Abstract
Longitudinal studies demonstrate that regular physical exercise extends longevity and reduces the risk of physical disability. Decline in physical activity with aging is associated with a decrease in exercise capacity that predisposes to frailty. Frailty syndrome includes lowered activity level, poor exercise tolerance, and loss of lean body and muscle mass. Poor exercise tolerance is related to aerobic endurance. Aerobic endurance training can significantly improve peak oxygen consumption by ~10–15%. Resistance training is the best way to increase muscle strength and mass. Although the increase in muscle mass in response to resistance training may be attenuated in frail older adults, resistance training can significantly improve muscle strength, particularly in institutionalized patients by ~110%. Because both aerobic and resistance training target specific components of frailty, studies combining aerobic and resistance training provide the most promising evidence with respect to successfully treating frailty. At the molecular level, exercise reduces frailty by decreasing muscle inflammation, increasing anabolism, and increasing muscle protein synthesis. More studies are needed to determine which exercises are best suited, most effective, and safe for this population. Based on the available studies, an individualized multicomponent exercise program that includes aerobic activity, strength exercises, and flexibility is recommended to treat frailty.
Introduction
The population age 65 and older is expected to more than double between 2012 and 2060, from 43.1 million to 92 million [1]. The continuing increase in the older population has generated interest toward investigations of older adults who are “frail”. Frailty is a state of vulnerability that carries an increased risk for adverse outcomes [2]; it can be viewed as a transition phase in older people between good health and poor health. Frail older adults are less capable of tolerating the stress of medical illness, hospitalization, and immobility. Common signs and symptoms are fatigue, weight loss, muscle weakness, and progressive decline in function. Frailty is more prevalent in older people and in those with multiple medical conditions.
Concomitant with age, there is decline in voluntary physical activity which is associated with decreases in numerous measures of exercise capacity including peak oxygen consumption (VO2pea), muscle strength, and fatigability which ultimately leads to frailty [3]. Recently it has been recognized that most older adults who are obese also meet criteria for frailty because of decrease muscle mass and strength that occurs with aging (sarcopenia) and a need to carry greater body mass due to obesity [4]. Because frailty increases the risk for loss of functional independence and decrease in quality of life, the identification of cost-effective interventions to prevent or ameliorate frailty is one of the most important public health challenges. Accordingly, exercise may be an effective strategy to prevent and treat frailty as it can target four of the five commonly used criteria: weakness, low physical activity, slowed motor performance, and poor exercise tolerance [5]. Frailty is not a contra-indication to physical activity, rather it maybe one of the most important indications to prescribe physical exercise. Longitudinal studies have demonstrated that regular physical activity extends longevity and reduces the risk of physical disability. In fact, cardiorespiratory fitness has been found to be a significant mortality predictor in older adults, independent of overall or abdominal obesity [6]. In more than 10,000 older adults participating in the Established Populations for Epidemiologic Studies of the Elderly (EPESE studies) an almost two-fold increased likelihood of dying without disability was found among those most physically active compared to those who were sedentary [7].
Aerobic endurance training
After age 30 y, aerobic capacity, often measured as VO2peak declines with age and contributes to a decrease in the older adult’s ability to perform activities of daily living. This is largely due to three major causes: 1) a decline in the ability of the cardiopulmonary system to deliver O2 2)a decline in the ability of the working muscle to extract O2, and 3) a decline in metabolic muscle mass and parallel increase in metabolically inactive fat mass [3]. Indeed, probably one of the most notable effects of endurance training is on VO2peak which is an important determinant of frailty in older adults [4] The improvement in VO2peak with endurance exercise training would be thought to reduce frailty in older adults and thus counter the decline in VO2peak with aging and physical inactivity. Whereas VO2peak declines ~1%/year in non-training individuals [8] this decline is ~0.5%/years in master athletes who participate in aerobic activities [3]. Another important adaptation to endurance exercise training is an increase in muscle oxidative capacity, which results in fatigue resistance or increased muscle endurance. In an interventional trial of 64 frail older men and women, a nine month program of strength training and walking exercise at 78% of peak heart rate increased endurance by improving VO2peak by ~14% [9]. A similar exercise program for 12 months in 107 frail obese older men and women also increased VO2peak by ~10% [10]. On the other hand, in healthy 77–87 years old, nine months of endurance training at 83% of peak heart rate increased VO2peak by 15%, as compared to increased VO2peak by 24–30% in healthy 60 to 71-yr-old, indicating that the adaptations in aerobic power may be attenuated in advancing age [11]. Data from met analyses [12] also showed that endurance training may help to conserve fat free mass (FFM) during weight loss, although it is probably less effective than resistance exercise. We recently reported that compared to weight loss induced by diet, weight loss induced by aerobic exercise preserved lower extremity muscle mass (measured by magnetic resonance imaging) and physical work capacity, although the amount of exercise was large [13].
