Abstract
Study Objectives:
Sleep is under the control of homeostatic and circadian processes, which interact to determine sleep timing and duration, but the mechanisms through which the circadian system modulates sleep are largely unknown. We therefore used adult-specific, temporally controlled neuronal activation and inhibition to identify an interaction between the circadian clock and a novel population of sleep-promoting neurons in Drosophila.
Methods:
Transgenic flies expressed either dTRPA1, a neuronal activator, or Shibirets1, an inhibitor of synaptic release, in small subsets of neurons. Sleep, as determined by activity monitoring and video tracking, was assessed before and after temperature-induced activation or inhibition using these effector molecules. We compared the effect of these manipulations in control flies and in mutant flies that lacked components of the molecular circadian clock.
Results:
Adult-specific activation or inhibition of a population of neurons that projects to the sleep-promoting dorsal Fan-Shaped Body resulted in bidirectional control over sleep. Interestingly, the magnitude of the sleep changes were time-of-day dependent. Activation of sleep-promoting neurons was maximally effective during the middle of the day and night, and was relatively ineffective during the day-to-night and night-to-day transitions. These time-ofday specific effects were absent in flies that lacked functional circadian clocks.
Conclusions:
We conclude that the circadian system functions to gate sleep through active inhibition at specific times of day. These data identify a mechanism through which the circadian system prevents premature sleep onset in the late evening, when homeostatic sleep drive is high.
Citation:
Cavanaugh DJ, Vigderman AS, Dean T, Garbe DS, Sehgal A. The Drosophila circadian clock gates sleep through time-of-day dependent modulation of sleep-promoting neurons. SLEEP 2016;39(2):345–356.
Keywords: sleep, circadian clock, Drosophila
Significance.
Our data provide direct physiological evidence for the long-held theory that the circadian system interacts with sleep homeostatic centers of the brain to produce consolidated nighttime sleep by contributing a time-of-day specific alerting signal. By enhancing our knowledge of the mechanisms through which the circadian system modulates sleep, our findings hold considerable relevance for understanding the severe behavioral, cognitive and metabolic consequences that result from circadian and sleep disorders.
INTRODUCTION
Sleep is an evolutionarily conserved behavior that is under the control of two independent but interrelated processes: a circadian mechanism, which determines the timing of sleep, and a homeostatic mechanism, which determines the amount and intensity of sleep.1 Circadian and homeostatic processes interact in complex ways to ensure that sleep occurs at optimal times. For example, in humans, the circadian system is thought to maximally drive wakefulness toward the end of the day, to prevent sleep at a time when homeostatic drive is high.2,3
In Drosophila, the circadian clock system is composed of ∼150 neurons that can be subdivided based on anatomical location and function. These include the small and large ventrolateral neurons (sLNvs and lLNvs, respectively), the dorsolateral neurons (LNds), and three groups of dorsal neurons (DN1, DN2, and DN3). Of these cells, the sLNvs and LNds appear to function as core oscillators, and control the morning and evening bouts of activity, respectively.4,5
Though the sleep-wake cycle is one of the most prominent circadian-regulated behaviors, the manner in which the circadian system controls sleep timing is unknown. It has been proposed that the lLNvs are involved in light-induced arousal,6 and that gamma-aminobutyric acid (GABA)-ergic inhibition of lLNvs controls sleep latency and sleep maintenance at night.7,8 The lLNv cells are likely under inhibitory control of the sLNvs, which promote nighttime sleep through release of sNPF.9 More recently, DN1 cells have been shown to promote late-night arousal through release of the peptide DH31.10 These studies have shown that circadian clock cells help to shape sleep architecture, primarily through sleep inhibition. However, connections between the circadian clock and sleep-regulatory regions of the brain are not known.
Few nonclock brain regions in the fly have been definitively demonstrated as sleep promoting. These include the pars inter-cerebralis (PI),11,12 mushroom body (MB),13–15 and dorsal fan-shaped body (dFSB).16,17 Here we identify a novel population of sleep-promoting neurons and show that the ability of these neurons to drive sleep varies with time of day. We further demonstrate that this time-of-day dependent effect persists in constant darkness, but is lost in mutants lacking components of the molecular clock, implicating circadian processes. These data show that the circadian system gates sleep timing by counteracting the influence of sleep-promoting brain regions at specific times of day.
METHODS
Fly Stocks
The following stocks were ordered from Bloomington Stock Center: UAS-mCD8::GFP (5137), Tub > GAL80 > (38881), Tub > stop > GAL80 (39213), L1/cyo;UAS-Denmark,UAS-syteGFP (33065) and 23E10-GAL4 (49032). 201y-GAL4 was used previously in the laboratory.13 UAS-dTrpA1 was a gift from L. Griffith. 20XUAS-IVS-Shibirets1-p10-INS (referred to as UAS-Shibirets1 in the text) was a gift from G. Rubin.18 MB247-FLP was a gift from T. Riemensperger.19 Cha-GAL80,20 cyc01 and per01 mutant flies were described previously.21,22 UASCD4::spGFP1-10 and LexAop-CD4::spGFP11 were gifts from M. Gordon and K. Scott. We recombined UAS-dTrpA1 with 201y-GAL4 to produce 201y-GAL4,UAS-dTrpA1 flies, and UAS-mCD8::GFP with UAS-dTrpA1 to create UAS-dTrpA1,UAS-CD8GFP flies. All flies used for behavioral testing were outcrossed seven times to the iso31 strain.
Sleep Analysis
Mated female flies were used for all sleep assays. Flies were raised on standard cornmeal-molasses medium at 18°C to prevent premature dTRPA1 or Shibirets1 effects. Following eclosion, flies were transferred to new vials and allowed to age for ∼1 w prior to sleep monitoring.
