To the Editor,
Cortisol fluctuations in Cushing syndrome were first detected in 1956 and named cyclic Cushing syndrome [1]. Cycle lengths have been reported, varying between 12 hours and 85 days with at least three peaks and two troughs [2]. To assess cortisol production cycles, repeated hormone tests are necessary. According to the literature [2,3], tests to measure the early morning first-voided urine free cortisol to creatinine ratio (UFCCR) or salivary free cortisol can be used as feasible alternatives to assess cyclic cortisol overproduction because they are more convenient to perform. Here, we report a case of cyclic Cushing syndrome that was diagnosed using consecutive UFCCR, reported for the first time in Korea.
An 18-year-old girl was admitted to the gastroenterology department with the chief complaints of nausea and weakness and was referred to an endocrinologist owing to high blood glucose and obesity in November 2009. Her body mass index was 31.24 kg/m2 (height 162 cm, weight 82 kg). Her blood pressure was 100/60 mmHg. She was obese, and her face was round (Fig. 1A), but she did not show other cushingoid features such as a buffalo hump or abdominal striae. On physical examination, hepatomegaly and mild tenderness on the right upper abdomen were found. She denied progressive weight gain. She had been consuming herbal medications and oral contraceptive pills during the previous 2 months owing to irregular menstrual cycles. She complained of episodic symptoms such as plethora, feelings of edema, and lethargy. She reported that the durations of episodic symptoms were approximately 5 to 7 days with or without menstruation. Her father was on renal replacement therapy for diabetic end-stage renal disease, and he was consuming medications for panhypopituitarism secondary to pituitary hemorrhage, which had occurred during hemorrhagic fever with renal failure. Her mother had a past medical history of chronic myeloid leukemia. Based on a fasting blood glucose level of 297 mg/dL and glycated hemoglobin (HbA1c) of 8.6%, diabetes mellitus was diagnosed for the first time. The fasting C-peptide level was 3.0 ng/mL, and autoantibodies, such as anti-glutamic acid decarboxylase antibody and islet cell antibody, were all negative. Total bilirubin was normal. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were elevated to 397 and 190 IU/L, respectively. Viral markers and autoantibodies for hepatitis were not detected. Total cholesterol, triglycerides, and high density lipoprotein cholesterol (HDL-C) levels were 330, 1,012, and 16.9 mg/dL, respectively. Her initial adrenocorticotropic hormone (ACTH) and cortisol levels were 17.81 pg/dL and 15.5 µg/dL, respectively. The result of the thyroid function test was normal. The prolactin level was 13.15 ng/mL; levels of luteinizing hormone, follicle stimulating hormone, and estradiol were 5.26, 6.79, and 5.00 IU/L, respectively. Growth hormone and insulin-like growth factor-1 levels were 0.46 and 40.2 ng/mL, respectively. Flat abdomen film and abdomen computed tomography (Fig. 1B) showed marked hepatosplenomegaly and diffuse gallbladder wall edema with normal adrenal glands. Her liver function and hepatomegaly slowly improved with conservative therapy. A total of 56 units of insulin daily were needed for glucose control at that time, and statin and fenofibrate were prescribed.
Figure 1.
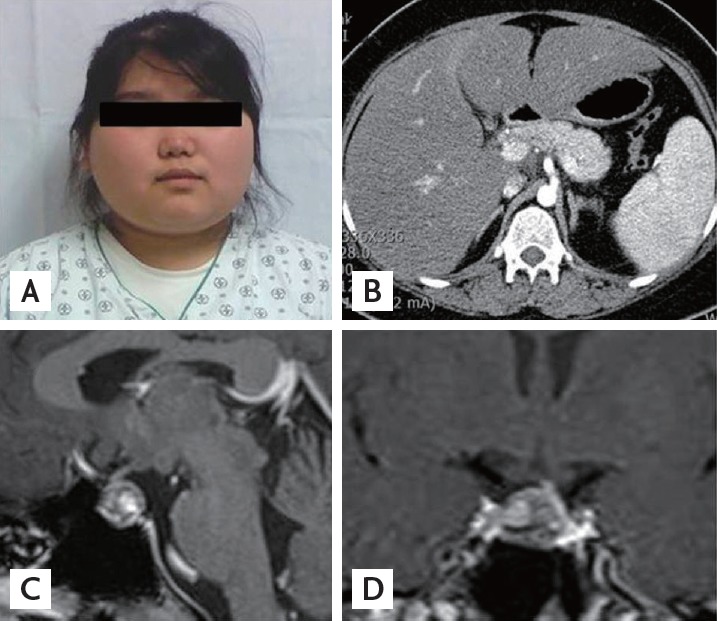
(A) Round and plethoric face of the patient. (B) Abdomen computed tomography of the patient showing normal adrenal glands. (C, D) Sellar magnetic resonance imaging of the patient showing a 1.5-cm, markedly heterogeneous pituitary macroadenoma with left-sided stalk deviation.
