Abstract
Purpose
Various gastrointestinal factors may contribute to maladaptive behavior in children with autism spectrum disorders (ASD). To determine the association between maladaptive behavior in children with ASD and gastrointestinal symptoms such as severity, intestinal microbiota, inflammation, enterocyte damage, permeability and absorption of opioid peptides.
Methods
This observational cross-sectional study compared children with ASD to healthy controls, aged 2-10 years. Maladaptive behavior was classified using the Approach Withdrawal Problems Composite subtest of the Pervasive Developmental Disorder Behavior Inventory. Dependent variables were gastrointestinal symptom severity index, fecal calprotectin, urinary D-lactate, urinary lactulose/mannitol excretion, urinary intestinal fatty acids binding protein (I-FABP) and urinary opioid peptide excretion.
Results
We did not find a significant difference between children with ASD with severe or mild maladaptive behavior and control subjects for gastrointestinal symptoms, fecal calprotectin, urinary D-lactate, and lactulose/mannitol ratio. Urinary opioid peptide excretion was absent in all children. Children with ASD with severe maladaptive behavior showed significantly higher urinary I-FABP levels compared to those with mild maladaptive behavior (p=0.019) and controls (p=0.015).
Conclusion
In our series, maladaptive behavior in ASD children was not associated with gastrointestinal symptoms, intestinal inflammation (no difference in calprotectin), microbiota (no difference in urinary D-lactate) and intestinal permeability (no difference in lactulose/manitol ratio). ASD children with severe maladaptive behavior have significantly more enterocyte damage (increased urinary I-FABP) than ASD children with mild maladaptive behavior and normal children.
Keywords: Autism spectrum disorder, Intestinal inflammation, Intestinal permeability, Enterocyte damage, Urinary opioids
INTRODUCTION
Autism spectrum disorders (ASD) comprise autism, pervasive developmental disorders not otherwise specified (PDD-NOS) and Asperger syndrome [1]. The global prevalence of autism has risen over the years. In 2012, its prevalence was estimated at 11.3 per 1,000 children [2]. The increased prevalence suggests that genetic and evironmental factors play a role [3,4,5]. Children with ASD also present with accompanying maladaptive behavior, further impairing the ability to learn and socialize [6].
Several theories have been postulated to explain the cause of maladaptive behavior in children with ASD, such as the behavioral theory, the sensory integration theory, the neurochemical theory and the biological theory. According to the biological theory, gastrointestinal (GI) symptoms may contribute to the maladaptive behavior [7]. However, other studies have not found an association between GI symptoms and maladaptive behavior in children with ASD [8,9]. According to the neurochemical theory, impaired intestinal permeability causes absorption of larger peptides t of dietary gluten and casein than normal. These peptides have a morphine-like effect on the brain, and are therefore called opioid peptides, and are hypothesized to cause maladaptive symptoms [10]. There is still much controversy surrounding both theories.
A link between ASD and GI disturbances has been reported in several papers, with a prevalence ranging from 23% to 70% [11,12]. These large variations in prevalence may be due to differences in criteria used to define GI symptoms, study design and characteristics of the study population. GI symptoms in children with ASD are not specific and may include food selectivity, regurgitation, constipation, chronic diarrhea and abdominal bloating which are hypothetically associated with an abnormal intestinal microbiota, intestinal inflammation and colitis [11,12]. Increased GI permeability has been demonstrated in children with ASD [13]. Exposure to viral or bacterial pathogens may trigger the disorder in some patients [14].
The prevalence of GI symptoms in children with ASD in Indonesia is not known. Therefore, we aimed in this study to determine if there is an association between the degree of maladaptive behavior in children with ASD and the severity of GI symptoms, intestinal bacteria and bacterial products, intestinal inflammation, intestinal permeability, enterocyte damage and urinary opioid peptide excretion. The understanding of the association of maladaptive behavior and severity of GI problems may provide new insights for an appropriate treatment of children with ASD.