Drosophila Activity Monitoring System Sleep Analysis
Individual flies were loaded into glass tubes containing 5% sucrose and 2% agar, and locomotor activity was monitored with the Drosophila Activity Monitoring system (DAMS) (Trikinetics, Waltham, MA). Sleep was defined as 5 consecutive min of inactivity. Sleep analysis was performed with PySolo software as described previously.23
For light-dark (LD) experiments, flies were entrained for 3 d to a 12 h:12 h (12:12) LD cycle at 21°C, then exposed to 28°C (for dTRPA1) or 29°C (for Shibirets1) for 2 d, and then returned to 21°C for 2 d. Temperature shifts occurred at Zeitgeber time (ZT) 0. For constant darkness (DD) experiments, flies were en-trained to 12:12 LD cycles for 3 d at 21°C, then exposed to DD for 2 d at 21°C, then DD for 2 d at 28°C, and finally returned to 21°C DD for 2 d. Temperature shifts occurred at circadian time (CT) 0. To determine sleep change, total, daytime and nighttime sleep at baseline (the final entrainment day at 21°C) were subtracted from the first day at 28°C or 29°C. For simplicity only the first day at 28°C or 29°C is depicted in most figures. In general, the effects persisted over the course of both days at elevated temperature, however, and were somewhat attenuated by the end of the second day, likely due to homeostatic mechanisms.
Video Tracking Sleep Analysis
For video tracking analysis, flies were loaded under CO2 anesthesia into individual wells of a Costar 24-well cell culture plate (Corning). To decrease shadows from the curvature of the plates, the sides of the plates were painted white. Each well was filled with a solution of 5% sucrose and 2% agar and air-dried. After loading, wells were sealed with TopSeal-A adhesive sealing film (PerkinElmer) and placed in a recording chamber with infrared lights. Flies were entrained for 3 d to a 12:12 LD cycle at 21°C, then exposed to 28°C (for dTRPA1) 1 d, and then returned to 21°C for 2 d. Temperature shifts occurred at ZT 0. Video recording was performed using a 1/2'' CCD Super Cube DSP Monochrome camera (Ikegami, Maywood, NJ) containing an infrared filter and fly tracking was carried out with Ethovision XT9 software (Noldus, Leesburg, VA). Flies with a minimum 50% body length change per second were considered active. Sleep was defined as a period of 5 min or longer of inactivity, and was calculated from the Ethovision tracking data using custom formulas in Microsoft Excel.
Arousal Threshold Assays
Arousal threshold was tested using DAMS activity analysis. Flies were raised and entrained as detailed previously and loaded into glass tubes containing 5% sucrose and 2% agar. For these experiments, large DAM2 monitors were used so that light and mechanical stimulation were equalized for all flies. Light arousal threshold was measured during the night by giving a 30-sec dim light pulse (100 lux) at ZT18-22. Mechanical stimulation was performed at ZT20. A 340 g rubber weight was dropped from 5 cm height onto a rack supporting the activity monitors. Locomotor activity was assayed before and after light pulse. Only flies that exhibited 5 min of inactivity prior to the light pulse were used for analysis. Arousal magnitude was calculated as the total number of beam breaks in the 5 min following light pulse or mechanical stimulation.
Negative Geotaxis Assay
∼1-w-old flies were entrained to a 12:12 LD cycle at 21°C and transferred to 28°C 1 h prior to testing. Assays were performed at ZT4. Ten flies of a given genotype were loaded into a conical vial, and five trials were conducted for each vial, with a minimum of 5 min between each trial. Each trial consisted of tapping the vial vigorously five times on the table, then placing the vial on the table and allowing the flies to climb for 10 sec. We determined the percentage of flies that successfully climbed past a 4-cm line during the 10-sec trial and averaged this across the five trials for each vial. For each genotype, we calculated the mean of three independent vials.
Immunohistochemistry
Primary antibodies were as follows: rabbit anti-green fluores-cent protein (GFP) 1:1000 (Molecular Probes A-11122), rat anti-red fluorescent protein (RFP) 1:1000 (Chromotek 5F8), and mouse anti-nc82 1:1000 (Developmental Studies Hybridoma Bank, deposited by Erich Buchner). Brains of adult flies were dissected in cold phosphate buffered saline with 0.1% Triton-X (PBST) and fixed in 4% formaldehyde for 30–60 min on ice. Brains were rinsed 3 × 10 min with PBST, blocked for 30–60 min in 5% normal donkey serum in PBST (NDST), and incubated overnight at 4°C in primary antibody diluted in NDST. Brains were then rinsed 4 × 10 min in PBST, incubated 2 h in fluorescein isothiocyanate (FITC) donkey anti-rabbit (Jackson 711-095-152), Cy3 donkey anti-rat (Jackson 712-16-150), or Cy5 donkey anti-mouse (Jackson 715-175-151) diluted 1:500 in NDST, rinsed 4 × 10 min in PBST, and mounted with Vecta-shield (Vector Laboratories, Burlingame, CA). Immunolabeled brains were visualized with a TCS SP5 confocal microscope (Leica). For GFP Reconstitution Across Synaptic Partners (GRASP) experiments we visualized endogenous GFP without staining.
Location Heat Maps
To make location heat maps, locomotor activity was recorded with DAMS multibeam monitors (Trikinetics), which contain 17 infrared beams spaced 3 mm apart. ∼1-w-old flies were loaded into multibeam monitors, entrained for 3 d to a 12:12 LD cycle at 21°C, then exposed to 28°C for 1 d. Temperature shift occurred at ZT 0. For every minute of the behavioral assay, we recorded the dwell time in each of the 17 beams of the multibeam monitor, which is determined by the number of seconds spent in a given beam during that minute.
For individual fly data, we plotted dwell time in each beam on a minute-by-minute basis. Each column represents 1 min of behavior, and each row represents one beam of the multi-beam monitor. Data are plotted for the final day at 21°C and the first day at 28°C using Microsoft Excel's three-color scale conditional formatting. The color scale reflects the percentage of time flies spent in each beam; red, yellow, and blue indicate that a fly spent 100%, 50%, or none of its time at a location during a given minute.
