,531 of the 2,892 were identified as having risk for insomnia (Table 2). Of these 1,531 individuals, clinical insomnia developed in 202 at 1-y follow-up, resulting in a positive predictive value (PPV) of 13.2%. Notably, PPV varies as a function of base rate, and the observed 13.2% PPV associated with a cut-point of 16 is 45% higher than our samples' 9.1% 1-y insomnia incidence rate. The negative predictive value (NPV) was 95.6%. In other words, of the 1,361 people who scored below 16 at baseline, insomnia developed in only 60 of those individuals.
Table 2.
Ford Insomnia Response to Stress Test ≥ 16 characteristics.
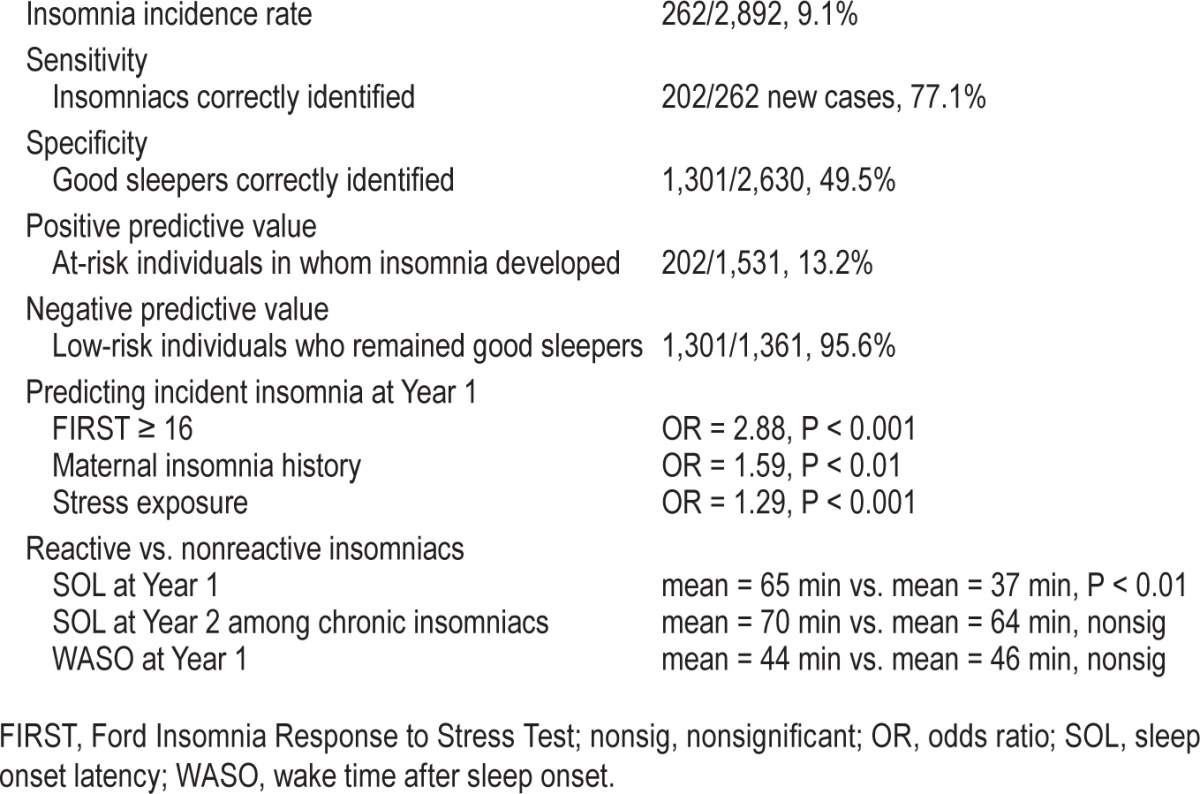
We then tested the risk for insomnia among individuals identified as having low versus elevated sleep reactivity based on the FIRST cutoff of 16 (Table 2). First, we fit a logistic regression predicting 1-y insomnia incidence and found that the risk of clinical insomnia developing in individuals who scored 16 or higher was nearly 300% higher than low-risk individuals (b = 1.06, OR = 2.88, 95% CI = 1.96–4.22, P < 0.001), while controlling for the effects of maternal insomnia history (OR = 1.59, P < 0.01) and stress exposure (OR = 1.29, P < 0.001). Because 37.9% of the sample's data were missing from the logistic regression model based on nonresponse rate regarding maternal history, we re-ran the model without this covariate to test whether the findings would replicate in the full sample. Analyses again revealed that reactive individuals were three times as likely to report insomnia at 1-y follow-up (OR = 3.00, P < 0.001). Next, as poor sleepers are at elevated risk for future insomnia,10 we evaluated whether the FIRST ≥ 16 cut-point predicted future insomnia in participants without insomnia at baseline who endorsed sleep disturbance, but did not meet insomnia criteria due to not endorsing the symptom frequency or daytime impairment criteria. In this subsample of disturbed baseline sleepers, analyses revealed that insomnia was twice as likely to develop in poor sleepers who scored 16 or higher on the FIRST than in nonreactive poor sleepers (OR = 2.09, P < 0.01) while controlling for maternal history (P = 0.40) and stress exposure (P < 0.001).