MATERIALS AND METHODS
Study design and subject recruitment
We conducted a cross-sectional observational study in children with ASD aged 2 to 10 years and age-matched normal controls. Subjects with ASD were consecutively recruited from the Anakku Clinic, Jakarta, and the Rumah Autis in Bekasi, Tangerang and Jakarta. Control subjects were found through a call among staff members and their family of the Anakku Clinic and Prodia Laboratories, located in the same building. Subjects were matched for age and socio-economic background. The study was conducted between July 2012 and February 2013.
Inclusion criteria for the children with ASD were children aged 2 to 10 years who had been diagnosed with autism, Asperger syndrome or PDD-NOS according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition text revision (DSM IV TR) criteria [15]. All patients had an abnormal or impaired development of social interaction and communication and a markedly restricted, repetitive, and monotonous (stereotypical) repertoire of activities and interests [1,15]. Subjects with ASD were further classified into ASD with severe maladaptive behavior ("severe maladaptive ASD") and ASD with mild maladaptive behavior ("mild maladaptive ASD") using the Approach Withdrawal Problem Composite-subtest of the Pervasive Developmental Disorder Behavior Inventory (Western Psychological Services, Torrance, CA, USA) [16]. Maladaptive behavior was classified as sensory perception impairment, ritualism and resistance to change, social pragmatic impairment, semantic pragmatic impairment, impairment in regulation of awareness to surroundings, specific phobias and aggressiveness [16].
The initial diagnosis of ASD and degree of maladaptive behavior was confirmed by the principal investigator and/or two child psychologists who have been working closely with the investigator at the Anakku Clinic.
The normal controls were physically and developmentally normal children aged 2 to 10 years, for whom a written parental informed consent had been obtained. Children were excluded if they had organic disorders.
The nutritional status was assessed based on the curve from the Center for Disease Control and Prevention and based on the percentage of the actual body weight (BW) in relation to to the ideal BW for body height (BH) as recommended by Indonesian Pediatric Society and the Waterlow classification [17,18,19].
Obesity=BW ≥120% of ideal BW for BH
Over-nourished=BW ≥110% and <120% of ideal BW for BH
Well-nourished=BW ≥90% and <110% of ideal BW for BH
Undernourished=BW ≥70% and <90% of ideal BW for BH
Malnourished=BW <70% of ideal BW for BH
The study protocol was approved by the Medical Research Ethics Committee of The University of Indonesia Medical School. For all participants, a written informed consent of one of the parents was obtained prior to data collection.
Gastrointestinal symptoms measurement
Parents were asked to complete a modified GI symptom severity index questionnaire. The cumulative scores from all items were recorded. All subjects were asked to provide fecal samples for measurement of fecal calprotectin using the calprotectin ELISA method (Immunodiagnostik AG, Bensheim, Germany). Urine samples were also collected to measure i) D-lactate to assess intestinal bacterial overgrowth (D-Lactate Colorimetric Assay Kit; AbcamBiochemicals, Cambridge, UK), ii) intestinal fatty acids binding protein (I-FABP) excretion to assess enterocyte damage (Human I-FABP ELISA Kit; Hycult Biotech, Uden, The Netherlands), iii) lactulose/mannitol excretion ratio to assess impaired intestinal permeability (Acetonitrile Liquid Chromatography Grade; Merck Inc., Whitehouse Station, NJ, USA), and iv) opioid peptides (ultra performance liquid chromatography-mass spectrometry [UPLC-MS], Xevo gen. 2; Waters, Milford, MA, USA).
Subjects were asked to fast for five hours prior to urine sampling, followed by the oral administration of a 2 mL/kg standard solution containing 5 g of lactulose, 2 g of mannitol and 40 g of sucrose per 100 mL (Novell Pharmaceutical Laboratories and Boehringer Ingelheim, Jakarta, Indonesia) to determine the lactulose/mannitol excretion ratio.
