Abstract
This study aimed to investigate factors affecting length of hospital stay and mortality of a specific group of patients with infected diabetic foot ulcer who underwent surgical drainage without major amputation, which is frequently encountered by orthopedic surgeons. Data on length of hospital stay, mortality, demographics, and other medical information were collected for 79 consecutive patients (60 men, 19 women; mean age, 66.1 [SD, 12.3] yr) with infected diabetic foot ulcer who underwent surgical drainage while retaining the heel between October 2003 and May 2013. Multiple linear regression analysis was performed to determine factors affecting length of hospital stay, while multiple Cox regression analysis was conducted to assess factors contributing to mortality. Erythrocyte sedimentation rate (ESR, P=0.034), glycated hemoglobin (HbA1c) level (P=0.021), body mass index (BMI, P=0.001), and major vascular disease (cerebrovascular accident or coronary artery disease, P=0.004) were significant factors affecting length of hospital stay, whereas age (P=0.005) and serum blood urea nitrogen (BUN) level (P=0.024) were significant factors contributing to mortality. In conclusion, as prognostic factors, the length of hospital stay was affected by the severity of inflammation, the recent control of blood glucose level, BMI, and major vascular disease, whereas patient mortality was affected by age and renal function in patients with infected diabetic foot ulcer undergoing surgical drainage and antibiotic treatment.
Keywords: Diabetic Foot, Foot Ulcer, Length of Stay, Mortality
Graphical Abstract
INTRODUCTION
Diabetic foot ulcer is a common and serious complication of diabetes mellitus (DM), which may require amputation or may lead to death (1,2,3,4,5,6,7). Concurrent infection with diabetic foot ulcer often aggravates disease progression and impedes wound healing and recovery, affecting patient prognosis (3). This condition frequently requires surgical drainage and subsequent antibiotic treatment, resulting in prolonged hospital stay; and despite these medical efforts and resources, treatment often results in death.
Orthopedic surgeons often encounter grave patients with diabetic foot ulcers and concomitant infections, requiring surgical treatment. Previous studies have reported that the length of hospital stay, as a surrogate of recovery time, and mortality are important outcomes of diabetic foot ulcers (2,3). However, factors affecting these outcomes have not been investigated in detail in patients with infected diabetic foot ulcers, requiring surgical treatment. Knowledge of these factors would enable orthopedic surgeons to better predict patient prognosis and to provide such patients with clinical information on controllable prognostic factors. These measures would help reduce a considerable sociomedical burden since DM is increasing in incidence, and infected diabetic foot ulcer is a serious complication of DM frequently requiring hospital admission.
Therefore, the aim of our study was to investigate factors affecting the length of hospital stay and mortality of patients with infected diabetic foot ulcers requiring surgical drainage.
MATERIALS AND METHODS
Subjects and data collection
Consecutive patients with diabetic foot ulcers were enrolled in our hospital between October 2003 and May 2013; of these, patients with infected diabetic foot ulcers who underwent surgical drainage were selected. Patients without apparent infections and those who had undergone immediate primary below-knee amputations were excluded from the study. Electronic patient medical records were reviewed, and data were collected on age, sex, body mass index (BMI), length of hospital stay, mortality, Wagner ulcer classification (Wagner stage), operative procedures (incision and debridement or primary major amputation), major vascular disease (cerebrovascular accident [CVA] or coronary artery disease [CAD]), ankle-brachial index [ABI] using Doppler, bacterial culture reports, and blood test results obtained on hospital admission (including hemoglobin, white blood cell count, total protein, erythrocyte sedimentation rate [ESR], C-reactive protein [CRP], blood urea nitrogen [BUN], serum creatinine [Cr], aspartate aminotransferase [AST], alanine aminotransferase [ALT], fasting blood glucose [Glu], and glycated hemoglobin [HbA1c]). When blood samples were obtained, they were immediately processed, refrigerated (if necessary), and transported to the central testing department in our hospital, where they were immediately analyzed.
The length of hospital stay was defined as the duration from the day of admission to the day of discharge, related only to the diagnosis and treatment of infected diabetic foot ulcers. We included Wagner stages III, IV, and V because stages I and II are not concurrent with infection according to the definition (6,8). We defined major amputation as amputation performed above the ankle level, such as Syme's amputation, below-knee amputation, and above-knee amputation.
