Abstract
Study Objectives:
Prospective data evaluating abnormal sleep quality and quantity with cognitive decline are limited because most studies used subjective data and/or had short follow-up. We hypothesized that, over 15 y of follow-up, participants with objectively measured obstructive sleep apnea (OSA) and other indices of poor sleep quantity and quality would experience greater decline in cognitive functioning than participants with normal sleep patterns.
Methods:
ARIC participants (n = 966; mean age 61 y, 55% women) with in-home polysomnography (1996–1998) and repeated cognitive testing were followed for 15 y. Three cognitive tests (Delayed Word Recall, Word Fluency, and Digit Symbol Substitution) were administered at two time points (1996–1998 and 2011–2013). Ten additional cognitive tests were administered at the 2011–2013 neurocognitive examination. OSA was modeled using established clinical OSA severity categories. Multivariable linear regression was used to explore associations of OSA and other sleep indices with change in cognitive tests between the two assessments.
Results:
A median of 14.9 y (max: 17.3) passed between the two cognitive assessments. OSA category and additional indices of sleep (other measures of hypoxemia and disordered breathing, sleep fragmentation, sleep duration) were not associated with change in any cognitive test. Analyses of OSA severity categories and 10 cognitive tests administered only in 2011–2013 also showed little evidence of an association.
Conclusions:
Overall, abnormal sleep quality and quantity at midlife was not related to cognitive decline and later-life cognition. The effect of adverse sleep quality and quantity on cognitive decline among the elderly remains to be determined.
Citation:
Lutsey PL, Bengtson LG, Punjabi NM, Shahar E, Mosley TH, Gottesman RF, Wruck LM, MacLehose RF, Alonso A. Obstructive sleep apnea and 15-year cognitive decline: the Atherosclerosis Risk in Communities (ARIC) study. SLEEP 2016;39(2):309–316.
Keywords: Atherosclerosis Risk in Communities (ARIC) study, cognitive decline, epidemiology, obstructive sleep apnea
Significance.
Cognitive decline among older adults is a major public health concern, yet effective interventions to prevent cognitive decline are elusive. Intriguing research conducted largely in cross-sectional samples and animal models has suggested that obstructive sleep apnea (OSA) and other indices of poor sleep quantity and quality may result in cognitive decline. Counter to prior evidence, using observational data from nearly 1,000 individuals who were followed for about 15 years, there was no relation between sleep characteristics and cognitive decline. Although these results dampen enthusiasm about the role of sleep in cognitive decline, there are important methodological considerations—namely selection bias, statistical power, and the length of follow-up—that need to be taken into account when interpreting these findings.
INTRODUCTION
Dementia and mild cognitive impairment (MCI) are common among US elderly.1 Yet, despite the immense and growing burden of these conditions, gaps exist in the understanding of characteristics leading to cognitive decline. Recent evidence, both epidemiological and pathophysiological, has suggested a possible association between abnormal sleep quality and quantity and cognitive impairment due to both cerebral vascular etiologies and Alzheimer disease.2–6 However, understanding of the underlying associations is incomplete.
In an acute setting, it is well established that poor sleep quality and curtailment of sleep duration is associated with worse cognitive function.3 However, evidence is limited regarding whether long-standing impairments in sleep quality and quantity have long-lasting effects on cognitive function. Dementia and MCI are believed to have a long preclinical phase and the etiologically relevant stage of life may be middle age, as has been suggested with other risk factors for cognitive decline (e.g., hypertension,7,8 diabetes,9–12 smoking13). Most prospective work exploring associations of sleep quantity and quality with cognitive decline has used subjective measures of sleep which have limited accuracy,14 had limited follow-up, and/or has focused on older individuals,15–18 in whom pathophysiological processes through which sleep results in cognitive decline may be well underway. There are several mechanisms through which poor sleep quality and habitual short sleep duration may affect cognitive decline,3,4 which include: cyclical nocturnal hypoxemia,19–21 sleep fragmentation,22 mediation through cardiovascular disease risk factors (e.g. hypertension, diabetes, inflammation), stroke (both clinical and subclinical),21,23,24 Aβ plaque buildup5, and interaction with the apolipoprotein E (APOE) ε4 risk allele.6,25
In a subset of the Atherosclerosis Risk in Communities (ARIC) study population who participated in the Sleep Heart Health Study (SHHS), we evaluated the associations between the severity of obstructive sleep apnea (OSA), degree of sleep fragmentation, and habitual sleep duration – assessed in middle age – with 15-y change in cognitive functioning test scores. We hypothesized that participants with disordered breathing during sleep, sleep disruption, and habitually short sleep duration would experience greater decline in cognitive function, as assessed by change on three cognitive tests [the Delayed Word Recall Test (DWRT), the Word Fluency Test (WFT), and the Digit Symbol Substitution Test (DSST)], which were administered at the time of the sleep exam (ARIC visit 4; 1996–1998) and repeated at the ARIC neurocognitive examination (ARIC visit 5; 2011–2013). Second, we examined the association between the severity of OSA and an additional 10 tests spanning several domains of cognitive functioning assessed as part of the follow-up neurocognitive examination.
METHODS
Study Design Overview
The ARIC study is a prospective cohort which in 1987–1989 enrolled 15,792 men and women aged 45–64 years using probability sampling from four US communities (visit 1).26 A total of four cohort reexaminations have taken place (visit 2: 1990– 1992, visit 3: 1993–1995, visit 4: 1996–1998, visit 5: 2011– 2013). Hospitalizations and mortality for ARIC participants have been tracked continuously since study enrollment though annual phone calls (semiannual since 2012), surveillance of local hospitals, and monitoring of state and national death indexes. Institutional review boards at each of the participating institutions approved the study protocols, and all participants provided informed consent at each study visit.
