Abstract
Background
One major problem of ABE (acetone, butanol and ethanol) fermentation is high oxygen sensitivity of Clostridium acetobutylicum. Currently, no single strain has been isolated or genetically engineered to produce butanol effectively under aerobic conditions. In our previous work, a symbiotic system TSH06 has been developed successfully by our group, and two strains, C. acetobutylicum TSH1 and Bacillus cereus TSH2, were isolated from TSH06.
Results
Compared with single culture, TSH06 showed promotion on cell growth and solvent accumulation under microaerobic conditions. To simulate TSH06, a new symbiotic system was successfully re-constructed by adding living cells of B. cereus TSH2 into C. acetobutylicum TSH1 cultures. During the fermentation process, the function of B. cereus TSH2 was found to deplete oxygen and provide anaerobic environment for C. acetobutylicum TSH1. Furthermore, inoculation ratio of C. acetobutylicum TSH1 and B. cereus TSH2 affected butanol production. In a batch fermentation with optimized inoculation ratio of 5 % C. acetobutylicum TSH1 and 0.5 % B. cereus TSH2, 11.0 g/L butanol and 18.1 g/L ABE were produced under microaerobic static condition. In contrast to the single culture of C. acetobutylicum TSH1, the symbiotic system became more aerotolerant and was able to produce 11.2 g/L butanol in a 5 L bioreactor even with continuous 0.15 L/min air sparging. In addition, qPCR assay demonstrated that the abundance of B. cereus TSH2 increased quickly at first and then decreased sharply to lower than 1 %, whereas C. acetobutylicum TSH1 accounted for more than 99 % of the whole population in solventogenic phase.
Conclusions
The characterization of a novel symbiotic system on butanol fermentation was studied. The new symbiotic system re-constructed by co-culture of C. acetobutylicum TSH1 and B. cereus TSH2 showed excellent performance on butanol production under microaerobic conditions. B. cereus TSH2 was a good partner for C. acetobutylicum TSH1 by providing an anaerobic environment. During fermentation process, the high ratio of Clostridium and low ratio of Bacillus composition indicated that this symbiotic system was an effective and easily controlled cultivation model for ABE fermentation under microaerobic conditions.
Keywords: ABE fermentation, Symbiotic system, Clostridium acetobutylicum TSH1, Bacillus cereus TSH2, Oxygen
Background
The endospore forming, gram-positive Clostridium acetobutylicum is a classic example of fermentative obligate anaerobes. Under anaerobic conditions, it ferments sugars or starch to acetate and butyrate and then shifts them to solvents such as butanol, acetone and ethanol. In these solvents, butanol has been regained attention in recent years as an attractive biofuel, because it exhibits superior performance in terms of energy content, blending ability, volatility, vapor pressure and corrosiveness [1, 2]. Thus, genetic engineering and bioprocess technologies are ranked highly among recent efforts to increase productivity and economic competitiveness of the fermentation route for butanol production [3–6]. Although many improvements and developments have been achieved over the past three decades,conventional ABE (acetone, butanol and ethanol) fermentation still has faced to a number of challenges [7]. One major problem is high oxygen sensitivity of C. acetobutylicum. O’Brien and Morris reported that glucose consumption rate decreased and cell growth was halted as well as DNA, RNA, protein syntheses were prevented if cells of C. acetobutylicum were exposed to high concentration of oxygen [8]. Compared with aerobes, the strict anaerobes need special equipment and complicated operation to eliminate oxygen in the culture medium, for example, adding reducing agents or flushing with N2 gas, which increased the total cost of ABE fermentation. Furthermore, aerobic metabolism could reach higher cell density without accumulating higher level of acids, and improve butanol productivity [9]. However, for batch fermentation, the productivity of butanol is often lower than 0.3 g/L h which decrease the economy of ABE fermentation [10]. So, high oxygen tolerance is believed to be one desirable behavior of C. acetobutylicum.
In nature, microorganisms often exist in highly diverse and complex communities rather than live in isolation. In these natural communities,microorganisms coexist stably by interacting with each other and exert various functions more effectively than single culture [11]. For example, natural cooperation were often observed in interspecies hydrogen transfer within methanogenic systems, and in the degradation of xenobiotics such as halogenated organic compounds [12]. As an obligate anaerobe, Clostridiums were not able to survive in aerobic environments, but were often detected with aerobic bacteria simultaneously in habitats which were exposed to oxygen environments such as rice paddy soils and water retting pond. In these habitats, aerobic bacteria were considered to be essential for Clostridiums by supplying an anaerobic environment and consuming harmful metabolites. The cooperation between Clostridiums and aerobic bacteria were observed not only in natural habitats but also in many artificial systems. For example, by controlling the volumetric transport rate of oxygen, an artificial symbiosis was established between C. phytofermentans and other two yeast species. In this system, both yeasts were capable of providing respiratory protection to C. phytofermentans, and the symbiosis was stable for almost 2 months under semi-aerobic conditions and produced more ethanol than any single cultures [11].
Co-culture of Clostridium and other bacteria which have enzymes capable of hydrolyzing cellulose and hemicellulose were induced in utilization of cellulosic biomass. In these co-culture systems, cellulose and hemicellulose were first hydrolyzed to glucose or butyric acid by cellulolytic strains, and then butanol was obtained subsequently by adding solvent-producing Clostridium species [13–16]. These researches were carried out under strict anaerobic conditions as all of the strains used were anaerobic bacteria. However, there were few reports on butanol fermentation by Clostridium and aerobic bacteria. Stevens used a co-culture of C. beijerinkii and Bacillus cereus to ferment cheese whey, and found that higher concentration of butanol was obtained in the mixed culture without sacrificing butyric acid [17]. In order to enhance solvent production from cassava starch, B. subtilis was co-cultured with C. butylicum. In this co-culture system, amylase activity was increased by tenfold and solvent production was enhanced by 6.5-fold compared to the pure culture of Clostridium [18]. Abd-Alla founded that co-culture of C. acetobutylicum and B. subtilis were able to use spoilage date palm fruits as a substrate to produce butanol without any anaerobic pretreatment [19]. In an artificial syntrophic co-culture system, Bacillus was founded to be a good partner for creating anaerobic environment and pre-saccharification of substrate for co-cultured Clostridium strain as it showed multiple extracellular enzyme activities including lipase, protease, a-amylase, pectinase and cellulose [20].