Progressive resistance training
It is well known that muscle strength and mass decreases with advancing age. A 30% reduction in strength between 50 and 70 years of age is generally found, with muscle strength losses being most dramatic after age 70 [14]. Most of the decline in strength can be explained by selective atrophy of type II muscle fibers and the loss of neuronal activation. Based on body composition techniques such as DXA and CT scan, the relative annual decline in muscle mass was estimated to be between −0.64 and −1.29% per year for older men and −0.53 and −0.84% per year for older women [15]. Although decline in muscle quality also contributes, several studies have found that the decline in strength in the older adult is primarily due to loss of muscle quantity with age. Several studies have shown that resistance exercise training increases muscle mass and thus muscle strength in both younger and older adults. However, the response to resistance training appears to be attenuated in older adults with mobility limitations or other co-morbidities. In healthy older adults, four months of progressive resistance training increased muscle mass by 16 to 23%, whereas it increased muscle mass by 2.0–9% in frail older adults [10, 16–18] Other studies showed that the gain in FFM in older women and men was only ~58% of that for younger men and women in response to resistance training [19]. Nonetheless, resistance training still has been found to significantly increase strength in older men and women. Several studies have demonstrated that these changes can occur even in the late stages in life [20, 21]. Indeed, based on two recent systemic reviews of randomized controlled trials (RCT) involving resistance training in older adults, it was concluded that resistance training results in significant improvement in muscle strength in older adults [22, 23]. These reviews included studies in both healthy and older adults. Of particular interest is that In frail institutionalized patients, Fiatarone et al demonstrated that 10 weeks of resistance exercise training increased muscle strength by ~113% as compared to ~3% in nonexercising subjects [16]. Moreover, our group has shown that in frail older men and women, resistance training added to diet reduced FFM loss (from 3.5 kg to 1.8 kg) during voluntary weight loss and increased both upper and lower extremity muscle strength (by 17–43%) despite FFM loss [24]. With respect to aspects of functional limitations, resistance training has been shown to improve gait speed in healthy and frail elders (weighted mean differences = 0.07 m/s based on 14 trials; n=798)[22]. Specifically, in frail older adults living in the nursing home and in the community, ten weeks of resistance training has been shown to significantly improve gait speed [16].
Combined aerobic and resistance training
Not only the physiologic adaptations to aerobic exercise and to resistance exercise are distinctly different but also both types of exercise target specific components of frailty. Therefore, the few exercise interventions conducted in frail older populations have mostly used combined aerobic and resistance exercise. A nine month RCT intervention of aerobic and resistance exercise concomitantly improved scores in the VO2peak (95% confidence bounds [CI] 0.9 to 3.6 mL/kg/min) and modified physical performance test (95% CI 1.0 to 5.2 points) [25]. In addition, a recent 12 month RCT of aerobic and resistance exercise also improved scores in the VO2peak and modified physical performance test in frail obese older adults, which were additive to the effects of diet-induced weight loss [10]. Finally, the Lifestyle Intervention and Independence for Elders (Life) pilot study also reported that a 12 month program of walking, resistance exercise, and flexibility training resulted in a clinically meaningful improvement of physical performance as assessed by using the Short Physical Performance Battery [26]. This study also presented promising evidence on the effectiveness of exercise in the prevention of the disability in walking as assessed by the capacity to complete a 400 meter work.