Statistical analysis
The minimum required sample size was calculated using the sample size formula for comparing the difference between means of two independent samples. Using a significance level of 0.05 and a power of 80%, we obtained a minimum sample size of 70 subjects in each group.
The objective data were correlated with the severity of the maladaptive behavior using one-way ANOVA for normally distributed data, and using the Kruskal-Wallis test for abnormally distributed data.
RESULTS
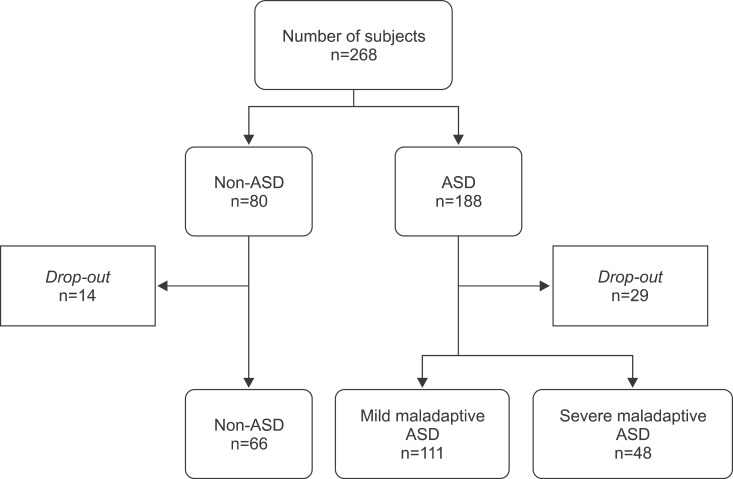
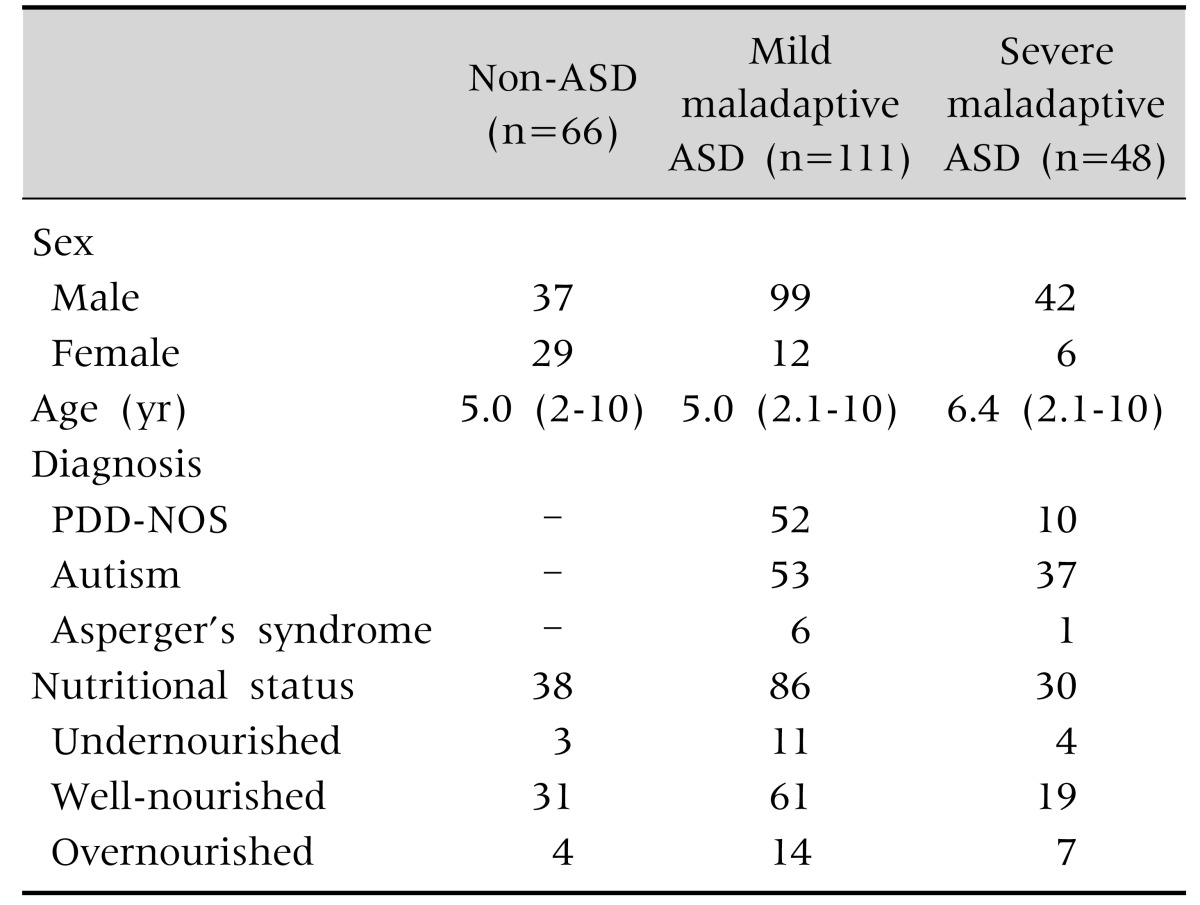
We recruited 268 subjects, of which 225 completed the study (Fig. 1). We obtained data from 66 healthy controls, 111 children with mild maladaptive ASD, and 48 children with severe maladaptive ASD. Subject characteristics at inclusion are shown in Table 1.