Surgical debridement and drainage were performed by eliminating all infected and nonviable tissues under spinal or general anesthesia. Intraoperative bacterial culture was performed using the tissue debris. If the toes were not viable, they were amputated at the time of debridement. Either a Penrose drain was placed to enhance postoperative drainage or the surgical wound remained open. Patients with suspicious ischemic foot were referred to the vascular surgery department preoperatively, and concurrent vascular intervention or bypass surgery was performed at the time of orthopedic surgery, based on the vascular surgeon's decision and ABI test.
Intravenous antibiotics were administered postoperatively according to the bacterial culture and antibiotic-sensitivity reports. Patients were discharged when the surgical wound was stable with minimal or no oozing, and blood test results (ESR and CRP) showed continuous improvement in inflammation.
Statistical analysis
To summarize patient demographics, descriptive statistics such as mean, SD, and proportion were used. The Kolmogorov-Smirnov test was used to verify the normality of the distribution of continuous variables. Multiple regression analysis with stepwise selection was performed to examine the effects of the baseline variables, in which length of hospital stay was used as the dependent variable. To determine the survival rate and the hazard ratio of mortality, we performed multiple Cox proportional hazards analysis with stepwise selection. The following factors were included in the analysis as possible predictors or confounders: age, sex, length of hospital stay, mortality, Wagner stage, ABI, ESR, CRP, BUN, Cr, AST, ALT, Glu, HbA1c, BMI, CVA, and CAD. All statistics were 2-tailed, and P values of <0.05 were considered significant. All analyses were performed using SPSS Statistics 20.0 for Windows (IBM Corporation, Armonk, NY, USA).
Ethics statement
This retrospective study was approved by the institutional review board of Seoul National University Bundang Hospital (IRB No. B-1308/216-109). The board waived the patients' informed consent.
RESULTS
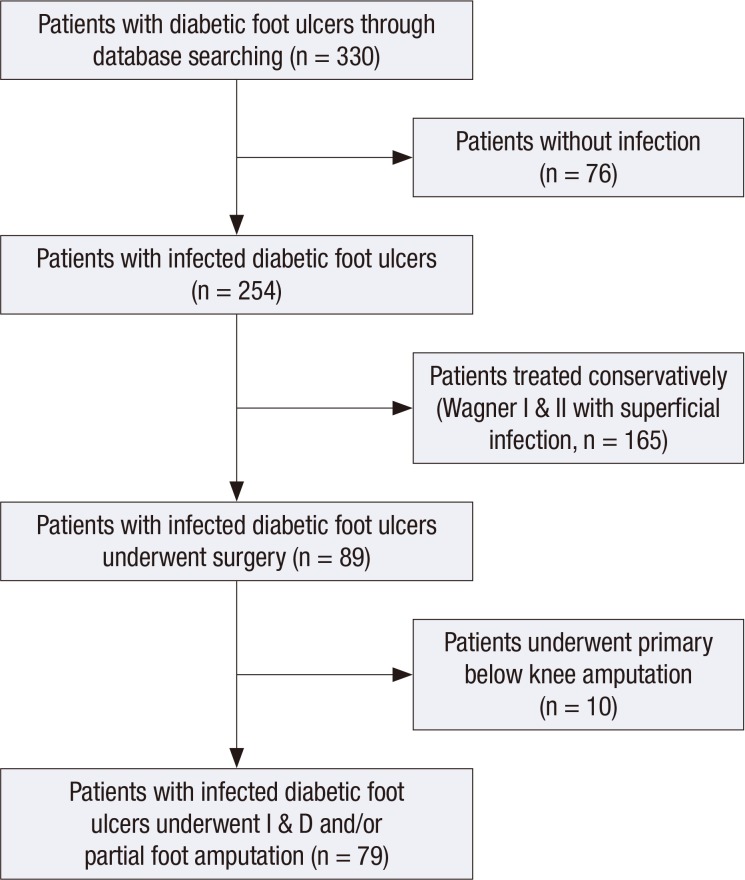
A total of 330 patients with diabetic foot ulcers were enrolled based on electronic medical records; of these, 76 were excluded because they had no sign of infection and were assigned to Wagner stage I or II. Of the 254 patients with infected diabetic foot ulcers, 165 were treated conservatively without surgery were excluded, which were assigned to Wagner stage I and II with superficial infection. Ten patients who underwent primary below-knee amputation also were excluded, leaving 79 patients (60 men, 19 women; mean age, 66.1 [SD, 12.3] yr) with infected diabetic foot ulcers to be included in the data analyses (Fig. 1).