For group data, we averaged the dwell time in each of the 17 beams for all flies of a given condition on a minute-by-minute basis, then plotted these average dwell times using three-color scale conditional formatting in Microsoft Excel. The color scale reflects the average percentage of time flies spent in each beam; red equals the highest average percentage of time spent in an individual beam across all experimental groups (43 sec = 71.7%), yellow equals 50% of the highest average percentage (35.8%), and blue indicates that flies spent no time in the beam.
We also used the multibeam data to determine food proximity preference index, which is the percentage of time each fly spent in the beam closest to the food source. For each individual fly, we determined food location preference index in 3-h bins throughout the length of the 2-d experiment. For group data, we determined the mean food proximity preference index for all flies of a given genotype for each 3-h bin.
Statistics
Analyses of variance (ANOVAs) with Tukey post hoc tests were used for all statistical comparisons except for sleep bout analysis. Because sleep bout data are not normally distributed, sleep bout duration and number were analyzed with Kruskal-Wallis with Dunn post hoc tests. A value of P < 0.05 was considered significant.
RESULTS
A Sleep-Promoting GAL4 Line
We reported previously that constitutive protein kinase A (PKA) activation in neurons labeled by 201y-GAL4 results in increased sleep duration.13 Because PKA activation is generally associated with increased neuronal activity, this result suggested that 201y-GAL4 is expressed in a sleep-promoting population of neurons. However, as constitutive manipulations can have developmental effects that indirectly influence adult behaviors, we decided to directly assess the contribution of 201y-GAL4+ neurons to adult sleep through ectopic expression of the warmth-activated dTRPA1 channel,7 which allows for temporal control over neuronal depolarization.
We found that adult-specific, dTRPA1-mediated activation of 201y-GAL4+ neurons robustly increased sleep duration, such that flies were sleeping ∼4.5 h more per day than at baseline (Figure 1A and 1B). While control flies also showed alterations in sleep duration when shifting between 21°C and 28°C, the day- and night-dependent effects were of similar, yet opposing magnitudes such that total sleep duration remained relatively constant, if not somewhat decreased, with the change in temperature (Figure 1A and 1B). In comparison to control flies, 201y-GAL4 > dTRPA1 flies exhibited greatly potentiated daytime sleep increases, and were protected from the nighttime sleep decreases demonstrated by controls, thus sleeping substantially more than controls during both day and night (Figure 1A and 1B). Activation of 201y-GAL4+ cells also resulted in more consolidated sleep, as transition to high temperatures resulted in fewer sleep bouts of increased duration, compared to control flies, which exhibited reduced sleep consolidation at high temperatures (Figure S1A–S1D, supplemental material). Furthermore, this sleep-promoting effect persisted over 2 days at elevated temperature, and exhibited dose-dependency, as inclusion of two copies of 201y-GAL4 and UAS-dTrpA1 resulted in even greater sleep duration (Figure 1A and 1B). Additionally, upon return to 21°C, 201y-GAL4 > dTRPA1 flies slept significantly less than control flies (Figure 1A and 1C), a phenomenon previously described as negative sleep rebound and proposed to reflect reduced sleep drive due to the accumulation of excess sleep.9
Figure 1.
201y-GAL4+ cells promote sleep. (A) Acute activation of 201y-GAL4+ cells with dTRPA1 induces sleep, as determined by Drosophila Activity Monitoring system (DAMS) monitoring. Sleep is plotted as a function of time at baseline (left panel), following transition to 28°C (middle panels) and following return to 21°C (right panel). White and black bars denote day and night, respectively, and pink shading indicates elevated temperature. (B) Change in sleep duration between baseline and day 1 28°C is plotted for total sleep, daytime specific sleep, and nighttime specific sleep, as determined by DAMS monitoring. (C) Sleep rebound, defined as the change in total daily sleep duration between baseline and day 1 of return to 21°C, as determined by DAMS monitoring. (D) Acute activation of 201y-GAL4+ cells with dTRPA1 induces sleep, as determined by video tracking. Sleep is plotted as a function of time at baseline (left panel), following transition to 28°C (middle panel) and following return to 21°C (right panel). White and black bars denote day and night, respectively, and pink shading indicates elevated temperature. (E) Change in sleep duration between baseline and day 1 28°C is plotted for total sleep, daytime specific sleep, and nighttime specific sleep, as determined by video tracking. (F) Sleep rebound, as determined by video tracking. (G) Activation of 201y-GAL4+ cells increases arousal threshold to a light stimulus. (H) Activation of 201y-GAL4+ cells increases arousal threshold to a mechanical stimulus. (I) Activation of 201y-GAL4+ cells does not affect climbing behavior in negative geotaxis assays. For all panels, analysis of variance with Tukey post hoc test was used. For B and C, **P < 0.0001, *P < 0.005 compared to both UAS and GAL4 controls, ∧∧P < 0.005 between indicated genotypes. For E and F, **P < 0.0001, *P < 0.05 compared to UAS-dTrpA1 controls. ∧P < 0.05 between indicated genotypes. For G and H, **P < 0.0001, ns, non-significant, compared to both UAS and GAL4 controls at a given temperature, n = 12–23 per genotype for E and n = 44–54 per genotype for F. For I, *P < 0.01, n = 3 independent replicates per genotype. For all graphs, means ± standard error of the mean are depicted. For simplicity, the terms GAL4 and UAS have been omitted from some genotype names.
Because traditional activity monitoring, in which fly activity is inferred from the breaking of an infrared beam that extends across the middle of a locomotor tube, lacks spatial resolution, we also independently verified sleep behavior using video tracking, which allowed us to monitor the location and activity of the fly throughout the entire behavioral arena. Importantly, these results confirmed that activation of 201y-GAL4+ cells robustly promotes sleep (Figure 1D and 1E). We also assayed arousal threshold to test whether TRPA1-mediated activation of 201y-GAL4+ cells altered depth of sleep, in addition to changing total sleep time. Notably, we found that sleeping 201y-GAL4 > dTRPA1 flies remained responsive to visual and mechanical stimuli, but at reduced levels compared to controls (Figure 1G and 1H). These data suggest that activation of these cells increases sleep depth, but does not render the flies unarousable.
