Abstract
The TNF Superfamily member LIGHT (TNFSF14) has recently emerged as a potential target for therapeutic interventions aiming to halt tissue fibrosis. In this perspective, we discuss how LIGHT may influence the inflammatory and remodeling steps that characterize fibrosis, relevant for many human diseases presenting with scarring such as Asthma, Idiopathic Pulmonary Fibrosis, Systemic sclerosis, and Atopic Dermatitis. LIGHT acts through two receptors in the TNF receptor superfamily, HVEM (TNFRSF14) and LTβR (TNFRSF3), which are broadly expressed on hematopoietic and non-hematopoietic cells. LIGHT can regulate infiltrating T cells, macrophages, and eosinophils, controlling their trafficking or retention in the inflamed tissue, their proliferation, and their ability to produce cytokines that amplify fibrotic processes. More interestingly, LIGHT can act on structural cells, namely epithelial cells, fibroblasts, smooth muscle cells, adipocytes, and endothelial cells. By signaling through either HVEM or LTβR expressed on these cells, LIGHT can contribute to their proliferation and expression of chemokines, growth factors, and metalloproteinases. This will lead to hyperplasia of epithelial cells, fibroblasts, and smooth muscle, deposition of extracellular matrix proteins, vascular damage, and further immune alterations that in concert constitute fibrosis. Because of its early expression by T cells, LIGHT may be an initiator of fibrotic diseases, but other sources in the immune system could also signify a role for LIGHT in maintaining or perpetuating fibrotic activity. LIGHT may then be an attractive prognostic marker as well as an appealing target for fibrosis therapies relevant to humans.
Keywords: TNF superfamily, TNFSF, LIGHT, HVEM, Lympotoxin beta receptor, fibrosis, remodeling
Graphical abstract
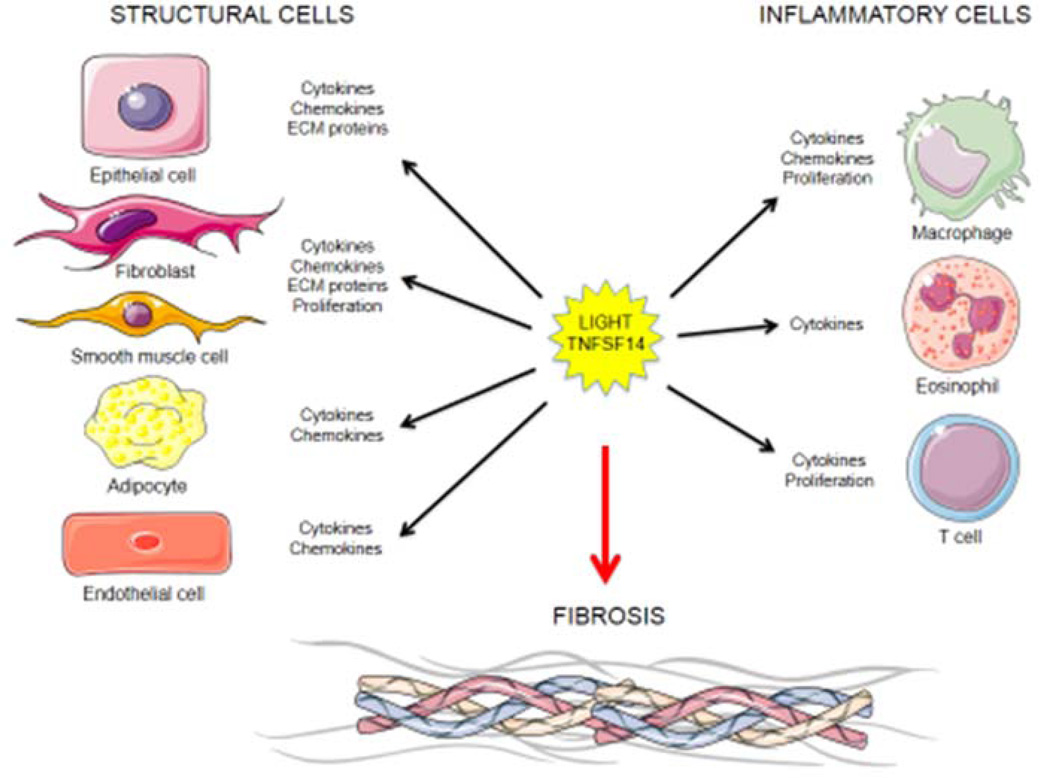
LIGHT can influence both structural and inflammatory cells to promote fibrosis. LIGHT controls the expression of pro-fibrotic factors like cytokines and chemokines driving cellular infiltration and activity in inflamed sites; induction of extracellular matrix protein synthesis and degradation; as well as proliferation, growth, differentiation and hyperplasia of cells involved in fibrosis. Cartoons designed from http://www.servier.com.