Effect on frailty as an outcome measure
Most exercise intervention trials studied the effects on features of frailty and the adverse outcomes of frailty. There have been relatively few studies designed to determine whether physical exercise can reverse frailty (frail reverse to nonfrail) or if older adults can convert from a greater state of frailty to a lesser state of frailty with exercise. The Frailty Intervention Trial (FIT) study examined whether a multifactorial intervention that included balance, strength, and endurance exercise could reduce frailty and improve mobility [27]. After 12 months of the intervention, there was a lower prevalence of frailty in the intervention group compared with the control group (between group difference 14.7%) which was associated with a significant improvement in the Short Physical Performance Battery (between group difference 1.44 pts), suggesting that it is possible to successfully “treat” frailty.
Molecular and cellular mechanisms underlying exercise training
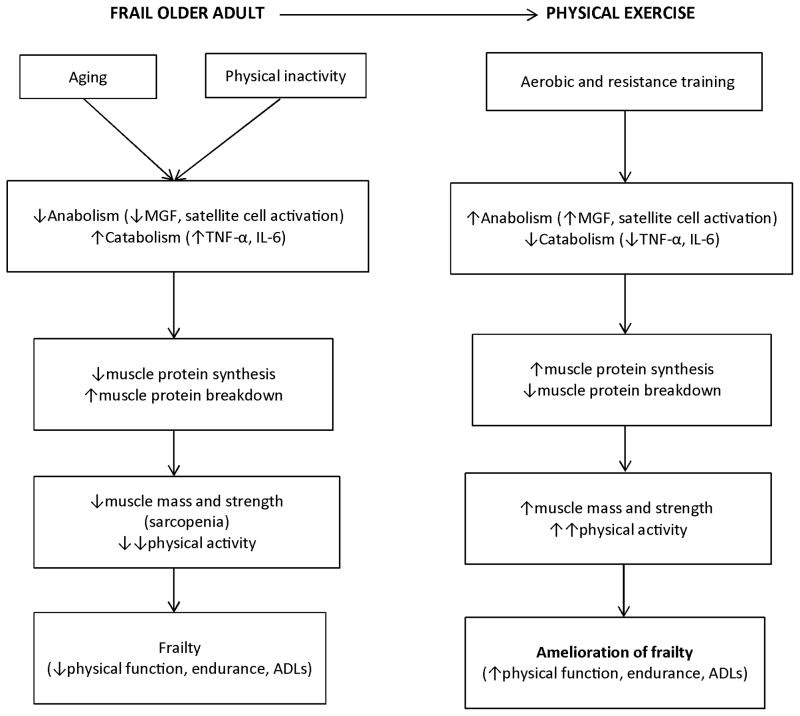
Aging and physical inactivity are associated with increased levels of chronic inflammation. Inflammatory cytokines have direct catabolic effects on skeletal muscle: Tumor necrosis factor-alpha (TNF-α) suppresses muscle protein synthesis (MPS) [28], while Interleuken-6 (IL-6) inhibits the anabolic effects of insulin-like growth factor-1 (IGF-1) [29]. These cytokines also induce insulin resistance, which contributes to sarcopenia and frailty by reducing MPS. High concentrations of TNF-α or IL-6 are associated with lower muscle mass or strength and mobility disability [30] and high IL-6 and low IGF-1 levels contribute synergistically to impaired mobility [31]. Accordingly, an important mechanism by which exercise training reduces frailty is by suppressing muscle inflammation and promoting anabolism which leads to an increase in MPS (Figure 1). We previously reported that in frail obese older adults 12 weeks of exercise (aerobic and resistance ) but not 12 weeks of weight loss (~7% reduction) decreased IL-6 and TNF-α and increased mechano growth factor mRNA of skeletal muscles, which was associated with positive effects on functional status [32]. Moreover, in these frail obese older adults, a multicomponent exercise program increased the mixed muscle protein fractional synthesis rate in the basal, postabsorptive state without affecting the magnitude of muscle protein anabolic response to feeding [33]. These changes in muscle protein anabolism were accompanied by increases in FFM, appendicular lean body mass, strength, and VO2peak, all of which are important determinants of frailty. There appears to be sexual dimorphism in muscle protein anabolism in that 1) older women have greater MPS rate in the basal state but less anabolic response to mixed meal than older men [34] and 2) older women have less MPS rate increase in response to exercise training in the basal state than older men [35]. These findings may explain not only the lower muscle mass in older women but also perhaps the need for greater exercise stimuli to achieve the same anabolic response seen in older men [36].