Fig. 1. Recruitment of study subjects. ASD: autism spectrum disorders.
Table 1. Subject Characteristics.
Values are presented as number only or median (range).
ASD: autism spectrum disorders, PDD-NOS: pervasive developmental disorders not otherwise specified.
Gastro-intestinal symptoms
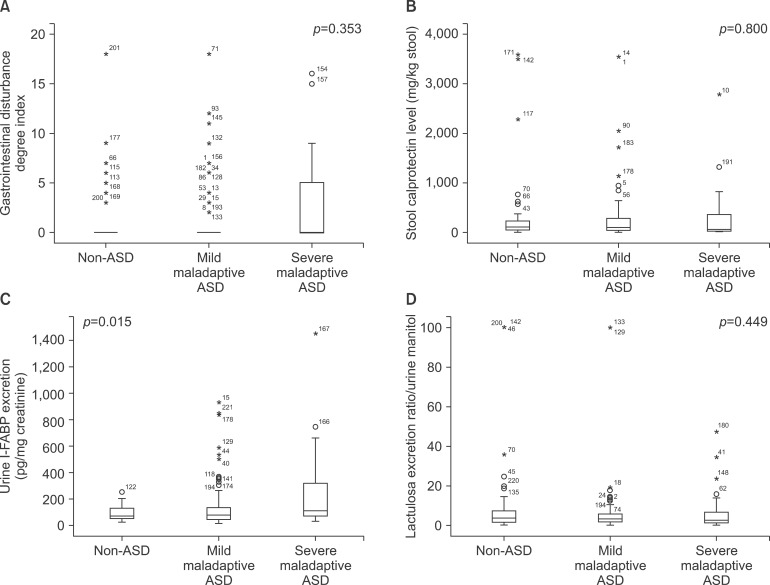
GI symptoms were reported in 14/66 (21.2%) children in the control group and in 36/159 (22.6%) in ASD children, of whom 22/111 (19.8%) had mild maladaptive ASD and 14/48 (29.1%) had severe maladaptive ASD (not significant). The median GI symptom severity index was 0 in all groups (p=0.35) (Fig. 2).
Fig. 2. Gastrointestinal disturbance, stool calprotectine level, urine intestinal fatty acids binding protein and lactulose excretion ratio in the autism spectrum disorders (ASD).