In 1996–1998 (visit 4, baseline for the current study) a subset of 1,920 ARIC participants from the Washington County, MD and suburban Minneapolis, MN field centers were recruited into the SHHS20 and underwent an in-home overnight polysomnogram (PSG). Virtually all participants recruited from the MD and MN centers were white. Cognitive function as measured by the DWRT, WFT, and DSST tests took place at ARIC visits 2, 4, and 5. As part of visit 5, also referred to here as the follow-up neurocognitive examination, extensive cognitive testing was done with the overall objective to determine the prevalence of cognitive impairment and dementia and the associations of midlife vascular risk factors and markers with later-life cognitive impairment, cognitive decline, and dementia.27 Participants were invited for examinations in the study clinic or in their homes or in long-term care facilities.
Of the 1,920 SHHS participants, we excluded 193 participants with incomplete data on OSA severity, 32 with central sleep apnea, 203 with incomplete data on cognitive tests and/ or covariates of interest, and 506 who did not participate in the follow-up neurocognitive examination due to either death (n = 277) or refusal to participate (n = 229). Our final analytic sample included 966 participants.
Sleep Measurements
The overnight unattended PSG was conducted using a portable monitor (PS-2 System; Compumedics Limited, Abbotsford, Victoria, Australia), using methods previously described.21 As in prior analyses of SHHS data, an apnea was considered present if there was an absence or near-absence of airflow (at least < 25% of baseline) for ≥ 10 sec.28,29 Hypopnea was defined as a decrease in the amplitude of the airflow below 70% of baseline for ≥ 10 sec. The apnea-hypopnea index (AHI) was defined as the average number of obstructive apneas (any apnea, regardless of the oxygen desaturation level) plus hypopneas (with at least a 4% decrease in oxygen saturation), per hour of sleep. As has been done previously in SHHS, participants were categorized into four OSA severity groups according to the AHI: < 5.0 events/h (normal), 5.0–14.9 events/h (mild sleep apnea), 15.0–29.9 events/h (moderate sleep apnea), and ≥ 30.0 events/h (severe sleep apnea). Central sleep apnea events, which were defined by the absence of airflow with no associated respiratory effort detected, were excluded from this definition. Information on measurement of other sleep related variables is provided in the supplemental material.
Cognitive Testing
Three cognitive tests were administered at the time of the sleep examination (1996–1998) and repeated at the follow-up neuro-cognitive examination (2011–2013): the DWRT, DSST, and WFT.
Digit Symbol Substitution Test (DSST)30
Tests executive function and processing speed; participants are asked to translate numbers to symbols using a key. The score is the total number of digits correctly translated to symbols within 90 sec and the range of possible scores is 0 to 93.
Delayed Word Recall Test (DWRT)31
Tests verbal learning and recent memory. Participants are presented with 10 common nouns that they are asked to use in a sentence. Two exposures to the words are given. After a 5-min delay, participants are given 60 sec to recall the words. The score for the DWRT is the number of words correctly recalled.
Word Fluency Test (WFT)32
Tests executive function and language. Participants are given 60 sec to generate as many words as possible beginning with the letters F, A, and S (60 sec for each letter), avoiding proper nouns. The WFT score is the total number of acceptable words generated for the three letters.
In general, low scores on the DWRT are more reflective of memory impairment and Alzheimer disease; whereas low scores on the WFT and DSST are more strongly associated with executive function and cerebral vascular dementia.33
The cognitive battery administered at the follow-up neuro-cognitive examination was much more extensive, and included most of the neuropsychological tests recommended for inclusion in the National Institute of Aging's National Alzheimer's Disease Coordinating Center's (NACC) Uniform Data Set Battery34: Trail Making Test, part A; Trail Making Test, part B; Digit Span Backwards, Logical Memory Test, part A; Logical Memory Test, part B; Incidental Learning, digit-symbol pairs; Clock Time Perception. Protocols for the tests, which have been described elsewhere, were standardized and examiners were trained centrally. The tests were administered in a fixed order during one session in a quiet room.
Statistical Analysis
Standardized z-scores for each cognitive test were calculated by subtracting the mean and dividing by the standard deviation (SD). For the DSST, DWRT, and WFT, z-scores were created based on the means and SD at visit 2 (when the first cognitive assessment was done), which was then applied to the distributions at the sleep (1996–1998) and follow-up neurocognitive (2011–2013) examinations. z-scores for the tests only administered at the follow-up neurocognitive examination were calculated using the means and SD among individuals eligible for this analysis. For the Trail Making tests z-scores were calculated using the log of the test scores. In addition, because a higher score on this test indicates worse performance, the z-scores were multiplied by −1 so that low z-scores indicated worse performance across all tests.
Participant characteristics (% or mean ± SD) are provided stratified by OSA severity and, separately, by follow-up neurocognitive examination participation status. For continuous variables, the linear trend across OSA categories was tested by modeling OSA categories ordinally. For categorical variables, the Cochran-Armitage trend test was used. To evaluate change in the DSST, DWRT, and WFT tests between the sleep and follow-up neurocognitive examinations, multivariable linear regression was used with the outcome being the follow-up neurocognitive examination z-score minus the sleep exam z-score. For cognitive function tests only measured at the follow-up neurocognitive examination, multivariable linear regression was also used, with the outcome being the follow-up neurocognitive examination z-score. OSA was modeled using established clinical OSA severity categories; dummy variables for each category were included in the regression model. Other indices of sleep quality and quantity were modeled as continuous variables, per 1 SD. Quadratic terms were included in the models to assess nonlinearity, and if evidence of nonlinearity was present, the sleep variables of interest were modeled as quartiles.
