Abstract
Background
There are no recent data on prevalence of coronary artery disease (CAD) in Indians. The last community based study from Kerala, the most advanced Indian state in epidemiological transition, was in 1993 that reported 1.4 % definite CAD prevalence. We studied the prevalence of CAD and its risk factors among adults in Kerala.
Methods
In a community-based cross sectional study, we selected 5167 adults (mean age 51 years, men 40.1 %) using a multistage cluster sampling method. Information on socio-demographics, smoking, alcohol use, physical activity, dietary habits and personal history of hypertension, diabetes, and CAD was collected using a structured interview schedule. Anthropometry, blood pressure, electrocardiogram, and biochemical investigations were done using standard protocols. CAD and its risk factors were defined using standard criteria. Comparisons of age adjusted prevalence were done using two tailed proportion tests.
Results
The overall age-adjusted prevalence of definite CAD was 3.5 %: men 4.8 %, women 2.6 % (p < 0.001). Prevalence of any CAD was 12.5 %: men 9.8 %, women 14.3 % (p < 0.001). There was no difference in definite CAD between urban and rural population. Physical inactivity was reported by 17.5 and 18 % reported family history of CAD. Other CAD risk factors detected in the study were: overweight or obese 59 %, abdominal obesity 57 %, hypertension 28 %, diabetes 15 %, high total cholesterol 52 % and low level of high density lipoprotein cholesterol 39 %. Current smoking was reported only be men (28 %).
Conclusion
The prevalence of definite CAD in Kerala increased nearly three times since 1993 without any difference in urban and rural areas. Most risk factors of CAD were highly prevalent in the state. Both population and individual level approaches are warranted to address the high level of CAD risk factors to reduce the increasing prevalence of CAD in this population.
Keywords: Coronary artery disease, Coronary risk factors, Prevalence, Kerala, India
Background
Coronary artery disease (CAD) is the foremost cause of disability and death the world over and is one of the top five causes of death in Indian population [1]. Mortality from CAD in Indians is predicted to increase rapidly and overtake that of the high-income countries. Among adults over 20 years of age, there has been a two-fold rise in CAD in rural areas and a 6-fold rise in urban areas during the period from 1960 to 2002 [2]. Previous studies have shown high prevalence of CAD in Asian Indians residing in the United States [3]. However there are no robust and contemporary data on CAD in native Indians. In a systematic review of CAD prevalence from India, Ahmed et al. commented that none of the studies conformed to the requirements of a high-quality epidemiologic study [4]. Kerala, with a population of over 33 million, is the most advanced state in epidemiological transition and has the highest prevalence of CAD risk factors in India [5]. The state has been reported to be the harbinger of what the rest of India is going to face in the near future [5]. The INTERHEART study reported the importance of conventional risk factors associated with CAD [6]. Although the CAD risk factor prevalence is the highest in the state of Kerala, there are no recent studies on the prevalence of CAD in this state. The only one community based study in 1993 from the rural area of the southernmost district of the state reported a CAD prevalence of 7.4 % [7]. The environment in Kerala is conducive for increasing the CAD risk factors [8] which is likely to result in an increase in the CAD prevalence. Therefore we wanted to study the current prevalence of CAD and its major risk factors in Kerala.
Methods
A detailed description of the design, sample, and methods of the study has already been published [9]. Briefly, this was a cross-sectional community-based study during the period from January to June 2011. We estimated the sample size based on an anticipated prevalence of 7.4 % of CAD for rural Kerala based on the data by Kutty et al. [7] and 11 % for urban Kerala based on the study by Mohan et al. in Chennai [10]. The total sample size was estimated to be 3000 in rural and 2400 in urban areas. Sample selection procedure is given in Fig. 1. Subjects aged 20–79 years were selected using a multistage cluster sampling procedure. In the first stage three out of the 14 districts of Kerala state were selected. The 14 districts were grouped into five northern, five southern and four central districts. From the northern group of districts, Kozhikode district was selected which included the city corporation of Kozhikode. In the southern group of districts, Thiruvananthapuram and Kollam districts had city corporations. Of these Thiruvananthapuram was selected randomly. In the central group of districts there were two districts which included corporations; Ernakulam and Thrissur. Of these Thrissur district was selected randomly. In the second stage, one ward was randomly selected from each of the three city corporation wards in the urban area. Each of the selected wards was divided into six geographic regions. One of these geographic regions was selected randomly as the study area. All the households in this area were eligible to be included in this sample. One subject between 20 and 59 years was selected using KISH method [11] and all the subjects between 60 and 79 years were included.
Fig. 1.
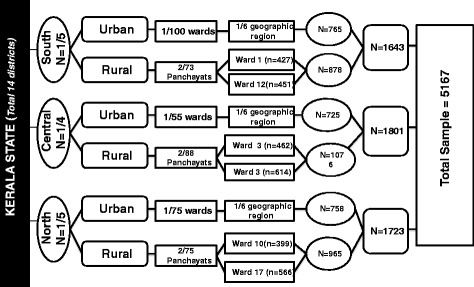
Sample selection procedure
In rural area, two of the Panchayats from the above three districts were randomly selected. From each of these Panchayats, one ward was randomly selected. All the households in the selected ward were eligible to be included on the sample. One subject between the age of 20 and 59 years was selected using KISH method and all subjects between 60 and 79 years were included.
Each selected individual was invited to visit an easily accessible facility with all past medical records after overnight fasting. Trained investigators collected all the relevant data.
Using a structured interview schedule, information on basic socio-economic and demographic details, smoking, physical activity, dietary habits, personal history of hypertension, dyslipidemia, diabetes mellitus and CAD were collected. The instrument also contained the Rose Angina Questionnaire (RAQ) [12, 13] and questions related to history of documented prior myocardial infarction, unstable angina, coronary artery bypass grafting (CABG) surgery, noninvasive investigations for CAD, coronary angiography, coronary angioplasty, documented use of drugs for CAD and hospital admission for CAD. Family history of ischemic heart disease, stroke and coronary risk factors was also recorded.
Anthropometric measurements
Height was measured by wall-mounted stadiometer (Model 206, Seca, Hamburg Germany) to the nearest centimeter. Subjects were asked to stand upright without shoes, with their back against the wall, heels together and eyes directed forward.
Weight was measured with a portable electronic weighing scale (Model HN 283, Omron Corporation, Shimogyo-ku, Kyoto, Japan) kept on a firm horizontal surface. The subjects were asked to wear light clothing and remove footwear. Weight was recorded in kilograms to the nearest 0.5Kg. Body mass index was calculated as weight in kg/(height in meter squared) [14].
Waist circumference was measured using a non-stretchable measuring tape. The subjects were asked to stand erect in a relaxed position with both feet together. Waist girth was measured at the midpoint between the iliac crest and the lower margin of the ribs at the end of expiration, to the nearest centimeter.