Inflammation
Fecal calprotectin was measured in 60 healthy controls, 102 children with mild maladaptive ASD, and 44 with severe maladaptive ASD. The median (range) fecal calprotectin excretion was 108.1 (5.3 to 3,595) in the control group, 100.54 (5.3 to 3,536) in the group with mild maladaptive ASD and 56.37 (5.3 to 2,778) µg/g feces in the group with severe maladaptive ASD (p=0.80).
Gastro-intestinal microbiota
Urinary D-lactate was measured in 66 controls, 109 subjects with mild maladaptive ASD, and 47 with severe maladaptive ASD. Median (range) urinary D-lactate was 100.11 (41.87 to 777.48) nmol/mg creatinine in the control group, 98.7 (34.86 to 1,441.14) nmol/mg creatinine in the mild maladaptive ASD group, and 89.26 (3.1 to 1,767.32) nmol/mg creatinine in the severe maladaptive ASD group (p=0.35).
Intestinal permeability
Urinary lactulose-mannitol excretion ratio was measured in 62 control children, 102 children with mild maladaptive ASD, and 45 children with severe maladaptive ASD. The median (range) urinary lactulose-mannitol excretion ratio was 3.63 (0 to 100) in the control, 3.05 (0 to 100) in the mild maladaptive ASD, and 2.53 (0 to 43.75) in the severe maladaptive ASD subjects (p=0.45).
Opioid peptide was absent in all urine samples.
Enterocyte damage
Urinary I-FABP was measured in 64 controls, 109 children with mild maladaptive ASD and 47 patients with severe maladaptive ASD. Median (range) urinary I-FABP was 69.63 (21.74 to 250.82) pg/mL in the controls, 76.02 (13.24 to 930.38) pg/mL in the mild maladaptive ASD and 109.08 (29.26 to 1,454.98) pg/mL in the severe maladaptive ASD group (p=0.01). The difference in urinary I-FABP was significant between the severe maladaptive ASD and the control groups (p=0.04) and between the severe and mild maladaptive ASD groups (p=0.019). There was no significant difference in urinary I-FABP between the mild maladaptive ASD and the control groups (p=0.94).
DISCUSSION
The goal of this study was to evaluate if an association could be found between maladaptive behavior in children with ASD children and GI symptoms, intestinal inflammation, intestinal bacterial products, increased intestinal permeability, and abnormal excretion of opioid peptides and enterocyte damage. We found that urinary I-FABP, a parameter evaluating enterocyte damage, was significantly increased in children with ASD and severe maladaptive behavior.
Urinary I-FABP was the only parameter that was significantly different between controls, children with mild maladaptive ASD and the group with severe maladaptive ASD. We found a significant difference between the severe maladaptive ASD and control groups (p=0.04) and between the severe and mild maladaptive ASD groups (p=0.02). The difference was not significant between the mild maladaptive ASD and the control groups (p=0.94). An increased I-FABP reflects the presence of enterocyte impairment or damage [20]. It is elevated in the early stages of enterocyte damage and normalizes as cell regeneration occurs [20,21,22]. A significantly higher urinary I-FABP without increased lactulose-mannitol excretion ratio suggests an enterocyte damage which is mild enough to preserve normal intestinal permeability.
We found that GI symptoms were present in about 20% of all children, without any difference between the healthy controls and the ASD children (p=0.35). In contrast to our study, Smith et al. [6] showed that children with ASD presented significantly more GI symptoms when compared to normal children, but reported a comparable prevalence in children with ASD children and children with other special-need. Another study did not find a significant difference in the incidence of constipation between children with and without ASD [8]. These results suggest that the presence of GI symptoms in children with ASD may be the result of a neurobehavioral artefact. At least three independent studies found the rs1858830 C variant in the MET gene promoter to be associated with autism [23]. The MET rs1858830 C allele was associated with both ASD and GI conditions. Disrupted MET signaling may contribute to increased risk for ASD that includes familial GI dysfunction [24].