Introduction
In this perspective, we discuss emerging data that suggests that TNF Superfamily member 14 (TNFSF14), also known as CD258 or its common name LIGHT (homologous to Lymphotoxins, shows Inducible expression, competes with HSV Glycoprotein D for HVEM, a receptor expressed on T cells), may control the severity of fibrotic disease and could be a suitable target for therapeutic interventions aiming to reduce fibrosis.
Fibrosis is a feature of a number of chronic diseases and is thought to result from an exaggerated or uncontrolled response to epithelial injury. It involves the accumulation of extracellular matrix proteins and fibers in tissues, such as collagen, elastin, periostin, fibronectin, smooth muscle actin, and glycosaminglycans, often leading to organ failure and death 1. Several autoimmune connective tissue disorders and inflammatory diseases display a fibroproliferative component characterized by tissue remodeling and scarring, including Asthma, Systemic sclerosis (SS), Atopic Dermatitis (AD), Psoriasis, Systemic lupus Erythematosus (SLE), Idiopathic Pulmonary fibrosis (IPF), Chronic Obstructive Pulmonary Disease (COPD), Graft versus Host disease (GvHD), Rheumatoid Arthritis (RA), Ankylosing Spondylitis (AS), Crohn’s disease (CD), Ulcerative colitis (UC), and Eosinophilic Esophagitis (EoE), as well as advanced liver and kidney diseases 2. Fibrosis can affect one or several organs depending on epithelial injury location and atopic march 1.
Inflammation is thought to initiate fibrosis 2. Several key immune cells are likely important in many diseases, especially macrophages, eosinophils, and T cells, with often contributions from other cell types including dendritic cells, ILC2, and NK cells 3. However, tissue structural or stromal cells are the main end-stage drivers and/or targets of the response, with epithelial cells, fibroblasts/myofibroblasts, smooth muscle cells, and endothelial cells all capable of undergoing hyperplasia or hypertrophy and contributing to the accumulation of extracellular matrix proteins and fibers 4. Several cytokines that can be derived from both immune cells and structural cells have been found in murine models of disease to be involved in the fibrotic process, and in particular, TGF-β, IL-13, and/or TSLP, have often been associated with driving the characteristic features of the disease 3, 5. As the initiation of fibrosis is thought to be a consequence of inflammation, anti-inflammatory drugs have been tested in patients, but with only moderate success. One possible explanation is that corticosteroids and other anti-inflammatory drug candidates do not decrease the production of fibrotic factors, or do not inhibit the activity of these factors that results in tissue remodeling 6, 7. Therefore an increased knowledge of specific molecules that start or maintain fibrotic activity is warranted.
We recently identified a novel member of the TNF Superfamily called LIGHT as a key regulator of fibrosis and a potential target for therapies aiming to halt development of this disease feature 8–10. LIGHT was initially described as a molecule made by T cells 11, 12, and it has two receptors that are ubiquitously expressed in several cell types, both inflammatory and structural. LIGHT can be either membrane-bound or produced soluble as a cytokine, and was originally described to enhance T cell activation through a specific receptor in the TNF receptor superfamily named the herpes virus entry mediator or HVEM (TNFRSF14) 11. LIGHT additionally binds another TNF superfamily receptor, named LTβR or lymphotoxin beta receptor (TNFRSF3). One or both receptors are now known to be broadly expressed on both stromal cells and hematopoietic cells, including lymphatic endothelial cells, fibroblastic reticular cells, follicular dendritic cells, dendritic cells, macrophages, mast cells, eosinophils, neutrophils, T and B cells, and NK cells 13. Importantly, one or both receptors are found on the primary cells that contribute to tissue remodeling and fibrosis, namely epithelial cells, fibroblasts, and smooth muscle cells.