Finally, to further rule out that activation of 201y-GAL4+ cells directly inhibited locomotion, which could be misinterpreted as sleep in our behavioral assays, we performed negative geotaxis assays. We found that heterozygous 201y-GAL4 > dTRPA1 flies exhibited normal climbing behavior at elevated temperatures (Figure 1I). We did note that flies with two copies of 201y-GAL4 and UAS-dTrpA1 had deficits in negative geotaxis, but this is likely because fewer of these flies were awakened during this assay as a result of their greatly increased arousal threshold. However, we cannot rule out the possibility that strong activation of 201y-GAL4+ cells has some direct effect on locomotion. Nevertheless, the fact that heterozygous flies show profound increases in sleep in the absence of any locomotor deficits unequivocally demonstrates that the sleep phenotype in these flies does not stem from motor paralysis. Further supporting this idea, analysis of activity index, a measure of waking activity, demonstrated that levels of locomotor activity were indistinguishable from that of controls at both 21°C and 28°C, and if anything slightly increased following transition to 28°C in 201y-GAL4 > dTRPA1 flies (Figure S1E).
Bidirectional Control of Sleep
If 201y-GAL4+ cells are indeed sleep promoting, then inhibition of these cells should lead to decreased sleep. To test this, we drove expression of Shibirets1, a temperature-sensitive dynamin mutant that leads to a rundown of synaptic vesicle release upon exposure to high temperature.18,24 As expected, inhibition of 201y-GAL4+ neurons resulted in decreased sleep duration. 201y-GAL4 > Shibirets1 flies slept ∼5 h less at 29°C compared to baseline, in contrast to control flies, which exhibited only small sleep decreases of < 1 h (Figure 2A and 2B). This was predominantly due to a substantial decrease in nighttime sleep, although a small effect was also observed during the day (Figure 2A and 2B). The nighttime selectivity of this effect suggests that 201y-GAL4+ sleep-promoting neurons are normally active at night. That we can drive sleep or wakefulness with adult-specific manipulations that have opposing effects on synaptic release strongly supports the idea that the 201y-GAL4+ cells are acutely sleep promoting.
Figure 2.
Inhibition of 201y-GAL4+ cells with Shibirets1 reduces sleep. (A) Sleep is plotted as a function of time at baseline (left panel), following transition to 29°C (middle panel), and following return to 21°C (right panel). White and black bars denote day and night, respectively, and pink shading indicates elevated temperature. (B) Change in sleep duration between baseline and day 1 29°C is plotted for total sleep, daytime specific sleep, and nighttime specific sleep for flies used in A. Data are depicted as mean ± standard error of the mean. Analysis of variance with Tukey post hoc test was used. **P < 0.0001, *P < 0.005 compared to both UAS and GAL4 controls.
Thermogenetically Induced Sleep Alters Food Proximity Place Preference Secondary to Changes in Sleep
To get a better idea of how activation of 201y-GAL4+ cells affects fly behavior, we analyzed place preference using multibeam DAMS analysis, in which infrared beams tran-sect the DAMS locomotor tubes every 3 mm. Through this analysis, we determined sleep duration, using our standard criteria, and also tracked the fly's location in relation to the food source, which is located on one end of the tube. These data served two important purposes: first, they allowed us to more accurately describe the behavior of the fly, especially during the time of the day-night transition, and second, they allowed us to dissociate the immobility associated with sleep from that produced by prolonged feeding, because inactivity bouts occurring away from the food source cannot be a result of feeding.
We found that at baseline, flies spent much of their time in the vicinity of the food. However, during the day and night transitions, flies actively explored the tube, traversing from one end to the other (Figure 3A and 3B). This is most clearly seen by the fact that the localization of individual flies is distributed throughout the tube at these times, which reflects their movement across multiple beams over the course of the minute of recording (Figure 3B). On a group level, this exploratory behavior is evidenced by a substantial increase in the amount of time spent in the beam farthest from the food source (Figure 3A), which results in a reduced food proximity preference index (a measure of the percentage of time flies spend in the beam closest to the food source) at dawn and dusk (Figure 3C). Notably, this exploratory behavior is also exhibited by heterozygous 201y-GAL4 > dTRPA1 flies during the day-night transition at 28°C (Figure 3B, arrow).
Figure 3.
Activation of 201y-GAL4+ cells alters food proximity preference secondary to changes in sleep. Fly location over time was determined using DAMS multibeam analysis. (A) Average location heat maps are plotted for the indicated genotypes at 21°C and 28°C. White and gray bars under each graph indicate day and night, respectively. The color scale reflects the average percentage of time flies spent in each beam; red equals the highest average percentage flies spent in an individual beam (71.6%), yellow equals 50% of the highest average percentage (35.8%), and blue equals zero. To the right of the plots is a schematic demonstrating the orientation of the locomotor tube, including the location of the food. (B) Location heat maps of representative individual flies of the indicated genotypes. Graphs are displayed as in A, with the addition of sleep histograms above the heat maps. Black ticks indicate that the fly was asleep for that minute. Arrow in B demonstrates that individual 201y-GAL4,UAS-dTrpA1 flies actively explore the locomotor tube during the sleep inhibition that occurs at day-night transition at 28°C. (C) Food proximity preference index is graphed as a function of time (in 3-h bins) for the indicated genotypes. **P < 0.0001, *P < 0.05 compared to both UAS and GAL4 controls. For simplicity, the terms GAL4 and UAS have been omitted from some genotype names.