Fig. 1. This flowchart shows the strategy to identify patients with infected diabetic foot ulcers who require surgical drainage.
The average length of hospital stay and the follow-up period were 37.8 (SD, 30.5) days and 20.0 (SD, 22.3) months, respectively. During the follow-up period, 10 patients (12.7%) died. The cause of deaths were pneumonia in three patients, CVA in two, heart failure in two, CAD in one, sepsis in one, and lung cancer in one. The survival rates at 12- and 28-months postoperatively were found to be 88.8% and 80.9%, respectively. There were 67 patients assigned to Wagner stage III and 12 to Wagner stage IV (Table 1). Bacterial culture reports revealed 58 patients had monomicrobial infection, 4 had polymicrobial infection, and 17 had sterile culture. Overall, 79 bacteria were isolated from 62 patients. Staphylococcus aureus was the most common pathogen isolated, followed by Pseudomonas aeruginosa, Morganella morganii, and Escherichia coli. Antibiotics that showed high sensitivity rate for Gram negative bacteria were; amikacin (92.9%), imipenem (92.9%), piperacillin/tazobactam (88.1%), ceftazidime (81.0%), cefotaxime (80.6%), and aztreonam (78.6%). Antibiotics that showed high sensitivity rate for Gram positive bacteria were; vancomycin (100%), teicoplanin (100%), rifampin (100%), trimethoprim/sulfamethoxazole (90.9%), and chloramphenicol (87.5%).
Table 1. Patient demographics and characteristics.
| Characteristics | Findings |
|---|---|
| Patients number | 79 |
| Mean age (yr) | 66.1 (SD 12.3) |
| Body mass index (kg/m2) | 22.2 (SD 3.5) |
| Sex | |
| Male | 60 (75.9%) |
| Female | 19 (24.1%) |
| Admission days | 37.8 (SD 30.5) |
| Mortality (dead/alive) | 10/69 |
| Follow up duration (mon) | 20.0 (SD 22.3) |
| Wagner classification | |
| Stage III | 67 (84.8%) |
| Stage IV | 12 (15.2%) |
| CVA and CAD | 14/79 (17.7%) |
| ABI | 0.98 (SD 0.31) |
| Hemoglobin (g/dL) | 10.7 (SD 1.7) |
| WBC count (103/µL) | 10.6 (SD 4.2) |
| Total protein (g/dL) | 6.6 (SD 0.8) |
| ESR (mm/h) | 68.6 (SD 29.3) |
| CRP (mg/L) | 7.8 (SD 7.6) |
| BUN (mg/dL) | 24.6 (SD 17.3) |
| Cr (mg/dL) | 2.5 (SD 2.7) |
| AST (IU/L) | 26.9 (SD 21.9) |
| ALT (IU/L) | 25.5 (SD 38.1) |
| Glucose (fasting) (mg/dL) | 185.5 (SD 112.6) |
| Hemoglobin A1c (%) | 8.6 (SD 2.0) |
CVA, cerebrovascular accident; CAD, coronary artery disease; ABI, ankle brachial index; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; BUN, blood urea nitrogen; Cr, serum creatinine; AST, aspartate aminotransferase; ALT, alanine aminotransferase. Normally distributed data recorded as mean (standard deviation).