We published the first reports that a genetic deficiency in LIGHT, and blocking LIGHT binding to both of its receptors, strongly reduced tissue remodeling and fibrosis in the lungs of allergen-challenged mice in models of severe asthma and in a model of idiopathic pulmonary fibrosis 8, 9. Moreover, injection of recombinant LIGHT into the lungs of mice reproduced the primary fibrotic features seen in severe asthmatics and IPF patients 8, 9. Furthermore, we recently published that recombinant LIGHT alone can also induce skin inflammation and dermal fibrosis, and in published and unpublished studies found that endogenous production of LIGHT is crucial to development of skin disease in mouse models of scleroderma and atopic dermatitis10. In line with the activity of LIGHT in fibrotic disease representing an exaggerated but normal physiological activity of this molecule, another study found that LIGHT-deficient animals displayed delayed closure of non-chronic wounds as well as exacerbated chronic wounding 14. In each report, collagen deposition and smooth muscle actin expression were reduced in the absence of LIGHT interactions with its receptors 8–10, 14. In this current perspective, we focus on the activity of LIGHT and discuss how this molecule can promote both inflammation and tissue remodeling/fibrosis, potentially making it a very attractive target for therapy of chronic inflammatory diseases that involve fibrotic activity. We specifically discuss the role of LIGHT on tissue infiltrating inflammatory cells (macrophages, eosinophils, and T cells) and structural cells (epithelial cells/keratinocytes, fibroblasts, smooth muscle cells, endothelial cells, and adipocytes).
LIGHT’s impact on inflammatory cells
LIGHT activity in T cells
T cells likely contribute to fibrotic disease by making cytokines that either enhance or recruit other inflammatory cells to the tissue sites, or by making cytokines that directly act on structural cells, like IL-13 or TNF, that promote pro-fibrotic activity. The connection between T cells and LIGHT may be significant to fibrotic disease in several ways. LIGHT is a stimulatory molecule for T cells, and can promote their proliferation and survival through binding to HVEM (LTβR is not expressed by T cells) 12, 15. In some cases, LIGHT activity can also promote expression of cytokines by T cells. This has been demonstrated for CD4 T cells of the Th2 and Th1 subsets, as well as for CD8 T cells. As such the absence of LIGHT in gene deficient mice has been shown to lead to reduced acute asthmatic lung inflammation driven by Th2 cells 8, and reduced GVHD driven by CD8 T cells or Th1 cells 16, 17. Equally importantly, T cells can be the source of LIGHT. Transgenic mice overexpressing LIGHT in T cells developed gut inflammation and Crohn’s like symptoms. Furthermore, transgenic LIGHT promoted enhanced atherosclerosis and Crohn’s disease when animals or cells derived from them were used under conditions conducive for such diseases 18, 19. Similarly, in a mouse model of IPF induced by bleomycin, we tested whether LIGHT from T cells was driving the disease. We observed that Rag-/- mice lacking T cells were resistant to disease, and that transfer of wild-type but not LIGHT-deficient T cells led to pronounced collagen deposition and smooth muscle hyperplasia (unpublished observations). Thus, LIGHT may both act on T cells to allow their accumulation at sites of fibrosis, and be produced by these T cells to contribute to fibrotic disease in other ways.
LIGHT signaling in macrophages
LIGHT may have several activities linked to macrophages that are relevant for development of tissue fibrosis. In concert with other cells, macrophages are known to be involved in many remodeling diseases, including asthma and COPD, through production of TGF-β. This cytokine is likely to be central to many fibrotic states as it can induce epithelial-mesenchymal transition (EMT) 20, differentiation of fibroblasts into myofibroblasts, production of extracellular matrix proteins including collagen, and enhance smooth muscle cell proliferation 21. LIGHT was shown to affect the accumulation of macrophages and up-regulate the expression of TGF-β by macrophages 8. Macrophages express LTβR and HVEM, and reside close to subepithelial regions, where smooth muscle hyperplasia and fibrosis often occur. We showed that lung macrophages, after chronic challenge with allergen, were impaired in accumulating in the lungs of animals where LIGHT was inactive, likely through a survival activity exhibited on these cells. Moreover, expression of latency-associated peptide, a marker for membrane-bound TGF-β, was decreased in macrophages from these mice. Suggesting this represented a direct activity of LIGHT on lung macrophages, stimulation of the LTβR on these cells in vitro was found to increase expression of TGF-β8. Other studies have suggested that LIGHT itself can signal into macrophages when membrane bound, and interestingly stimulating the monocytic THP-1 cell line with an agonistic antibody to LIGHT promoted the expression of MMP-9 and IL-822. MMP-9 is a positive regulator of inflammation that functions through degradation of ECM proteins to enable the migration of immune cells to inflamed sites, and activation of cytokines and chemokines 23. IL-8 can recruit neutrophils into inflammatory sites, enhances blood vessel formation, and regulates phagocytosis by neutrophils and macrophages, all of which could contribute to fibrotic activity 23. Therefore, LIGHT might drive fibrosis by enhancing the survival and accumulation of macrophages in the inflamed sites, as well as increasing their expression of TGF-β, MMP-9, and IL-8.