Blood pressure
Blood pressure was recorded with electronic apparatus (model 1A2, Omron Corporation, Shimogyo-ku, Kyoto, Japan) in sitting position, on the left arm resting on a table at heart level, after the subject having rested for at least 15 min. Three readings were taken 3 min apart and the mean of the last two readings was recorded as the BP. Heart rate was also recorded.
Electrocardiogram
Resting 12 lead electrocardiogram (ECG) was performed on all subjects by trained technicians with three-channel digital ECG recorders with facility for display and measured parameters. For each lead, five consecutive complexes were recorded. Minnesota coding [15] and application of CAD criteria were performed by an experienced cardiologist for the respective region; those subjects diagnosed to have CAD were re-evaluated by another cardiologist in a blinded manner. Disagreements were resolved by consensus. Similar procedure was followed for 10 % of randomly selected subjects without a diagnosis of CAD.
Biochemical investigations
Blood samples were drawn from individuals after 10–12 h of fasting. Plasma glucose was estimated immediately, onsite using the Glucose oxidase/ peroxidase- phenol-4-amenophenazone method (GOD-PAP). Blood samples for lipid profile measurement were transported on ice packs (4–6 °C) to an accredited core laboratory (Thyrocare Technologies Ltd, Navi Mumbai, India) on the same day. Estimation was carried out within 48 h in clinical chemistry instruments (Olympus AU2700) and Advia 1800 chemistry system (Siemens) using standard commercially available kits (Agappe Diagnostics). Photometry technology was used for lipid profile. Serum cholesterol was measured by cholesterol oxidase phenol 4- aminoantipyrine peroxidase (CHOD-PAP) method and serum triglycerides by glycerol-3 -phosphate oxidase -p - aminophenzone peroxidase (GPO-PAP) method. High density lipoprotein (HDL) cholesterol was estimated by enzyme selective protection method. The reaction between cholesterol assays is suppressed by the electrostatic interaction between polyanions & cationic substances. Low-density lipoprotein (LDL) cholesterol was calculated by using Friedwald’s equation.
Ethical clearance
The present study was in compliance with the Helsinki Declaration. The study was approved by the Ethics Committee of Cardiological Society of India, Kerala Chapter. Informed written consent was obtained from all participants.
Definitions used
We defined coronary artery disease as: (a) Definite CAD based on any of: documented evidence of prior acute coronary syndrome (ACS) or treatment for CAD, documented history of undergoing coronary angioplasty or CABG, more than 50 % epicardial coronary stenosis by invasive coronary angiography, ECG showing pathological Q waves (any of Minnesota code 1-1-1 to 1-1-7 or 1-2-1 to 1-2-5 or 1-2-7), imaging evidence of a region of loss of viable myocardium that is thinned and has a motion abnormality, in the absence of a non-ischemic cause [16], RAQ angina plus ECG changes (any of Minnesota codes 4-1-1, 4-1-2, 4-2 or 5-l, 5-2), or RAQ angina plus positive treadmill ECG (exercise-induced horizontal or down-sloping ST depression of ≥ 1 mm at 80 ms from J point), or inducible ischemia on stress imaging;(b) Probable CAD based on any of (in the absence of any of the definite criteria): RAQ angina without significant ECG changes, ECG changes (any of Minnesota Code 4-1-1,4-1-2, 4-2 or 5-1, 5-2) without RAQ angina, or positive treadmill ECG without RAQ angina. Any CAD was defined as those who satisfied either definite or probable CAD criteria.
We defined diabetes mellitus as fasting blood glucose value of ≥ 7 mmol/L and/or if there was current use of medications for diabetes [17], hypertension as blood pressure ≥140 mm of Hg systolic and/or ≥90 mm of Hg diastolic and/or currently on drugs for high blood pressure [18], and dyslipidemia as any of: serum total cholesterol ≥ 5.18 mmol/L, serum LDL cholesterol ≥ 3.37 mmol/L, serum HDL cholesterol <1.04 mmol/L in men or < 1.29 mmol/L in women, or serum triglycerides ≥1.69 mmol /L [19]. We categorized body mass index (BMI) as normal (18.0–22.9 kg/m2), overweight (23.0–24.9 kg/m2), or obesity (≥25 kg/m2). Abdominal obesity was defined as a waist circumference of ≥90 cm in men or ≥80 cm in women [20]. Physical activity levels were classified into sedentary and non-sedentary. All subjects reporting physical activity for at least 30 min a day for a minimum of 5 days a week (household activities involving physical effort, walking to and from work involving at least 30 min., manual workers, those performing leisure-time physical activity) were considered non-sedentary. All others were classified as sedentary [21]. We determined the socioeconomic status of the participants using the validated standard of living Index tool developed by National Family Health Survey [22]. We divided our entire sample into tertiles, based upon the index score. Participants in the upper tertile were classified as high socioeconomic group, those in the middle tertile as intermediate socioeconomic group and lower tertile as low socioeconomic group.
Statistical analysis
We entered data into CS Pro software (the US Census Bureau) version 4 · 0 for Windows. Data cleaning and statistical analysis were performed using Stata (Stata Corp, Texas, USA) version 13 · 0 for Windows. All proportions were age-adjusted using WHO population data. Frequency distribution was done for categorical variables. Comparisons of age-adjusted prevalence between different categories were done using two-tailed proportion test. The difference in the age-adjusted prevalence and its 95 % confidence intervals are provided. P value <0 · 05 defined the level of statistical significance.
Results
Of the total 6477 individuals contacted, we could evaluate 5167 (men 40.1 %); overall response rate was 79.8 % (men 75.0 %, women 83.3 %). There were no missing data in the interview schedule, ECG or anthropometrics. Data on blood sugar and serum cholesterol were missing in 32 and 47 subjects respectively; these were excluded from analysis.
Table 1 depicts the demographic and behavioural characteristics of the participants by area. There were greater proportion of participants without formal education in rural area as compared to urban; those with >10 years of education were more in urban as compared to rural area. The proportion of subjects that reported current smoking was similar in rural and urban men. There was larger proportion of vegetarianism, obesity and abdominal obesity among urban participants.
Table 1.