A series of nested models were explored; covariates came from the sleep examination, unless otherwise noted. Model 1 adjusted for age, sex, field center, and educational attainment (visit 1). Model 2 further adjusted for ethanol intake, smoking status, leisure time physical activity (visit 3), and APOE ε4 risk allele. Model 3 additionally adjusted for body mass index (BMI). Model 4 also adjusted for characteristics believed to be on the causal pathway between OSA and cognitive decline (i.e., high-sensitivity C-reactive protein (CRP), diabetes mellitus, hypertension, and prevalent coronary heart disease, heart failure, and stroke).
In sensitivity analyses, to account for attrition due to either death or failure to attend the follow-up neurocognitive examination (censoring), we incorporated inverse probability of attrition weighting (IPAW) methods,35 as has been done previously with the ARIC follow-up neurocognitive data.27 Weights for each individual were the inverse of the product of the estimated probability of being alive and the probability of participating in the study (conditional on being alive). Characteristics included in the IPAW weighting models are provided in the supplemental material. We also calculated stabilized weights35 by multiplying our original weights by probabilities of follow-up neurocognitive examination participation calculated using only baseline age, sex, and educational level.
RESULTS
Of the original 1,472 SHHS participants, 966 participated in the ARIC follow-up neurocognitive examination and were included in our final analytic sample, whereas 506 (34%) were not included due to death, attrition, or incomplete cognitive testing information. Relative to those who did not participate in the follow-up neurocognitive examination, those who participated tended, at the time of the sleep examination, to be younger, have higher educational attainment, and have a lower prevalence of cardiovascular diseases and risk factors (Table 1). Overall, when stratified by OSA category, characteristics of the entire SHHS sample were similar to those of our final analytic sample (Table S1, supplemental material versus Table 2).
Table 1.
Participant characteristics at the sleep examination (visit 4; 1996–1998), stratified by neurocognitive follow-up examination (visit 5; 2011–2013) participation status.
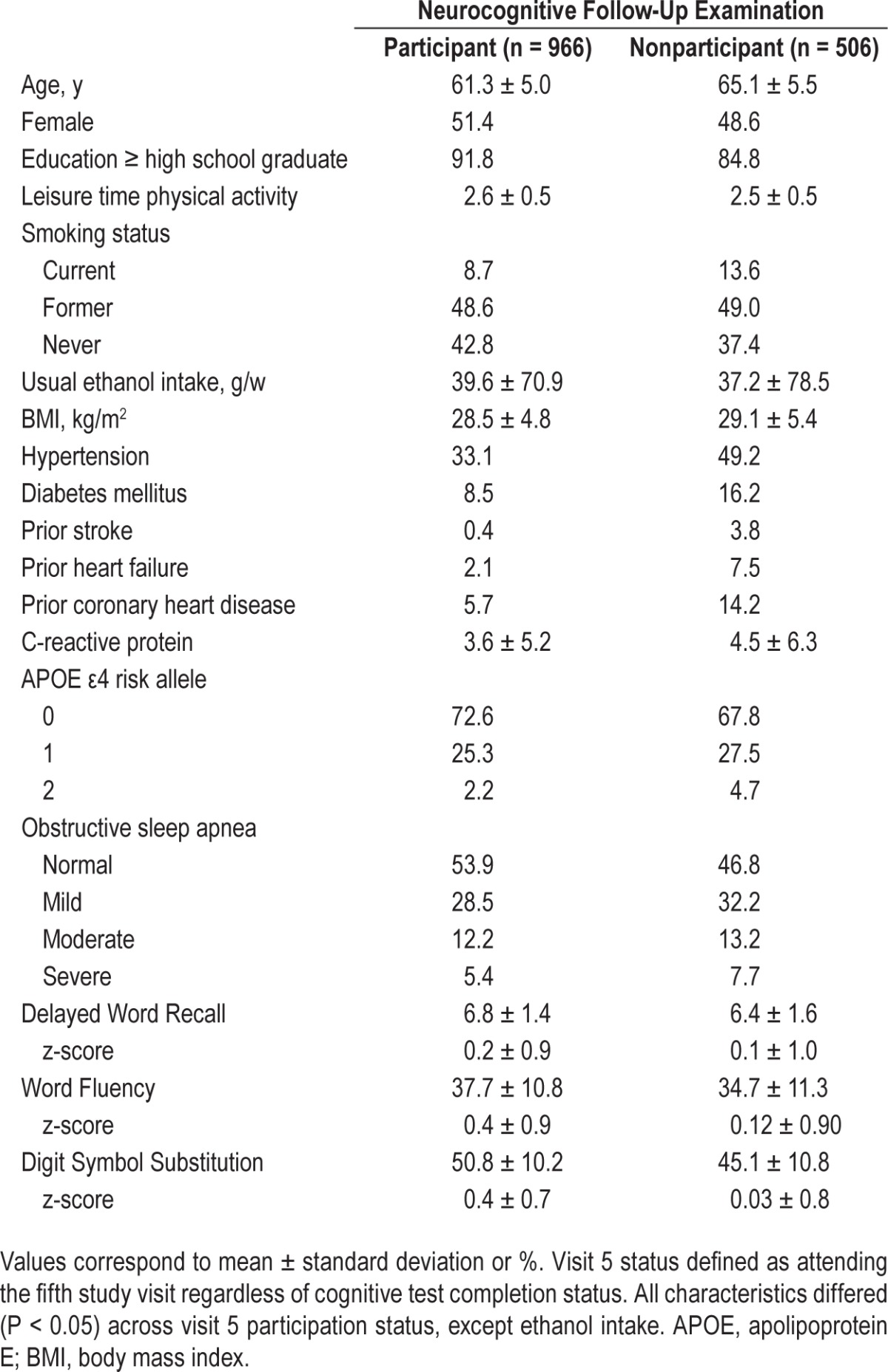
Table 2.
