Abstract
Purpose of review
To analyze the evidence that glutamine supplementation increases citrulline production. To determine the contribution of glutamine to the synthesis of citrulline.
Recent findings
Glutamine supplementation has been proposed in the treatment of critically ill patients; however, a recent large multicenter randomized controlled trial resulted in increased mortality in the glutamine supplemented group. Within this context, defining the contribution of glutamine to the production of citrulline, and thus to de novo arginine synthesis, has become a pressing issue.
Summary
The beneficial effects of glutamine supplementation may be partially mediated by the effects of glutamine on citrulline synthesis by the gut and the de novo synthesis of arginine by the kidney and other tissues. Although there is no strong evidence to support that glutamine is a major precursor for citrulline synthesis in humans, glutamine has the potential to increase overall gut function and in this way increase citrulline production.
Keywords: arginine, citrulline, glutamine, stable isotopes
Introduction
Low plasma glutamine concentration is an independent risk factor for mortality in critically ill pediatric and adult patients. For this reason glutamine has been postulated to be an essential amino acid in the critically ill and glutamine supplementation a beneficial intervention [1]. Furthermore, it has been hypothesized that most of the positive effect of glutamine supplementation is mediated by an increase in citrulline and arginine production [2]. However, recent data suggest that the tracer techniques used to determine the contribution of glutamine to citrulline synthesis overestimate the contribution of glutamine.
Glutamine supplementation in critical care setting
Recent meta-analyses in critically ill patients supplemented with glutamine have shown a reduction in overall mortality, hospital mortality and length of stay (LOS) [3▪], a reduction in infection rate and LOS [4] and a reduction in infection rate, but not in LOS [5]. In patients undergoing abdominal surgery a reduction in LOS and infection rate [6] and LOS [7▪] due to glutamine supplementation has been reported. This apparent consensus on the benefits of glutamine supplementation is due, to a large extent, on the fact that these meta-analyses share many trials, particularly the larger more rigorous ones. A temporal trend analysis, however, seem to indicate that whereas earlier studies showed positive outcomes, more recent trials found no differences between the glutamine supplemented and control groups [8]. This may be due to a lower quality of early trials [8,9] or to the failure of a recent large study to find any benefit of glutamine supplementation [10]. More recently a large multicenter randomized controlled trial in critically ill patients (REducing Deaths Due to Oxidative Stress, REDOX) not only failed to find any benefit of glutamine supplementation, but also showed an increase in mortality [11]. Although, different reasons (route and dose of glutamine, inclusion criteria) have been proposed to explain this negative outcome [3▪], a better understanding of the mechanism by which glutamine exerts its action is needed [12]. Because it has been hypothesized that the beneficial effects of glutamine supplementation are partly mediated by the effects of glutamine on citrulline synthesis and the de novo synthesis of arginine [2], the role of glutamine in the production of citrulline warrants further discussion.
Glutamine utilization by the gut
The pioneering work of Windmueller and Spaeth [13] established the uptake and metabolism of glutamine by the small intestine. The gut utilized arterial and luminal glutamine which was metabolized to CO2, organic acids, and other amino acids [13]. Other findings of this early work were the high first pass extraction of glutamine (and glutamate) and the release of citrulline by the gut. These observations have been interpreted by many researchers as evidence that glutamine is the precursor for the synthesis of citrulline. Whereas citrulline accounted for 34% of the glutamine nitrogen, it accounted for only 6% of its carbon. Furthermore, because the origin of the carbamoyl carbon incorporated to generate citrulline from ornithine is CO2, it is possible that the oxidation of the [U-14C5] glutamine used in these studies generated most of this non-specific carbon. Also of note is that both the oxidation of glutamine and the synthesis of carbamoyl phosphate take place in the same sub-compartment (enterocyte mitochondria, Fig. 1). Regardless, the proper interpretation of Windmueller and Spaeth's early findings (“intestinally derived citrulline, an end product of glutamine nitrogen metabolism…”) [13] can be found in a review article by these same authors [14].