Baseline demographic and behavioural characteristics of the participants by area
| Variable | Urban | Rural | Total | |||||
|---|---|---|---|---|---|---|---|---|
| (N = 2248) | (N = 2919) | (N = 5167) | ||||||
| n | (%) | n | (%) | n | (%) | P value | ||
| Age | ||||||||
| 20–29 | 111 | 4.94 | 225 | 7.71 | 336 | 6.50 | <0.001 | |
| 30–39 | 345 | 15.35 | 543 | 18.60 | 888 | 17.19 | ||
| 40–49 | 507 | 22.55 | 692 | 23.71 | 1199 | 23.20 | ||
| 50–59 | 493 | 21.93 | 531 | 18.19 | 1024 | 19.82 | ||
| 60–69 | 566 | 25.18 | 650 | 22.27 | 1216 | 23.53 | ||
| 70–79 | 226 | 10.05 | 278 | 9.52 | 504 | 9.75 | ||
| Sex | ||||||||
| Men | 971 | 43.19 | 1101 | 37.72 | 2072 | 40.10 | <0.001 | |
| Women | 1277 | 56.81 | 1818 | 62.28 | 3095 | 59.90 | ||
| Socio-economic status | ||||||||
| Low | 630 | 28.02 | 1303 | 44.64 | 1933 | 37.41 | <0.001 | |
| Middle | 792 | 35.23 | 743 | 25.45 | 1535 | 29.71 | ||
| High | 826 | 36.74 | 873 | 29.91 | 1699 | 32.88 | ||
| Educational status | ||||||||
| No formal education | 87 | 3.87 | 203 | 6.95 | 290 | 5.61 | <0.001 | |
| 1–4 years | 208 | 9.25 | 437 | 14.97 | 645 | 12.48 | ||
| 5–10 years | 1294 | 57.56 | 1729 | 59.23 | 3023 | 58.51 | ||
| >10 years | 659 | 29.31 | 550 | 18.84 | 1209 | 23.40 | ||
| Smokinga | ||||||||
| Never | 523 | 55.52 | 443 | 44.04 | 966 | 49.59 | <0.001 | |
| Past | 111 | 11.78 | 220 | 21.87 | 331 | 16.99 | ||
| Current | 308 | 32.70 | 343 | 34.10 | 651 | 33.42 | ||
| Physical activity | ||||||||
| Sedentary | 1770 | 78.74 | 2328 | 79.75 | 4098 | 79.31 | 0.371 | |
| Non-sedentary | 478 | 21.26 | 591 | 20.25 | 1069 | 20.69 | ||
| Dietary habits | ||||||||
| Vegetarian | 161 | 7.16 | 110 | 3.77 | 271 | 5.24 | <0.001 | |
| Non-vegetarian | 2087 | 92.84 | 2809 | 96.23 | 4896 | 94.76 | ||
| BMI | ||||||||
| Low | 111 | 4.95 | 244 | 8.37 | 355 | 6.88 | <0.001 | |
| Normal | 685 | 30.53 | 1042 | 35.76 | 1727 | 33.48 | ||
| Overweight | 450 | 20.05 | 527 | 18.09 | 977 | 18.94 | ||
| Obese | 998 | 44.47 | 1101 | 37.78 | 2099 | 40.69 | ||
| Abdominal Obesity | ||||||||
| Yes | 1394 | 62.20 | 1709 | 58.67 | 3103 | 60.21 | <0.001 | |
| No | 847 | 37.80 | 1204 | 41.33 | 2051 | 39.79 | ||
a Smoking in men only
Prevalence of coronary artery disease
The crude and age-adjusted prevalence of definite CAD in Kerala was 5.8 and 3.5 % respectively. The figures for men were 8.0 % and 4.8 % and for women 4.2 and 2.6 % respectively; the differences were significant across gender (p < 0.001). The overall crude and age-adjusted prevalence of any CAD in Kerala was 16.6 and 12.5 % respectively. The crude and age-adjusted prevalence of any CAD in men were 14.6 and 9.8 % and in women were 17.9 and 14.3 % respectively; the prevalence was significantly higher in women as compared to men (p < 0.001). Table 2 outlines the prevalence of CAD (any & definite) among different age groups and gender. As age increased, the prevalence of CAD increased in both gender.
Table 2.
Crude and age-adjusted prevalence of CAD by age group and gender
| Age group (yrs.) | Men | Women | Difference (%) | 95 % CI | p value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Any CAD | n | P (%) | SE | n | P (%) | SE | |||
| 20–29 | 144 | 2.78 | 1.37 | 192 | 7.29 | 1.88 | −4.51 | (−9.07,0.04) | 0.069 |
| 30–39 | 306 | 6.54 | 1.42 | 582 | 8.93 | 1.18 | −2.40 | (−6.01,1.21) | 0.213 |
| 40–49 | 465 | 7.10 | 1.19 | 734 | 14.44 | 1.30 | −7.34 | (−10.80,−3.89) | <0.001 |
| 50–59 | 386 | 12.44 | 1.68 | 638 | 19.91 | 1.58 | −7.47 | (−11.99,−2.95) | 0.002 |
| 60–69 | 537 | 22.72 | 1.81 | 679 | 25.77 | 1.68 | −3.05 | (−7.89,1.78) | 0.218 |
| 70–79 | 234 | 32.05 | 3.06 | 270 | 29.26 | 2.77 | 2.79 | (−5.28,10.87) | 0.497 |
| All | 2072 | 14.58 | 0.78 | 3095 | 17.87 | 0.69 | −3.29 | (−5.32,−1.26) | 0.002 |
| Age adjusted | 2072 | 9.80 | 0.65 | 3095 | 14.26 | 0.69 | −4.46 | (−6.24,−2.69) | <0.001 |
| Definite CAD | |||||||||
| 20–29 | 144 | 0.00 | 0.00 | 192 | 0.00 | 0.00 | - | - | - |
| 30–39 | 306 | 2.29 | 0.86 | 582 | 0.52 | 0.30 | 1.77 | (0.00,3.55) | 0.017 |
| 40–49 | 465 | 3.87 | 0.90 | 734 | 1.77 | 0.49 | 2.10 | (0.10,4.10) | 0.026 |
| 50–59 | 386 | 6.48 | 1.25 | 638 | 3.13 | 0.69 | 3.34 | (0.54,6.14) | 0.012 |
| 60–69 | 537 | 13.41 | 1.47 | 679 | 8.98 | 1.10 | 4.42 | (0.83,8.02) | 0.014 |
| 70–79 | 234 | 18.80 | 2.56 | 270 | 12.59 | 2.02 | 6.21 | (−0.17,12.59) | 0.055 |
| All | 2072 | 8.01 | 0.60 | 3095 | 4.23 | 0.36 | 3.78 | (2.41,5.15) | <0.001 |
| Age adjusted | 2072 | 4.80 | 0.39 | 3095 | 2.63 | 0.23 | 2.17 | (1.09,3.25) | <0.001 |
CAD coronary artery disease, P prevalence, SE standard error
The prevalence of definite CAD was similar irrespective of region or religion. The prevalence of any CAD was lowest among Christians and highest among Muslims (10 vs.15.8 % p = 0 · 002). Of the three geographical regions of Kerala, Thirvananthapuram had lower prevalence of any CAD compared to Thrissur and Kozhikode (8.3 vs. 14.4 and 14.7 % p < 0.001).