Demographic and clinical characteristics of the final analytic sample (n = 966) at the sleep examination (visit 4; 1996–1998), stratified by sleep examination obstructive sleep apnea categories.
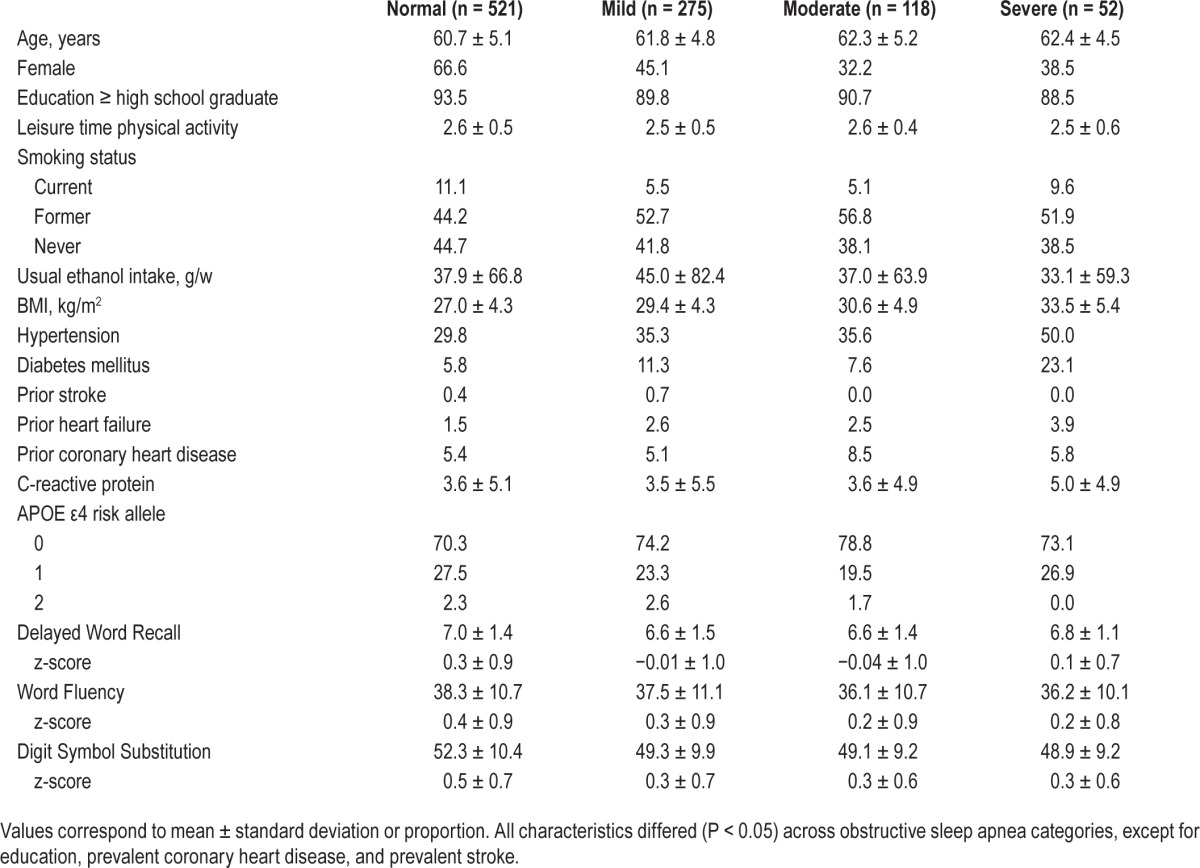
The 966 ARIC participants included in our final analytic sample were on average 61.3 ± 5.0 y old and 51.4% female when the baseline sleep study (1996–1998) was conducted. Of these participants, 5.4% (n = 52) were classified as having severe OSA, 12.2% (n = 118) as having moderate OSA, 28.5% (n = 275) as having mild OSA, and 53.9% (n = 521) as having a normal sleep breathing pattern. Relative to participants classified as normal, those with severe OSA were more likely to be male, have a higher BMI, have a higher CRP level, and to be hypertensive, diabetic, and have prevalent heart failure or coronary heart disease (Table 2).