LIGHT signaling in eosinophils
In humans, the importance of eosinophils to fibrosis has been assessed when treating asthmatics with anti-IL-5 antibodies, which in select populations has led to a reduction of eosinophils in the lungs accompanied by a reduction in airway deposition of extracellular matrix proteins 24. Eosinophils might contribute to fibrotic disease in several ways, but their ability to make IL-13 and TGF-β could be central to any activity in this regard. IL-13 has been reported to possess a number of pro-fibrotic activities, including inducing epithelial cell proliferation, myofibroblast proliferation, and smooth muscle actin expression, and IL-13 can synergize with TGF-β to promote certain features associated with EMT 25–27. Eosinophils have been found to upregulate IL-13 production after chronic challenge with allergens in mouse severe asthma models correlating with the development of fibrosis or accumulation of smooth muscle cells or alpha smooth muscle actin. Suggesting that LIGHT contributes to this activity, we showed that lung eosinophils express HVEM, and when they were analyzed in allergen-challenged LIGHT-deficient animals, or mice where LIGHT interactions were blocked, they had reduced IL-13 expression. Showing this was a direct activity, recombinant LIGHT upregulated IL-13 expression in eosinophils cultured in vitro 8. In line with this, in models of IPF, the effect of recombinant LIGHT in driving some aspects of lung tissue remodeling was also found to be dependent on signaling through the receptor for IL-13 9. Thus, LIGHT-HVEM signaling in eosinophils can promote fibrotic activity by maximizing the expression of the pro-remodeling cytokine IL-13. Whether LIGHT also enhances TGF-β production by eosinophils has not been studied, but this may be another way in which it could contribute to tissue remodeling.
LIGHT’s impact on structural cells
LIGHT function in epithelial cells
Epithelial cells can contribute to fibrosis and tissue remodeling in several ways, through undergoing EMT and making extracellular matrix proteins, producing chemokines, and secreting inflammatory cytokines 20, 28, 29. Recent work from our laboratory and elsewhere has focused on the function of LIGHT signaling in epithelial cells. Airway epithelial cells (bronchial and alveolar origin) and epidermal keratinocytes, thought to be key players in development of asthma and atopic dermatitis, were found to express both LTβR and HVEM on their surface 10, 30. These cells are considered important sources of inflammatory molecules and extracellular matrix proteins that contribute to lung and skin disease. LIGHT induced a limited but distinct set of inflammatory genes, namely adhesion molecules (ICAM-1, VCAM-1), chemokines (CCL5, CCL20, CXCL1, CXCL3, CXCL5 and CXCL11), cytokines (IL-6, Activin A and GM-CSF), and metalloproteinases (matrix metalloproteinase-9 and a desintegrin and metalloproteinase domain-8) in lung epithelial cells 30, 31 and many of these are upregulated in keratinocytes (unpublished observations). The induction of most of these genes was resistant to steroid treatment in lung epithelial cells 30. ICAM-1 and VCAM-1 play an important role in the recruitment, infiltration and retention of leucocytes to the lung and skin. This could emphasize an indirect activity of LIGHT on epithelial cells to perpetuate fibrosis. LIGHT’s modulation of genes involved in chemotaxis appears to be very specific, and may primarily impact migration of T cells, macrophages, neutrophils, and eosinophils. GM-CSF expression was also strongly induced upon LIGHT stimulation. GM-CSF is a modulator of growth of myeloid cells such as dendritic cells and monocytes/macrophages and granulocytes. Like GM-CSF, IL-6 can control growth and differentiation of macrophages, T cells and dendritic cells 32. MMP-9 is elevated in asthmatics and positively correlates with disease progression 33–36. In line with MMP-9 playing a role in fibrosis development, mice deficient in this molecule presented with less eosinophilia, lower levels of CCL5, and reduced collagen deposition in their lungs after allergen challenge 37. Activin A has also been implicated in lung fibrosis, since it was elevated in sera of asthmatics and COPD patients, as well as mice induced with allergens 38, 39. However its exact contribution to inflammation and remodelling processes remains unclear and needs further investigation.