Table 3 depicts the age-adjusted prevalence of CAD (any and definite) by gender, age group (age ≤ 45 and >45 years), and by area. Age-adjusted prevalence of any CAD in participants ≤45 years and >45 years was 7.8 and 18.7 % respectively. Similarly the age-adjusted prevalence of definite CAD was 0.9 % in participants ≤45 years and 6.8 % in >45 years. Age-adjusted prevalence of CAD (any and definite) was significantly higher in participants >45 years (p < 0.001). The prevalence of any CAD was significantly higher in rural women (15.6 %) as compared to urban women (11.7 %) (p < 0.01). The overall prevalence of any CAD was higher in rural (13.2 %) than urban (11 · 3 %) area (p = 0.038). However, there was no difference between urban and rural areas in the overall prevalence of definite CAD (3.4 vs. 3.6 %) (p = 0.72).
Table 3.
Age-adjusted prevalence of CAD by gender, age, and area
| Gender | =/<45 years | >45 years | Difference (%) | 95 % CI | p value | |||||
| n | P (%) | SE | n | P (%) | SE | |||||
| Any CAD | Men | 728 | 5.12 | 0.82 | 1344 | 15.05 | 1.04 | −9.92 | (−12.42,−7.43) | <0.001 |
| Women | 1239 | 9.48 | 0.92 | 1856 | 21.08 | 1.10 | −11.60 | (−14.07,−9.13) | <0.001 | |
| All | 1967 | 7.78 | 0.64 | 3200 | 18.68 | 0.78 | −10.90 | (−12.69,−9.10) | <0.001 | |
| Definite CAD | Men | 728 | 1.82 | 0.43 | 1344 | 8.43 | 0.80 | −6.61 | (−8.39,−4.84) | <0.001 |
| Women | 1239 | 0.36 | 0.15 | 1856 | 5.61 | 0.58 | −5.25 | (−6.35,−4.15) | <0.001 | |
| All | 1967 | 0.89 | 0.18 | 3200 | 6.80 | 0.47 | −5.91 | (−6.88,−4.95) | <0.001 | |
| Urban | Rural | |||||||||
| Any CAD | Men | 971 | 10.82 | 1.06 | 1101 | 8.94 | 0.81 | 1.88 | (−0.70,4.46) | 0.151 |
| Women | 1277 | 11.66 | 0.88 | 1818 | 15.63 | 0.94 | −3.97 | (−6.40,−1.55) | 0.002 | |
| All | 2248 | 11.31 | 0.68 | 2919 | 13.23 | 0.66 | −1.92 | (−3.72,−0.12) | 0.038 | |
| Definite CAD | Men | 971 | 4.92 | 0.57 | 1101 | 4.69 | 0.54 | 0.23 | (−1.62,2.08) | 0.806 |
| Women | 1277 | 2.24 | 0.29 | 1818 | 2.84 | 0.33 | −0.60 | (−1.72,0.51) | 0.299 | |
| All | 2248 | 3.38 | 0.29 | 2919 | 3.57 | 0.28 | −0.18 | (−1.19,0.82) | 0.722 | |
CAD coronary artery disease, P prevalence, SE standard error
Table 4 shows the age-adjusted prevalence of various criteria for diagnosis of CAD. ST code, T code, and RAQ angina were significantly higher in women; all other criteria were higher in men (p < 0.01). Table 5 represents the age-adjusted prevalence of various criteria for diagnosis of CAD among participants with any CAD. Again, most of the definite criteria for CAD were higher in men. Except ST code, T code, and RAQ angina, there was higher prevalence of various coronary artery criteria among men as compared to women (p < 0.001); ST code was higher in women, while T code and RAQ angina showed no significant difference. Among participants diagnosed to have any CAD, the prevalence of known CAD was 7.8 and 2.7 % in men and women respectively. Symptomatic CAD (sum of those with known CAD and RAQ angina) was prevalent in 54 % with any CAD.
Table 4.
Age-adjusted prevalence of various CAD parameters among the total participant population
| Total | Men | Women | Difference (%) | 95 % CI | p value | ||||
|---|---|---|---|---|---|---|---|---|---|
| n = 5167 | n = 2072 | n = 3095 | |||||||
| P (%) | SE | P (%) | SE | P (%) | SE | ||||
| Q codes | 0.87 | 0.11 | 1.65 | 0.24 | 0.32 | 0.08 | 1.33 | (0.74,1.91) | <0.001 |
| ST Code | 1.87 | 0.19 | 1.00 | 0.18 | 2.46 | 0.29 | −1.47 | (−2.16,−0.77) | <0.001 |
| T code | 5.46 | 0.35 | 3.86 | 0.46 | 6.44 | 0.49 | −2.59 | (−3.79,−1.39) | <0.001 |
| Documented ACS | 1.59 | 0.14 | 2.70 | 0.30 | 0.84 | 0.13 | 1.86 | (1.09,2.63) | <0.001 |
| Documented treatment for CAD | 2.26 | 0.16 | 3.55 | 0.34 | 1.39 | 0.16 | 2.17 | (1.27,3.07) | <0.001 |
| Angioplasty/CABG or Fibrinolysis | 0.44 | 0.07 | 0.96 | 0.17 | 0.05 | 0.03 | 0.91 | (0.48,1.34) | <0.001 |
| Angiographic Coronary stenosis | 0.63 | 0.09 | 1.15 | 0.19 | 0.27 | 0.07 | 0.88 | (0.39,1.37) | <0.001 |
| Imaging evidence of RWMA | 0.06 | 0.09 | 1.34 | 0.20 | 0.10 | 0.05 | 1.24 | (0.73,1.74) | <0.001 |
| RAQ angina | 6.38 | 0.35 | 4.71 | 0.44 | 7.47 | 0.51 | −2.76 | (−4.06,−1.46) | <0.001 |
| Positive treadmill ECG | 0.54 | 0.08 | 0.98 | 0.17 | 0.22 | 0.07 | 0.76 | (0.30,1.21) | <0.001 |
Table 5.