Though some reports confirmed that aerobic bacteria, such as B. subtilis and B. cereus, had a positive interaction with anaerobic Clostridium, the role of aerobic bacteria was not established clearly. Firstly, aerobic bacteria in the co-culture system were considered to sustain anaerobic environment for Clostridiums, however, there was lack of oxygen variation data during fermentation process in these literatures. Secondly, different micro-organisms in the co-culture system may compete for substrates, therefore, the product yield and productivity would be influenced compared with single cultures. Moreover, the complicated community interactions between the different microorganisms in co-culture systems were still unclear. So, further study would be necessary.
In our previous work, a stable symbiotic system TSH06 was developed and anaerobic C. acetobutylicum TSH1 and aerobic B. cereus TSH2 were isolated from TSH06 [21]. In this study, a new symbiotic system was successfully re-constructed by adding living cells of B. cereus TSH2 to C. acetobutylicum TSH1 culture, and some important characters of ABE fermentation by this symbiotic system, such as dissolved oxygen and inoculation ratio, were investigated. In addition, butanol production and abundance of each strain during the fermentation process were also discussed.
Results and discussion
Cell growth and solvent production with selected different strains under microaerobic conditions
In general, symbiotic system or co-culture system appears to be advantageous over single culture because of the potential for cooperative utilization of the metabolic pathways of all involved organisms [22]. Cell growth of one micro-organism may be enhanced by activities of other micro-organisms. Figure 1a shows the cell growth of symbiotic system TSH06, C. acetobutylicum TSH1 and B. cereus TSH2 under microaerobic static conditions, respectively. Like most of Clostridiums, C. acetobutylicum TSH1 cannot grow in P2 medium under microaerobic conditions, and OD600 was still below 0.3 after 30 h. Different from C. acetobutylicum TSH1, B. cereus TSH2 survived with a low biomass in P2 medium, OD600 reached 1.2 at 12 h and then remained steady. Both of C. acetobutylicum TSH1 and B. cereus TSH2 displayed poor growth when they were cultured individually, however, promotion of cell growth occurred in symbiotic system TSH06. At the initial 12 h, cell growth rate of TSH06 was comparable with that of B. cereus TSH2. After that, OD600 of TSH06 increased quickly and reached 3.7 at 48 h, while OD600 of B. cereus TSH2 remained at 1.2. After 48 h, OD600 of TSH06 decreased owing to the butanol toxicity [5].
Fig. 1.
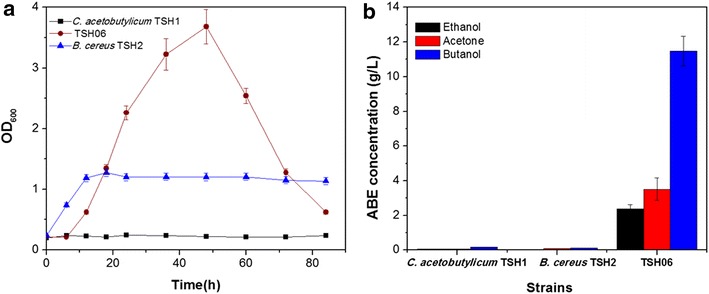
Cell growth and ABE solvents production by TSH06, C. acetobutylicum TSH1 and B. cereus TSH2. a Time course of cell growth. b Acetone, butanol and ethanol observed in final broth. The cultures were carried out in 100 mL shaken flasks with 60 mL P2 medium, and incubated statically at 37 °C without anaerobic treatment for 84 h
In symbiotic system TSH06,not only cell growth but also solvent producing ability was enhanced. Figure 1b shows solvent production in ABE fermentation under microaerobic conditions. With TSH06, 11.5 g/L butanol, 3.5 g/L acetone, and 2.4 g/L ethanol were obtained. However, ABE couldn’t be produced in single culture of C. acetobutylicum TSH1 or B. cereus TSH2 respectively. These results demonstrated that the symbiotic system had potential advantage in ABE fermentation, as it didn’t need any anaerobic pretreatment, such as reducing agent addition or N2 flushing. That made ABE fermentation more economical and easier to be operated.
Re-construction of symbiotic system by adding various B. cereus TSH2 cultures into C. acetobutylicum TSH1
In symbiotic system TSH06, there were only two micro-organisms, C. acetobutylicum TSH1 and B. cereus TSH2. C. acetobutylicum TSH1 was an anaerobic, solvent-producing strain, and B. cereus TSH2 was an aerobic, non-solvent-producing strain. Two positive interactions may exist in TSH06. One interaction is B. cereus TSH2 protecting C. acetobutylicum TSH1 from environmental influences, such as oxygen toxicity; another is the products of B. cereus TSH2 supplying C. acetobutylicum TSH1 as substrate. To explore the interactions mentioned above, symbiotic systems were re-constructed by adding various components of B. cereus TSH2 cultures into C. acetobutylicum TSH1 culture. B. cereus TSH2 cultures included cells, cell-free cultures, cell-free enzymes, sterilized cultures and whole cultures. In cell-free cultures, cell-free enzymes and sterilized cultures, no living cells were existed. Except cells, the adding volume of all liquid cultures was 1 mL. Cells were harvested from 10 mL broth, and added into C. acetobutylicum TSH1 directly.
Table 1 shows cell growth and solvent production under both anaerobic and microaerobic conditions by re-constructed symbiotic systems. Under anaerobic conditions, cells grew well and solvents (ABE) were produced for all the cases. However, under microaerobic conditions, solvents were produced only when living B. cereus TSH2 cells were supplied, i.e. with cells or whole cultures addition. The results indicated that living cells of B. cereus TSH2 offered C. acetobutylicum TSH1 to grow and produce butanol under microaerobic conditions, but the products and enzymes of B. cereus TSH2 didn’t have this ability. So, it was the B. cereus TSH2 cells protecting C. acetobutylicum TSH1 from environmental influences, but not the products or components of B. cereus TSH2. As an aerobic bacteria, B. cereus TSH2 could consume the residual oxygen and sustain an anaerobic environment. Dead cells or parts of cell lost the ability to consume oxygen and failed to rescue C. acetobutylicum TSH1 under microaerobic conditions.
Table 1.