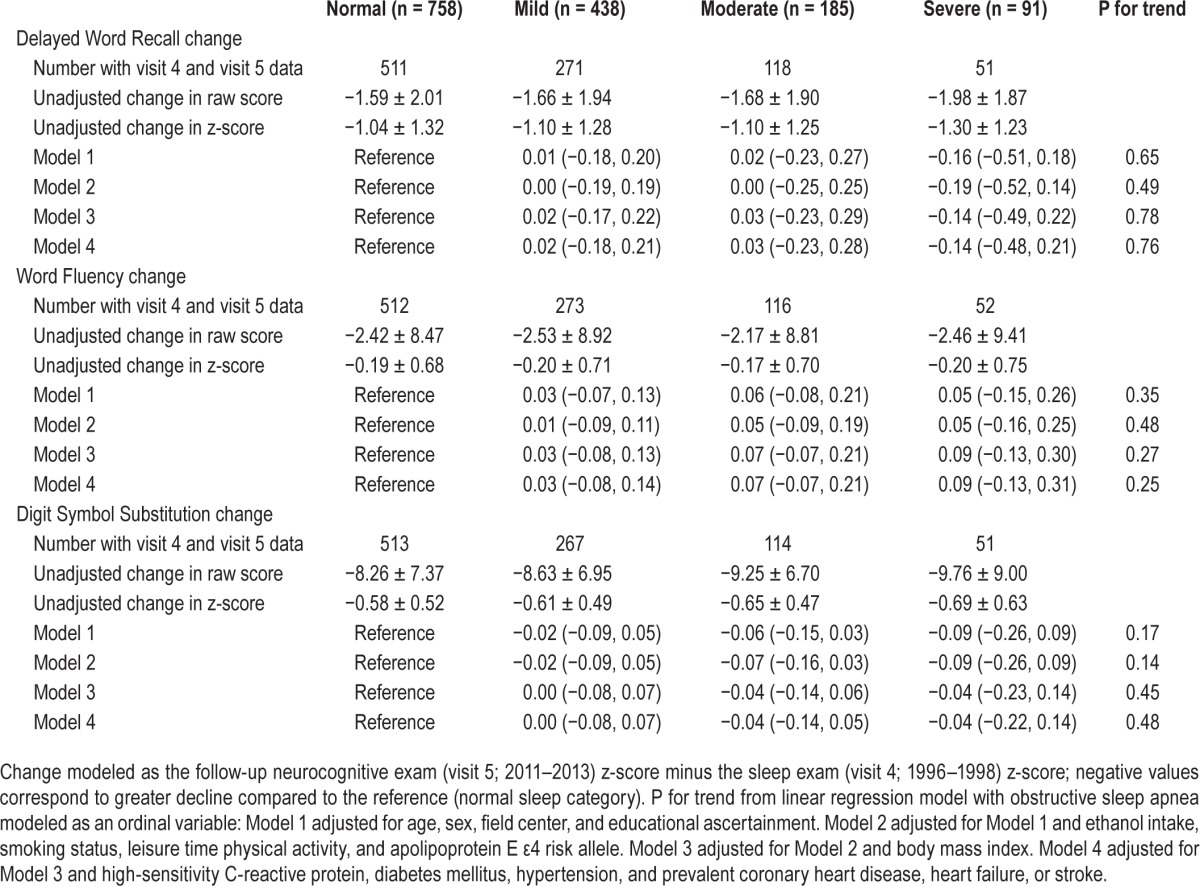
A median of 14.9 y (max: 17.3 y) passed between the cognitive assessments at the sleep and follow-up neurocognitive examinations. Over this time cognitive performance decreased, according to mean (± SD) scores (raw score / z-score) on the DWRT (−1.64 ± 1.97 / −1.08 ± 1.30), WFT (−2.42 ± 8.68 / −0.19 ± 0.69), and DSST (−8.57 ± 7.27 / −0.60 ± 0.51). Associations between OSA severity categories and linear change in cognitive test scores between the sleep and follow-up neurocognitive examinations are presented in Table 3. There was no association between OSA category and change in DWRT, WFT, or DSST, regardless of degree of adjustment. Results were similar when we accounted for attrition by employing inverse probability weighting and stabilized inverse probability weighting models (Tables S2 and S3, supplemental material).
Table 3.
Adjusted mean change and 95% confidence intervals from linear regression models for change in cognitive score from sleep exam (visit 4; 1996–1998) to the neurocognitive follow-up examination (visit 5; 2011–2013) (z-scores standardized to visit 2; 1990–1992) stratified by obstructive sleep apnea categories.
We also examined associations between measures of disordered breathing, including severity of nocturnal hypoxemia and sleep fragmentation, habitual sleep duration, and change in scores on the DWRT, WFT, and DSST tests. Overall, there was no evidence of an association (Table 4). For measures of disordered breathing including nocturnal hypoxemia and sleep fragmentation, which were modeled continuously, there also was no evidence of nonlinearity. The only evidence of an association was that, across all models, participants with habitually short sleep duration (< 7 h) experienced a slightly greater decline in DSST scores relative to participants who slept 7 to 8 h: mean change (95% confidence interval [CI]): Model 1: −0.10 (−0.18, −0.02); Model 2: −0.11 (−0.19, −0.03); Model 3: −0.10 (−0.19, −0.02); Model 4: −0.10 (−0.19, −0.02). Overall, results were similar when IPAW or stabilized IPAW were used (Table S4, supplemental material). For the association between sleep duration and DSST, estimates which accounted for attrition were slightly stronger; the Model 1 IPAW estimated change was −0.15 (−0.26, −0.05), while the Model 1 stabilized IPAW estimate was −0.14 (−0.23, −0.05).
Table 4.
Adjusted mean change and 95% confidence intervals of sleep disordered breathing, sleep architecture, sleep fragmentation, and sleep duration from linear regression models for change in cognitive score from the sleep examination (visit 4; 1996–1998) to the follow-up neurocognitive examination (visit 5; 2011–2013) (z-scores standardized to visit 2; 1990–1992).