Fecal calprotectin was not significantly different between controls, children with mild and severe maladaptive ASD (p=0.800). The median levels of calprotectin were similar in the three groups and in each group there were some children with clearly elevated levels. Age may be one of the contributory factors as infants and young children below 2 years of age have been reported with increased fecal calprotectin concentrations in the absence of any disease [25]. Also, major differences exist between specificity and absolute values between different tests [26]. Other factors which may explain the wide range of fecal calprotectin may be related to the handling of the samples and the fact that a number of these children may have chronic intestinal inflammation caused by different microorganisms. Anyway, these results suggest that maladaptive behavior in children with ASD is not associated with intestinal inflammation. Similar results have been reported in a study that failed to find elevated fecal calprotectin and rectal nitric oxide in children with autism [27,28]. However, other investigators have found endoscopic and laboratory evidence of intestinal inflammation in children with ASD [29].
The lack of a significant difference in urinary D-lactate between controls and patients with mild and severe maladaptive ASD subjects (p=0.35) suggests that there is no increase in bacteria or bacterial products in the GI tract of children with ASD. Similarly, another study did not find a difference in intestinal microflora between ASD children with and without GI symptoms and controls [30].
The urinary lactulose-mannitol excretion ratio was not significantly different between controls and subjects with mild and severe maladaptive ASD (p=0.45). These results suggest that maladaptive behavior in children with ASD is not associated with impaired intestinal permeability. Similar results have been reported previously in ASD children [13]. Acute diarrhea and malnutrition may impair intestinal permeability and act as confounders [31]. In our study, malnutrition was present in only a few patients.
No subject showed opioid peptide excretion in the urine. These results are in contrast with those reported by some other investigators [13,26]. However, these studies used the standard high-performance liquid chromatography technique what may be subject to false positive results as this method is unable to differentiate spectrometry peaks caused by opioids and other substances [10]. We used the more specific UPLC-MS method. Two other studies using the same method did not find opioid peptides in the urine of ASD children [31,32].
Gut and dietary factors are reported to possibly worsen or improve transiently behavioral symptoms in a subset of persons with ASD. Propionic acid, a major short chain fatty acid produced by ASD-associated GI bacteria (clostridia, Bacteroides, Desulfovibrio) and also a common food preservative, can produce reversible behavioral, electrographic, neuroinflammatory, metabolic, and epigenetic changes closely resembling those found in ASD when administered to rodents. More studies on the impact of the microbiota on behavioral changes are needed [33].
In conclusion, according to our results maladaptive behavior in ASD children seems not associated with GI symptoms, intestinal inflammation, microbiota and intestinal permeability. However, we found arguments for a more important enterocyte damage in ASD children with severe maladaptive behavior than in ASD children with mild adaptive behavior disturbace and normal children. There is no evidence of urinary opioid peptide excretion in ASD and non-ASD children. Further research is needed on the pathogenesis of enterocyte damage in ASD children.