Cell growth and solvents (ABE) production by re-constructed systems
| Re-constructed systems | Aerobic conditions | Anaerobic conditions | |||
|---|---|---|---|---|---|
| C. acetobutylicum cultures | B. cereus TSH2 cultures | Cell growth [21] | ABE (g/L) | Cell growth | ABE (g/L) |
| C. acetobutylicum TSH1 | Cells a | + | 13.9 ± 0.9 | + | 15.6 ± 0.4 |
| Cell-free culturesb | − | − | + | 16.0 ± 0.8 | |
| Cell-free enzymesc | − | − | + | 16.6 ± 1.2 | |
| Sterilized culturesd | − | − | + | 15.1 ± 0.3 | |
| Whole culturese | + | 16.5 ± 1.2 | + | 16.8 ± 0.5 | |
aCells were harvested from 10 mL broth by centrifugation at 10,625g for 5 min
bCultures were filtered using a 0.22 μm pore size filter
cEnzymes were obtained by cells subjected to ultrasonic treatment at 200 W on ice for 10 min (10 s interval every 30 s), and filtered with a 0.22 μm pore size filter
dCultures were obtained by the whole culture of B. cereus TSH2 autoclaved at 121 °C for 20 min
eCultures were the fresh broth of B. cereus TSH2
Dynamic dissolved oxygen variation during fermentation process
Dynamic dissolved oxygen variation of symbiotic system during fermentation process was studied in 5 L bioreactor. The symbiotic system was re-constructed by co-culture of C. acetobutylicum TSH1 and B. cereus TSH2, with inoculum ratio of 5 and 0.5 % respectively. A single culture of C. acetobutylicum TSH1 was carried out in the same bioreactor as control. The results showed in Fig. 2. For the single culture of C. acetobutylicum TSH1, dissolved oxygen decreased gradually after inoculating and kept at 33.1 % after 24 h. In contrast, dissolved oxygen was exhausted quickly within 1 h in the symbiotic system. Further study was carried out in a system in which C. acetobutylicum TSH1 was inoculated at the beginning and then B. cereus TSH2 was inoculated after 1 h of the fermentation. Under such condition, dissolved oxygen decreased to 76.3 % and kept at this level, however, it decreased sharply to zero in 1 h after B. cereus TSH2 addition. The results demonstrated clearly that B. cereus TSH2 consumed dissolved oxygen in the culture and offered anaerobic condition for C. acetobutylicum TSH1, thus oxygen tolerance of the symbiotic system was guaranteed by B. cereus TSH2 addition.
Fig. 2.
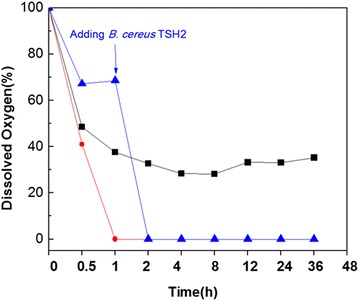
Time courses of dissolved O2 concentrations in different cultures. Filled square C. acetobutylicum TSH1, filled circle C. acetobutylicum TSH1+ B. cereus TSH2/0 h (adding B. cereus TSH2 at the beginning), filled triangle C. acetobutylicum TSH1+ B. cereus TSH2/1 h (adding B. cereus TSH2 at 1 h). The experiment was performed in 5 L bioreactor at 37 °C, the seed of C. acetobutylicum TSH1 was from a culture of P2 medium under 37 °C for 48 h, the seed of B. cereus TSH2 was from a culture of LB medium under 37 °C for 24 h
Clostridiums are commonly classified as strict anaerobes, and oxygen is toxic or lethal to these bacteria. However, some of Clostridiums showed tolerance to oxygen and could survive for a short time when exposed to air [23, 24]. It has been reported that an oxygen scavenging system existed in C. acetobutylicum, which included many proteins, such as NADH oxidase, superoxide reductase (SOR), NADH:rubredoxin oxidoreductase (NROR), rubredoxin (Rd), flavoprotein A2 (FprA2), desulfoferrodoxin (Dsr), and rubperoxin (Rpr) [25–27]. These proteins constituted an alternative detoxification system for C. acetobutylicum to survive in short period of aeration. To scavenge oxygen,this alternative pathway used the ‘reducing power’ (NADH and NADPH) in cell as substrate. If cell activity was high and ‘reducing power’ was sufficient, anaerobic conditions would be established, and cell growth and metabolism would be started on [8]. Unfortunately, C. acetobutylicum TSH1 usually failed to build anaerobic conditions for itself. Adding aerobic B. cereus TSH2 to medium would help C. acetobutylicum TSH1 to consume residual oxygen and set up anaerobic condition (Fig. 2). The symbiotic system constructed by co-culture of C. acetobutylicum TSH1 and B. cereus TSH2 does not require addition of any costly reducing agent or flushing with N2 to ensure anaerobic conditions, which makes ABE fermentation more economy.
Effect of inoculation ratio of C. acetobutylicum TSH1 and B. cereus TSH2 on butanol production
In symbiotic system, B. cereus TSH2 acted as an oxygen scavenger and maintained anaerobic condition for C. acetobutylicum TSH1. However, B. cereus TSH2 didn’t produce butanol in the fermentation process. To study the effects of B. cereus TSH2 on butanol production, fermentation with different inoculation ratios were performed in 100 mL shake flasks. C. acetobutylicum TSH1 inoculation ratio of 5 % was fixed and B. cereus TSH2 inoculation ratio was changed from 0 to 5 %.
As shown in Table 2, the concentration, yield and productivity of butanol were decreased with inoculation ratio of B. cereus TSH2 increasing. When the inoculation ratio of B. cereus TSH2 was 0.5 %, butanol concentration reached 11.9 g/L. At B. cereus TSH2 ratio of 5 %, only 9.1 g/L butanol was produced. The butanol yield and productivity also decreased from 0.23 g/g to 0.20 g/g and 0.16 g/L h to 0.11 g/L h, respectively. The possible reason was attributed to nutrients competition and products inhibition which was produced by B. cereus TSH2. Though B. cereus TSH2 offered the condition for C. acetobutylicum TSH1 cell growth and butanol production under microaerobic conditions, excessive presence of B. cereus TSH2 would be disadvantage to butanol fermentation. The optimized inoculation ratio of B. cereus TSH2 was 0.5 %, which was adopted in the following studies.
Table 2.