We then tested whether the 16 cut-point differentiated between individuals with chronic insomnia (defined as having insomnia at Years 1 and 2) versus individuals who were never classified as having insomnia. Analyses revealed that insomnia was six times as likely to develop in reactive individuals (b = 1.80, OR = 6.06, 95% CI = 2.52–14.57, P < 0.001). Of note, though stress exposure predicted chronic insomnia (OR = 1.28, P < 0.001), maternal history did not differentiate between chronic insomniacs and never-insomniacs (OR = 1.53, P = 0.18).
Last, we sought to compare sleep reactive versus nonreactive insomniacs to investigate whether these groups differed in symptom presentation and disease course. Analyses revealed that sleep reactive insomniacs were more than twice as likely to endorse difficulty initiating sleep (b = 0.99, OR = 2.70, 95% CI = 1.49–4.90, P < 0.01) such that sleep reactive insomniacs reported nearly double the SOL as nonreactive insomniacs, t(260) = 2.88, P < 0.01, Cohen d = 0.49 corresponding to a medium effect (Table 2). However, this SOL-related difference between sleep reactive and nonreactive insomniacs became nonsignificant when insomnia became chronic at Year 2 (OR = 1.68, P = 0.50). As expected, sleep reactive and nonreactive insomniacs were above quantitative threshold in regard to WASO,26 though no significant difference was observed between sleep reactive insomniacs (44 min) and nonreactive insomniacs (46 min), t(270) = 0.41, P = 0.80. No group differences were found in frequency of nighttime symptoms, non-refreshing sleep, daytime impairment, or subjective severity of insomnia. Finally, sleep reactive and nonreactive insomniacs did not differ in disease course from Year 1 to Year 2 (OR = 1.98, P = 0.09).
FIRST ≥ 18
When using FIRST ≥ 18 as the cut-point, 1,035 of 2,892 baseline individuals (35.8%) were identified as being at risk for insomnia (Table 3). Of those 1,035 individuals, 163 met criteria for clinical insomnia at 1-y follow-up. The observed 15.7% PPV was 73% higher than the samples' 9.1% incidence rate. In comparison, insomnia developed in only 5.3% (i.e., 99/1,857) of the individuals identified as low risk (NPV: 94.7%).
Table 3.
Ford Insomnia Response to Stress Test ≥ 18 characteristics.