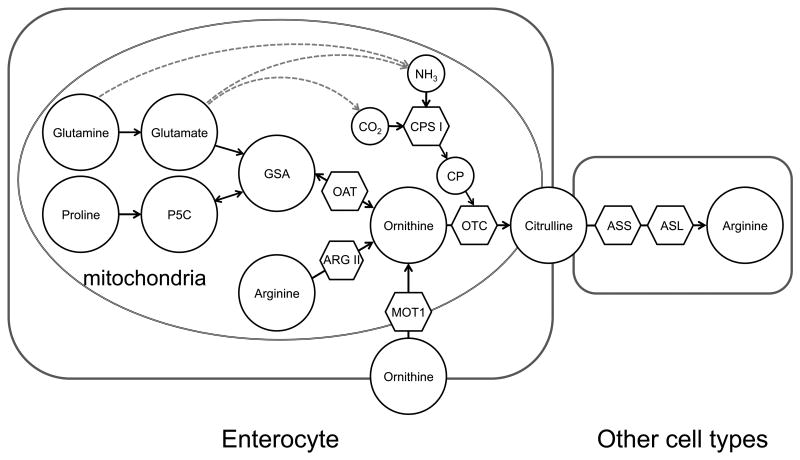
Figure 1.
Metabolites (circles) and enzymes (hexagons) involved in the synthesis of citrulline and arginine. Citrulline synthesis takes place in the mitochondria of the enterocyte and it is utilized by other tissues where ASS and ASL are expressed. Note that glutamine and glutamate can provide non-specific carbon and nitrogen to citrulline synthesis. In addition glutamate provides the δ–nitrogen in the synthesis of ornithine (not shown). Abbreviations: P5C (pyrroline-5-carboxylate), GSA (glutamate semialdehyde), NH3 (ammonia), CO2 (carbon dioxide), CP (carbamoyl phosphate), OAT (ornithine aminotransferase), ARG II (arginase II), MOT1 (mitochondrial ornithine transporter 1), CPS I (carbamoyl phosphate synthase I), OTC (ornithine transcarbamylase), ASS (argininosuccinate synthase), ASL (arginine succinate lyase).
Glutamine utilization and citrulline production
The role of glutamine as a main fuel source for the gut is well established [13] as well as the role of citrulline as a marker for gut mass and function [15▪▪]. A strong correlation between glutamine uptake by the gut and release of citrulline has been observed [16▪]. For these reasons glutamine supplementation has the potential to maintain or increase gut metabolic function and thus increase citrulline production and plasma concentrations [15]. An increase in citrulline concentration after glutamine supplementation has been reported in rats and humans [2,16▪]. Furthermore a citrulline generation test after a single dose of glutamine has been proposed [17]. Although it is likely that this increase in plasma citrulline was due to a real increase in citrulline production, a reduction in the removal rate of citrulline is an alternative explanation. Because glutamine and citrulline share some of the same transporters [16▪] the sudden increase in glutamine concentration may reduce the clearance of citrulline from the circulation resulting in an increase in its plasma concentration. In fact an increase in plasma citrulline concentration, without an apparent increase in citrulline production has been reported [2].
Origin of Circulating Citrulline
The synthesis of citrulline by carbamylation of ornithine takes place in only two cell types: the hepatocyte and the enterocyte, where the enzymes carbamoyl phosphate synthase (CPS1) and ornithine transcarbamylase (OTC) are expressed (Fig. 1). In the liver, the citrulline produced is channeled into argininosuccinate synthase (ASS) and lyase (ASL) for the synthesis of arginine within the urea cycle. For this reason little or no citrulline is exported into the circulation under normal conditions; however, when the urea cycle is impaired (argininosuccinate aciduria in humans [18] or ASL knockout mice [19]) the rate of appearance of citrulline increases many fold suggesting that under these conditions the liver contributes to the circulating citrulline pool.
The citrulline produced by the enterocytes enters the portal circulation, bypasses the liver, enters the general circulation and is utilized by the kidney and other tissues for the synthesis of arginine [20▪,21▪]. Small amounts of circulating citrulline are produced as co-product in the synthesis of nitric oxide and in the turnover of posttranslational modified arginine (citrullinated and methylated). Although these sources are difficult to quantify, it is unlikely that they exceed ∼10% of the citrulline flux.