Butanol concentration, yield and productivity with different B. cereus TSH2 inoculum ratios
| Inoculum ratio | Butanol concentration (g/L) | Butanol yield (g/g) | Butanol productivity (g/L h) | |
|---|---|---|---|---|
| C. acetobutylicum TSH1 (%) | B. cereus TSH2 (%) | |||
| 5 | 0 | – | – | – |
| 0.5 | 11.9 ± 0.4 | 0.23 ± 0.01 | 0.16 ± 0.01 | |
| 1 | 11.3 ± 0.9 | 0.23 ± 0.01 | 0.15 ± 0.01 | |
| 2 | 10.1 ± 0.7 | 0.22 ± 0.01 | 0.15 ± 0.01 | |
| 5 | 9.1 ± 0.5 | 0.20 ± 0.02 | 0.11 ± 0.01 | |
Unlike B. cereus TSH2, the role of C. acetobutylicum TSH1 in symbiotic system was butanol production. Therefore, butanol producing ability of the symbiotic system depended on vitality of C. acetobutylicum TSH1. Table 3 showed butanol production with different C. acetobutylicum TSH1 inoculation ratios. With inoculation ratio increased from 1 to 7 %, there was no significantly change on butanol concentration and yield, but butanol productivity was increased from 0.13 g/L h to 0.18 g/L h. Considering the butanol concentration, yield and productivity, the optimized inoculation ratio of C. acetobutylicum TSH1 was 5 %. Under this inoculation ratio, butanol concentration, yield and productivity reached 11.9 g/L, 0.23 g/g and 0.16 g/L h. The lag phase period was shortened and fermentation process was accelerated with the C. acetobutylicum TSH1 inoculation ratio increasing. For example, the fermentation was ceased at 88, 80, 72 and 64 h when the inoculation is 1, 3, 5 and 7 %, respectively. Because C. acetobutylicum TSH1 still was the main contributor for butanol production in the symbiotic system, the similar butanol concentrations and yields were obtained with different inoculation ratios. Based on the above results, the optimized inoculation ratio of 0.5 % B. cereus TSH2 and 5 % C. acetobutylicum TSH1 was adopted.
Table 3.
Butanol concentration, yield and productivity with different C. acetobutylicum TSH1 inoculum volume
| Inoculum volume | Butanol concentration (g/L) | Butanol yield (g/g) | Butanol productivity (g/L h) | |
|---|---|---|---|---|
| B. cereus TSH2 (%) | C. acetobutylicum TSH1 (%) | |||
| 0.5 | 0 | – | – | – |
| 1 | 11.2 ± 0.7 | 0.22 ± 0.01 | 0.13 ± 0.01 | |
| 3 | 11.8 + 0.6 | 0.23 ± 0.01 | 0.15 ± 0.01 | |
| 5 | 11.9 ± 0.8 | 0.23 ± 0.01 | 0.16 ± 0.01 | |
| 7 | 11.5 ± 0.5 | 0.22 ± 0.01 | 0.18 ± 0.01 | |
Batch fermentation of symbiotic system under microaerobic conditions
Based on the above results, batch fermentation of symbiotic system under microaerobic conditions was studied with 3 L P2 broth in 5 L bioreactor. For symbiotic system, the inoculation ratio of C. acetobutylicum TSH1 and B. cereus TSH2 was 5 and 0.5 %, respectively. Fermentation was performed under microaerobic conditions including two treatment. One was static culture without any anaerobic pretreatment and another was the culture with air sparging at a rate of 0.15 L/min (0.05 vvm). In addition, single culture of C. acetobutylicum TSH1 with and without anaerobic pretreatment (N2 flushing) were introduced as control.
C. acetobutylicum TSH1 didn’t grow in P2 medium without anaerobic pretreatment. OD600 was still below 0.3 after 30 h (Fig. 3a). With anaerobic pretreatment by N2 flushing, C. acetobutylicum TSH1 grew well and OD600 reached 10.03 at 30 h. At 42 h, 18.3 g/L ABE was produced and 49.5 g/L glucose was consumed (Fig. 3a, b). The results proved that C. acetobutylicum TSH1 was obligate anaerobic bacteria. It was unable to grow and produce butanol under microaerobic conditions. In contrast, 18.1 g/L ABE was produced and 50.4 g/L glucose was consumed by the symbiotic system under static culture without anaerobic pretreatment. Furthermore, cells grew well and produced 17.8 g/L ABE in symbiotic system with air sparging. Though some papers have reported that aerating a small amounts of air into the fermentation broth didn’t affect the cell growth of Clostridium, these researches either used periodic aeration to control ORP (oxidoreduction potential) level or aerated with 5 % O2/95 % N2 mixed gas [28, 29]. In this study, even when air was continuously aerated into bioreactor, the symbiotic system still produced ABE solvents (Fig. 3b). To our knowledge, this is the first report about butanol production by a symbiotic system with continuous air sparging. The results also confirmed that oxygen tolerance of symbiotic system was much higher than that of the single culture. Compared with the condition of anaerobic pretreatment, fermentation rate of symbiotic system were slower. The fermentation of C. acetobutylicum TSH1 with nitrogen pretreatment finished at 42 h. And the fermentation of with symbiotic system air sparging and without nitrogen pretreatment finished at 48 h.
Fig. 3.
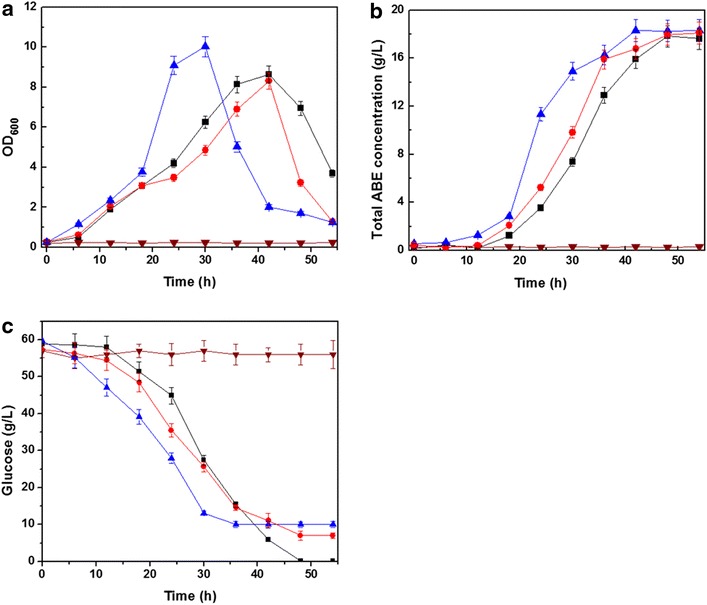
Biomass, ABE solvents, glucose concentration by symbiotic system and single culture of C. acetobutylicum TSH1. a Time courses of biomass (OD600), b Time courses of ABE solvents concentration, c Time courses of glucose consumption. Filled triangle C. acetobutylicum TSH1 with anaerobic treatment, filled inverted triangle C. acetobutylicum TSH1 without anaerobic treatment, filled square symbiotic system with 0.15 L/min (0.05 vvm) air flushing filled circle symbiotic system without anaerobic treatment
Product titers, yields, and productivities were shown in Table 4. Butanol titers obtained in these cultures were similar, but yields and productivities were slightly different. For C. acetobutylicum TSH1, about 11.1 g/L butanol was obtained, and for the symbiotic system, 11.0 g/L and 11.2 g/L butanol was obtained, respectively. Butanol yield of C. acetobutylicum TSH1 was 0.22 g/g, which was similar with artificial symbiotic system without anaerobic pretreatment. But yield of symbiotic system with air flushing was 0.18 g/g. Compared with the nitrogen pretreatment and static culture, continuous air flushing could take away a little solvent from the broth, so the solvent loss rate was higher. And another possible reason is excessive oxygen is disadvantage to the butanol production under microaerobic condition. Butanol productivity of C. acetobutylicum TSH1 with anaerobic pretreatment was 0.26 g/L h. In contrast, productivities of symbiotic system without anaerobic pretreatment and with air flushing were 0.21 and 0.23 g/L h, respectively. This phenomenon was corresponding to the slower cell growth and glucose consumption in symbiotic system cultures (Fig. 3). The slower fermentation rate of symbiotic system cultures could be attributed to inhibition of oxygen, as both of the cultures were carried out without anaerobic pretreatment. These results were agreed with the observations of Dwidar M et al. [30] and indicated that co-culture of C.acetobutylicum and Bacillus produced butyric acid after a longer lag time compared with nitrogen purging pretreatment.