Age-adjusted prevalence of various CAD parameters among any CAD
| Total | Men | Women | Difference (%) | 95 % CI | p value | ||||
|---|---|---|---|---|---|---|---|---|---|
| n = 855 | n = 302 | n = 553 | |||||||
| P (%) | SE | P (%) | SE | P (%) | SE | ||||
| Q codes | 4.88 | 0.79 | 12.22 | 2.48 | 1.49 | 0.40 | 10.74 | (6.91,14.57) | <0.001 |
| ST Code | 12.94 | 2.16 | 7.08 | 1.51 | 15.14 | 2.74 | −8.07 | (−12.23,−3.91) | <0.001 |
| T code | 43.06 | 3.44 | 45.84 | 6.35 | 42.61 | 3.92 | 3.23 | (−3.74,10.20) | 0.3625 |
| Documented ACS | 8.68 | 0.98 | 20.36 | 2.85 | 3.80 | 0.71 | 16.56 | (11.75,21.37) | <0.001 |
| Documented treatment for CAD | 11.88 | 1.05 | 26.56 | 2.95 | 5.98 | 0.79 | 20.59 | (15.23,25.94) | <0.001 |
| Angioplasty/CABG or Fibrinolysis | 2.18 | 0.41 | 6.63 | 1.44 | 0.19 | 0.11 | 6.43 | (3.60,9.26) | <0.001 |
| Angiographic Coronary stenosis | 3.21 | 0.55 | 8.07 | 1.81 | 1.10 | 0.31 | 6.98 | (3.78,10.17) | <0.001 |
| Imaging evidence of RWMA | 3.03 | 0.46 | 8.75 | 1.56 | 0.40 | 0.19 | 8.34 | (5.11,11.57) | <0.001 |
| RAQ angina | 49.69 | 3.44 | 43.98 | 6.43 | 51.60 | 3.96 | −7.62 | (−14.60,−0.64) | 0.033 |
| Positive treadmill ECG | 2.74 | 0.52 | 6.26 | 1.33 | 1.12 | 0.51 | 5.14 | (2.27,8.01) | <0.001 |
Q code-any of the Minnesota Q code 1-1-1 to 1-1-7 or 1-2-1 to 1-2-5 or 1-2-7; ST code-any of the Minnesota ST code 4-1-1,4-1-2 or 4–2; T code -any of the Minnesota T code 5-1 or 5-2; ACS acute coronary syndrome, CABG coronary artery bypass graft surgery, RWMA regional wall motion abnormality, ECG electrocardiogram, P prevalence, SE standard error
Coronary risk factors
Age-adjusted prevalence of major risk factors of CAD by gender and area is presented in Table 6. Conventional risk factors like diabetes mellitus, hypertension, high serum cholesterol, low serum HDL cholesterol, smoking, physical inactivity, and family history of CAD were highly prevalent in the state. Except low HDL cholesterol (23.9 % in urban men vs. 27.6 % in rural men), all other major risk factors were higher in urban men as compared to rural men. This pattern was also observed in urban women as compared to rural women except high serum cholesterol (49.2 vs. 52.4 %; p = 0.089) and low HDL cholesterol (43.4 vs. 48.7 %; p =0.004).
Table 6.
Age adjusted prevalence of major risk factors of CAD by gender and area
| Risk factor | All | Men | Women | p value** | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urban | Rural | p value* | Urban | Rural | p value* | |||||||||||||
| n | % | SE | n | % | SE | n | % | SE | n | % | SE | n | % | SE | ||||
| Diabetes | 5135 | 15.23 | 0.50 | 962 | 19.14 | 1.40 | 1097 | 16.23 | 1.16 | 0.0831 | 1272 | 15.40 | 0.95 | 1804 | 12.47 | 0.73 | 0.020 | <0.001 |
| Hypertension | 5153 | 28.44 | 0.63 | 967 | 39.99 | 2.04 | 1095 | 26.24 | 1.33 | <0.001 | 1275 | 30.15 | 1.29 | 1816 | 23.42 | 0.85 | <0.001 | <0.001 |
| High cholesterol | 5121 | 52.31 | 0.88 | 960 | 56.54 | 2.22 | 1092 | 53.36 | 1.85 | 0.1487 | 1264 | 49.23 | 1.76 | 1805 | 52.35 | 1.35 | 0.089 | 0.023 |
| Low HDL | 5120 | 38.55 | 0.88 | 959 | 23.92 | 1.94 | 1092 | 27.56 | 1.69 | 0.0603 | 1264 | 43.41 | 2.02 | 1805 | 48.73 | 1.44 | 0.004 | <0.001 |
| Smoking | 2072 | 28.05 | 1.19 | 971 | 30.45 | 2.00 | 1101 | 26.88 | 1.48 | 0.0721 | - | - | - | - | - | - | - | - |
| Physical inactivity | 5167 | 17.45 | 0.64 | 971 | 30.72 | 2.06 | 1101 | 28.77 | 1.64 | 0.3315 | 1277 | 11.19 | 1.19 | 1818 | 9.38 | 0.64 | 0.101 | <0.001 |
| Family history | 5167 | 18.35 | 0.64 | 971 | 18.97 | 1.47 | 1101 | 17.37 | 1.39 | 0.3467 | 1277 | 20.37 | 1.39 | 1818 | 16.92 | 1.02 | 0.015 | 0.939 |
P prevalence, SE standard error, *p value between urban and rural; **p value between men and women
Risk factor analysis of any and definite CAD is presented in Table 7. The age adjusted prevalence of risk factors such as hypertension, low HDL and family history were significantly higher in any CAD group (p value <0.01). However, higher cholesterol and smoking were higher in no CAD group. In definite CAD, all risk factors except low HDL were statistically significant (p value <0.01). In addition, excluding higher cholesterol all age adjusted prevalence of these conventional risk factors was higher in definite CAD as compared to no CAD.
Table 7.