Origin of the Ornithine Utilized for Citrulline Synthesis
There is considerable debate surrounding the precursors utilized for citrulline synthesis [22-26]. The ornithine used for citrulline production in the gut has three possible sources: 1) it can originate from proline and glutamate/glutamine through glutamate semialdehyde (GSA) and by action of ornithine aminotransferase (OAT), 2) it can result from arginase activity in the enterocyte, or 3) plasma ornithine can be transported from the circulation (Fig. 1). Thus many different precursors can contribute to the synthesis of citrulline. In fact glutamine has been reported to be the main precursor in humans and mice [2,27]; proline, in infants [28,29] and neonatal pigs [30]; and arginine, in mice [31,32]. Although species differences may exist the following methodological issues have fueled the controversy regarding what precursors are used for citrulline synthesis: 1) the choice of tracer, 2) the mathematical model to interpret the isotope data, and 3) the developmental stage of the subjects or animals studied (neonate vs. adult).
Choice of tracer to determine the contribution of glutamine to citrulline synthesis
[2-15N] (amino) glutamine has been used extensively to determine precursor-product relationship between glutamine and citrulline [2,16,27]. The limitation of the 15N tracer is that it can label any of the three nitrogen atoms of citrulline [33▪▪]. Because in these reports the enrichment of citrulline was determined by measuring the relative abundance of M+1(m/z+1) ions, the contribution of glutamine to citrulline synthesis has the potential of being overestimated. We have co-infused isomolar rates of [2-15N] (amino) and [2,3,3,4,4 2H5] glutamine in mice and the measured enrichment of the M+1 citrulline was ∼20 fold higher than the enrichment of the [2,3,3,4,4 2H5] isotopologue [33▪▪]. Furthermore, in a different set of mice, [U-13C5] glutamine showed very little incorporation and production of [1,2,3,4,5 13C5] citrulline [33▪▪]. This shows that, in fact, measuring M+1 results in a gross overestimation of the contribution of glutamine to citrulline synthesis. To determine the incorporation of glutamine nitrogen to citrulline, we have developed an analytical method that is able to quantify the enrichment of the individual nitrogen atoms [34]. However, when we used this method the enrichment of the [2-15N] citrulline was still 5 fold higher than the enrichment of the [2,3,3,4,4 2H5] citrulline [33▪▪]. Thus, it seems that the use of labeled nitrogen to determine the contribution of a precursor does not properly trace the fate of the carbon backbone of glutamine.
Mathematical models to interpret tracer enrichments
The classical equation for the determination of precursor-product relationship relies on the ratio between the enrichment of the product (derived from the precursor) and the enrichment of the precursor itself at the site of synthesis [31]. Because it is assumed that most of the citrulline is of enteral origin, circulating citrulline enrichment is a good approximation for the enrichment of citrulline at the site of its synthesis. For intravenous infusions, the plasma enrichment of the precursor can be utilized in the calculations. Note that in this case what is being determined is the contribution of the plasma precursor to the synthesis of citrulline. The total contribution of the precursor cannot be determined because the extent of the intracellular dilution of the precursor at the site of utilization is unknown.
Whereas the intravenous infusion of tracers provides a partial answer, this classical model cannot be extended to enteral tracers [28,29,35]. Otherwise the underlying assumption is that the enteral tracer is not used during first pass metabolism, but after entering the peripheral circulation and returning to the gut is then utilized for the synthesis of citrulline. Because of the large first pass extraction of glutamine (∼75%) and subsequent dilution by unlabeled glutamine of endogenous origin, the plasma enrichment grossly underestimates the enrichment of the precursor at the site of citrulline synthesis. Since the denominator becomes smaller, the apparent contribution of the precursor to the synthesis of the product becomes larger. An additional limitation in these studies is that only one precursor was studied at a time and thus this over-accounting has not been apparent.
To overcome these limitations we have developed a model to determine the contribution of enteral tracers and thus quantify the contribution of different dietary precursors [30,31]. This new model relies on mass balance between precursor and product and thus it does not depend on the enrichment of the precursor at the site of citrulline synthesis. In fact, when used for intravenous infusions the model simplifies to the classic precursor-product equation. Using this new model we were able to estimate the contribution of multiple enteral and arterial precursors in mice [31] and neonatal pigs [30] using U-13C tracers. We have compared the two models and, as expected, the classical precursor-product equation overestimated the contribution of the enteral precursors (247% vs 42% of the citrulline flux) [31]. In this model comparison, the contribution of glutamate using the classical equation was 72% of the citrulline flux; however, this was due to the small actual contribution estimated by the new model (∼1% citrulline flux) compounded with the large disappearance of glutamate during first pass (first pass extraction 94%). Had we only infused glutamate enterally and used the classical equation, our conclusion would have been that dietary glutamate was the main precursor for the synthesis of citrulline, instead of a minor source.