Table 4.
Comparison of batch ABE fermentations by C. acetobutylicum TSH1 and symbiotic system
| C. acetobutylicum TSH1 with pretreatment | Symbiotic system without pretreatment | Symbiotic system with air sparging | |
|---|---|---|---|
| Glucose consumed (g/L) | 49.5 ± 1.0 | 50.4 ± 1.8 | 58.6 ± 1.0 |
| Acetone (g/L) | 4.9 ± 0.1 | 4.9 ± 0.1 | 3.1 ± 0.2 |
| Butanol (g/L) | 11.1 ± 0.9 | 11.0 ± 0.5 | 11.2 ± 0.7 |
| Ethanol (g/L) | 2.3 ± 0.04 | 2.2 ± 0.1 | 2.6 ± 0.07 |
| Total ABE (g/L) | 18.3 ± 1.8 | 18.1 ± 1.2 | 16.9 ± 0.9 |
| Butanol yield (g/g) | 0.22 ± 0.01 | 0.22 ± 0.01 | 0.18 ± 0.01 |
| ABE yield (g/g) | 0.37 ± 0.01 | 0.36 ± 0.01 | 0.29 ± 0.01 |
| Butanol productivity (g/L h) | 0.26 ± 0.01 | 0.21 ± 0.01 | 0.23 ± 0.01 |
| ABE productivity (g/L h) | 0.44 ± 0.01 | 0.38 ± 0.01 | 0.37 ± 0.01 |
Dynamics of abundance of C. acetobutylicum TSH1 and B. cereus TSH2 in the symbiotic system
Under microaerobic condition, B. cereus TSH2 provided anaerobic environment for C. acetobutylicum TSH1. However, the mutual mechanism of these two strains was not clear yet. In order to illustrate the interspecies relationship between C. acetobutylicum TSH1 and B. cereus TSH2, dynamic relative abundance during fermentation process under microaerobic static conditions were quantified by real-time PCR. The initial inoculation ratio of C. acetobutylicum TSH1/B. cereus TSH2 (C/B) was 10:1(5 % C. acetobutylicum TSH1: 0.5 % B. cereus TSH2), 1:1(2.5 % C. acetobutylicum TSH1: 2.5 % B. cereus TSH2) and 1:10 (5 % C. acetobutylicum TSH1: 0.5 B. cereus TSH2), respectively.
When C/B ratio was 10:1, C. acetobutylicum TSH1 accounted for 94.5 % of the whole population at the beginning, whereas B. cereus TSH2 was only 5.5 % (Fig. 4a). However, the ratio of B. cereus TSH2 increased fast and reached 59.2 % at 4 h, while ratio C. acetobutylicum TSH1 declined to 40.2 %. Correspondingly, dissolved oxygen was totally exhausted in this period (Fig. 2). The abundance of B. cereus TSH2 started to decline sharply from 4 to 12 h, and maintained at a very low level of 0.15 % after 24 h. At the same time, abundance of C. acetobutylicum TSH1 increased to 99.85 % of the whole population. The high ratio of Clostridium and low ratio of Bacillus composition in the symbiotic system was beneficial for butanol production.
Fig. 4.
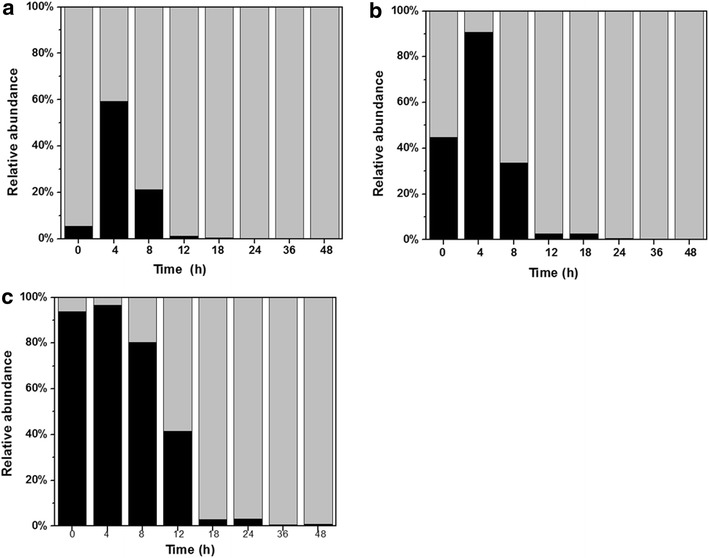
Dynamics of relative abundance for C. acetobutylicum TSH1 and B. cereus TSH2 at different inoculation ratios. a inoculation ratio of C. acetobutylicum TSH1/B. cereus TSH2 was 10:1, b inoculation ratio of C. acetobutylicum TSH1/B. cereus TSH2 was 1:1, c inoculation ratio of C. acetobutylicum TSH1/B. cereus TSH2 was 10:1. Gray, relative abundance of C. acetobutylicum TSH1, Black, relative abundance of B. cereus TSH2
When C/B ratio was changed from 10:1 to 1:1 and 1:10, the abundance of B. cereus TSH2 increased and abundance of C. acetobutylicum TSH1 decreased correspondingly, especially in the initial 4 h after inoculation. The highest proportion of B. cereus TSH2 was 96.5 % when the C/B ratio was 1:10. However, the abundance of B. cereus TSH2 sharply decreased to 0.92 % in the later phase of fermentation which was similar to the case of C/B ratio at 10:1, and C. acetobutylicum TSH1 finally became dominant species in the symbiotic system. The difference between these two conditions was that the abundance decline rate of B. cereus TSH2 at C/B ratio 10:1 was lower than that of C/B ratio at 1:10. Higher proportion and longer declining period indicated that more nutrients were consumed by B. cereus TSH2. The results explained why excessive of B. cereus TSH2 in the symbiotic system had a negative effect on butanol production.