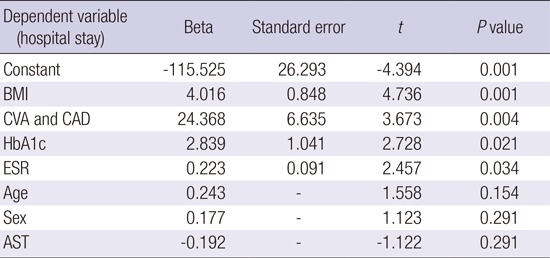
In multiple linear regression analysis, BMI (P=0.001), presence of major vascular disease (CVA or CAD) (P=0.004), HbA1c (P=0.021), and ESR (P=0.034) were found to be significant independent factors affecting the length of hospital stay (Table 2).
Table 2. Effects of BMI, CVA&CAD, HbA1c and ESR on hospital stay in multiple linear regression model with stepwise selection method.
| Dependent variable (hospital stay) | Beta | Standard error | t | P value |
|---|---|---|---|---|
| Constant | -115.525 | 26.293 | -4.394 | 0.001 |
| BMI | 4.016 | 0.848 | 4.736 | 0.001 |
| CVA and CAD | 24.368 | 6.635 | 3.673 | 0.004 |
| HbA1c | 2.839 | 1.041 | 2.728 | 0.021 |
| ESR | 0.223 | 0.091 | 2.457 | 0.034 |
| Age | 0.243 | - | 1.558 | 0.154 |
| Sex | 0.177 | - | 1.123 | 0.291 |
| AST | -0.192 | - | -1.122 | 0.291 |
R2=0.742. BMI, body mass index; CVA and CAD, cerebrovascular accident and coronary artery disease; ESR, erythrocyte sedimentation rate.
Multiple Cox regression analysis showed that age (P=0.005) and serum BUN (P=0.024) were significant independent factors affecting mortality (Table 3).
Table 3. Effects of age and BUN on mortality in multiple Cox regression model with stepwise selection method.
| Dependent variable (mortality) | HR | 95% CI | SE | P value |
|---|---|---|---|---|
| Age | 1.557 | 1.141 to 2.216 | 0.159 | 0.005 |
| BUN | 1.112 | 1.014 to 1.220 | 0.047 | 0.024 |
| BMI | 0.867 | 0.671 to 1.120 | 0.130 | 0.274 |
HR, hazard ratio; SE, standard error; CI, confidence interval; BUN, blood urea nitrogen.
DISCUSSION
This study investigated factors affecting the length of hospital stay and mortality in the management of infected diabetic foot ulcers requiring orthopedic surgery without major amputation. Our study involved a different cohort than that used in previous studies investigating the prognosis of diabetic foot ulcers in that, our patients underwent orthopedic surgery (drainage and/or partial foot amputation) for infected diabetic foot ulcers. Our results showed that the length of hospital stay was affected by the severity of inflammatory reaction (ESR), the recent control of blood glucose level (HbA1c), BMI, and major vascular disease (CVA or CAD). Patient mortality was affected by age and renal function (BUN).
In this study, length of hospital stay was used as a surrogate of recovery time from infected diabetic foot ulcer. Our study showed a greater length of hospital stay than that reported in other studies (2,7). This is because our cohort included severer diabetic foot ulcers with concomitant infection than that taken in a previous study (2), and we endeavored to salvage the limb without major amputation, which required subsequent intravenous antibiotic treatment causing prolonged hospital admission compared with that in another study (7). On the other hand, it should be considered that the length of hospital stay can be affected by differences in medical care and socioeconomic systems used in different hospitals (1,9). This study was performed in South Korea, where more than 95% of the population are insured by Natioal Health Insurance System and are considered to easily access medical system.
In our study, length of hospital stay was affected by severity of inflammatory reaction (ESR), the recent control of blood glucose level (HbA1c), BMI, and major vascular disease (CVA or CAD) at the time of hospital admission. These results are concurrent with those of a previous study, which reported that HbA1c level is an independent predictor for length of hospital stay in diabetic patients with sepsis (10). Another previous study showed that BMI was associated with immunologic dysfunction (11), which could explain our result that the patients with higher BMI showed longer length of hospital stay in our infected diabetic foot cohort. There is little supportive evidence on whether the presence of major vascular disease (CVA or CAD) increases the length of hospital stay in patients with infected diabetic foot ulcers. One possible explanation for this result is that patients with major vascular diseases are likely to take anticoagulant medication, which can increase the incidence of excessive oozing and hematoma at the surgical site, providing a media for bacterial growth and impeding infection control, as observed in other orthopedic surgeries (4,12). Another explanation could be the higher incidence of concurrent peripheral arterial disease in patients with CVA and CAD, which impedes wound healing progression (13). However, this issue requires further investigation in patients with diabetes.