Whether LIGHT strongly contributes to the production of extracellular matrix proteins by epithelial cells is not clear. Certainly in isolation, we found that LIGHT only had a weak activity in this regard 30, but it remains to be determined if LIGHT can synergize with other fibrotic factors to directly drive collagen or similar molecules. A more obvious link between LIGHT’s activity in epithelial cells and induction of fibrosis was revealed when we showed that LIGHT stimulation of either lung or skin epithelial cells promoted the expression of TSLP 9, 10. TSLP has been described as a master regulator of fibrotic disease associated with allergic inflammation, and has been tightly associated with several autoimmune and inflammatory diseases with a fibrotic component such as idiopathic pulmonary fibrosis, asthma, scleroderma and atopic dermatitis 5, 40. We found that much of the fibrotic activity of recombinant LIGHT in the lungs and skin was reduced when LIGHT was injected into animals lacking the receptor for TSLP. This suggests that LIGHT not only controls expression of TSLP but also likely synergizes with TSLP for certain pro-fibrotic activities 9, 10.
LIGHT function in fibroblasts and smooth muscle cells
Fibroblasts contribute to tissue fibrosis by proliferating and forming bundles of cells thereby decreasing tissue elasticity, as well as by producing extracellular matrix proteins and by secreting inflammatory cytokines and chemokines. Fibroblasts from several organs have long been known to express LTβR (e.g. mouse embryonic fibroblasts, human synovial fibroblasts), and we recently found in unpublished data that both smooth muscle cells and fibroblasts from the lungs and skin express LTβR as well as HVEM. There is presently a paucity of data on the activity of LIGHT in fibroblasts and smooth muscle cells, but several reports suggest that its activities on these cells are likely to contribute to tissue fibrosis. One study that used synovial fibroblasts showed modulation of MMP-9 and IL-6 expression by LIGHT in these cells 41, similar to that we observed in epithelial cells 30. LIGHT also promoted fibroblast proliferation and upregulated several chemokines 41. Additionally, LTβR signaling induced the expression of ICAM-1, several chemokines including CCL5, and several MMPs, in synovial fibroblasts 42. This suggests the activity of LIGHT in fibroblasts might be quite similar to that in epithelial cells. From our in vivo studies in mice, recombinant LIGHT administration led to smooth muscle hyperplasia and collagen accumulation in both the lung and skin. However, as yet we have not found any strong direct activity of LIGHT on lung or skin fibroblasts or smooth muscle cells in terms of upregulation of collagen or alpha smooth muscle actin. In contrast, LIGHT was able to induce proliferation of these cells (unpublished observations), which may contribute to the increase in the former features seen in vivo.
LIGHT activity in adipocytes
Adipose tissue inflammatory activity can also lead to fibrosis whereby accumulation of collagen covers the adipose tissue as well as the connective tissue below the adipose layer. HVEM was found to be expressed in mature human adipocytes and has been suggested to be important for their homeostasis. The interaction between soluble LIGHT and HVEM can contribute to adipose tissue inflammation by enhancing T cell and macrophage accumulation and activation at these sites 43, 44. Serum levels of LIGHT were described to be increased in obese patients, and soluble LIGHT increased the release of the inflammatory cytokines and chemokine, TNF, IL-6, and MCP-1, from human adipocytes. Treatment with anti-HVEM antibody, or the genetic deletion of HVEM, markedly blunted the infiltration of CD4 or CD8 T cells and macrophages, and the release of cytokines, in adipose tissue. Furthermore, LIGHT was also found elevated in the serum of Non Alcoholic Fatty Liver Disease patients that suffer from dyslipidemia and metabolic disorders 45. Moreover, blocking LIGHT reduced dislypidemia in mice deficient in LDLR, which lack the ability to control lipid levels in the blood rendering its host at high risk of coronary heart disease 46. Therefore, by directly modulating adipose cell activity, and regulating inflammatory cell recruitment, LIGHT may also contribute to a fibrotic response in this tissue.
LIGHT effects in endothelial cells
Vascular damage and increased angiogenesis is also one of the main characteristics of tissues where fibrosis is found. Endothelial cells play a critical role in the control of vascular function and additionally can participate in inflammatory and immune reactions. LIGHT signaling through LTβR expressed in endothelial cells has been described to promote T cell adhesion through upregulation of E-selectin, ICAM-1, and VCAM-1 47, 48. Additionally, LIGHT can induce IL-8 47 and CXCL12 49 expression in these cells that would further facilitate the migration of leucocytes to areas of inflammation, and indirectly promote the environment conducive for promoting tissue fibrosis.