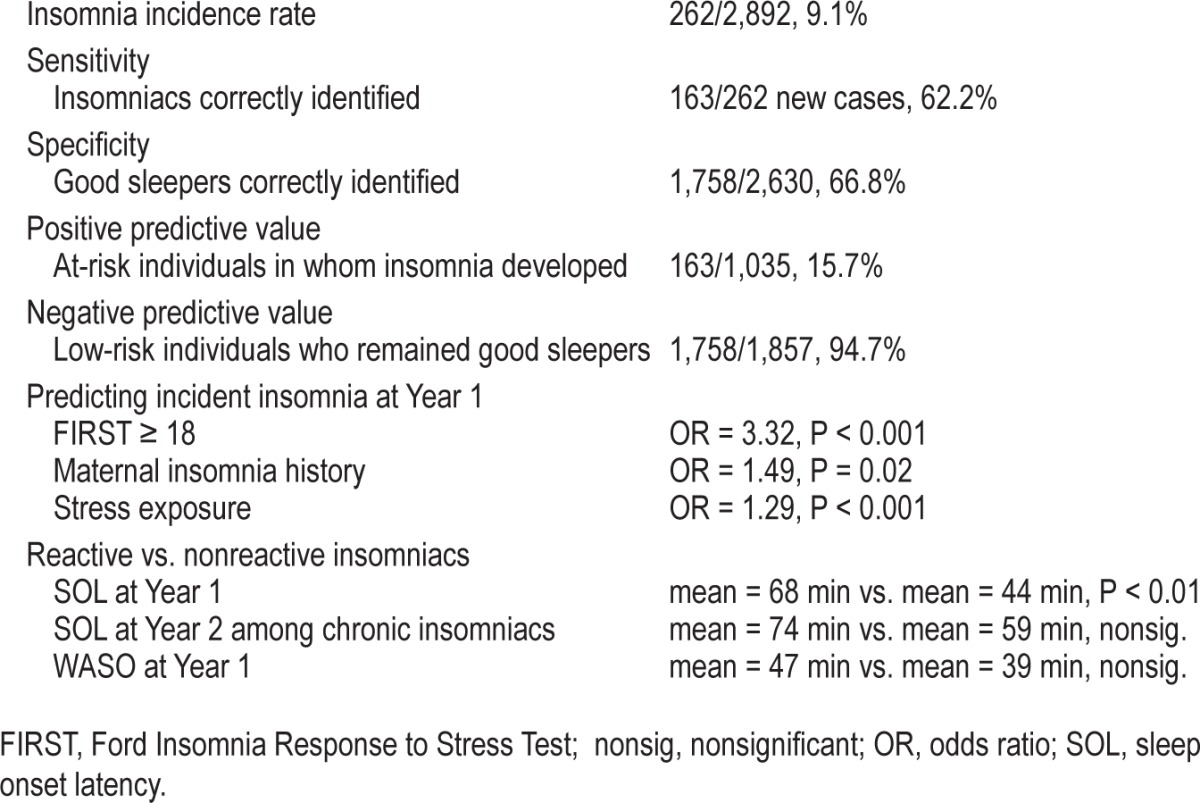
We then tested the risk of insomnia incidence for individuals identified as having low versus high sleep reactivity using the 18-point cutoff (Table 3). Logistic regression revealed that that the odds of insomnia onset were more than 300% greater among at-risk individuals (b = 1.20, OR = 3.32, 95% CI = 2.35– 4.68, P < 0.001) while controlling for the effects of maternal history (OR = 1.49, P = 0.02) and stress exposure (OR = 1.29, P < 0.001). To demonstrate this finding once again in the full sample, we re-ran the model excluding the maternal history covariate, which replicated the effect size: OR = 3.03, P < 0.001. Next, we evaluated the utility of the FIRST ≥ 18 cutoff among poor sleepers to rule out reactivity-based insomnia risk as an artifact of baseline sleep disturbance. Analyses revealed that insomnia was almost three times more likely to develop in poor sleepers with elevated sleep reactivity than in nonreactive poor sleepers (OR = 2.73, P < 0.001) while controlling for maternal history (P = 0.58) and stress exposure (P < 0.001).
We then tested whether the 18-point cutoff differentiated between chronic insomniacs versus never-insomniacs. Analyses revealed that chronic insomnia was five times as likely to develop in individuals who scored at or above the FIRST 18 cut-point at baseline (b = 1.68, OR = 5.39, 95% CI = 2.70–10.72, P < 0.001) while controlling for stress exposure (OR = 1.29, P < 0.001). Once again, however, maternal history of insomnia did not differentiate between chronic insomniacs and never-insomniacs (OR = 1.44, P = 0.26). Analyses revealed that sleep reactive insomniacs were twice as likely to complain of difficulty initiating sleep (b = 70.99, OR = 2.02, 95% CI = 1.18– 3.45, P < 0.01), which corresponded to a 24-min longer SOL for sleep reactive insomniacs compared to nonreactive insomniacs, t(260) = 2.75, P < 0.01, Cohen d = 0.36 corresponding to a small to medium effect. Once again, this SOL-related difference between sleep reactive and nonreactive insomniacs became nonsignificant when insomnia became chronic (b = 0.36, OR = 1.43, 95% CI = 0.41–5.01, P = 0.58). Both sleep reactive and nonreactive insomniacs reported sleep maintenance difficulties and were above threshold in regard to WASO,26 with no group difference observed between sleep reactive insomniacs (47 min) and nonreactive insomniacs (39 min), t(270) = 1.08, P = 0.28. No other group differences in symptom presentation were found between sleep reactive and nonreactive insomniacs. Finally, sleep reactive and nonreactive insomniacs did not differ in disease course from Year 1 to Year 2 (OR = 1.51, P = 0.22).