Risk factors analysis of any and definite CAD
| Risk factor | Any CAD | Definite CAD | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Difference (%) | 95 % CI | p value | Yes | No | Difference (%) | 95 % CI | p value | |||||||||
| n | % | SE | n | % | SE | n | % | SE | n | % | SE | |||||||
| Diabetes | 851 | 17.07 | 1.97 | 4284 | 14.89 | 0.52 | 2.18 | (−0.56,4.93) | 0.106 | 296 | 33.30 | 5.24 | 4839 | 14.73 | 0.50 | 18.57 | (13.11,24.03) | <0.001 |
| Hypertension | 852 | 32.19 | 2.04 | 4301 | 27.67 | 0.67 | 4.51 | (1.10,7.92) | 0.008 | 295 | 51.32 | 5.34 | 4858 | 28.04 | 0.64 | 23.29 | (17.44,29.13) | <0.001 |
| High cholesterol | 849 | 47.63 | 3.24 | 4272 | 52.82 | 0.92 | −5.19 | (−8.86,−1.51) | 0.006 | 293 | 41.01 | 5.68 | 4828 | 53.01 | 0.88 | −12.00 | (−17.81,−6.20) | <0.001 |
| Low HDL | 849 | 43.62 | 3.53 | 4271 | 37.59 | 0.92 | 6.04 | (2.40,9.68) | 0.001 | 293 | 42.76 | 5.90 | 4827 | 38.33 | 0.89 | 4.44 | (−1.39,10.26) | 0.13 |
| Smoking | 855 | 6.91 | 1.02 | 4312 | 11.80 | 0.56 | −4.89 | (−6.85,−2.94) | <0.001 | 297 | 15.69 | 4.42 | 4870 | 11.22 | 0.53 | 4.46 | (0.23,8.69) | 0.019 |
| Physical inactivity | 855 | 15.55 | 2.17 | 4312 | 17.49 | 0.68 | −1.94 | (−4.62,0.74) | 0.170 | 297 | 31.02 | 5.32 | 4870 | 17.13 | 0.65 | 13.89 | (8.52,19.25) | <0.001 |
| Family history | 855 | 27.06 | 3.09 | 4312 | 17.37 | 0.66 | 9.70 | (6.51,12.88) | <0.001 | 297 | 25.29 | 4.06 | 4870 | 18.03 | 0.65 | 7.27 | (2.21,12.33) | 0.002 |
Discussion
Prevalence of coronary artery disease
Coronary artery disease has been gaining importance as a major public health problem in India. In 2003, the prevalence of CAD was estimated to be 3–4 % in rural areas and 8–10 % in urban areas according to a population-based cross sectional survey [23]. Our study also showed high prevalence of CAD in Kerala. The age-adjusted overall prevalence of definite CAD was 3.5 % and any CAD was 12.5 %. We chose criteria for any CAD to detect all possible cases of CAD; for definite CAD we have avoided less specific criteria like RAQ angina, positive treadmill electrocardiogram or minor electrocardiographic changes in isolation and used combined criteria when these less specific components were incorporated.
Age-adjusted prevalence of definite CAD was higher in men. However, any CAD prevalence in women was significantly higher than in men. It is well known that women commonly have ECG with nonspecific ST –T changes and angina with normal coronary arteries; this may have resulted in overestimation of any CAD prevalence in women. In our study the prevalence of RAQ angina and ST-T changes were significantly higher among women whose proportion in rural area (62 %) was higher than that of urban area (57 %). Earlier population studies also consistently recorded much higher prevalence of CAD in women using RAQ angina and ST-T criteria for diagnosis of CAD [24–27]. It has also been noted that RAQ angina is less reliable in women [28] and non-white population [29]. Moreover, Patel et al. in a study from migrant South Asians observed that the value of ST-T changes alone as indicator of CAD was questionable, especially in women [30].
This study showed no difference in the prevalence of definite CAD between urban and rural population of Kerala while any CAD was slightly more in the rural population. Previous studies from other parts of India have highlighted the marked urban preponderance of CAD [25, 31, 32]. Some of the risk factors such as general obesity and abdominal obesity were higher in urban areas, whereas smoking was higher in rural area. There was no difference in physical inactivity in rural and urban areas. In most other Indian states there are huge differences between urban and rural areas. However, in Kerala these differences are either minimal or some risk factors are higher in urban while others are higher in rural as noted above. This could be the reason for not having significant difference of definite CAD prevalence in urban and rural areas of Kerala.
The overall age-adjusted prevalence of any CAD in subjects at or below the age of 45 years was 7.8 %. Our study showed a higher prevalence of CAD among young subjects compared to a previous study [7]; in this 1993 study, there were no subjects with definite CAD below the age of 45 years where as we found definite CAD in 0.9 %. It is well known that coronary artery disease occurs in Indians 5–10 years earlier [33, 34]. The average age of first myocardial infarction in Asians is 53 years; among the three Asian populations, Chinese, Malay, and Indian, the highest age-adjusted incidence of coronary events in both sexes was in Indians [6]. The age-adjusted prevalence of CAD in young subjects in our study was similar to the US data on prevalence of CAD in the young (1.2 %).
Comparisons with previous Indian studies on CAD prevalence may be problematic due to heterogeneity in period of study, sample characteristics, and criteria for diagnosis of CAD. We did a systematic search for articles in PubMed and EMBASE published during the period from 1990 to 2014 in English language on the prevalence of CAD in India by community-based surveys. We chose only studies that had a minimum sample size of 1000, and that based the diagnosis on documented history of CAD and ECG criteria. We could collect 11original articles, one systematic review [4] and one meta-analysis [35]. The studies were heterogeneous in terms of sampling methods, subject groups, and criteria for diagnosis. Most of the studies were from northern India. Only one study mentioned the period of field survey [25]. Only one study analyzed the data for definite CAD and probable CAD separately [7]. Most studies were on either rural or urban population; only four covered both the groups [25, 31, 32, 36]. Sample size calculations were reported in three studies [7, 25, 31]. In a study from Delhi during the period from 1984 to 1987, Chadda et al. reported unadjusted prevalence of 9.7 % in urban and 2.7 % in rural inhabitants [25]. The sample consisted of men and women aged 24–64 years; they used clinical history of CAD and electrocardiographic criteria for diagnosis of CAD. Gupta et al. published an epidemiological survey of urban inhabitants over 20 years of age in Rajasthan, India [27]. The prevalence of CAD was 7.6 % (6 % in men and 10.4 % in women). The age distribution of the sample was consistent with that of the population. Singh et al. in 1997 surveyed an urban and rural sample of 3575 subjects between the ages 25 and 64 years from North India and reported CAD prevalence of 9 and 3.3 % respectively [31]. Mohan and colleagues reported 9 % age-adjusted prevalence of CAD in an urban sample of Chennai, South India [10]. More recently, another study from India noted 12.6 % crude CAD prevalence in an urban population [37]. All these studies had used less specific criteria for diagnosis of CAD; definite CAD as we had analyzed had not been computed in these studies. A study from Pakistan showed a crude prevalence of definite CAD in 6.1 men and 4 % women; they found ischemic ECG changes much more prevalent in women [38].
The US statistics reported 6 % age-adjusted CAD prevalence in respondents above 18 years by survey based on self-reported CAD by telephonic interview [39]. The prevalence between ages 18–44 years was 1.2 %. The update on heart disease and stroke in the US reported CAD prevalence of 6.4%in adults ≥ 20 years of age. The prevalence of CAD was 7.9 for men and 5.1 % for women [40]. The higher figure in the US data may be due to differences in age, sample, survey methods, and criteria for diagnosis. The reported prevalence of CAD in the United Kingdom was 3.5 % [41]; this figure was similar to our data on definite CAD.
As to the figures for definite CAD, only one study [7] reported the prevalence (1.4 %); they found no participant below the age of 45 years with definite CAD. However, it should be noted that the age range in this study was 25–64 years and the population was rural only. Compared to this, our data show an increase in the prevalence of definite CAD (0.9 %). Any CAD prevalence of 11.3 % in urban and 13.2 % in rural area respectively in our study was higher compared to some previous studies in the same age group and similar definition for CAD (8.1–9 % for urban and 3.5–5 % for rural) [10, 27, 42, 43]. These data indicate an increase in the prevalence of any CAD over the past two decades. The increase in prevalence of any CAD may partially be due to improvement of treatment of CAD thus allowing better survival.