References
- 1.Steyaert JG, De la Marche W. What's new in autism? Eur J Pediatr. 2008;167:1091–1101. doi: 10.1007/s00431-008-0764-4. [DOI] [PubMed] [Google Scholar]
- 2.Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators; Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders--Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61:1–19. [PubMed] [Google Scholar]
- 3.Stilp RL, Gernsbacher MA, Schweigert EK, Arneson CL, Goldsmith HH. Genetic variance for autism screening items in an unselected sample of toddler-age twins. J Am Acad Child Adolesc Psychiatry. 2010;49:267–276. doi: 10.1016/j.jaac.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect. 2006;114:1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Currenti SA. Understanding and determining the etiology of autism. Cell Mol Neurobiol. 2010;30:161–171. doi: 10.1007/s10571-009-9453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith RA, Farnworth H, Wright B, Allgar V. Are there more bowel symptoms in children with autism compared to normal children and children with other developmental and neurological disorders?: A case control study. Autism. 2009;13:343–355. doi: 10.1177/1362361309106418. [DOI] [PubMed] [Google Scholar]
- 7.Gorrindo P, Williams KC, Lee EB, Walker LS, McGrew SG, Levitt P. Gastrointestinal dysfunction in autism: parental report, clinical evaluation, and associated factors. Autism Res. 2012;5:101–108. doi: 10.1002/aur.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibrahim SH, Voigt RG, Katusic SK, Weaver AL, Barbaresi WJ. Incidence of gastrointestinal symptoms in children with autism: a population-based study. Pediatrics. 2009;124:680–686. doi: 10.1542/peds.2008-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mouridsen SE, Rich B, Isager T. A longitudinal study of gastrointestinal diseases in individuals diagnosed with infantile autism as children. Child Care Health Dev. 2010;36:437–443. doi: 10.1111/j.1365-2214.2009.01021.x. [DOI] [PubMed] [Google Scholar]
- 10.Reichelt KL, Knivsberg AM. Can the pathophysiology of autism be explained by the nature of the discovered urine peptides? Nutr Neurosci. 2003;6:19–28. doi: 10.1080/1028415021000042839. [DOI] [PubMed] [Google Scholar]
- 11.Chaidez V, Hansen RL, Hertz-Picciotto I. Gastrointestinal problems in children with autism, developmental delays or typical development. J Autism Dev Disord. 2014;44:1117–1127. doi: 10.1007/s10803-013-1973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatr. 2011;32:351–360. doi: 10.1097/DBP.0b013e31821bd06a. [DOI] [PubMed] [Google Scholar]
- 13.Kemperman RF, Muskiet FD, Boutier AI, Kema IP, Muskiet FA. Brief report: normal intestinal permeability at elevated platelet serotonin levels in a subgroup of children with pervasive developmental disorders in Curaçao (The Netherlands antilles) J Autism Dev Disord. 2008;38:401–406. doi: 10.1007/s10803-007-0399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Handen BL, Melmed RD, Hansen RL, Aman MG, Burnham DL, Bruss JB, et al. A double-blind, placebo-controlled trial of oral human immunoglobulin for gastrointestinal dysfunction in children with autistic disorder. J Autism Dev Disord. 2009;39:796–805. doi: 10.1007/s10803-008-0687-y. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. Diagnostic and statistical manual of mental disorders IV-TR. Washington DC: American Psychiatric Association; 2000. [Google Scholar]
- 16.Cohen IL, Sudhalter V. PDD behavior inventory. Professional manual. Florida: Psychological Assessment Resources, Inc.; 2005. [Google Scholar]
- 17.Centers for Disease Control and Prevention; National Center for Health Statistics. Clinical Growth Chart: United States [Internet] Atlanta (GA): Centers for Disease Control and Prevention; 2000. [cited 2012 June 1]. Available from: http://www.cdc.gov/growthcharts. [Google Scholar]
- 18.Sjarif DR, Lestari ED, Mexitalia M, Nasar SS. Textbook of pediatric nutrition and metabolic diseases. Jakarta: Indonesian Pediatric Society; 2011. pp. 36–48. [Google Scholar]