Comparing Cutoff Values for Relative Risk
Last, we evaluated relative risk associated with the FIRST cut-points among reactive sleepers by comparing insomnia rates at Year 1 between two groups: (1) moderately reactive sleepers as indicated by scoring 16 or 17 on the FIRST (i.e., above threshold, but below the 18-point value), and (2) highly reactive sleepers as indicated by scoring 18 or above on the FIRST. A significant 2 × 2 chi-square analysis showed that 7.7% of moderately reactive sleepers (n = 39/505), developed insomnia at Year 1, whereas insomnia developed in 16.1% of highly reactive sleepers (n = 170/1,055) (OR = 2.30, χ2(1) = 20.73, P < 0.001). Notably, this finding was replicated using logistic regression and controlling for maternal history and stress exposure, and the odds of the development of insomnia were greater for highly reactive sleepers (OR = 2.61, P < 0.001). Moreover, relative risk estimates were then calculated among nonreactive (FIRST scores ≤ 15), moderately reactive, and highly reactive sleepers. Insomnia developed in 4.4% of nonreactive sleepers (60/1,361). Therefore, the risk for insomnia was 1.8 (0.077/0.044) times higher for moderately reactive sleepers compared to nonreactive sleepers. Further, the risk for insomnia was 3.7 (16.1/4.4) and 2.1 (16.1/7.7) times higher for highly reactive sleepers as compared to nonreactive and moderately reactive sleepers, respectively.
DISCUSSION
This investigation sought to use a trait measure of sleep reactivity to identify individuals at elevated risk for insomnia using web-based epidemiological survey data from a large community-based sample with no lifetime history of the disorder. Results identified two cut-points for the FIRST indicating elevated risk for initial onset of insomnia. Importantly, the FIRST cutoffs outperformed participant-reported parental history of insomnia. Not only did the FIRST cutoff values correspond to insomnia incidence, they differentiated between chronic insomniacs and never-insomniacs, which known parental history failed to do. Notably, symptom presentation differed between sleep reactive and nonreactive insomniacs at the time of insomnia incidence (1-y post initial assessment) such that individuals with sensitive sleep systems were more likely to complain of prolonged sleep onset latency. Together, these findings offer strong empirical support for the utility of the FIRST for identifying individuals at risk for developing acute and chronic insomnia.
We identified two clinically relevant cut-points for the FIRST: ≥ 16 and ≥ 18. The 16-point cutoff value was the more sensitive of the two identified cut-points, and insomnia was almost three times more likely to develop in individuals who scored ≥ 16 over the follow-up period than individuals who scored below threshold (Table 2). Moreover, chronic insomniacs were six times more likely to score above the 16-point cutoff compared to never-insomniacs. Seventy-seven percent of insomniacs at 1-y follow-up were identified as at-risk for insomnia (sensitivity) prior to onset. However, only half of sleepers in whom insomnia did not develop at 1-y follow-up were correctly identified at baseline (specificity). In this context, we believe it is important to note that sensitivity and specificity values are traditionally presented to characterize a test's ability to detect current disease status. This investigation differs in that these values represent predictive qualities 1 y prior to disease detection. Finally, some evidence was found to suggest that individuals with moderate levels of sleep reactivity (operationalized as reporting FIRST scores of 16 and 17, i.e., above threshold, but below the 18-point cutoff) were at twice the risk for insomnia than nonreactive sleepers (i.e., FIRST ≤ 15), but just half of the risk of highly reactive sleepers (i.e., FIRST ≥ 18). Thus, individuals who score in this range may be interpreted as having moderate risk for insomnia.