Exposure to high temperature substantially altered food proximity place preference in 201y-GAL4 > dTRPA1 flies (Figure 3C). This was especially obvious in homozygous flies, which spent most of their time in close proximity to the food source during the nighttime at 28°C (Figure 3A–3C). Inspection of food proximity preference index plots revealed that this altered localization was secondary to changes in sleep, however, because exposure to elevated temperature induced short-onset increases in sleep behavior that occurred during the first day of elevated temperature, and preference for food location was not different from control lines until nighttime (Figure 3C). In fact, homozygous 201y-GAL4 > dTRPA1 flies actually exhibited decreased food proximity preference during the first few hours at 28°C (Figure 3C). These data rule out the possibility that activation of 201y-GAL4+ cells directly induces feeding behavior, because the initial localization of 201y-GAL4 > dTRPA1 flies following activation is incompatible with feeding.
Interestingly, many individual flies with two copies of 201y-GAL4 and UAS-dTrpA1 demonstrated progressive localization toward food (Figure 3B), as has been found with other thermogenetic manipulations that increase sleep.9 Thus, it is likely that 201y-GAL4+ neuronal activation causes prolonged sleep, which initially precludes feeding behavior. Over time, however, increased hunger slowly drives flies toward the food source, where they remain for the duration of the experiment.9
Non-MB Cells Underlie the Sleep-Promoting Effect of 201y-GAL4
201y-GAL4 shows prominent expression in the MB (Figure 4A), an area that we and others have identified as a major sleep-regulating region of the fly.13–15 However, it also expresses in several non-MB areas. To test whether selective activation of 201y-GAL4+ MB neurons is sufficient to promote sleep, we employed an intersectional strategy through which expression of 201y-GAL4 was suppressed in non-MB cells by GAL80 (a suppressor of GAL4). We used a ubiquitously expressed Tub > GAL80 > transgene that allowed for excision of the GAL80 construct upon expression of the flippase (FLP) gene,25 and combined it with a MB-specific FLP transgene.19 Through excision of GAL80 in the MB, 201y-GAL4 expression was effectively restricted to the MB (Figure 4A). Unexpectedly, we found dTRPA1-mediated activation of this restricted subset of 201y-GAL4+ cells was unable to increase sleep above control levels (Figure 4B and 4C).
Figure 4.
The non-mushroom body (MB) subset of 201-GAL4+ cells underlies the sleep-promoting effect of 201y-GAL4+ neuronal activation. (A) Individual 201y-GAL4/UAS-dTrpA1,UAS-CD8GFP ; MB-FLP/+ (left panels), 201y-GAL4/UAS-dTrpA1,UAS-CD8GFP;MB-FLP/Tub > GAL80 > (middle panels), and 201y-GAL4/UAS-dTrpA1,UAS-CD8GFP;MB-FLP/Tub > stop > GAL80 (right panels) brains were stained for GFP immunofluorescence. Maximum projections of the anterior and posterior fly brain are shown as indicated. Note that expression was restricted to either exclusively MB cells (middle) or to non-MB cells (right panels) with these manipulations. Arrowheads point to residual alpha/beta core MB expression in 201y-GAL4/UAS-dTrpA1,UAS-CD8GFP;MB-FLP/Tub > stop > GAL80 flies. Fly brains in A were dissected and stained following behavioral experiments depicted in B and C. (B) Sleep is plotted for the aforementioned genotypes at baseline (left), following transition to 28°C (middle), and following return to 21°C (right). White and black bars denote day and night, respectively, and pink shading indicates elevated temperature. (C) Change in sleep duration between baseline (21°C) and day 1 28°C is plotted for total sleep, daytime specific sleep, and nighttime specific sleep. An analysis of variance with Tukey post hoc test was used in C. **P < 0.0001, *P < 0.001 compared to both UAS and GAL4 controls. ∧∧P < 0.0001 between indicated genotypes. (D) Restriction of 201y-GAL4 with Cha-GAL80. A maximum confocal image of 201y-GAL4/UAS-CD8GFP;Cha-GAL80/+ brain stained for GFP immunofluorescence. (E) Driving dTRPA1 expression in alpha and beta core of MB is insufficient to promote sleep. Sleep is plotted for the first day at 28°C. (F) Change in sleep duration between baseline (21°C) and day 1 28°C is plotted for total sleep, daytime specific sleep, and nighttime specific sleep for the flies depicted in E. An analysis of variance with Tukey post hoc test was used in F. **P < 0.0001 compared to UAS-ChaGAL80 controls. For all graphs, means ± standard error of the mean are depicted. For simplicity, the terms GAL4 and UAS have been omitted from some genotype names.
We also restricted 201y-GAL4 to non-MB cells by replacing the Tub > GAL80 > transgene with Tub > stop > GAL80, in which the stop codon is targeted by MB-FLP so GAL80 is expressed only in the MB. We found that activation of this non-MB subset of 201y-GAL4+ cells was as effective as when we activated the entire 201y-GAL4+ population (Figure 4B and 4C), suggesting that the non-MB population of 201y-GAL4+ cells is the relevant sleep-promoting population. However, we did notice a small amount of residual MB labeling in the core region of the alpha and beta lobes in this line (Figure 4A, arrowheads), which appeared to be absent in our MB-restricted line (Figure 4A, middle panels). It was therefore formally possible that the residual alpha/beta core expression was responsible for the sleep-promoting effect of activating 201y-GAL4+ cells. To test this possibility, we sought other means to restrict 201y-GAL4 expression, including other GAL80 lines. Among those tested, we found that Cha-GAL80, which expresses in a widespread population of cholinergic neurons,20 effectively restricted 201y-GAL4 to the alpha/beta core region as well as a population of neurons in the pars intercerebralis (Figure 4D). As the addition of Cha-GAL80 completely suppressed the ability of 201y-GAL4+ cell activation to promote sleep (Figure 4E and 4F), we conclude that activation of alpha/ beta core MB neurons is not sleep promoting, and therefore that the non-MB subset of 201y-GAL4 cells underlies its sleep-promoting effect.