- 19.Waterlow JC. Classification and definition of protein-calorie malnutrition. Br Med J. 1972;3:566–569. doi: 10.1136/bmj.3.5826.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vreugdenhil AC, Wolters VM, Adriaanse MP, Van den Neucker AM, van Bijnen AA, Houwen R, et al. Additional value of serum I-FABP levels for evaluating celiac disease activity in children. Scand J Gastroenterol. 2011;46:1435–1441. doi: 10.3109/00365521.2011.627447. [DOI] [PubMed] [Google Scholar]
- 21.Grootjans J, Thuijls G, Verdam F, Derikx JP, Lenaerts K, Buurman WA. Non-invasive assessment of barrier integrity and function of the human gut. World J Gastrointest Surg. 2010;2:61–69. doi: 10.4240/wjgs.v2.i3.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dettmer K, Hanna D, Whetstone P, Hansen R, Hammock BD. Autism and urinary exogenous neuropeptides: development of an on-line SPE-HPLC-tandem mass spectrometry method to test the opioid excess theory. Anal Bioanal Chem. 2007;388:1643–1651. doi: 10.1007/s00216-007-1301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson PB, Boccuto L, Skinner C, Collins JS, Neri G, Gurrieri F, et al. Further evidence that the rs1858830 C variant in the promoter region of the MET gene is associated with autistic disorder. Autism Res. 2009;2:232–236. doi: 10.1002/aur.87. [DOI] [PubMed] [Google Scholar]
- 24.Campbell DB, Buie TM, Winter H, Bauman M, Sutcliffe JS, Perrin JM, et al. Distinct genetic risk based on association of MET in families with co-occurring autism and gastrointestinal conditions. Pediatrics. 2009;123:1018–1024. doi: 10.1542/peds.2008-0819. [DOI] [PubMed] [Google Scholar]
- 25.Savino F, Castagno E, Calabrese R, Viola S, Oggero R, Miniero R. High faecal calprotectin levels in healthy, exclusively breast-fed infants. Neonatology. 2010;97:299–304. doi: 10.1159/000255161. [DOI] [PubMed] [Google Scholar]
- 26.Prell C, Nagel D, Freudenberg F, Schwarzer A, Koletzko S. Comparison of three tests for faecal calprotectin in children and young adults: a retrospective monocentric study. BMJ Open. 2014;4:e004558. doi: 10.1136/bmjopen-2013-004558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernell E, Fagerberg UL, Hellström PM. No evidence for a clear link between active intestinal inflammation and autism based on analyses of faecal calprotectin and rectal nitric oxide. Acta Paediatr. 2007;96:1076–1079. doi: 10.1111/j.1651-2227.2007.00298.x. [DOI] [PubMed] [Google Scholar]
- 28.Buie T, Campbell DB, Fuchs GJ, 3rd, Furuta GT, Levy J, Vandewater J, et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. 2010;125(Suppl 1):S1–S18. doi: 10.1542/peds.2009-1878C. [DOI] [PubMed] [Google Scholar]
- 29.de Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, Iardino P, et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr. 2010;51:418–424. doi: 10.1097/MPG.0b013e3181dcc4a5. [DOI] [PubMed] [Google Scholar]
- 30.Gondalia SV, Palombo EA, Knowles SR, Cox SB, Meyer D, Austin DW. Molecular characterisation of gastrointestinal microbiota of children with autism (with and without gastrointestinal dysfunction) and their neurotypical siblings. Autism Res. 2012;5:419–427. doi: 10.1002/aur.1253. [DOI] [PubMed] [Google Scholar]
- 31.Boaz RT, Joseph AJ, Kang G, Bose A. Intestinal permeability in normally nourished and malnourished children with and without diarrhea. Indian Pediatr. 2013;50:152–153. doi: 10.1007/s13312-013-0030-3. [DOI] [PubMed] [Google Scholar]
- 32.Cass H, Gringras P, March J, McKendrick I, O'Hare AE, Owen L, et al. Absence of urinary opioid peptides in children with autism. Arch Dis Child. 2008;93:745–750. doi: 10.1136/adc.2006.114389. [DOI] [PubMed] [Google Scholar]
- 33.MacFabe DF. Enteric short-chain fatty acids: microbial messengers of metabolism, mitochondria, and mind: implications in autism spectrum disorders. Microb Ecol Health Dis. 2015;26:28177. doi: 10.3402/mehd.v26.28177. [DOI] [PMC free article] [PubMed] [Google Scholar]