The value of FIRST ≥ 18 resulted in a more conservative test (Table 3). In these individuals, insomnia was 300% as likely to develop at 1-y follow-up, and they were more than five times more likely to be chronic insomniacs than never-insomniacs. Moreover, the insomnia risk for these highly reactive individuals was approximately two to four times as high when compared to moderately reactive and nonreactive sleepers, respectively. As such, individuals who score 18 or higher on the FIRST may be described as being at high risk for insomnia. Of note, 62.2% of insomniacs at Year 1 were identified as at high risk for insomnia at baseline, whereas 66.8% of noninsomniacs were correctly identified as having low risk using this cut-point. In comparison to the 16-point cutoff, these values reflect a less sensitive test, but also a markedly lower false positive rate and higher PPV. Overall, we contend that neither cut-point is universally preferable over the other. Rather, we urge clinicians and researchers to use either cutoff based on their needs and preferences regarding sensitivity and specificity. Future research may investigate the benefits associated with each cutoff in predicting future sleep pathology.
Not only did the FIRST reliably identify at-risk individuals, it was a stronger predictor than known biological parental history of insomnia. Specifically, prior research has identified maternal history of insomnia to accurately predict elevated risk for this disorder13,14; a finding that was replicated in our study. However, the effect size of participant-reported maternal history substantially smaller than the effect sizes associated with the FIRST cutoff values. Unexpectedly, maternal history did not differentiate between chronic insomniacs and never-insomniacs, whereas both FIRST cut-points distinguished between the two groups. It is important to note that more than one-third of our sample indicated not knowing whether or not their biological mother or father had a history of insomnia. This is a serious limitation that critically restricts the utility of solely relying on patient- or participant-reported biological family history as an indicator of future insomnia risk. Significant method error is likely involved when individuals are asked to report on familial history of clinical sleep disorders, as most individuals are not trained to identify these disorders nor may be privy to family members' health history. Given the heritability of sleep reactivity,15,20 it is likely that parental history may have served as a proxy for sleep reactivity in prior studies. Indeed, recent research by Fernandez-Mendoza and colleagues20 has shown that FIRST scores aggregate within nuclear families with heritability estimated at 29%.
Importantly, the FIRST demonstrated good clinical utility in a subsample of poor baseline sleepers. Within these individuals—those who endorsed nocturnal symptoms but did not meet insomnia criteria due to not endorsing frequency or daytime impairment criteria—sleep reactivity played an important role in the risk for insomnia. Though reactive sleepers may be more innately predisposed to experience sleeping problems, finding that insomnia was two to three times more likely to develop in reactive poor sleepers than in nonreactive poor sleepers suggests that the effect of sleep reactivity on insomnia risk is independent of premorbid sleep disturbance.
Sleep Reactivity and Future Insomnia Research
The current study found that SOL was nearly twice as long in duration at disease onset among reactive insomniacs (see Tables 2 and 3), a finding that is highly consistent with prior research on acute sleep onset insomnia as a gateway to chronic insomnia.27,28 Germane to this presleep wakefulness, prior research has demonstrated that heightened cognitive arousal is associated with trait sleep reactivity,12 and that cognitive arousal even moderates the relationship between sleep reactivity and incident insomnia22 such that sleep reactive individuals are especially sensitive to cognitive intrusions (e.g., worry, rumination). Thus, a mechanism by which sleep reactivity initiates and perpetuates insomnia disorder may center on the cognitive processes during the presleep period. In a recent review, Riemann and colleagues29 demonstrated that cognitive arousal is not only a common complaint among insomniacs, but also that presleep cognitive content often reappears in dreams. The authors presented these findings in the context of highlighting rapid eye movement (REM) instability (i.e., frequent micro-arousals and macroarousals during REM sleep) as a possible pathway to and biomarker of insomnia. Thus, future studies can determine whether REM instability presages insomnia by examining whether such premorbid polysomnogram abnormalities are evident in sleep reactive individuals. Additionally, risk for incident insomnia may be greatly minimized should cognitive arousal be identified among premorbid samples with high sleep reactivity. For instance, both behavioral interventions to override nocturnal wakefulness (e.g., restricting time in bed30) coupled with techniques to defuse cognitive arousal (e.g., mindfulness meditation31), may preempt the evolution of transient sleep disturbance into clinical insomnia.