Figure 1.
Theoretical framework for the molecular and cellular mechanisms by which physical exercise ameliorates frailty in older adults. Abbreviations: MGF, mechano growth factor; TNF-α, Tumor necrosis factor-alpha, IGF-1, Insulin-like growth factor 1; ADL, activities of daily living
Recommendations and future directions
In a systemic review of the effectiveness of exercise interventions for the management of frailty, it was found that even though the participants were frail, the exercise adherence was high with no adverse events in most reported studies, supporting that exercise was safe and feasible in this older population [37]. Although exercise uniformly had a positive impact on functional measurements, exercise seemed to be more beneficial in frail people living in long-term care facilities compared to the community (probably due to floor and ceiling effects of some outcome measurements) and in the earlier stages of frailty compared to the later stages of frailty (probably due to less ability to exercise with greater degree of frailty). With respect to specific type of exercise program, a multicomponent training was found to have a more positive effect on the functional ability and adverse health consequences of the frail people. Interventions lasting longer than five months seemed to result in greater benefits on the adverse health consequences of the frail people. The duration for each session of exercise that was most beneficial was 30–45 minutes, which is less than what is usually recommended for healthier older adults. Clearly, more RCTs are needed that include robust sample sizes and participants with different degrees of frailty, and examine age and potential sex dimorphism of the positive effects of exercise in frail older adults. More studies are also needed to determine which exercises are best suited, most effective, and safe (type, setting, duration, frequency, and intensity) for this population. Whether these exercise interventions would require supervision by rehabilitation personnel or could be safely and effectively conducted in the community or even at home needs further investigation. Based on currently available evidence, a multicomponent exercise program that includes aerobic activity, strength exercises, and flexibility is recommended in frail older adults (Table 1)
Table 1.
Exercise Recommendations for frail older adults*
|
Aerobic exercise Moderate to vigorous activity enough to raise the pulse rate to 70–80% of the maximum heart rate. Activity performed for a minimum of 20–30 minutes at least three days per week |
|
Resistance exercise The progressive resistance program should involve all major muscle groups of the upper and lower extremities and trunk. One set of 8 to 10 different exercise, with 10 to 15 repetitions per set, performed 2–3 nonsecutive days per week. Moderate-high intensity training is recommended, in which moderate intensity is 5 or 6 on a 0 to 10 scale. |
|
Flexiblity and balance exercise Stretching to the point of tightness and holding the position for a few seconds.. Flexibility activities are performed on all days that aerobic or muscle strengthening activity is performed. Balance training exercise 2–3 times per week. |
The exercise program should be individualized according to an older individual’s medical conditions and disability. The program should start at a low-to-moderate intensity, duration, and frequency to promote compliance and minimize musculoskeletal injuries.
It is worth mentioning that changes in the lifestyle habits of frail, older persons may present special challenges. Multiple medical problems, depression, sensory impairments, and cognitive dysfunction may make it difficult to change lifestyle. The increase in chronic disabilities with aging reduces physical activity and exercise capacity. To facilitate adherence to lifestyle change that includes regular physical exercise, program participation by spouse or caregivers may need to be encouraged. In addition, special consideration should be given to hurdles faced during learning by frail older adults, such as impaired vision and hearing, orthopedic conditions, multiple comorbidities, and limited financial resources.