Developmental stage and citrulline production
OAT is a crucial enzyme that provides ornithine for the synthesis of citrulline and thus determines what precursors are used. However, OAT is a bidirectional enzyme that catalyzes both the synthesis and disposal of ornithine [36]. It has been shown in vivo that OAT inhibition increased plasma ornithine concentration in adult mice. Likewise, the inhibition of OAT in transgenic mice results in a paradoxical neonatal hypo-ornithemia and hyper-ornithemia after weaning, which mimics similar findings reported in humans with gyrate atrophy. Thus, in adults the catabolic route predominates, whereas in the neonate the synthesis of ornithine prevails [36]. Based on these findings it would be expected for glutamine and proline to be the main precursors for citrulline synthesis in neonates, while in adulthood plasma ornithine and arginine would predominate. Our studies in neonatal pigs [30] and adult mice [31] seem to support these assumptions, and suggest that any species differences may be minimal.
Conclusion
The role of glutamine in the synthesis of citrulline in humans remains elusive despite the multiple supplementation trials and studies utilizing stable isotopes. We have shown that in animal models (mice and pigs) the contribution of glutamine to citrulline synthesis is minor, and it is not unlikely that the same is true in humans. Glutamine supplementation, however, has the potential to increase citrulline synthesis. This can be either by mass action (by providing more precursors for its synthesis) or by enhancing overall gut function. Both hypotheses can be tested using stable isotopes. However, carbon or deuterium labeled glutamine tracers should be used to trace the carbon skeleton of glutamine and the appropriate mathematical models should be used to interpret the tracer data.
Key Points.
Although many articles have concluded that glutamine is the main precursor for citrulline synthesis, this remains controversial due to methodological issues
Nitrogen labeled tracers are not appropriate to trace the carbon skeleton of glutamine
The classic precursor-product equation cannot be used when tracers are given enterally
There seem to be differences between neonates and adults regarding the precursors utilized for citrulline synthesis.
Acknowledgments
None.
Financial support and sponsorship: This work was supported by NIH grant R01GM108940.
Footnotes
Conflicts of interest: There are no conflicts of interest.
References and Recommended Reading
- 1.Cynober L, De Bandt JP. Glutamine in the intensive care unit. Curr Opin Clin Nutr Metab Care. 2014;17:98–104. doi: 10.1097/MCO.0000000000000014. [DOI] [PubMed] [Google Scholar]
- 2.Buijs N, Brinkmann SJH, Oosterink JE, et al. Intravenous glutamine supplementation enhances renal de novo arginine synthesis in humans: A stable isotope study. Am J Clin Nutr. 2014;100:1385–1391. doi: 10.3945/ajcn.113.081547. [DOI] [PubMed] [Google Scholar]
- 3▪.Wischmeyer PE, Dhaliwal R, McCall M, et al. Parenteral glutamine supplementation in critical illness: A systematic review. Crit Care. 2014;18:R76. doi: 10.1186/cc13836. This is the most recent review on the effect of parenteral glutamine in critically ill patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bollhalder L, Pfeil AM, Tomonaga Y, Schwenkglenks M. A systematic literature review and meta-analysis of randomized clinical trials of parenteral glutamine supplementation. Clin Nutr. 2013;32:213–223. doi: 10.1016/j.clnu.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Chen QH, Yang Y, He HL, et al. The effect of glutamine therapy on outcomes in critically ill patients: A meta-analysis of randomized controlled trials. Crit Care. 2014;18:R8. doi: 10.1186/cc13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yue C, Tian W, Wang W, et al. The impact of perioperative glutamine-supplemented parenteral nutrition on outcomes of patients undergoing abdominal surgery: A meta-analysis of randomized clinical trials. Am Surg. 2013;79:506–513. [PubMed] [Google Scholar]