Though most insomniacs reported high baseline sleep reactivity and initially presented with pronounced sleep latency, a subgroup of insomniacs was described as having low sleep reactivity prior to the development of the disorder, among whom no clear insomnia phenotype was observed. Despite difficulties falling and staying asleep among nonreactive insomniacs exceeding quantitative criteria only modestly to moderately,26 symptom presentation, other than the aforementioned differences in sleep latency, was highly similar and disease course did not differ between reactive and nonreactive insomnia subgroups. Even so, the onset of insomnia in low-reactivity individuals highlights the multifactorial and complex etiology of sleep pathology, and consequent limitations in the ability of the FIRST to detect disease risk in this smaller, less premorbidly reactive subpopulation of future insomniacs.
Study Limitations
We believe the current study adds to the literature on the development of insomnia, but must be interpreted in the context of some methodological limitations. First, we must acknowledge the potential threats to validity due to the use of self-report instruments. Second, participants were not screened for comorbid psychopathology (other than depression). However, comorbid insomnia is the norm in the general population, thus adding to the generalizability of results. Finally, older adults were under-represented in the current study, and our sample consisted of no participants older than 67 y. As such, it is unclear whether these findings generalize to older adults. Despite these limitations, we demonstrated the FIRST's capability of predicting initial onset insomnia in individuals without history of insomnia or depression, thus affording opportunity to preempt the disease process, and allowing for more personalized treatment.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by an NIMH Grant (R01 MH082785; PI: Drake) and an investigator initiated research award from Merck & Co (PI: Drake). Dr. Drake has served as a consultant for Teva; has received research support from Merck and Teva; and has served on a speakers bureau for Jazz, Purdue, and Teva. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; international statistical classification of diseases and related health problems, tenth revision; and research diagnostic criteria/international classification of sleep disorders, criteria: results from the America insomnia survey. Biol Psychiatry. 2011;69:592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Morin CM, LeBlanc M, Daley M, Gregoire J, Merette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123–30. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 3.LeBlanc M, Merette C, Savard J, Ivers H, Baillargeon L, Morin CM. Incidence and risk factors of insomnia in a population-based sample. Sleep. 2009;32:1027–37. doi: 10.1093/sleep/32.8.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singareddy R, Vgontzas AN, Fernandez-Mendoza J, et al. Risk factors for incident chronic insomnia: a general population prospective study. Sleep Med. 2012;13:346–53. doi: 10.1016/j.sleep.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor DJ, Mallory LJ, Lichstein KL, Durrence H, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30:213–8. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- 6.Riemann D, Voderholzer U. Primary insomnia: a risk factor to develop depression? J Affect Dis. 2003;76:255–9. doi: 10.1016/s0165-0327(02)00072-1. [DOI] [PubMed] [Google Scholar]
- 7.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Dis. 2011;135:10–9. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Taylor DJ, Lichstein KL, Durrence HH. Insomnia as a health risk factor. Behav Sleep Med. 2003;1:227–47. doi: 10.1207/S15402010BSM0104_5. [DOI] [PubMed] [Google Scholar]
- 9.Lichstein KL, Taylor DJ, McCrae CS, Ruiter ME. Insomnia: epidemiology and risk factors. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5th ed. St. Louis, MO: Elsevier; 2011. [Google Scholar]