In general, as shown by qPCR, both C. acetobutylicum TSH1 and B. cereus TSH2 were coexisted during the fermentation process under microaerobic static conditions. At the beginning of fermentation, there was still a certain amount of dissolved oxygen in the fermenter, aerobic B. cereus TSH2 grew primarily and consumed oxygen. In this period, B. cereus TSH2 was an assistant for C. acetobutylicum TSH1. Once anaerobic condition established, C. acetobutylicum TSH1 grew quickly and became the dominant species. During the fermentation process, C. acetobutylicum TSH1 released large amounts of gas which prevented air into the fermenter and maintained anaerobic condition by itself. Furthermore, nutrient components of P2 medium and anaerobic condition were more favorable for Clostridium. So, C. acetobutylicum TSH1 became dominant species during fermentation process. The results of qPCR indicated that symbiotic system was able to reach a balance with high ratio of Clostridium and low ratio of Bacillus composition by natural cooperation and competition between the two strains. This was another advantage in using symbiotic system for ABE fermentation.
Conclusions
Based on symbiotic system TSH06, a new symbiotic system was successfully re-constructed by co-culture of two different specified strains, anaerobic, solventogenic C. acetobutylicum TSH1 and aerobic, non-solventogenic B. cereus TSH2. Different from the traditional butanol fermentation strains, this symbiotic system showed higher oxygen tolerance and was able to produce butanol under microaerobic conditions. In 5 L bioreactor with 0.15 L/min (0.05 vvm) air sparging, 11.2 g/L butanol and 17.8 g/L ABE was obtained by the symbiotic system. This was the first report on butanol production by symbiotic system with continuous air sparging.
In this symbiotic system, B. cereus TSH2 was confirmed to be a good partner for C. acetobutylicum TSH1 by supplying an anaerobic environment. At the initial of fermentation, B. cereus TSH2 grew firstly and exhausted the residual oxygen in fermenter, then C. acetobutylicum TSH1 started to grow and produce solvents. Along with the fermentation, C. acetobutylicum TSH1 became dominant species and accounted for 99.85 % of the whole population in solventogenic phase. Increasing inoculation ratio of B. cereus TSH2 would affect butanol producing ability of the symbiotic system, but the relative abundance of each strain was not affected in the final broth, and C. acetobutylicum TSH1 was dominant species no matter how inoculation ratio was changed. The high ratio of Clostridium and low ratio of Bacillus composition during fermentation process indicated that this symbiotic system was an effective and easily controlled cultivation model for ABE fermentation under microaerobic conditions. It may be an attractive approach for butanol production.
Methods
Strains and medium
Both of C. acetobutylicum TSH1 and B. cereus TSH2 were isolated from TSH06, which was isolated from corn powder in the agricultural areas around the campus [21]. The stock culture was maintained in the form of a spore suspension in 25 % glycerol and frozen at −80 °C.
C. acetobutylicum TSH1 was anaerobically pre-cultured in a corn mash medium (6.5 % corn boiling at 100 °C for 30 min with constant stirring, and then autoclave at 121 °C for 20 min). It was incubated under static condition in an anaerobic chamber (Plus labs 855AC, US) at 37 °C for 18–24 h. The subculture was prepared in hemi-synthesis P2 medium containing: glucose (30 g/L), yeast extract(1 g/L), KH2PO4 (0.5 g/L), K2HPO4 (0.5 g/L), ammonium acetate (2.2 g/L), vitamins (1 mg/L para-amino-benzoic acid, 1 mg/L thiamin and 0.01 mg/L biotin), and mineral salts (0.2 g/L MgSO4•7H2O, 0.01 g/L MnSO4•7H2O, 0.01 g/L FeSO4•7H2O, 0.01 g/L NaCl), prepared according to the procedures described previously [31], incubated under static condition at 37 °C for 48 h. B. cereus TSH2 was aerobically pre-cultured in a Luria–Bertani (LB) medium, under shaking condition at 200 rpm and 37 °C for 12 h.
Culture conditions
In the symbiotic system, inoculum volume of C. acetobutylicum TSH1 was 5 %, and B. cereus TSH2 was 0.5 % if not otherwise indicated. All of the inoculum size used volume ratio (vol %). To keep constant inoculation volume, the seed cultures were diluted to similar OD value before inoculation. Shake flask culture was carried out in 100 mL shaken flask with a rubber stopper, and working volume was 60 mL. The fermentation was performed statically at 37 °C without anaerobic treatment. Bioreactor fermentation was carried out in 5 L Biostat B plus fermenter (Sartorius company, Germany) containing 3 L P2 medium (55–60 g/L initial glucose) at 37 °C. Anaerobic fermentation was performed with N2 flushing pretreatment. Microaerobic fermentation was achieved by static culture or flushing with air at a rate of 0.15 L/min (0.05 vvm). Samples were taken at regular intervals to analyze biomass, substrate and solvents concentration.
qPCR analysis
Genomic DNA was extracted according to the manufacturer’s instructions (TaKaRa MiniBest Bacterial Genome DNA Extraction Kit Ver. 3.0). The purity of DNA was checked by using a NanoDrop ND-2000 spectrophotometer (NanoDrop technologies, Wilmington, DE) and electrophoresis. Primers were designed according to 16S rRNA sequence difference between B. cereus and C. acetobutylicum listed in Table 5.
Table 5.
Primers used for qPCR in this study
| Primer | Sequence (5′–3′) | Targeted strain |
|---|---|---|
| B16SF | GTTGAATAAGCTGGCACC | B. cereus TSH2 |
| B16SR | CGTGGGCTTTCACATCAGA | B. cereus TSH2 |
| C16SF | GGGCTGCATTTCAAACTGGA | C. acetobutylicum TSH1 |
| C16SR | GGGCTGCATTTCAAACTGGA | C. acetobutylicum TSH1 |
The qPCR was conducted using Bio-Rad CFX Connect Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Richmond, CA). The reaction mixture for each assay contained 12.5 μL of SYBR Premic EX Taq (TaKaRa Biotechnology Co., Dalian, China), 0.5 μL of each primer, and 1 μL (20 ng) of genomic DNA in a final volume of 25 μL. The PCR was performed using the following protocol: 95 °C for 2 min, following by 40 cycles at 95 °C for 10 s, and 55 °C for 30 s. After the PCR was over, a temperature gradient between 65 and 95 °C was performed for analysis of dissociation curves. The assay was performed at least three times for each sample.