An overnight dexamethasone suppression test (DST) revealed that the cortisol level at 8:00 AM was 17.3 µg/dL. During low-dose DST, serum cortisol and 24-hour urine free cortisol (UFC) were not sufficiently suppressed (serum cortisol 6.63 µg/dL, UFC 17.5 µg/day), and cortisol was paradoxically elevated after high-dose DST. The baseline cortisol level was 15.14 µg/dL, and, after high-dose DST, the cortisol level increased to 63.44 µg/dL. The basal plasma ACTH level was 23.33 pg/mL, and the ACTH level after high-dose DST was 86.02 pg/mL. The baseline 24-hour UFC was 28.8 µg/day (normal reference range, 20 to 90) and strikingly increased to 4,387 µg/day on the last day of the high-dose DST. During high-dose DST, she complained of headache, plethora, lethargy, edema, and an unpleasant feeling similar to her previous episodic symptoms. Sellar magnetic resonance imaging showed a 1.5-cm heterogeneous pituitary macroadenoma, indicating recent hemorrhage (Fig. 1C and 1D). We recommended an operation to treat this atypical pituitary Cushing syndrome. However, she wanted to be referred to Severance Hospital for treatment of the pituitary tumor. The doctors at that hospital decided to observe the patient instead of immediate surgery after repeating the low- and high-dose DST. They discontinued all medications during the observation period as her clinical condition had improved, and she remained well without any medications or insulin for a period of time. The results of her blood test at our outpatient department in March 2010 were as follows: ACTH, 56.95 pg/mL; cortisol, 13.41 µg/dL; HbA1c, 6.1%; AST, 54 IU/L; ALT, 88 IU/L; total cholesterol, 253 mg/dL; triglycerides, 214 mg/dL; HDL-C, 32.1 mg/dL; and low density lipoprotein cholesterol 193.4 mg/dL. We randomly performed serial early morning UFCCR for 7 days under the suspicion of cyclic Cushing syndrome and detected a 7-fold increase in UFCCR from her usual UFCCR (Fig. 2). One month later, a 30-day sequential early morning UFCCR was performed, and the results showed that the cortisol peak between days 22 and 26 was 200-fold higher than that of the other days (Fig. 2). She also described episodic symptoms, as she had experienced previously, during the peak UFCCR period. It appeared that the cortisol levels cyclically increased approximately once a month. In November 2010, she underwent a pituitary operation via the transsphenoidal approach at Severance Hospital. Histopathology revealed that the tumor cells were arranged in a trabecular and diffuse pattern (Fig. 3A), with granular eosinophilic cytoplasm and occasionally forming focal pseudovascular rosettes (Fig. 3B). Immunohistochemistry for pituitary hormones was positive only for ACTH (Fig. 3C and 3D). The postoperative combined pituitary test showed panhypopituitarism. After the operation, hormone replacement therapy was commenced with corticosteroids and L-thyroxine. She did not experience any further episodic symptoms, and her diabetes was controlled well with metformin. She remained amenorrheic after the operation, and was prescribed cyclic hormone replacement therapy. She lost approximately 10 kg over a period of 3 years postoperatively.
Figure 2.
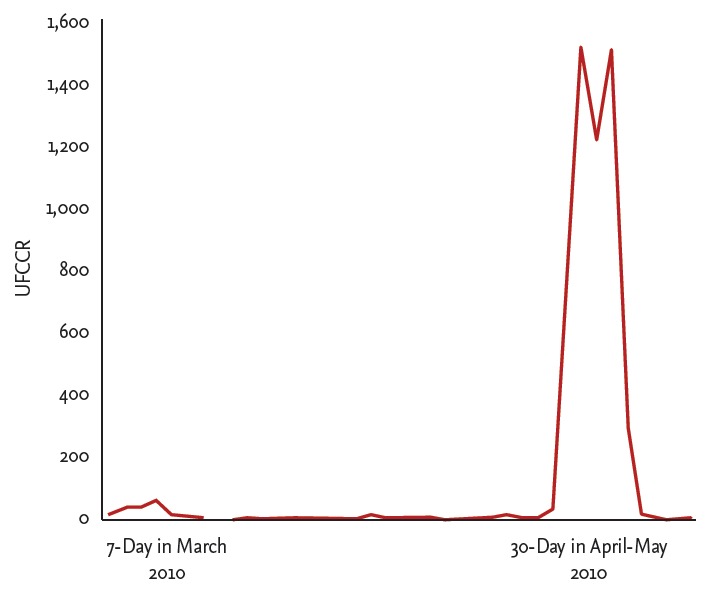
Sequential morning urine free cortisol to creatinine ratio (UFCCR) in the patient for 7 days in March and 30 days from April to May 2010.
Figure 3.