- 10.Fernandez-Mendoza J, Vgontzas AN, Bixler EO, et al. Clinical and polysomnographic predictors of the natural history of poor sleep in the general population. Sleep. 2012;35:689–97. doi: 10.5665/sleep.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drake CL, Pillai V, Roth T. Stress and sleep reactivity: a prospective investigation of the stress-diathesis model of insomnia. Sleep. 2013;37:1295–304. doi: 10.5665/sleep.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernández-Mendoza J, Vela-Bueno A, Vgontzas AN, et al. Cognitive-emotional hyperarousal as a premorbid characteristic of individuals vulnerable to insomnia. Psychosom Med. 2010;72:397–403. doi: 10.1097/PSY.0b013e3181d75319. [DOI] [PubMed] [Google Scholar]
- 13.Bastien CH, Morin CM. Familial incidence of insomnia. J Sleep Res. 2000;9:49–54. doi: 10.1046/j.1365-2869.2000.00182.x. [DOI] [PubMed] [Google Scholar]
- 14.Dauvilliers Y, Morin C, Cervena K, et al. Family studies in insomnia. J Psychosom Res. 2005;58:271–8. doi: 10.1016/j.jpsychores.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Drake CL, Friedman NP, Wright KP, Jr, Roth T. Sleep reactivity and insomnia: genetic and environmental influences. Sleep. 2011;34:1179–88. doi: 10.5665/SLEEP.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drake CL, Roth T. Predisposition in the evolution of insomnia: evidence, potential mechanisms, and future directions. Sleep Med Clin. 2006;1:333–49. [Google Scholar]
- 17.Drake C, Richardson G, Roehrs T, Scofield H, Roth T. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 2004;27:285–92. doi: 10.1093/sleep/27.2.285. [DOI] [PubMed] [Google Scholar]
- 18.Drake CL, Jefferson C, Roehrs T, Roth T. Stress-related sleep disturbance and polysomnographic response to caffeine. Sleep Med. 2006;7:567–72. doi: 10.1016/j.sleep.2006.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonnet MH, Arand DL. Situational insomnia: consistency, predictors, and outcomes. Sleep. 2003;26:1029–37. doi: 10.1093/sleep/26.8.1029. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Mendoza J, Shaffer ML, Olavarrieta-Bernardino S, et al. Cognitive–emotional hyperarousal in the offspring of parents vulnerable to insomnia: a nuclear family study. J Sleep Res. 2014;23:489–98. doi: 10.1111/jsr.12168. [DOI] [PubMed] [Google Scholar]
- 21.Kalmbach DA, Pillai V, Chen P, Arnedt JT, Drake C. Shift work disorder, depression, and anxiety in the transition to rotating shifts: the role of sleep reactivity. Sleep Med. 2015;16:1532–8. doi: 10.1016/j.sleep.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pillai V, Roth T, Mullins HM, Drake CL. Moderators and mediators of the relationship between stress and insomnia: stressor chronicity, cognitive intrusion, and coping. Sleep. 2013;37:1199–208. doi: 10.5665/sleep.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Psychiatric Association. Washington, DC: American Psychiatric Association; 2000. Diagnostic and statistical manual of mental disorders, 4th ed., text revision. [Google Scholar]
- 24.Hobson CJ, Kamen J, Szostek J, Nethercut CM, Tiedmann JW, Wojnarowicz S. Stressful life events: a revision and update of the social readjustment rating scale. Int J Stress Manage. 1998;5:1–23. [Google Scholar]
- 25.Scully JA, Tosi H, Banning K. Life event checklists: revisiting the social readjustment rating scale after 30 years. Educ Psychol Meas. 2000;60:864–76. [Google Scholar]
- 26.Lichstein K, Durrence H, Taylor D, Bush A, Riedel B. Quantitative criteria for insomnia. Behav Res Ther. 2003;41:427–45. doi: 10.1016/s0005-7967(02)00023-2. [DOI] [PubMed] [Google Scholar]
- 27.Green MJ, Espie CA, Hunt K, Benzeval M. The longitudinal course of insomnia symptoms: inequalities by sex and occupational class among two different age cohorts followed for 20 years in the west of Scotland. Sleep. 2012;35:815–23. doi: 10.5665/sleep.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pillai V, Roth T, Drake C. The nature of stable insomnia phenotypes. Sleep. 2015;38:127–38. doi: 10.5665/sleep.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riemann D, Spiegelhalder K, Nissen C, Hirscher V, Baglioni C, Feige B. REM sleep instability-a new pathway for insomnia? Pharmacopsychiatry. 2012;45:167. doi: 10.1055/s-0031-1299721. [DOI] [PubMed] [Google Scholar]
- 30.Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10:45–56. [PubMed] [Google Scholar]
- 31.Querstret D, Cropley M. Assessing treatments used to reduce rumination and/or worry: a systematic review. Clin Psychol Rev. 2013;33:996–1009. doi: 10.1016/j.cpr.2013.08.004. [DOI] [PubMed] [Google Scholar]