The mortality rate of patients with infected diabetic foot ulcers was associated with age and renal function (BUN) in our study, which is supported by the results of a previous study (14). Regarding renal function, Lewis et al. (15) showed that the presence of end-stage renal disease and chronic kidney disease were independent risk factors for the development of diabetic foot ulcers, and patients with renal diseases also had higher mortality rates than patients without renal diseases. Previous studies have reported that patients with diabetic foot ulcers may experience higher mortality rates than expected (16,17). Boyko et al. (18) noted that diabetic foot ulceration serves as a marker for other associated diseases that increase mortality risks.
Our bacterial culture specimens were obtained intraoperatively to reduce the contamination of normal skin flora. A considerable portion of our specimens showed sterile culture, but this is comparable to the results of a previous study reporting sterile culture in 21% of the patients (19). This high rate of sterile culture might have been caused by preoperative exposure to antibiotic treatment before the culture specimens were obtained. The most common pathogens identified were Staphylococcus aureus and Pseudomonas aeruginosa, which suggests that they should be the targets of empirical antibiotic treatment for diabetic foot infection. However, the culture results did not affect the length of hospital stay or mortality of patients with infected diabetic foot ulcers in this study. Antibiotics sensitivity test showed amikacin (92.9%) and imipenem (92.9%) were drug of choice for Gram negative bacteria, and vancomycin (100%), teicoplanin (100%), and rifampin (100%) were that for Gram positive bacteria. Amikacin and vancomycin need to be used cautiously for patients with diabetic nephropathy.
There are some limitations to be addressed in this study. First, this study was retrospective; therefore, the data were not strictly controlled, and the accuracy of the clinical data depended on medical record review. Second, our hospital is a tertiary referral center for complex foot and ankle diseases; therefore, our results may not be applied to the general diabetic population. Third, the follow-up period was somewhat short, which could have affected accurate analysis of mortality data. Fourth, data on diabetic neuropathy was not included in the data analysis, which is considered to be one of important factor in the development and progression of diabetic foot ulcer.
The length of hospital stay was affected by the severity of inflammation (ESR), the recent control of blood glucose level (HbA1c), BMI, and major vascular disease (CVA or CAD). Patient mortality was affected by age and kidney function (BUN). These results provide useful clinical information regarding the prognosis of patients with infected diabetic foot ulcers who undergo surgical drainage and antibiotic treatment.
ACKNOWLEDGMENTS
The authors wish to thank Mi Seon Yoo, BS, Seoul National University Bundang Hospital and Hyun Mi Kim, BS, Seoul National University Bundang Hospital for their technical support and advice.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conception and coordination: Kim TG, Park MS, Lee KM, Lee TS, Kim BK. Acquisition of data: Kwon SS, Yoon C, Jung KJ, Lee TS, Kim BK. Analysis of data: Moon SY, Park MS, Kwon SS, Yoon C, Jung KJ. Manuscript preparation: Kim TG, Moon SY, and Lee KM. Manuscript approval: all authors.