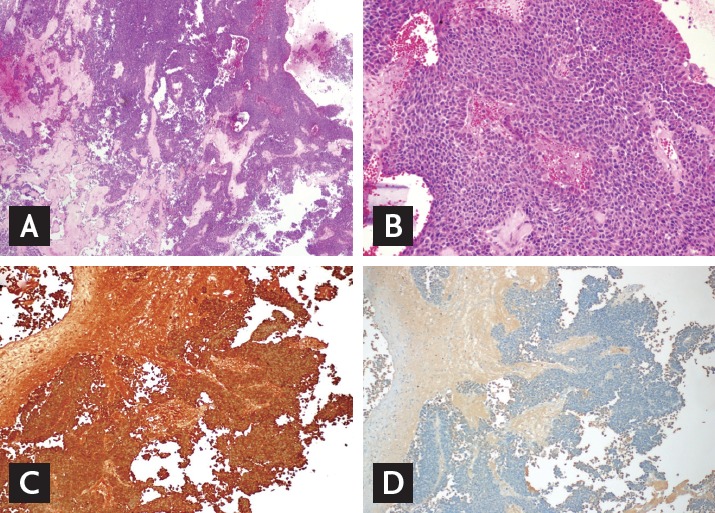
Tissue pathology of the pituitary tumor. The tumor cells were arranged in a trabecular and diffuse pattern (A: H&E, ×40). They were with granular eosinophilic cytoplasm, occasionally forming focal pseudovascular rosettes (B: H&E, ×200). On immunohistochemistry, the tumor was positive for adrenocorticotropic hormone (C: ACTH, ×100), but growth hormone (D: GH, ×100), prolactin, thyroid stimulating hormone, follicle stimulating hormone, and luteinizing hormone were all negative (data not shown). These pictures were provided by courtesy of Dr. Se Hoon Kim in Yonsei University.
The prevalence of cyclic Cushing syndrome is unknown, but that of cyclic hypercortisolism is estimated at approximately 20% to 40%. ACTH-dependent pituitary adenoma is the most frequent cause of cyclic Cushing syndrome [4]. To diagnose cyclic Cushing syndrome, it is important to confirm cyclic cortisol secretion. However, existing screening tests for Cushing syndrome, such as the 24-hour UFC and 1-mg overnight DST, are not ideal to assess cyclic hypercortisolism. Based on the need for a more convenient, repeatable test, early morning first-voided UFCCR has been used to diagnose cyclic Cushing syndrome [2,3]. This test reflects a mixture of diurnal nadir to the peak secretion of cortisol because it uses urine collected in the bladder from midnight to early morning. The sensitivity and specificity of spot urine cortisol to creatinine ratio are unknown. Only Atkinson et al, with 46 samples, reported a positive correlation between early morning UFCCR and 24-hour UFC levels (r = 0.92) and determined that the upper range of the normal cortisol to creatinine ratio was 50 [3].
The excellent repeatability is a major advantage of the UFCCR. If the patient has to urinate frequently during the night, the first-voided UFCCR level will not accurately reflect the serum cortisol level. Therefore, clear instructions to refrain from urinating during the night should be provided. According to a previous study, 9.33% of patients with simple obesity were diagnosed with Cushing syndrome [5]. Therefore, if there is a clinical suspicion of Cushing syndrome, even due to simple obesity, screening for excess cortisol production should be performed, and tests for cyclical production of cortisol may also be performed despite normal screening test results for Cushing syndrome. For this reason, a simple and repeatable screening test for Cushing syndrome is necessary, and early morning UFCCR could be an alternative tool for this purpose.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Birke G, Diczfalusy E. Fluctuation in the excretion of adrenocortical steroids in a case of Cushing’s syndrome. J Clin Endocrinol Metab. 1956;16:286–290. doi: 10.1210/jcem-16-2-286. [DOI] [PubMed] [Google Scholar]
- 2.Mullan KR, Atkinson AB, Sheridan B. Cyclical Cushing’s syndrome: an update. Curr Opin Endocrinol Diabetes Obes. 2007;14:317–322. doi: 10.1097/MED.0b013e3281a477b3. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson AB, Kennedy AL, Carson DJ, Hadden DR, Weaver JA, Sheridan B. Five cases of cyclical Cushing’s syndrome. Br Med J (Clin Res Ed) 1985;291:1453–1457. doi: 10.1136/bmj.291.6507.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meinardi JR, Wolffenbuttel BH, Dullaart RP. Cyclic Cushing’s syndrome: a clinical challenge. Eur J Endocrinol. 2007;157:245–254. doi: 10.1530/EJE-07-0262. [DOI] [PubMed] [Google Scholar]
- 5.Tiryakioglu O, Ugurlu S, Yalin S, et al. Screening for Cushing’s syndrome in obese patients. Clinics (Sao Paulo) 2010;65:9–13. doi: 10.1590/S1807-59322010000100003. [DOI] [PMC free article] [PubMed] [Google Scholar]