The Circadian System Gates the Sleep-Promoting Effect of Activation of 201y-GAL4+ Neurons
We noted that the sleep-promoting effect of activating 201y-GAL4+ neurons was time-of-day dependent. Thus, dTRPA1-mediated activation of these cells increased sleep to near ceiling levels during much of the day and night. However, around the times of the day and night transitions, activation was unable to effectively drive sleep increases (Figure 1A and 1B). Although the delayed sleep increase on the morning of the initial temperature step is likely artifactual due to the temperature kinetics of the incubator, we observed fluctuations in sleep magnitude over the course of the subsequent 2 d at constant elevated temperature, with sleep dipping leading into the day-night transition, and then again in the late evening and early morning of the second day at 28°C. Because the effectiveness of dTRPA1-mediated activation showed some attenuation following extended periods at 28°C, we have focused our analysis on the day-night transition of the first day at elevated temperature.
To quantify this effect, we calculated total sleep from ZT10-ZT14, a timeframe spanning the day-night transition (Figure 5A and 5B). Control flies are normally awake from ZT10-ZT12, and then gradually fall asleep following night onset, from ZT12-ZT14 (Figure 5A). Although 201y-GAL4 > dTRPA1 flies sleep more than control flies during this time (Figure 5A and 5B), their sleep amount is dynamically regulated such that it reaches a minimum around ZT12, so at this time, sleep amount is indistinguishable from controls. This is despite the fact that dTRPA1-mediated activation is ostensibly constant over this time. We interpret these results as indicating that the ability of 201y-GAL4+ cells to drive sleep is gated by active sleep inhibition during certain times of day. This time-of-day dependent inhibition is common to other manipulations that lead to increased sleep,11,13,15 which indicates that it is a general phenomenon and not specific to activation of 201y-GAL4+ cells. Notably, we were able to largely overcome the overriding sleep inhibition when we maximally activated 201y-GAL4+ cells (using homozygous 201y-GAL4,UAS-dTrpA1 flies) (Figure 1A and 1B). The time-of-day modulation persisted under conditions of constant darkness (Figure 5C and 5D), which ruled out a direct effect of light and suggested that the circadian system might be responsible.
Figure 5.
The sleep-promoting effect of 201y-GAL4 activation is time-of-day dependent. (A) Sleep is plotted from ZT10–ZT14 on day 1 28°C for the indicated genotypes. White and black bars denote light and dark, respectively, and pink shading indicates elevated temperature. (B) Total sleep from ZT10-ZT14 is plotted for the indicated genotypes. (C) Acute activation of 201y-GAL4+ cells with dTRPA1 induces sleep in dark-dark conditions. Sleep is plotted at baseline (left panel), following transition to 28°C (middle panel) and following return to 21°C (right panel). (D) Change in sleep duration between baseline and day 1 28°C is plotted for total sleep, daytime specific sleep, and nighttime specific sleep for flies used in C. For all panels, analysis of variance with Tukey post hoc test was used. **P < 0.0001 compared to both UAS and GAL4 controls. ∧∧P < 0.0001 between indicated genotypes. For all graphs, means ± standard error of the mean are depicted. For simplicity, the terms GAL4 and UAS have been omitted from some genotype names.
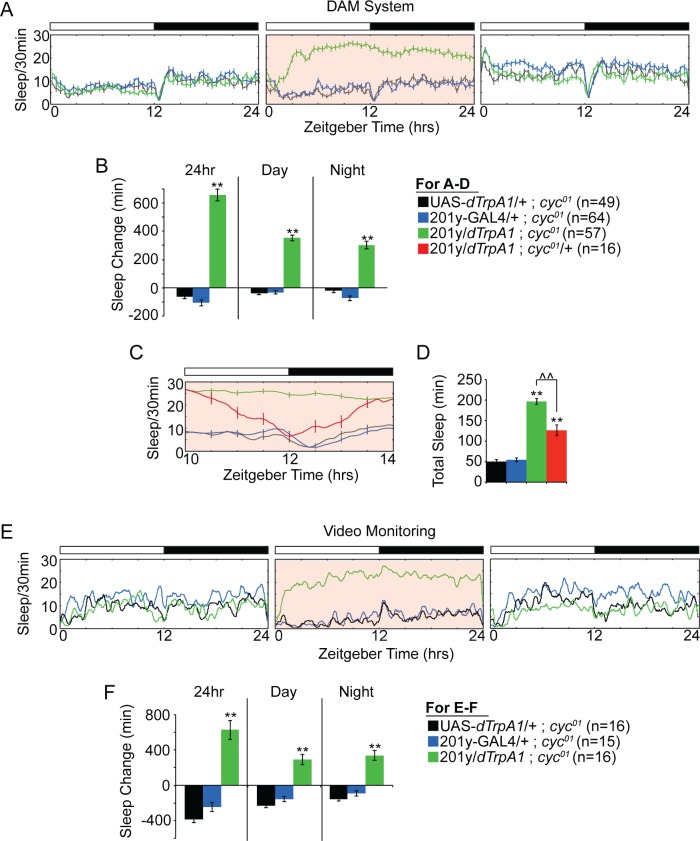
To directly test for a circadian component, we assayed 201y-GAL4+ neuronal activation in cyc01 mutants, which lack a functional circadian clock. Importantly, we found that the time-of-day dependency was eliminated in cyc01 mutants. Thus, in a cyc01 mutant background, dTRPA1-mediated activation of 201y-GAL4+ cells caused constant sleep increases throughout the day (Figure 6A), including from ZT10-Z14, a time during which 201y-GAL4+ neuronal activation in cyc01 heterozygous flies was unable to maximally drive sleep (Figure 6C and 6D). These effects were corroborated with video monitoring experiments (Figure 6E and 6F). Similar results were also obtained with per01 mutant flies (Figure S2, supplemental material), thus demonstrating that elimination of either the positive or negative arm of the molecular clock is sufficient to prevent the circadian sleep-inhibitory effect during the day-night transition.
Figure 6.