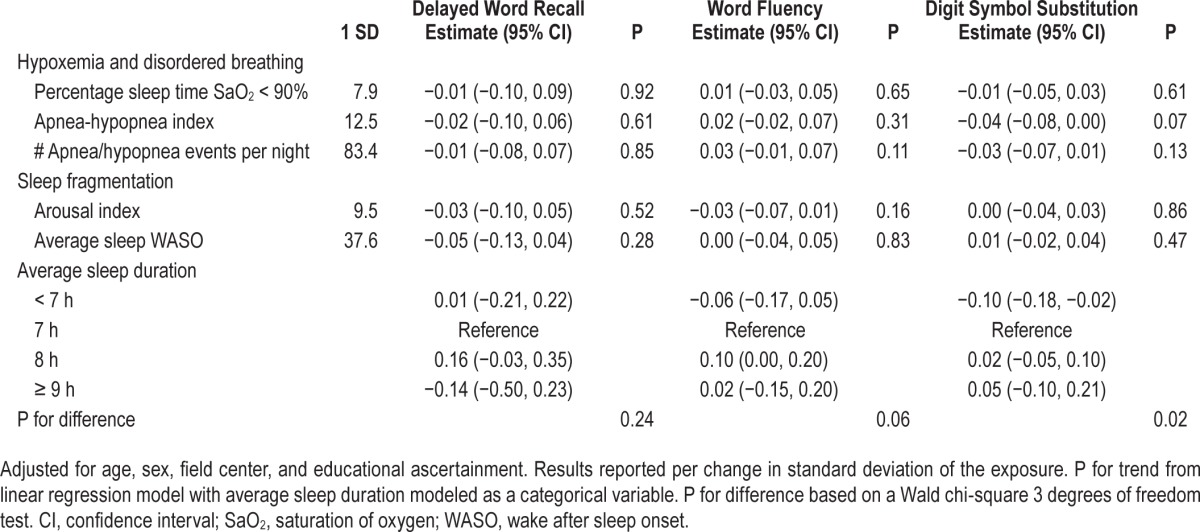
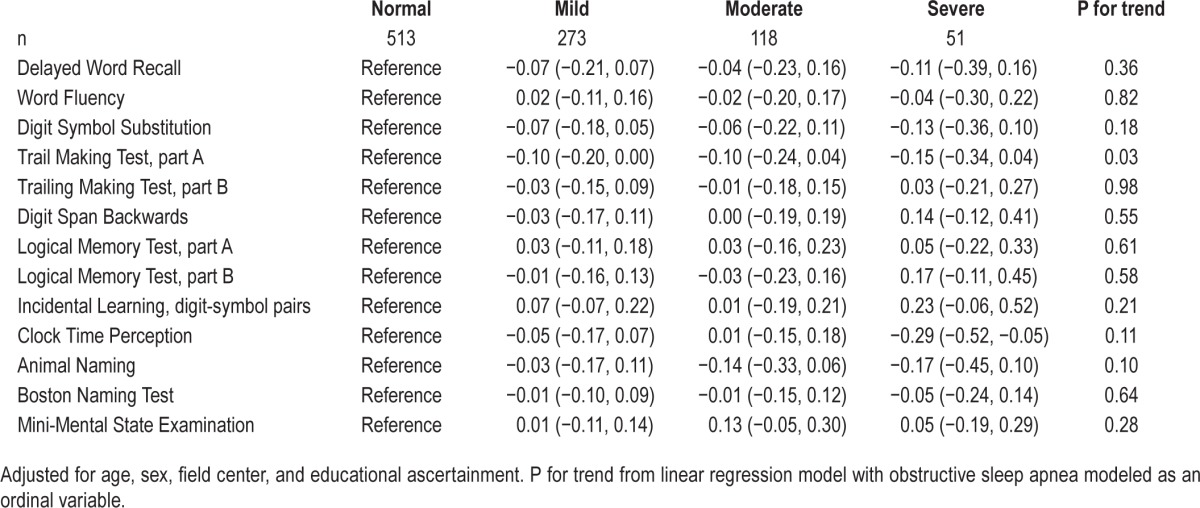
Table 5 presents associations between sleep examination OSA severity categories and 10 cognitive tests administered only at the follow-up neurocognitive examination, as well as the follow-up neurocognitive examination values (not change) for the DWRT, WFT, and DSST. For the Trail Making Test, part A, there was evidence of a linear trend (P trend = 0.03) whereby participants with greater OSA severity at the sleep examination had worse scores on this test. This association persisted across all statistical models (data not shown). Those with severe OSA also had lower scores on the Clock Time Perception tests (−0.29 [−0.52, −0.05]) relative to those who had no evidence of OSA (Table 5). This association was also present across all models (data not shown).
Table 5.
Estimates and 95% confidence intervals per standard deviation of cognitive test score (2011–2013) by obstructive sleep apnea category (1996–1998).
DISCUSSION
In a community-based sample of nearly 1,000 individuals we evaluated the association between abnormal indices of sleep quality and quantity, measured objectively in middle age, with 15-y change in cognitive test scores. Overall, we found no evidence that severity of OSA, degree of nocturnal hypoxemia, sleep fragmentation, or habitual sleep duration were associated with cognitive decline. In secondary analyses exploring the association between OSA severity and tests spanning multiple cognitive domains, which were only administered 15 y after the sleep study, there was also no evidence of an association between midlife OSA and later life cognition. The lack of a prospective association of sleep quality and quantity with later-life cognition was counter to our hypothesis, which considered midlife as the etiologically relevant frame for evaluating the association between OSA and cognitive decline.