- 7▪.Sandini M, Nespoli L, Oldani M, et al. Effect of glutamine dipeptide supplementation on primary outcomes for elective major surgery: Systematic review and meta-analysis. Nutrients. 2015;7:481–499. doi: 10.3390/nu7010481. This is the most recent and extensive meta-analysis on the effect of glutamine on the outcome of elective surgery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fadda V, Maratea D, Trippoli S, Messori A. Temporal trend of short-term mortality in severely ill patients receiving parenteral glutamine supplementation. Clin Nutr. 2013;32:492–493. doi: 10.1016/j.clnu.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Van Den Berghe G. Low glutamine levels during critical illness - adaptive or maladaptive? N Engl J Med. 2013;368:1549–1550. doi: 10.1056/NEJMe1302301. [DOI] [PubMed] [Google Scholar]
- 10.Andrews PJD, Avenell A, Noble DW, et al. Randomised trial of glutamine, selenium, or both, to supplement parenteral nutrition for critically ill patients. BMJ. 2011;342:695. doi: 10.1136/bmj.d1542. [DOI] [PubMed] [Google Scholar]
- 11.Heyland D, Muscedere J, Wischmeyer PE, et al. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med. 2013;368:1489–1497. doi: 10.1056/NEJMoa1212722. [DOI] [PubMed] [Google Scholar]
- 12.Wernerman J. Glutamine - from conditionally essential to totally dispensable? Crit Care. 2014;18:162. doi: 10.1186/cc13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Windmueller HG, Spaeth AE. Uptake and metabolism of plasma glutamine by the small intestine. J Biol Chem. 1974;249:5070–5079. [PubMed] [Google Scholar]
- 14.Windmueller HG, Spaeth AE. Source and fate of circulating citrulline. Am J Physiol. 1981;241:E473–480. doi: 10.1152/ajpendo.1981.241.6.E473. [DOI] [PubMed] [Google Scholar]
- 15▪▪.Beaufrere AM, Neveux N, Patureau Mirand P, et al. Long-term intermittent glutamine supplementation repairs intestinal damage (structure and functional mass) with advanced age: Assessment with plasma citrulline in a rodent model. J Nutr Health Aging. 2014;18:814–819. doi: 10.1007/s12603-014-0554-9. In this article glutamine supplementation maintained gut structure and mass in ageing rats. As consequenceplasma citrulline concentration was greater than in non-supplemented animals. [DOI] [PubMed] [Google Scholar]
- 16▪.Wijnands KA, Castermans TMR, Hommen MPJ, et al. Arginine and citrulline and the immune response in sepsis. Nutrients. 2015;7:1426–1463. doi: 10.3390/nu7031426. This is a comprehensive review on the metabolism of arginine and citrulline in sepsis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papadia C, Sabatino AD, Corazza GR, Forbes A. Diagnosing small bowel malabsorption: A review. Intern Emerg Med. 2014;9:3–8. doi: 10.1007/s11739-012-0877-7. [DOI] [PubMed] [Google Scholar]
- 18.Nagamani SCS, Shchelochkov OA, Mullins MA, et al. A randomized controlled trial to evaluate the effects of high-dose versus low-dose of arginine therapy on hepatic function tests in argininosuccinic aciduria. Mol Genet Metab. 2012;107:315–321. doi: 10.1016/j.ymgme.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erez A, Nagamani SCS, Shchelochkov OA, et al. Requirement of argininosuccinate lyase for systemic nitric oxide production. Nat Med. 2011;17:1619–1626. doi: 10.1038/nm.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪.Marini JC, Didelija IC, Fiorotto ML. Extrarenal citrulline disposal in mice with impaired renal function. Am J Physiol. 2014;307:F660–F665. doi: 10.1152/ajprenal.00289.2014. This study shows that citrulline can be utilized directly by many tissues and organs to generate arginine when renal function is impaired. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21▪.Marini JC, Didelija IC. Arginine depletion by arginine deiminase does not affect whole protein metabolism or muscle fractional protein synthesis rate in mice. PLoS ONE. 2015:10. doi: 10.1371/journal.pone.0119801. In this article it was shown in mice with no circulating arginine that the ability to convert citrulline into arginine in different tissues is sufficient to maintain protein synthesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ligthart-Melis GC, Vermeulen MAR, Van Leeuwen PAM, Deutz NEP. Glutamine: Precursor or nitrogen donor for citrulline synthesis? Am J Physiol. 2010;299:E683. doi: 10.1152/ajpendo.00425.2010. [DOI] [PubMed] [Google Scholar]