(A) The time-of-day dependency of 201y-GAL > dTRPA1-mediated sleep increase is eliminated in cyc01 mutant flies. Sleep, as determined by DAMS monitoring, is plotted as a function of time at baseline (left panel), following transition to 28°C (middle panel) and following return to 21°C (right panel). White and black bars denote day and night, respectively, and pink shading indicates elevated temperature. (B) Change in sleep duration between baseline and day 1 28°C is plotted for total sleep, daytime specific sleep, and nighttime specific sleep for flies used in A. (C) Sleep is plotted from ZT10-ZT14 on day 1 28°C for flies used in A. (D) Total sleep from ZT10-ZT14 is plotted for the indicated genotypes. (E) Sleep, as determined by video monitoring, is plotted as a function of time at baseline (left panel), following transition to 28°C (middle panel) and following return to 21°C (right panel). (F) Change in sleep duration between baseline and day 1 28°C is plotted for total sleep, daytime specific sleep, and nighttime specific sleep, as determined by video monitoring. For all graphs, means ± standard error of the mean are depicted. Analysis of variance with Tukey post hoc test was used. **P < 0.0001 compared to both UAS and GAL4 controls. ∧∧P = 0.0001 between indicated genotypes. For simplicity, the terms GAL4 and UAS have been omitted from some genotype names.
Sleep-Promoting 201y-GAL4+ Neurons Project to the dFSB
To understand how 201y-GAL4+ neurons might affect known sleep-promoting areas, we performed GRASP analysis to identify synaptic partners of these neurons. We focused on the dFSB given its role in sleep homeostasis in the fly.17 We found that 201y-GAL4+ cells contact dFSB neurons in two discrete brain regions: one near the MB calyx, and the other in the superior medial protocerebrum (15/15 brains analyzed; Figure 7A, upper panels). Additional analyses revealed that these two areas could be dissociated based on the subset of 201y-GAL4+ cells that contacts the dFSB. Thus, when we restricted 201y-GAL4 to the MB subset, GRASP signal was only observed near the MB calyx (7/8 brains analyzed; Figure 7A, middle panels). In contrast, when we excluded 201y-GAL4 from the MB, GRASP signal was selectively observed in the superior medial protocerebrum (12/12 brains analyzed; Figure 7A, bottom panels). Notably, this brain region is enriched for dendritic components of dFSB neurons (Figure 7B), which suggests that the dFSB is downstream of the non-MB population of 201y-GAL4+ neurons in a circuit controlling sleep.
Figure 7.
Sleep-promoting 201y-GAL4+ neurons contact the dendritic compartment of the dorsal fan-shaped body (dFSB). (A) Representative 201y-GAL4,23E10-LexA/LexAop-GFP11;UAS-GFP1-10/+ (all 201y-GAL4+ cells; top panels), 201y,23E10-LexA/LexAop-GFP11;Tub > GAL80 >, UAS-GFP1-10/ MB-FLP (MB subset of 201y-GAL4+ cells; middle panels), and 201y-GAL4,23E10-LexA/LexAop-GFP11; > stop > GAL80,UAS-GFP1-10/MB-FLP fly brains (non-MB subset of 201y-GAL4+ cells; bottom panels) imaged for endogenous GFP Reconstitution Across Synaptic Partners (GRASP) signal (green; left), and co-stained for nc82 (magenta; center), which labels neuropil. Merged images are shown on the right. GRASP signal is observed in the superior medial protocerebrum (arrows), and near the mushroom body calyx (arrowheads). (B) Representative maximum projection image of the dendritic (red; left) and axonal (green; center) components of dFSB neurons. A merged image is shown on the right. The 23E10-GAL4 line, which selectively marks dFSB neurons, was used to drive simultaneous expression of UAS-Denmark, which labels dendrites, and UAS-syt-eGFP, which labels the presynaptic compartment of axons. Note the relative concentration of Denmark signal in the dorsal projection of dFSB neurons (arrows).
DISCUSSION
Circadian and homeostatic mechanisms interact to control sleep, and sleep timing is one of the most prominent behavioral outputs of the circadian system. The importance of the circadian system for sleep timing is underscored by the fact that mutations in core clock genes lead to Familial Advanced Sleep Phase Syndrome in humans, which is characterized by aberrant sleep timing.26,27 Notably, mutations in homologous genes in Drosophila also lead to sleep timing defects. However, the mechanisms through which the circadian system controls sleep timing are largely unknown. Here we have used inducible genetic manipulations to demonstrate that the circadian system dynamically regulates the efficacy of sleep-promoting brain regions by actively inhibiting sleep at specific times of day.
When we used two copies of 201y-GAL4 and UAS-dTrpA1, activation resulted in near-maximal sleep throughout the day. This is similar to the effects observed following activation of other sleep-promoting populations, including cells of the dFSB,16 and sNPF neurons.9 In contrast, we found that flies expressing just one copy of each transgene exhibited submaximal sleep increases. This serendipitous finding allowed us to uncover an important interaction between these sleep-promoting neurons and the circadian system, which had not previously been appreciated because the sleep increases produced by manipulations of other sleep-promoting cells are so strong that they largely overwhelm any circadian influences.
Notably, the attenuated sleep increase in flies carrying just one copy of each transgene was only evident at certain times of day. Thus, during most of the day and night, sleep increased to near-maximal levels, in a manner indistinguishable from flies with two copies of the transgenes. However, around the time of the day-night transition, sleep in single copy flies was substantially reduced. We propose that circadian cells normally control sleep timing by gating the ability of sleep-promoting brain regions to drive sleep, especially around the time of the day-night transition. This is reminiscent of the proposed function of the circadian system in humans, which is thought to maximally drive wakefulness in the evening, to prevent sleep from prematurely occurring at a time when homeostatic sleep drive is high.2,3 It is also in line with the idea that the influence of the circadian system on sleep is generally inhibitory,1 although direct evidence for this is minimal. In flies, it has been proposed that the lLNvs promote arousal,6,7 and are likely regulated by sLNvs via the release of sNPF.9 Further, the DN1s have recently been shown to promote late-night arousal through the release of the peptide DH31.10 Whether these populations contribute to the circadian-controlled inhibition of sleep suggested by our studies remains to be determined.