The strongest existing epidemiologic evidence that sleep may be associated prospectively with cognitive decline comes from the Study of Osteoporotic Fractures, which has evaluated prospectively the relation between objectively measured sleep and risk of incident MCI and dementia (combined outcome).4 Over a median 4.7 y, after multivariable adjustment women (median age, 82.3 y) with moderate or severe OSA, were 85% more likely to develop MCI or dementia.4 The relation between subjectively measured sleep quality and quantity with incident cognitive decline has been evaluated in several prior studies.15–18 Recently, self-reported short and long sleep duration was associated with higher risk of MCI and dementia in a large prospective study of older women who were followed from 1995–2008.36 Results of the other studies were mixed, but they were limited by short follow-up (at most 3 y), and making direct comparisons is difficult as they used varying measures of sleep disturbances and cognition.
Although several other risk factors for cognitive decline show stronger associations when measured in midlife,7,9–11,13 it is possible that if poor sleep quality and/or quantity and disorders such as OSA are causally associated with cognition, their effect may be more acute. As has been reviewed by others,37 several small, short, randomized trials of patients with OSA have reported modest improvements in cognitive functioning after continuous positive airway pressure therapy for OSA, with the greatest gains observed in the executive function domain.38 Cross-sectional studies have suggested that poor sleep quality and quantity are most closely linked to lower cognition in the executive function and attention domains.38–40 Notably, a prior cross-sectional analysis of the ARIC data revealed no association between the severity of OSA and cognitive performance.41 Yet, ARIC participants were 53–74 y old at the time, and the analysis may have taken place too early in the natural history of the disease.
Limitations of the current study are the single assessment of sleep, incomplete information on continuous positive airway pressure intervention during the follow-up period, that the etiologically relevant timeframe for the association between sleep and cognitive decline could be outside the examined window, that all cognitive tests were not administered at the time of the SHHS, and that selection bias may have been present. Analyzing only those persons who attended a visit, when the likelihood of attending the visit is associated with both abnormal sleep characteristics and cognition, may provide biased results. Of the original SHHS participants, one-third did not attend the follow-up neurocognitive examination due to either death or attrition. We attempted to account for selection bias by using IPAW models. Results of these analyses were similar to those from standard analyses. Our study also has important strengths, including objectively measured sleep assessments, a relatively large sample size, the availability of information on numerous potential confounders, and the assessment of an extensive battery of cognitive tests, which were administered by centrally trained staff.
To summarize, in this community-based study of nearly 1,000 men and women the associations of the severity of OSA, sleep fragmentation, nocturnal hypoxemia, and habitual sleep duration with cognitive decline over 15 y of follow-up were resoundingly null. However, although we observed no significant associations, it remains possible that in more acute settings poor sleep quality may be associated with cognitive decline, particularly among the elderly.
DISCLOSURE STATEMENT
This was not an industry supported study. The ARIC portion of the SHHS was supported by National Heart, Lung, and Blood Institute cooperative agreements U01HL53934 (University of Minnesota) and U01HL64360 (Johns Hopkins University).The ARIC is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Neurocognitive data is collected by U01 HL096812, HL096814, HL096899, HL096902, HL096917 from the NHLBI and the National Institute of Neurological Disorders and Stroke, and with previous brain MRI examinations funded by R01-HL70825 from the NHLBI. This study was additionally supported by grant R21 HL121412 to Dr. Pamela Lutsey. The authors have indicated no financial conflicts of interest. Statistical analyses took place at the University of Minnesota. All authors reviewed the manuscript at their respective institutions.
ACKNOWLEDGMENTS
The authors thank the staff and participants of the ARIC study for their important contributions.
REFERENCES
- 1.Fitzpatrick AL, Kuller LH, Ives DG, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52:195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 2.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing in collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118:1080–111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 3.Amlander C, Fuller P. Westchester, IL: Sleep Research Society; 2009. Basics of sleep guide. [Google Scholar]
- 4.Yaffe K, Laffan AM, Harrison S, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–9. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–7. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gottlieb DJ, DeStefano AL, Foley DJ, et al. APOE epsilon4 is associated with obstructive sleep apnea/hypopnea: the Sleep Heart Health Study. Neurology. 2004;63:664–8. doi: 10.1212/01.wnl.0000134671.99649.32. [DOI] [PubMed] [Google Scholar]
- 7.Staessen JA, Richart T, Birkenhäger WH. Less atherosclerosis and lower blood pressure for a meaningful life perspective with more brain. Hypertension. 2007;49:389–400. doi: 10.1161/01.HYP.0000258151.00728.d8. [DOI] [PubMed] [Google Scholar]