The urban prevalence data in this study may be comparable to other cities of India; however, the rural figures for prevalence may not be comparable to most rural areas in rest of India since rural Kerala is different from rest of rural India in social and economic development.
Prevalence of coronary risk factors
We found high prevalence of major conventional risk factors for CAD in our survey. The prevalence of various risk factors in our study like diabetes (15.2 %), hypertension (28.4 %), high cholesterol (52.3 %) and smoking in men (28.1 %) was comparable to the figures in a recent large study from Kerala, by Thankappan et al. [5]. The figures were also similar in the US adult population [44]. Physical activity in our study seems to be an overestimate due to the problems of self reports as has been reported by a recent study from Kerala [45].
Limitations of the study
Our study was the largest and most comprehensive contemporary community-based survey on the prevalence of CAD and its risk factors from India. The geographic spread of the survey enabled us to derive meaningful inferences of prevalence from the state. We could capture all the necessary data from participants with very little missing variables. One of the limitations for our study was the sub-optimal response rate, which could reach only ~ 80 %; the response rate was lower in rural men. We could not capture the reasons for the poor response in men although we surmise that, being manual laborers many of them would be working on Sundays. As a result the sample consisted of higher proportion of women than in the general population. Sex disaggregated data will partly take care of this problem. There was significantly higher proportion of older individuals compared to the population of Kerala although age-adjustment in analysis would have removed this bias.
Conclusion
Our study revealed a high age-adjusted prevalence of CAD and coronary risk factors in Kerala, South India. Our study did not show any difference in the CAD prevalence in urban and rural population. Our data on prevalence of CAD are comparable to the figures from the US and the United Kingdom. Compared to previous Indian studies and the 1993 study of Kerala, this study showed higher prevalence of CAD in Kerala. The prevalence of definite CAD in Kerala increased nearly three times since 1993 without any difference in urban and rural areas. Most risk factors of CAD were highly prevalent in the state. Both population and individual level approaches are warranted to address the high level of CAD risk factors to reduce the increasing prevalence of CAD in this population.
Acknowledgements
We thankfully acknowledge the Cardiological Society of India Kerala Chapter for the generous and exclusive funding of the study. We also appreciate the important intellectual input provided by Dr. Mark Huffman.
Dedication
For the Cardiological Society of India Kerala Chapter Coronary Artery Disease and Risk Factors Prevalence (CSI Kerala-CRP) Study Investigators.
Funding
The study was funded exclusively by the Cardiological Society of India, Kerala Chapter; this is the professional organization of cardiologists in this region. The funding source had no role in the design, data collection, analysis or interpretation or writing up of the article.
Abbreviations
- ACS
acute coronary syndrome
- CHOD-PAP
cholesterol oxidase phenol 4-aminoantipyrine peroxidase
- CABG
coronary artery bypass grafting
- CAD
coronary artery disease
- ECG
electrocardiogram
- GOD-PAP
glucose oxidase/ peroxidase-phenol-4-amenophenazone method
- HDL
high density lipoprotein
- LDL)
low-density lipoprotein
- RAQ
rose angina questionnaire
Footnotes
Competing interests
There has been no financial relationship with any company or any other funding source. The authors declare that they have no competing interest.
Authors’ contributions
MNK, GZ, KV, PPM and SH conceptualized and designed the study. MNK lead the study in northern region, GZ in Central region, and SH in southern region. LJ did the statistical analysis. All authors contributed to the writing of the manuscript, read the final version and approved it for submission.
Contributor Information
M. N. Krishnan, Email: dr.mn.krishnan@gmail.com
G. Zachariah, Email: geevarzachariah@gmail.com
K. Venugopal, Email: venugopalknair@gmail.com
P. P. Mohanan, Email: drmohanan@gmail.com
S. Harikrishnan, Email: drharikrishnan@hotmail.com
G. Sanjay, Email: drsanjayganesh@gmail.com
L. Jeyaseelan, Email: ljey@cmcvellore.ac.in
K. R. Thankappan, Phone: 91-471-2524231, Email: kavumpurathu@yahoo.com, Email: kr.thankappan@gmail.com
References
- 1.Gupta R, Guptha S, Sharma KK, Gupta A, Deedwania P. Regional variations in cardiovascular risk factors in India: India Heart Watch. World J Cardiol. 2012;4:112–20. doi: 10.4330/wjc.v4.i4.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta R, Joshi P, Mohan V, Reddy KS, Yusuf S. Epidemiology and causation of coronary heart disease and stroke in India. Heart. 2008;94:16–26. doi: 10.1136/hrt.2007.132951. [DOI] [PubMed] [Google Scholar]
- 3.Enas EA, Garg A, Davidson MA, Nair VM, Huet BA, Yusuf S. Coronary heart disease and its risk factors in first-generation immigrant Asian Indians to the United States of America. Indian Heart J. 1996;48:343–53. [PubMed] [Google Scholar]
- 4.Ahmed N, Bhopal R. Is coronary heart disease rising in India? A systematic review based on ECG defined coronary heart disease. Heart. 2005;91:719–25. doi: 10.1136/hrt.2003.031047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thankappan KR, Shah B, Mathur P, Sarma PS, Srinivas G, Mini GK, et al. Risk factor profile for chronic non-communicable diseases: results of a community-based study in Kerala, India. Indian J Med Res. 2010;131:53–63. [PubMed] [Google Scholar]
- 6.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 7.Kutty VR, Balakrishnan KG, Jayasree AK, Thomas J. Prevalence of coronary heart disease in the rural population of Thiruvananthapuram district, Kerala, India. Int J Cardiol. 1993;39:59–70. doi: 10.1016/0167-5273(93)90297-T. [DOI] [PubMed] [Google Scholar]
- 8.Sivasankaran S, Thankappan KR. Prevention of non-communicable diseases requires a life course approach: a case study from Kerala. Indian J Med Res. 2013;137:874–7. [PMC free article] [PubMed] [Google Scholar]
- 9.Zachariah G, Harikrishnan S, Krishnan MN, Mohanan PP, Sanjay G, Venugopal K, et al. Prevalence of coronary artery disease and coronary risk factors in Kerala, South India: a population survey-design and methods. Indian Heart J. 2013;65:243–9. doi: 10.1016/j.ihj.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohan V, Deepa R, Rani SS, Premalatha G. Prevalence of coronary artery disease and its relationship to lipids in a selected population in South India: The Chennai Urban Population Study (CUPS No. 5) J Am Coll Cardiol. 2001;38:682–7. doi: 10.1016/S0735-1097(01)01415-2. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . WHO STEPS Manual. 2008. pp. 2-2-24–25. [Google Scholar]
- 12.Rose GA. The diagnosis of ischemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962;27:645–58. [PMC free article] [PubMed] [Google Scholar]
- 13.Rose GA, Blackburn H. Cardiovascular survey methods. Monogr Ser World Health Organ. 1968;56:1–188. [PubMed] [Google Scholar]
- 14.World Health Organization . WHO STEPS Part 3. 2008. pp. 3-3-1–3-3-14. [Google Scholar]
- 15.Ronald JP, Richards SC, Zhu-Ming Z. The Minnesota Code manual of electrocardiographic findings. Second edition Springer. 2010.