To generate standard curves for quantification, two standard plasmids were used. Standard plasmid was constructed by pEASY-T1 vector ligation with PCR product of 16S rRNA, and then transformed to E. coli DH5α. Copy number was calculated by Eq. (1).
| 1 |
qPCR reactions were run on serial dilutions of each standard plasmid to relate threshold cycle number(Ct value) to copy numbers of the target sequence and to generate standard curves for quantification in unknown samples. Typically, standard curves were linear across five orders of magnitude (107–102 copies, R2 = 1–0.98).
Since 16S DNA is expressed with 11 and 13 copies in the genomes of C. acetobutylicum and B. cereus, respectively, the abundance of each strain in the co-culture system was determined by Eqs. (2) and (3).
| 2 |
| 3 |
Analytical methods
Cell density was determined by measuring OD600 using a UV–visible spectrophotometer (UV-2802PC; Unico, Shanghai, China). During the fermentation period, 1.0 mL sample was taken on time and centrifuged at 10,625g for 5 min. The supernatant was used for ABE andglucose concentrations analyses. ABE were measured by gas chromatography (GC 2010, Shimadzu Co., Kyoto, Japan) equipped with a flame ionization detector (FID) and a glass column (KB-5MS,25 m × 0.53 mm × 1.00 μm, Kromat Corporration, US). The temperature of the detector and injector were maintained at 260 and 240 °C, respectively.
Glucose concentration was determined by high-pressure liquid chromatography (HPLC) analysis (LC-20AT, ShiShimadzu Co., Kyoto, Japan). A HPX-87 H column (300 × 7.8 mm) (Bio-Rad, USA) was used with a mobile phase of 5 mM sulfuric acid and a flow rate of 0.80 mL/min at 65 °C. The oxygen concentration in the medium was measured with an oxygen electrode (Hamilton OXYFERM VP 225, Bonaduz, Switzerland). Butanol concentration was calculated as butanol produced in g per L of broth. Butanol yield was calculated as g of butanol produced per g of sugar utilized and was expressed in g/g, butanol productivity was calculated as butanol produced in g per liter of broth divided by the fermentation time and was expressed in g/L h.
Authors’ contributions
PW performed the experiments and drafted the manuscript. JZ was in charge of project and design the experiments. GW assisted in qPCR analysis. JZ, HL, GW revised the manuscript. GW, BTB gave some suggestions. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21176141), Tsinghua University Initiative Scientific Research Program (No. 2012THZ02289), the Foundation of Key Laboratory for Industrial Biocatalysis (Tsinghua University), Ministry of Education (No. 2015302), National Energy Administration (No. 20131448926) and Norway Statoil Petroleum AS.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Pengfei Wu, Email: wpf5101@163.com.
Genyu Wang, Email: wgymap@126.com.
Gehua Wang, Email: wanggehua@tsinghua.edu.cn.
Børre Tore Børresen, Email: btbo@statoil.com.
Hongjuan Liu, Email: liuhongjuan@mail.tsinghua.edu.cn.
Jianan Zhang, Email: zhangja@tsinghua.edu.cn.
References
- 1.Lee SY, Park JH, Jang SH, Nielsen LK, Kim J, Jung KS. Fermentative butanol production by clostridia. Biotechnol Bioeng. 2008;101:209–228. doi: 10.1002/bit.22003. [DOI] [PubMed] [Google Scholar]
- 2.Dürre P. Biobutanol: an attractive biofuel. Biotechnol J. 2007;2:1525–1534. doi: 10.1002/biot.200700168. [DOI] [PubMed] [Google Scholar]
- 3.Alsaker KV, Spitzer TR, Papoutsakis ET. Transcriptional analysis of spo0A overexpression in Clostridium acetobutylicum and its effect on the cell’s response to butanol stress. J Bacteriol. 2004;186:1959–1971. doi: 10.1128/JB.186.7.1959-1971.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen CR, Lan EI, Dekishima Y, Baez A, Cho KM, Liao JC. Driving forces enable high-titer anaerobic 1-butanol synthesis in Escherichia coli. Appl Environ Microbiol. 2011;77:2905–2915. doi: 10.1128/AEM.03034-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xue C, Zhao J, Lu C, Yang S, Bai F, Tang IC. High-titer n-butanol production by Clostridium acetobutylicum JB200 in fed-batch fermentation with intermittent gas stripping. Biotechnol Bioeng. 2012;109:2746–2756. doi: 10.1002/bit.24563. [DOI] [PubMed] [Google Scholar]
- 6.Chen C, Xiao Z, Tang X, Cui H, Zhang J, Li W, Ying C. Acetone–butanol–ethanol fermentation in a continuous and closed-circulating fermentation system with PDMS membrane bioreactor. Bioresour Technol. 2013;128:246–251. doi: 10.1016/j.biortech.2012.10.077. [DOI] [PubMed] [Google Scholar]
- 7.García V, Päkkilä J, Ojamo H, Muurinen E, Keiski RL. Challenges in biobutanol production: how to improve the efficiency? Renew Sust Energ Rev. 2011;15:964–980. doi: 10.1016/j.rser.2010.11.008. [DOI] [Google Scholar]
- 8.O’Brien RW, Morris JG. Oxygen and the growth and metabolism of Clostridium acetobutylicum. J Gen Microbiol. 1971;68:307–318. doi: 10.1099/00221287-68-3-307. [DOI] [PubMed] [Google Scholar]
- 9.Papoutsakis ET. Engineering solventogenic clostridia. Curr Opin Biotechnol. 2008;19:420–429. doi: 10.1016/j.copbio.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi N, Blaschek HP. Recent advances in ABE fermentation: hyper-butanol producing Clostridium beijerinckii BA101. J Ind Microbiol Biotechnol. 2001;27:287–291. doi: 10.1038/sj.jim.7000114. [DOI] [PubMed] [Google Scholar]