- 8.Gottesman RF, Schneider AC, Albert M, et al. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014;71:1218–27. doi: 10.1001/jamaneurol.2014.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elias PK, Elias MF, D'Agostino RB, et al. NIDDM and blood pressure as risk factors for poor cognitive performance. The Framingham Study. Diabetes Care. 1997;20:1388–95. doi: 10.2337/diacare.20.9.1388. [DOI] [PubMed] [Google Scholar]
- 10.Gregg EW, Yaffe K, Cauley JA, et al. Is diabetes associated with cognitive impairment and cognitive decline among older women? Arch Intern Med. 2000;160:174–80. doi: 10.1001/archinte.160.2.174. [DOI] [PubMed] [Google Scholar]
- 11.Logroscino G, Kang JH, Grodstein F. Prospective study of type 2 diabetes and cognitive decline in women aged 70-81 years. BMJ. 2004;328:548. doi: 10.1136/bmj.37977.495729.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rawlings AM, Sharrett AR, Schneider ALC, et al. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med. 2014;161:785–93. doi: 10.7326/M14-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernan MA, Alonso A, Logroscino G. Cigarette smoking and dementia: potential selection bias in the elderly. Epidemiology. 2008;19:448–50. doi: 10.1097/EDE.0b013e31816bbe14. [DOI] [PubMed] [Google Scholar]
- 14.Wolfson AR, Carskadon MA, Acebo C, et al. Evidence for the validity of a sleep habits survey for adolescents. Sleep. 2003;26:213–6. doi: 10.1093/sleep/26.2.213. [DOI] [PubMed] [Google Scholar]
- 15.Potvin O, Lorrain D, Forget H, et al. Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep. 2012;35:491–9. doi: 10.5665/sleep.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc. 2001;49:1185–9. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- 17.Tworoger SS, Lee S, Schernhammer ES, Grodstein F. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis Assoc Dis. 2006;20:41–8. doi: 10.1097/01.wad.0000201850.52707.80. [DOI] [PubMed] [Google Scholar]
- 18.Jelicic M, Bosma H, Ponds RWHM, Van Boxtel MPJ, Houx PJ, Jolles J. Subjective sleep problems in later life as predictors of cognitive decline. Report from the Maastricht Ageing Study (MAAS) Int J Geriatr Psychiatry. 2002;17:73–7. doi: 10.1002/gps.529. [DOI] [PubMed] [Google Scholar]
- 19.Bliwise DL. Sleep apnea, APOE4 and Alzheimer's disease 20 years and counting? J Psychosom Res. 2002;53:539–46. doi: 10.1016/s0022-3999(02)00436-1. [DOI] [PubMed] [Google Scholar]
- 20.Durgan DJ, Bryan RM. Cerebrovascular consequences of obstructive sleep apnea. J Am Heart Assoc. 2012:1. doi: 10.1161/JAHA.111.000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea–hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2010;182:269–77. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Born J, Wilhelm I. System consolidation of memory during sleep. Psychol Res. 2012;76:192–203. doi: 10.1007/s00426-011-0335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 24.Munoz R, Duran-Cantolla J, Martínez-Vila E, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37:2317–21. doi: 10.1161/01.STR.0000236560.15735.0f. [DOI] [PubMed] [Google Scholar]
- 25.Kadotani H, Kadotani T, Young T, et al. Association between apolipoprotein e ε4 and sleep-disordered breathing in adults. JAMA. 2001;285:2888–90. doi: 10.1001/jama.285.22.2888. [DOI] [PubMed] [Google Scholar]
- 26.The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 27.Gottesman RF, Rawlings AM, Sharrett AR, et al. Impact of differential attrition on the association of education with cognitive change over 20 years of follow-up: the ARIC Neurocognitive Study. Am J Epidemiol. 2014;179:956–66. doi: 10.1093/aje/kwu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–85. [PubMed] [Google Scholar]
- 29.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–67. [PubMed] [Google Scholar]
- 30.Wechsler D. San Antonio, TX: Psychological Corporation; 1981. WAIS-R manual: Wechsler adult intelligence scale-revised. [Google Scholar]
- 31.Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol. 1989;46:141–5. doi: 10.1001/archneur.1989.00520380041011. [DOI] [PubMed] [Google Scholar]
- 32.Benton AL, Eslinger PJ, Damasio AR. Normative observations on neuropsychological test performances in old age. J Clin Neuropsychol. 1981;3:33–42. doi: 10.1080/01688638108403111. [DOI] [PubMed] [Google Scholar]
- 33.Selnes OA, Vinters HV. Vascular cognitive impairment. Nat Clin Pract Neurol. 2006;2:538–47. doi: 10.1038/ncpneuro0294. [DOI] [PubMed] [Google Scholar]
- 34.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–6. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 35.Weuve J, Tchetgen Tchetgen EJ, Glymour MM, et al. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology. 2012;23:119–28. doi: 10.1097/EDE.0b013e318230e861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen JC, Espeland MA, Brunner RL, et al. Sleep duration, cognitive decline, and dementia risk in older women. Alzheimer Dement. 2015 Jun 15; doi: 10.1016/j.jalz.2015.03.004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez AI, Martinez P, Miro E, Bardwell WA, Buela-Casal G. CPAP and behavioral therapies in patients with obstructive sleep apnea: effects on daytime sleepiness, mood, and cognitive function. Sleep Med Rev. 2009;13:223–33. doi: 10.1016/j.smrv.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Saunamäki T, Jehkonen M. A review of executive functions in obstructive sleep apnea syndrome. Acta Neurol Scand. 2007;115:1–11. doi: 10.1111/j.1600-0404.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 39.Sforza E. Sleep apnea syndrome and cognition. Front Neurol. 2012:3. doi: 10.3389/fneur.2012.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackson ML, Howard ME, Barnes M. Cognition and daytime functioning in sleep-related breathing disorders. Progress in brain research. 2011;190:53–68. doi: 10.1016/B978-0-444-53817-8.00003-7. [DOI] [PubMed] [Google Scholar]
- 41.Boland LL, Shahar E, Iber C, et al. Measures of cognitive function in persons with varying degrees of sleep-disordered breathing: the Sleep Heart Health Study. J Sleep Res. 2002;11:265–72. doi: 10.1046/j.1365-2869.2002.00308.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