Acknowledgments
Supported by grant RO1AG031176 and resources at the New Mexico VA Health Care System
Reference List
- 1.U.S.Department of Commerce. US Census Bureau Projections Show a Slower Growing, Older, More Diverse Nation a Half Century from Now. United States Census Bureau; 2014. [cited 2014 Mar 1];Available from: : http://www.census.gov/newsroom/releases/archives/ [Google Scholar]
- 2.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013 Mar 2;381(9868):752–62. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambert CP, Evans WJ. Adaptations to aerobic and resistance exercise in the elderly. Rev Endocr Metab Disord. 2005 May;6(2):137–43. doi: 10.1007/s11154-005-6726-5. [DOI] [PubMed] [Google Scholar]
- 4.Villareal DT, Banks M, Siener C, Sinacore DR, Klein S. Physical Frailty and Body Composition in Obese Elderly Men and Women. Obes Res. 2004 Jun 1;12(6):913–20. doi: 10.1038/oby.2004.111. [DOI] [PubMed] [Google Scholar]
- 5.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 6.Sui X, LaMonte MJ, Laditka JN, Hardin JW, Chase N, Hooker SP, et al. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA. 2007 Dec 5;298(21):2507–16. doi: 10.1001/jama.298.21.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leveille SG, Guralnik JM, Ferrucci L, Langlois JA. Aging successfully until death in old age: opportunities for increasing active life expectancy. Am J Epidemiol. 1999 Apr 1;149(7):654–64. doi: 10.1093/oxfordjournals.aje.a009866. [DOI] [PubMed] [Google Scholar]
- 8.Heath GW, Hagberg JM, Ehsani AA, Holloszy JO. A physiological comparison of young and older endurance athletes. J Appl Physiol Respir Environ Exerc Physiol. 1981 Sep;51(3):634–40. doi: 10.1152/jappl.1981.51.3.634. [DOI] [PubMed] [Google Scholar]
- 9.Ehsani AA, Spina RJ, Peterson LR, Rinder MR, Glover KL, Villareal DT, et al. Attenuation of cardiovascular adaptations to exercise in frail octogenarians. J Appl Physiol. 2003 Nov;95(5):1781–8. doi: 10.1152/japplphysiol.00194.2003. [DOI] [PubMed] [Google Scholar]
- 10.Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011 Mar 31;364(13):1218–29. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans EM, Racette SB, Peterson LR, Villareal DT, Greiwe JS, Holloszy JO. Aerobic power and insulin action improve in response to endurance exercise training in healthy 77–87 yr olds. J Appl Physiol. 2005 Jan;98(1):40–5. doi: 10.1152/japplphysiol.00928.2004. [DOI] [PubMed] [Google Scholar]
- 12.Garrow JS, Summerbell CD. Meta-analysis: effect of exercise, with or without dieting, on the body composition of overweight subjects. Eur J Clin Nutr. 1995 Jan;49(1):1–10. [PubMed] [Google Scholar]
- 13.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, et al. Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J Appl Physiol. 2007 Feb;102(2):634–40. doi: 10.1152/japplphysiol.00853.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol Respir Environ Exerc Physiol. 1979 Mar;46(3):451–6. doi: 10.1152/jappl.1979.46.3.451. [DOI] [PubMed] [Google Scholar]
- 15.Visser M. Epidemiology of Muscle Mass Loss with Age. In: Cruz-jentoft A, Morley JE, editors. Sarcopenia. 1. West Sussex, UK: John Wiley & Sons, Ltdll; 2012. pp. 1–7. [Google Scholar]
- 16.Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994 Jun 23;330(25):1769–75. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 17.Binder EF, Yarasheski KE, Steger-May K, Sinacore DR, Brown M, Schechtman KB, et al. Effects of progressive resistance training on body composition in frail older adults: results of a randomized, controlled trial. J Gerontol A Biol Sci Med Sci. 2005 Nov;60(11):1425–31. doi: 10.1093/gerona/60.11.1425. [DOI] [PubMed] [Google Scholar]
- 18.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990 Jun 13;263(22):3029–34. [PubMed] [Google Scholar]
- 19.Lemmer JT, Ivey FM, Ryan AS, Martel GF, Hurlbut DE, Metter JE, et al. Effect of strength training on resting metabolic rate and physical activity: age and gender comparisons. Med Sci Sports Exerc. 2001 Apr;33(4):532–41. doi: 10.1097/00005768-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 20.McCartney N, Hicks AL, Martin J, Webber CE. A longitudinal trial of weight training in the elderly: continued improvements in year 2. J Gerontol A Biol Sci Med Sci. 1996 Nov;51(6):B425–B433. doi: 10.1093/gerona/51a.6.b425. [DOI] [PubMed] [Google Scholar]