Because constitutive activation of 201y-GAL4+ cells does not affect behavioral rhythms (data not shown), it is unlikely that the sleep increases are occurring upstream of the circadian clock network. Our results therefore support a model in which independent homeostatic and circadian sleep regulatory circuits exist in the fly brain. We propose that sleep homeo-static drive is dependent on input from multiple brain regions, including those non-MB cells labeled by 201y-GAL4. These multiple inputs are likely integrated somewhere downstream of the 201y-GAL4+ cells. Further experiments are necessary to determine the cellular identity of this putative integrator; however, one candidate region is the dFSB. Interestingly, our GRASP data show that the non-MB subset of 201y-GAL4+ cells contacts dendritic components of dFSB cells in the superior medial protocerebrum. Activation of the dFSB is acutely sleep promoting, and neuronal excitability of the dFSB is increased in response to sleep deprivation, consistent with homeostatic inputs.17 It will be of interest to test whether this region, or any other sleep-promoting brain area, also exhibits circadian modulation.
Although our experiments have demonstrated that a subset of non-MB 201y-GAL4+ neurons is sleep promoting, future studies are necessary to determine the specific non-MB cells that underlie this effect. Despite the relatively restricted distribution of 201y-GAL4+ cells, there are 50–100 non-MB cells per hemisphere, and our efforts to further narrow down the sleep-promoting population have been unsuccessful. However, our GRASP data suggest that a detailed assessment of the 201y-GAL4+ cells that project to the dFSB in the superior me-dial protocerebrum could help to pinpoint the sleep-promoting population.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by NIH grant 5F32GM100555 to Dr. Cavanaugh and 2R01NS048471 to Dr. Sehgal. Dr. Sehgal is an Investigator of the HHMI. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Drs. Thomas Clandinin, Leslie Griffith, Paul Hardin, Thomas Riemensperger, Michael Rosbash, and Gerald Rubin for fly stocks, and members of the Sehgal laboratory for helpful discussions.
REFERENCES
- 1.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 2.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–38. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 4.Grima B, Chélot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–73. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- 5.Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–8. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- 6.Shang Y, Griffith LC, Rosbash M. Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proc Natl Acad Sci U S A. 2008;105:19587–94. doi: 10.1073/pnas.0809577105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parisky KM, Agosto J, Pulver SR, et al. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60:672–82. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu S, Lamaze A, Liu Q, et al. WIDE AWAKE Mediates the Circadian Timing of Sleep Onset. Neuron. 2014;82:151–66. doi: 10.1016/j.neuron.2014.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shang Y, Donelson NC, Vecsey CG, Guo F, Rosbash M, Griffith LC. Short neuropeptide F is a sleep-promoting inhibitory modulator. Neuron. 2013;80:171–83. doi: 10.1016/j.neuron.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunst M, Hughes ME, Raccuglia D, et al. Calcitonin gene-related peptide neurons mediate sleep-specific circadian output in Drosophila. Curr Biol. 2014;24:2652–64. doi: 10.1016/j.cub.2014.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foltenyi K, Greenspan RJ, Newport JW. Activation of EGFR and ERK by rhomboid signaling regulates the consolidation and maintenance of sleep in Drosophila. Nat Neurosci. 2007;10:1160–7. doi: 10.1038/nn1957. [DOI] [PubMed] [Google Scholar]
- 12.Park S, Sonn JY, Oh Y, Lim C, Choe J. SIFamide and SIFamide receptor defines a novel neuropeptide signaling to promote sleep in Drosophila. Mol Cells. 2014;37:295–301. doi: 10.14348/molcells.2014.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441:757–60. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- 14.Pitman JL, McGill JJ, Keegan KP, Allada R. A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature. 2006;441:753–6. doi: 10.1038/nature04739. [DOI] [PubMed] [Google Scholar]
- 15.Aso Y, Sitaraman D, Ichinose T, et al. Mushroom body output neurons encode valence and guide memory-based action selection in Drosophila. Elife. 2014;23:e04580. doi: 10.7554/eLife.04580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donlea JM, Thimgan MS, Suzuki Y, Gottschalk L, Shaw PJ. Inducing sleep by remote control facilitates memory consolidation in Drosophila. Science. 2011;332:1571–6. doi: 10.1126/science.1202249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donlea JM, Pimentel D, Miesenböck G. Neuronal machinery of sleep homeostasis in Drosophila. Neuron. 2014;81:860–72. doi: 10.1016/j.neuron.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfeiffer BD, Truman JW, Rubin GM. Using translational enhancers to increase transgene expression in Drosophila. Proc Natl Acad Sci U S A. 2012;109:6626–31. doi: 10.1073/pnas.1204520109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pech U, Dipt S, Barth J, et al. Mushroom body miscellanea: transgenic Drosophila strains expressing anatomical and physiological sensor proteins in Kenyon cells. Front Neural Circuits. 2013;7:147. doi: 10.3389/fncir.2013.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitamoto T. Conditional disruption of synaptic transmission induces male-male courtship behavior in Drosophila. Proc Natl Acad Sci U S A. 2002;99:13232–7. doi: 10.1073/pnas.202489099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rutila JE, Suri V, Le M, So WV, Rosbash M, Hall JC. CYCLE is a second bHLH-PAS clock protein essential for circadian rhythmicity and transcription of Drosophila period and timeless. Cell. 1998;93:805–14. doi: 10.1016/s0092-8674(00)81441-5. [DOI] [PubMed] [Google Scholar]
- 22.Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971;68:2112–6. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilestro GF, Cirelli C. pySolo: a complete suite for sleep analysis in Drosophila. Bioinformatics. 2009;25:1466–7. doi: 10.1093/bioinformatics/btp237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- 25.Bohm RA, Welch WP, Goodnight LK, et al. A genetic mosaic approach for neural circuit mapping in Drosophila. Proc Natl Acad Sci U S A. 2010;107:16378–83. doi: 10.1073/pnas.1004669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toh KL, Jones CR, He Y, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–3. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y, Padiath QS, Shapiro RE, et al. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005;434:640–4. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.