Conclusion
Recent data suggest that LIGHT is an important driver of fibrosis and tissue remodeling. By signaling concomitantly on inflammatory and structural cells, through its receptors LTβR and HVEM, LIGHT is able to control the expression of major pro-fibrotic factors such as TGF-β, IL-13 and TSLP, and these factors combined can subsequently regulate hyperplasia of fibroblasts, epithelial cells, and smooth muscle cells, and promote deposition of extracellular matrix proteins such as collagen. Additionally, LIGHT can regulate accumulation of Th2 cells, chemokines that attract these and other immune cells, adhesion molecules that will maintain the inflammatory environment, and other factors such as metalloproteinases that can participate in the fibrotic response. In some model systems, the expression of LIGHT is early, likely derived from activated T cells, and it can therefore be viewed as a potential prognostic marker and initiator of fibrosis. However, there may be other scenarios where LIGHT is made by T cells or other immune cells at a later time, once the fibrotic response is ongoing and chronic, and LIGHT then participates in maintaining this response through continued production of the factors above, such as IL-13 and TGF-β. Although there is a paucity of data at present in humans, LIGHT has been found elevated in the serum or sputum of patients suffering from a number of inflammatory diseases with a fibrotic component, including asthma 50, atopic dermatitis 51, rheumatoid arthritis 41, non alcoholic fatty liver disease 45, atherosclerosis 48 and colitis 19. Polymorphisms in the gene encoding LIGHT have also been associated with Crohn’s disease. Blocking LIGHT can be achieved with either neutralizing reagents such as antibodies or soluble decoy receptors (e.g. LTβR.Ig) that bind LIGHT, or by using specific antibodies to either of its receptors that prevent their binding to LIGHT. Given the state of research on LIGHT at present, there is a reasonable expectation that such reagents might provide significant therapeutic benefit in patients whose diseases present with scarring and tissue remodeling.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rana Herro, Email: rherro@lji.org.
Michael Croft, Email: mick@lji.org.
References
- 1.Ramming A, Dees C, Distler JH. From pathogenesis to therapy - Perspective on treatment strategies in fibrotic diseases. Pharmacol Res. 2015;100:93–100. doi: 10.1016/j.phrs.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borthwick LA, Wynn TA, Fisher AJ. Cytokine mediated tissue fibrosis. Biochim Biophys Acta. 2013;1832:1049–1060. doi: 10.1016/j.bbadis.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thannickal VJ, Toews GB, White ES, Lynch JP, 3rd, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 5.Yoo J, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rafii R, Juarez MM, Albertson TE, Chan AL. A review of current and novel therapies for idiopathic pulmonary fibrosis. J Thorac Dis. 2013;5:48–73. doi: 10.3978/j.issn.2072-1439.2012.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thannickal VJ, Flaherty KR, Martinez FJ, Lynch JP., 3rd Idiopathic pulmonary fibrosis: emerging concepts on pharmacotherapy. Expert Opin Pharmacother. 2004;5:1671–1686. doi: 10.1517/14656566.5.8.1671. [DOI] [PubMed] [Google Scholar]
- 8.Doherty TA, et al. The tumor necrosis factor family member LIGHT is a target for asthmatic airway remodeling. Nat Med. 2011;17:596–603. doi: 10.1038/nm.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herro R, Da Silva Antunes R, Aguilera AR, Tamada K, Croft M. Tumor necrosis factor superfamily 14 (LIGHT) controls thymic stromal lymphopoietin to drive pulmonary fibrosis. J Allergy Clin Immunol. 2015;136:757–768. doi: 10.1016/j.jaci.2014.12.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herro R, Antunes Rda S, Aguilera AR, Tamada K, Croft M. The Tumor Necrosis Factor Superfamily Molecule LIGHT Promotes Keratinocyte Activity and Skin Fibrosis. J Invest Dermatol. 2015;135:2109–2118. doi: 10.1038/jid.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mauri DN, et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1998;8:21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 12.Harrop JA, et al. Herpesvirus entry mediator ligand (HVEM-L), a novel ligand for HVEM/TR2, stimulates proliferation of T cells and inhibits HT29 cell growth. J Biol Chem. 1998;273:27548–27556. doi: 10.1074/jbc.273.42.27548. [DOI] [PubMed] [Google Scholar]