- 16.Mendis S, Thygesen K, Kuulasmaa K, Giampaoli S, Mähönen M, Ngu Blackett K, et al. World Health Organization definition of myocardial infarction:2008–09 revision. Int J Epidemiol. 2011;40:139–46. doi: 10.1093/ije/dyq165. [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(1):S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 Report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 19.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 20.Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D, et al. Consensus Statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–70. [PubMed] [Google Scholar]
- 21.World Health Organization . Global recommendations on physical activity for health. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 22.International Institute of Population Sciences (IIPS) ORC Macro . National family health survey (NFHS −2), India, 1998–99. Mumbai: International Institute of Population Sciences; 2000. [Google Scholar]
- 23.Gupta R. Burden of coronary heart disease in India. Indian Heart J. 2005;57:632–8. [PubMed] [Google Scholar]
- 24.Sarvotham SG, Berry JN. Prevalence of coronary heart disease in an urban population in northern India. Circulation. 1968;37:939–53. doi: 10.1161/01.CIR.37.6.939. [DOI] [PubMed] [Google Scholar]
- 25.Chadha SL, Radhakrishnan S, Ramachandran K, Kaul U, Gopinath N. Epidemiological study of coronary heart disease in urban population of Delhi. Indian J Med Res. 1990;92:424–30. [PubMed] [Google Scholar]
- 26.Gupta R, Prakash H, Majumdar S, Sharma SC, Gupta VP. Prevalence of coronary heart disease and coronary risk factors in an urban population of Rajasthan. Indian Heart J. 1995;47:331–8. [PubMed] [Google Scholar]
- 27.Gupta R, Gupta VP, Sarna M, Bhatnagar S, Thanvi J, Sharma V, et al. Prevalence of coronary heart disease and risk factors in an urban Indian population: Jaipur Heart Watch-2. Indian Heart J. 2002;54:59–66. [PubMed] [Google Scholar]
- 28.Harris RB, Weissfeld LA. Gender differences in the reliability of reporting symptoms of angina pectoris. J Clin Epidemiol. 1991;44:1071–8. doi: 10.1016/0895-4356(91)90009-X. [DOI] [PubMed] [Google Scholar]
- 29.Fischbacher CM, Bhopal R, Unwin N, White M, Alberti KG. The performance of the Rose angina questionnaire in South Asian and European origin populations: a comparative study in Newcastle. UK Int J Epidemiol. 2001;30:1009–16. doi: 10.1093/ije/30.5.1009. [DOI] [PubMed] [Google Scholar]
- 30.Patel DJ, Winterbotham M, Sutherland SE, Britt RG, Keil JE, Sutton GC. Comparison of methods to assess coronary heart disease prevalence in South Asians. Natl Med J India. 1997;10:210–3. [PubMed] [Google Scholar]
- 31.Singh RB, Sharma JP, Rastogi V, Raghuvanshi RS, Moshiri M, Verma SP, et al. Prevalence of coronary artery disease and coronary risk factors in rural and urban populations of north India. Eur Heart J. 1997;18:1728–35. doi: 10.1093/oxfordjournals.eurheartj.a015167. [DOI] [PubMed] [Google Scholar]
- 32.Kumar R, Singh MC, Singh MC, Ahlawat SK, Thakur JS, Srivastava A, et al. Urbanization and coronary heart disease: a study of urban–rural differences in northern India. Indian Heart J. 2006;58:126–30. [PubMed] [Google Scholar]
- 33.Hughes LO, Raval U, Raftery EB. First myocardial infarctions in Asian and white men. BMJ. 1989;298:1345–50. doi: 10.1136/bmj.298.6684.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Enas EA, Dhawan J, Petkar S. Coronary artery disease in Asian Indians: lessons learnt and the role of lipoprotein(a) Indian Heart J. 1997;49:25–34. [PubMed] [Google Scholar]
- 35.Gupta R, Gupta VP. Meta-analysis of coronary heart disease prevalence in India. Indian Heart J. 1996;48:241–5. [PubMed] [Google Scholar]
- 36.Kamili M, Dar I, Ali G, Wazir H, Hussain S. Prevalence of coronary heart disease in Kashmiris. Indian Heart J. 2007;59:44–9. [PubMed] [Google Scholar]
- 37.Latheef SA, Subramanyam G. Prevalence of coronary artery disease and coronary risk factors in an urban population of Tirupati. Indian Heart J. 2007;59:157–64. [PubMed] [Google Scholar]
- 38.Jafar TH, Qadri Z, Chaturvedi N. Coronary artery disease epidemic in Pakistan: more electrocardiographic evidence of ischemia in women than in men. Heart. 2008;94:408–13. doi: 10.1136/hrt.2007.120774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention (CDC) Prevalence of coronary heart disease --United States, 2006–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1377–81. [PubMed] [Google Scholar]
- 40.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 41.Townsend N, Wickramasinghe K, Bhatnagar P. Coronary heart disease statistics. London: British Heart Foundation; 2012. p. 82. [Google Scholar]
- 42.Gupta R, Gupta HP, Keswani P, Sharma S, Gupta VP, Gupta KD. Coronary heart disease and coronary risk factors in rural Rajasthan. J Assoc Physicians India. 1994;42:24–6. [PubMed] [Google Scholar]
- 43.Gupta AK, Bharadwaj A, Ashotra S, Gupta BP. Feasibility and training of multipurpose workers in detection, prevention and control of coronary artery disease in apple-belt of Shimla hills. South Asian J Prev Cardiol. 2002;6:17–22. [Google Scholar]
- 44.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics-2014 update: a report from the American heart association. Circulation. 2014;129:e28–292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathews E, Pratt M, Jissa VT, Thankappan KR. Self-reported physical activity and its correlates among adult women in the expanded part of Thiruvananthapuram City, India. Indian J Public Health. 2015;59:136–40. doi: 10.4103/0019-557X.157535. [DOI] [PMC free article] [PubMed] [Google Scholar]