References
- 1.Borghans I, Kool RB, Lagoe RJ, Westert GP. Fifty ways to reduce length of stay: an inventory of how hospital staff would reduce the length of stay in their hospital. Health Policy. 2012;104:222–233. doi: 10.1016/j.healthpol.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Frykberg RG, Piaggesi A, Donaghue VM, Schipani E, Habershaw GM, Navalesi R, Veves A. Difference in treatment of foot ulcerations in Boston, USA and Pisa, Italy. Diabetes Res Clin Pract. 1997;35:21–26. doi: 10.1016/s0168-8227(96)01359-9. [DOI] [PubMed] [Google Scholar]
- 3.Ogbera AO, Chinenye S, Onyekwere A, Fasanmade O. Prognostic indices of diabetes mortality. Ethn Dis. 2007;17:721–725. [PubMed] [Google Scholar]
- 4.Nelson JP. Deep infection following total hip arthroplasty. J Bone Joint Surg Am. 1977;59:1042–1044. [PubMed] [Google Scholar]
- 5.Pollard J, Hamilton GA, Rush SM, Ford LA. Mortality and morbidity after transmetatarsal amputation: retrospective review of 101 cases. J Foot Ankle Surg. 2006;45:91–97. doi: 10.1053/j.jfas.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Sun JH, Tsai JS, Huang CH, Lin CH, Yang HM, Chan YS, Hsieh SH, Hsu BR, Huang YY. Risk factors for lower extremity amputation in diabetic foot disease categorized by Wagner classification. Diabetes Res Clin Pract. 2012;95:358–363. doi: 10.1016/j.diabres.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 7.Wukich DK, Hobizal KB, Brooks MM. Severity of diabetic foot infection and rate of limb salvage. Foot Ankle Int. 2013;34:351–358. doi: 10.1177/1071100712467980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharp CS, Bessman AN, Wagner FW, Jr, Garland D. Microbiology of deep tissue in diabetic gangrene. Diabetes Care. 1978;1:289–292. doi: 10.2337/diacare.1.5.289. [DOI] [PubMed] [Google Scholar]
- 9.Perelman J, Shmueli A, Closon MC. Deriving a risk-adjustment formula for hospital financing: integrating the impact of socio-economic status on length of stay. Soc Sci Med. 2008;66:88–98. doi: 10.1016/j.socscimed.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Gornik I, Gornik O, Gasparović V. HbA1c is outcome predictor in diabetic patients with sepsis. Diabetes Res Clin Pract. 2007;77:120–125. doi: 10.1016/j.diabres.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Ilavská S, Horváthová M, Szabová M, Nemessányi T, Jahnová E, Tulinská J, Líšková A, Wsolová L, Staruchová M, Volkovová K. Association between the human immune response and body mass index. Hum Immunol. 2012;73:480–485. doi: 10.1016/j.humimm.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 12.McDougall CJ, Gray HS, Simpson PM, Whitehouse SL, Crawford RW, Donnelly WJ. Complications related to therapeutic anticoagulation in total hip arthroplasty. J Arthroplasty. 2013;28:187–192. doi: 10.1016/j.arth.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Ahn S, Park YJ, Min SI, Kim SY, Ha J, Kim SJ, Kim HS, Yoon BW, Min SK. High prevalence of peripheral arterial disease in Korean patients with coronary or cerebrovascular disease. J Korean Med Sci. 2012;27:625–629. doi: 10.3346/jkms.2012.27.6.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morbach S, Furchert H, Gröblinghoff U, Hoffmeier H, Kersten K, Klauke GT, Klemp U, Roden T, Icks A, Haastert B, et al. Long-term prognosis of diabetic foot patients and their limbs: amputation and death over the course of a decade. Diabetes Care. 2012;35:2021–2027. doi: 10.2337/dc12-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis S, Raj D, Guzman NJ. Renal failure: implications of chronic kidney disease in the management of the diabetic foot. Semin Vasc Surg. 2012;25:82–88. doi: 10.1053/j.semvascsurg.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Lee JS, Lu M, Lee VS, Russell D, Bahr C, Lee ET. Lower-extremity amputation. Incidence, risk factors, and mortality in the Oklahoma Indian Diabetes Study. Diabetes. 1993;42:876–882. doi: 10.2337/diab.42.6.876. [DOI] [PubMed] [Google Scholar]
- 17.Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care. 2003;26:491–494. doi: 10.2337/diacare.26.2.491. [DOI] [PubMed] [Google Scholar]
- 18.Boyko EJ, Ahroni JH, Smith DG, Davignon D. Increased mortality associated with diabetic foot ulcer. Diabet Med. 1996;13:967–972. doi: 10.1002/(SICI)1096-9136(199611)13:11<967::AID-DIA266>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 19.Tiwari S, Pratyush DD, Dwivedi A, Gupta SK, Rai M, Singh SK. Microbiological and clinical characteristics of diabetic foot infections in northern India. J Infect Dev Ctries. 2012;6:329–332. doi: 10.3855/jidc.1827. [DOI] [PubMed] [Google Scholar]