- 23.Marini JC, Didelija IC, Lee B. Reply to Ligthart-Melis et al. Am J Physiol. 2010;299:E684. [Google Scholar]
- 24.Ligthart-Melis GC, Marini JC, Engelen MPKJ, Deutz NEP. Glutamine supplementation, citrulline production, and de novo arginine synthesis: Is there a relation? Am J Clin Nutr. 2015;101:890–892. doi: 10.3945/ajcn.114.104679. [DOI] [PubMed] [Google Scholar]
- 25.Buijs N, Brinkmann SJH, Oosterink JE, et al. Reply to GC Ligthart-Melis et al. Am J Clin Nutr. 2015;101:892–893. doi: 10.3945/ajcn.114.104778. [DOI] [PubMed] [Google Scholar]
- 26.Ligthart-Melis GC, Deutz NEP. Is glutamine still an important precursor of citrulline? Am J Physiol. 2011;301:E264–E266. doi: 10.1152/ajpendo.00223.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kao C, Hsu J, Bandi V, Jahoor F. Alterations in glutamine metabolism and its conversion to citrulline in sepsis. Am J Physiol. 2013;304:E1359–1364. doi: 10.1152/ajpendo.00628.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomlinson C, Rafii M, Ball RO, Pencharz PB. Arginine can be synthesized from enteral proline in healthy adult humans. J Nutr. 2011;141:1432–1436. doi: 10.3945/jn.110.137224. [DOI] [PubMed] [Google Scholar]
- 29.Tomlinson C, Rafii M, Sgro M, et al. Arginine is synthesized from proline, not glutamate, in enterally fed human preterm neonates. Pediatr Res. 2011;69:46–50. doi: 10.1203/PDR.0b013e3181fc6ab7. [DOI] [PubMed] [Google Scholar]
- 30.Marini JC, Stoll B, Didelija IC, Burrin DG. De novo synthesis is the main source of ornithine for citrulline production in neonatal pigs. Am J Physiol. 2012;303:E1348–E1353. doi: 10.1152/ajpendo.00399.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marini JC. Arginine and ornithine are the main precursors for citrulline synthesis in mice. J Nutr. 2012;142:572–580. doi: 10.3945/jn.111.153825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marini JC, Didelija IC, Castillo L, Lee B. Plasma arginine and ornithine are the main citrulline precursors in mice infused with arginine-free diets. J Nutr. 2010;140:1432–1437. doi: 10.3945/jn.110.125377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33▪▪.Marini JC, Didelija IC, Castillo L, Lee B. Glutamine: Precursor or nitrogen donor for citrulline synthesis? Am J Physiol. 2010;299:E69–E79. doi: 10.1152/ajpendo.00080.2010. In this study different glutamine tracers were used to determine the contribution of glutamine to citrulline synthesis. Only tracers that follow the carbon skeleton of glutamine should be used to determine precursor-product relationship. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marini JC. Quantitative analysis of 15n-labeled positional isomers of glutamine and citrulline via electrospray ionization tandem mass spectrometry of their dansyl derivatives. Rapid Commun Mass Spectrom. 2011;25:1291–1296. doi: 10.1002/rcm.5007. [DOI] [PubMed] [Google Scholar]
- 35.Tomlinson C, Rafii M, Ball RO, Pencharz P. Arginine synthesis from enteral glutamine in healthy adults in the fed state. Am J Physiol. 2011;301:E267–E273. doi: 10.1152/ajpendo.00006.2011. [DOI] [PubMed] [Google Scholar]
- 36.Marini JC. Citrulline and urea metabolism in the gut. In: Energy and protein metabolism and nutrition. In: Crovetto GM, editor. EAAP Scientific Series. Vol. 127. Wageningen: Academic Publishers EAAP Scientific Series; 2010. pp. 87–98. [Google Scholar]