- 11.Zuroff TR, Xiques SB, Curtis WR. Consortia-mediated bioprocessing of cellulose to ethanol with a symbiotic Clostridium phytofermentans/yeast co-culture. Biotechnol Biofuels. 2013;6:59. doi: 10.1186/1754-6834-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato S, Haruta S, Cui ZJ, Ishii M, Igarashi Y. Effective cellulose degradation by a mixed-culture system composed of a cellulolytic Clostridium and aerobic non-cellulolytic bacteria. FEMS Microbiol Ecol. 2004;51:133–142. doi: 10.1016/j.femsec.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Harvey BG, Meylemans HA. The role of butanol in the development of sustainable fuel technologies. J Chem Technol Biotechnol. 2011;86:2–9. doi: 10.1002/jctb.2540. [DOI] [Google Scholar]
- 14.Wen Z, Wu M, Lin Y, Yang L, Lin J, Cen P. Artificial symbiosis for acetone-butanol-ethanol (ABE) fermentation from alkali extracted deshelled corn cobs by co-culture of Clostridium beijerinckii and Clostridium cellulovorans. Microb Cell Fact. 2014;13:92. doi: 10.1186/s12934-014-0092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geng A, He Y, Qian C, Yan X, Zhou Z. Effect of key factors on hydrogen production from cellulose in a co-culture of Clostridium thermocellum and Clostridium thermopalmarium. Bioresour Technol. 2010;101:4029–4033. doi: 10.1016/j.biortech.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 16.Liu XG, Zhu Y, Yang ST. Butyric acid and hydrogen production by Clostridium tyrobutyricum ATCC 25755 and mutants. Enzyme Microb Technol. 2006;38:521–528. doi: 10.1016/j.enzmictec.2005.07.008. [DOI] [Google Scholar]
- 17.Stevens D, Alam S, Bajpai R. Fermentation of cheese whey by a mixed culture of Clostridium beijerinckii and Bacillus cereus. J Ind Microbiol. 1988;3:15–19. doi: 10.1007/BF01569437. [DOI] [Google Scholar]
- 18.Tran HTM, Cheirsilp B, Hodgson B, Umsakul K. Potential use of Bacillus subtilis in a co-culture with Clostridium butylicum for acetone-butanol-ethanol production from cassava starch. Biochem Eng J. 2010;48:260–267. doi: 10.1016/j.bej.2009.11.001. [DOI] [Google Scholar]
- 19.Abd-Alla MH, El-Enany A. A. Production of acetone-butanol-ethanol from spoilage date palm (Phoenix dactylifera L.) fruits by mixed culture of Clostridium acetobutylicum and Bacillus subtilis. Biomass Bioenerg. 2012;42:172–178. doi: 10.1016/j.biombioe.2012.03.006. [DOI] [Google Scholar]
- 20.Chang J, Chou C, Ho C, Chen W, Lay J, Huang C. Syntrophic co-culture of aerobic Bacillus and anaerobic Clostridium for bio-fuels and bio-hydrogen production. Int J Hydrogen Energ. 2008;33:5137–5146. doi: 10.1016/j.ijhydene.2008.05.021. [DOI] [Google Scholar]
- 21.Wang G, Wu P, Liu Y, Mi S, Mai S, Gu C, Wang G, Liu H, Zhang J, Børresen BT, et al. Isolation and characterisation of non-anaerobic butanol-producing symbiotic system TSH06. Appl Microbiol Biotechnol. 2015;99:8803–8813. doi: 10.1007/s00253-015-6864-y. [DOI] [PubMed] [Google Scholar]
- 22.Bader J, Mast-Gerlach E, Popovic MK, Bajpai R, Stahl U. Relevance of microbial coculture fermentations in biotechnology. J Appl Microbiol. 2010;109:371–387. doi: 10.1111/j.1365-2672.2009.04659.x. [DOI] [PubMed] [Google Scholar]
- 23.Kawasaki S, Nakagawa T, Nishiyama Y, Benno Y, Uchimura T, Komagata K, Kozaki K, Niimura Y. Effect of oxygen on the growth of Clostridium butyricum (type species of the genus Clostridium), and the distribution of enzymes for oxygen and for active oxygen species in Clostridia. J Ferment Bioeng. 1998;86:368–372. doi: 10.1016/S0922-338X(99)89006-0. [DOI] [Google Scholar]
- 24.Kawasaki S, Watamura Y, Ono M, Watanabe T, Takeda K, Niimura Y. Adaptive responses to oxygen stress in obligatory anaerobes Clostridium acetobutylicum and Clostridium aminovalericum. Appl Environ Microbiol. 2005;71:8442–8450. doi: 10.1128/AEM.71.12.8442-8450.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawasaki S, Sakai Y, Takahashi T, Suzuki I, Niimura Y. O2 and reactive oxygen species detoxification complex, composed of O2-responsive NADH: rubredoxin oxidoreductase-flavoprotein A2-desulfoferrodoxin operon enzymes, Rubperoxin, and Rubredoxin, in Clostridium acetobutylicum. Appl Environ Microbiol. 2009;75:1021–1029. doi: 10.1128/AEM.01425-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riebe O, Fischer RJ, Wampler DA, Kurtz DM, Bahl H. Pathway for H2O2 and O2 detoxification in Clostridium acetobutylicum. Microbiology. 2009;155:16–24. doi: 10.1099/mic.0.022756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hillmann F, Fischer RR, Saint-Prix F, Girbal L, Bahl H. PerR acts as a switch for oxygen tolerance in the strict anaerobe Clostridium acetobutylicum. Mol Microbiol. 2008;68:848–860. doi: 10.1111/j.1365-2958.2008.06192.x. [DOI] [PubMed] [Google Scholar]
- 28.Kawasaki S, Ishikura J, Watamura Y, Niimura Y. Identification of O2 -induced peptides in an obligatory anaerobe Clostridium acetobutylicum. FEBS Lett. 2004;571:21–25. doi: 10.1016/j.febslet.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Zhu Y, Zhang Y, Li Y. Controlling the oxidoreduction potential of the culture of Clostridium acetobutylicum leads to an earlier initiation of solventogenesis, thus increasing solvent productivity. Appl Microbiol Biotechnol. 2012;93:1021–1030. doi: 10.1007/s00253-011-3570-2. [DOI] [PubMed] [Google Scholar]
- 30.Dwidar M, Kim S, Jeon BS, Um Y, Mitchell RJ, Sang BI. Co-culturing a novel Bacillus strain with Clostridium tyrobutyricum ATCC 25755 to produce butyric acid from sucrose. Biotechnol Biofuels. 2013;6:35. doi: 10.1186/1754-6834-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monot F, Martin J, Petitdemange H, Gay R. Acetone and butanol production by Clostridium acetobutylicum in a synthetic medium. Appl Environ Microbiol. 1982;44:1318–1324. doi: 10.1128/aem.44.6.1318-1324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