- 21.Adams KJ, Swank AM, Berning JM, Sevene-Adams PG, Barnard KL, Shimp-Bowerman J. Progressive strength training in sedentary, older African American women. Med Sci Sports Exerc. 2001 Sep;33(9):1567–76. doi: 10.1097/00005768-200109000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Latham NK, Bennett DA, Stretton CM, Anderson CS. Systematic review of progressive resistance strength training in older adults. J Gerontol A Biol Sci Med Sci. 2004 Jan;59(1):48–61. doi: 10.1093/gerona/59.1.m48. [DOI] [PubMed] [Google Scholar]
- 23.Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009;(3):CD002759. doi: 10.1002/14651858.CD002759.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frimel TN, Sinacore DR, Villareal DT. Exercise attenuates the weight-loss-induced reduction in muscle mass in frail obese older adults. Med Sci Sports Exerc. 2008;40(7):1213–9. doi: 10.1249/MSS.0b013e31816a85ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Binder EF, Schechtman KB, Ehsani AA, Steger-May K, Brown M, Sinacore DR, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. Journal of the American Geriatrics Society. 2002 Dec;50(12):1921–8. doi: 10.1046/j.1532-5415.2002.50601.x. [DOI] [PubMed] [Google Scholar]
- 26.Pahor M, Blair SN, Espeland M, Fielding R, Gill TM, Guralnik JM, et al. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006 Nov;61(11):1157–65. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 27.Cameron ID, Fairhall N, Langron C, Lockwood K, Monaghan N, Aggar C, et al. A multifactorial interdisciplinary intervention reduces frailty in older people: randomized trial. BMC Med. 2013;11:65. doi: 10.1186/1741-7015-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frost RA, Lang CH, Gelato MC. Transient exposure of human myoblasts to tumor necrosis factor-alpha inhibits serum and insulin-like growth factor-I stimulated protein synthesis. Endocrinology. 1997 Oct;138(10):4153–9. doi: 10.1210/endo.138.10.5450. [DOI] [PubMed] [Google Scholar]
- 29.De BF, Alonzi T, Moretta A, Lazzaro D, Costa P, Poli V, et al. Interleukin 6 causes growth impairment in transgenic mice through a decrease in insulin-like growth factor-I. A model for stunted growth in children with chronic inflammation. J Clin Invest. 1997 Feb 15;99(4):643–50. doi: 10.1172/JCI119207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002 May;57(5):M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 31.Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, Fried LP. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab. 2003 May;88(5):2019–25. doi: 10.1210/jc.2002-021694. [DOI] [PubMed] [Google Scholar]
- 32.Lambert CP, Wright NR, Finck BN, Villareal DT. Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J Appl Physiol. 2008 Aug;105(2):473–8. doi: 10.1152/japplphysiol.00006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villareal DT, Smith GI, Sinacore DR, Shah K, Mittendorfer B. Regular multicomponent exercise increases physical fitness and muscle protein anabolism in frail, obese, older adults. Obesity (Silver Spring) 2011 Feb;19(2):312–8. doi: 10.1038/oby.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith GI, Atherton P, Villareal DT, Frimel TN, Rankin D, Rennie MJ, et al. Differences in muscle protein synthesis and anabolic signaling in the postabsorptive state and in response to food in 65–80 year old men and women. PLoS ONE. 2008;3(3):e1875. doi: 10.1371/journal.pone.0001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith GI, Villareal DT, Sinacore DR, Shah K, Mittendorfer B. Muscle protein synthesis response to exercise training in obese, older men and women. Med Sci Sports Exerc. 2012 Jul;44(7):1259–66. doi: 10.1249/MSS.0b013e3182496a41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bamman MM, Hill VJ, Adams GR, Haddad F, Wetzstein CJ, Gower BA, et al. Gender differences in resistance-training-induced myofiber hypertrophy among older adults. J Gerontol A Biol Sci Med Sci. 2003 Feb;58(2):108–16. doi: 10.1093/gerona/58.2.b108. [DOI] [PubMed] [Google Scholar]
- 37.Theou O, Stathokostas L, Roland KP, Jakobi JM, Patterson C, Vandervoort AA, et al. The effectiveness of exercise interventions for the management of frailty: a systematic review. J Aging Res. 2011;2011:569194. doi: 10.4061/2011/569194. [DOI] [PMC free article] [PubMed] [Google Scholar]