- 13.Murphy M, et al. Expression of the lymphotoxin beta receptor on follicular stromal cells in human lymphoid tissues. Cell Death Differ. 1998;5:497–505. doi: 10.1038/sj.cdd.4400374. [DOI] [PubMed] [Google Scholar]
- 14.Petreaca ML, et al. Deletion of a tumor necrosis superfamily gene in mice leads to impaired healing that mimics chronic wounds in humans. Wound Repair Regen. 2012;20:353–366. doi: 10.1111/j.1524-475X.2012.00785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamada K, et al. LIGHT, a TNF-like molecule, costimulates T cell proliferation and is required for dendritic cell-mediated allogeneic T cell response. J Immunol. 2000;164:4105–4110. doi: 10.4049/jimmunol.164.8.4105. [DOI] [PubMed] [Google Scholar]
- 16.Tamada K, et al. Modulation of T-cell-mediated immunity in tumor and graft-versus-host disease models through the LIGHT co-stimulatory pathway. Nat Med. 2000;6:283–289. doi: 10.1038/73136. [DOI] [PubMed] [Google Scholar]
- 17.del Rio ML, et al. Therapeutic blockade of LIGHT interaction with herpesvirus entry mediator and lymphotoxin beta receptor attenuates in vivo cytotoxic allogeneic responses. Transplantation. 2014;98:1165–1174. doi: 10.1097/TP.0000000000000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaikh RB, et al. Constitutive expression of LIGHT on T cells leads to lymphocyte activation, inflammation, and tissue destruction. J Immunol. 2001;167:6330–6337. doi: 10.4049/jimmunol.167.11.6330. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, et al. The critical role of LIGHT in promoting intestinal inflammation and Crohn's disease. J Immunol. 2005;174:8173–8182. doi: 10.4049/jimmunol.174.12.8173. [DOI] [PubMed] [Google Scholar]
- 20.Hackett TL, et al. Induction of epithelial-mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-beta1. Am J Respir Crit Care Med. 2009;180:122–133. doi: 10.1164/rccm.200811-1730OC. [DOI] [PubMed] [Google Scholar]
- 21.Duffield JS, Lupher M, Thannickal VJ, Wynn TA. Host responses in tissue repair and fibrosis. Annu Rev Pathol. 2013;8:241–276. doi: 10.1146/annurev-pathol-020712-163930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee WH, et al. Tumor necrosis factor receptor superfamily 14 is involved in atherogenesis by inducing proinflammatory cytokines and matrix metalloproteinases. Arterioscler Thromb Vasc Biol. 2001;21:2004–2010. doi: 10.1161/hq1201.098945. [DOI] [PubMed] [Google Scholar]
- 23.Lim SG, Suk K, Lee WH. Reverse signaling from LIGHT promotes proinflammatory responses in the human monocytic leukemia cell line, THP-1. Cell Immunol. 2013;285:10–17. doi: 10.1016/j.cellimm.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Flood-Page P, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–1036. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev. 2004;202:175–190. doi: 10.1111/j.0105-2896.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 26.Kaminska M, et al. Airway remodeling in subjects with severe asthma with or without chronic persistent airflow obstruction. J Allergy Clin Immunol. 2009;124:45–51. doi: 10.1016/j.jaci.2009.03.049. e41-44. [DOI] [PubMed] [Google Scholar]
- 27.Woodruff PG, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hackett TL. Epithelial-mesenchymal transition in the pathophysiology of airway remodelling in asthma. Curr Opin Allergy Clin Immunol. 2012;12:53–59. doi: 10.1097/ACI.0b013e32834ec6eb. [DOI] [PubMed] [Google Scholar]
- 29.Camelo A, Dunmore R, Sleeman MA, Clarke DL. The epithelium in idiopathic pulmonary fibrosis: breaking the barrier. Front Pharmacol. 2014;4:173. doi: 10.3389/fphar.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.da Silva Antunes R, Madge L, Soroosh P, Tocker J, Croft M. The TNF Family Molecules LIGHT and Lymphotoxin alphabeta Induce a Distinct Steroid-Resistant Inflammatory Phenotype in Human Lung Epithelial Cells. J Immunol. 2015;195:2429–2441. doi: 10.4049/jimmunol.1500356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikami Y, et al. Tumor necrosis factor superfamily member LIGHT induces epithelial-mesenchymal transition in A549 human alveolar epithelial cells. Biochem Biophys Res Commun. 2012;428:451–457. doi: 10.1016/j.bbrc.2012.10.097. [DOI] [PubMed] [Google Scholar]
- 32.Rincon M, Irvin CG. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int J Biol Sci. 2012;8:1281–1290. doi: 10.7150/ijbs.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee KS, et al. Vascular endothelial growth factor modulates matrix metalloproteinase-9 expression in asthma. Am J Respir Crit Care Med. 2006;174:161–170. doi: 10.1164/rccm.200510-1558OC. [DOI] [PubMed] [Google Scholar]
- 34.Oshita Y, et al. Increased circulating 92 kDa matrix metalloproteinase (MMP-9) activity in exacerbations of asthma. Thorax. 2003;58:757–760. doi: 10.1136/thorax.58.9.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demedts IK, Brusselle GG, Bracke KR, Vermaelen KY, Pauwels RA. Matrix metalloproteinases in asthma and COPD. Curr Opin Pharmacol. 2005;5:257–263. doi: 10.1016/j.coph.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki R, Miyazaki Y, Takagi K, Torii K, Taniguchi H. Matrix metalloproteinases in the pathogenesis of asthma and COPD: implications for therapy. Treat Respir Med. 2004;3:17–27. doi: 10.2165/00151829-200403010-00003. [DOI] [PubMed] [Google Scholar]
- 37.Lim DH, et al. Reduced peribronchial fibrosis in allergen-challenged MMP-9-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2006;291:L265–L271. doi: 10.1152/ajplung.00305.2005. [DOI] [PubMed] [Google Scholar]
- 38.Karagiannidis C, et al. Activin A is an acute allergen-responsive cytokine and provides a link to TGF-beta-mediated airway remodeling in asthma. J Allergy Clin Immunol. 2006;117:111–118. doi: 10.1016/j.jaci.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 39.Verhamme FM, et al. Role of activin-A in cigarette smoke-induced inflammation and COPD. Eur Respir J. 2014;43:1028–1041. doi: 10.1183/09031936.00082413. [DOI] [PubMed] [Google Scholar]
- 40.Comeau MR, Ziegler SF. The influence of TSLP on the allergic response. Mucosal Immunol. 2010;3:138–147. doi: 10.1038/mi.2009.134. [DOI] [PubMed] [Google Scholar]
- 41.Pierer M, et al. The TNF superfamily member LIGHT contributes to survival and activation of synovial fibroblasts in rheumatoid arthritis. Rheumatology (Oxford) 2007;46:1063–1070. doi: 10.1093/rheumatology/kem063. [DOI] [PubMed] [Google Scholar]
- 42.Braun A, Takemura S, Vallejo AN, Goronzy JJ, Weyand CM. Lymphotoxin beta-mediated stimulation of synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2004;50:2140–2150. doi: 10.1002/art.20356. [DOI] [PubMed] [Google Scholar]
- 43.Kim HM, Jeong CS, Choi HS, Kawada T, Yu R. LIGHT/TNFSF14 enhances adipose tissue inflammatory responses through its interaction with HVEM. FEBS Lett. 2011;585:579–584. doi: 10.1016/j.febslet.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 44.Kim HJ, et al. HVEM-deficient mice fed a high-fat diet are protected from adipose tissue inflammation and glucose intolerance. FEBS Lett. 2011;585:2285–2290. doi: 10.1016/j.febslet.2011.05.057. [DOI] [PubMed] [Google Scholar]
- 45.Otterdal K, et al. Increased Serum Levels of LIGHT/TNFSF14 in Nonalcoholic Fatty Liver Disease: Possible Role in Hepatic Inflammation. Clin Transl Gastroenterol. 2015;6:e95. doi: 10.1038/ctg.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lo JC, et al. Lymphotoxin beta receptor-dependent control of lipid homeostasis. Science. 2007;316:285–288. doi: 10.1126/science.1137221. [DOI] [PubMed] [Google Scholar]
- 47.Chang YH, Hsieh SL, Chao Y, Chou YC, Lin WW. Proinflammatory effects of LIGHT through HVEM and LTbetaR interactions in cultured human umbilical vein endothelial cells. J Biomed Sci. 2005;12:363–375. doi: 10.1007/s11373-005-1360-5. [DOI] [PubMed] [Google Scholar]
- 48.Celik S, et al. Platelet-associated LIGHT (TNFSF14) mediates adhesion of platelets to human vascular endothelium. Thromb Haemost. 2007;98:798–805. [PubMed] [Google Scholar]
- 49.Madge LA, May MJ. Classical NF-kappaB activation negatively regulates noncanonical NF-kappaB-dependent CXCL12 expression. J Biol Chem. 2010;285:38069–38077. doi: 10.1074/jbc.M110.147207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hastie AT, et al. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol. 2010;125:1028–1036. doi: 10.1016/j.jaci.2010.02.008. e1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kotani H, et al. Increased plasma LIGHT levels in patients with atopic dermatitis. Clin Exp Immunol. 2012;168:318–324. doi: 10.1111/j.1365-2249.2012.04576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]