Abstract
Boiled silkworm pupa is a traditional food in Asia, and patients with silkworm pupa food allergy are common in these regions. Still now only one allergen from silkworm, arginine kinase, has been identified. The purpose of this study was to identify novel food allergens in silkworm pupa by analyzing a protein extract after heat treatment. Heat treated extracts were examined by proteomic analysis. A 27-kDa glycoprotein was identified, expressed in Escherichia coli, and purified. IgE reactivity of the recombinant protein was investigated by ELISA. High molecular weight proteins (above 100 kDa) elicited increased IgE binding after heat treatment compared to that before heat treatment. The molecular identities of these proteins, however, could not be determined. IgE reactivity toward a 27-kDa glycoprotein was also increased after heating the protein extract. The recombinant protein was recognized by IgE antibodies from allergic subjects (33.3%). Glycation or aggregation of protein by heating may create new IgE binding epitopes. Heat stable allergens are shown to be important in silkworm allergy. Sensitization to the 27-kDa glycoprotein from silkworm may contribute to elevation of IgE to silkworm.
Keywords: Allergens, Bombyx mori, Hemolymph, 27-kDa Glycoprotein
Graphical Abstract
INTRODUCTION
Sericulture is important industry in Asia from thousands years ago. Silkworm can induce various kind of allergic disease. Silkworms (Bombyx mori) transform from a larva to a pupa and the thread of silk (a major component of cocoon) is made from the pupa. Various materials from silkworms such as urine of silkworm, scales of silkworm moth, and silk gum can induce respiratory allergies, such as occupational asthma (1), asthma (2,3,4), and allergic rhinitis (5,6,7).
The silkworm pupa is also utilized in dietary supplements, and medicines, and for disposal of food and animal waste (8). Approximately 1,500-2,000 insect species are consumed worldwide as a food source (9). In Korea, silkworm pupae are a traditional food. Boiled and seasoned silkworm pupae are often sold as snacks. Their high protein content and reasonable nutrient composition make the pupae candidate for dietary use on spacecrafts (10). In China, roasted silkworm pupae are also eaten and dried bodies of silkworm pupae are utilized to relieve flatulence, muscular spasms, as well as phlegm expectorant.
Silkworm pupae are recognized as an important cause of food allergies (11). In Korea, 9.4% of allergic patients were found to be sensitized to silkworm pupa, and it was the most frequently sensitized allergen among the 62 frequent culprit food allergens by skin prick test (12). Allergic responses to the silkworm pupa are serious and it is recognized as a frequent cause of anaphylaxis in East Asia (13).
Molecular identities of silkworm allergens have been reported scarcely. In a recent study, Bomb m 1 (arginine kinase) was described as a major allergen (14). Arginine kinase has been proposed to be an invertebrate pan-allergen due to its cross-reactivity and versatility (15).
In this study, we investigated novel food allergens in silkworm pupa. As food allergens are known to be heat stable (16), and boiled silkworm pupae induce food allergy, IgE binding components that were stable after heat treatment of a protein extract from a silkworm pupa were analyzed by a proteomic approach. A 27-kDa glycoprotein was found to be a heat stable allergen. We therefore generated recombinant protein and investigated its allergenicity.
MATERIALS AND METHODS
Serum samples
Serum samples were obtained from 15 patients (age range 17-43 yr; average 34) who visited the Allergy-Asthma Clinic at Severance Hospital, Seoul, Korea. These individuals were diagnosed with silkworm allergy based on temporal relation between silkworm pupa uptake and onset of cutaneous allergic symptoms and confirmed in eight cases with positive skin prick test to silkworm pupa (Table 1). However, we could not do skin prick test in the other seven silkworm pupa allergy patients, as the patients took antihistamines or the skin prick test reagent is not available. These cutaneous allergic symptoms included urticaria and anaphylaxis, and these symptoms occurred within 30 min after the uptake. Serum samples from 7 individuals (age range 3-49 yr; average 25) who were not allergic to silkworms and showed no positive reaction to the allergen tested by skin prick test and ImmunoCAP (Phadia, Uppsala, Sweden) were also used as negative control in this study. Serum samples from 8 individuals (age range 14-62 yr; average 38) who had specific IgE to house dust mite (>0.7 kU/L) were also used as a control in order to evaluate the possible cross-reaction without genuine sensitization.
Table 1. Clinical features of subjects enrolled in this study.
| Patient No. | Age (yr) | Sex | SPT wheal | Symptoms | Last event (years ago) | ELISA (positivity) | Optical density to 27-kDa glycoprotein |
|---|---|---|---|---|---|---|---|
| 1 | 39 | F | ND | Anaphylaxis | 11 | - | 0.07925 |
| 2 | 35 | F | 3.5 | Urticaria | 10 | - | 0.08265 |
| 3 | 36 | F | ND | Urticaria | Unknown | - | 0.0878 |
| 4 | 37 | F | 4.5 | Urticaria | Unknown | + | 0.10075 |
| 5 | 40 | M | ND | Urticaria | Unknown | - | 0.07725 |
| 6 | 25 | M | ND | Urticaria | Unknown | + | 0.229 |
| 7 | 43 | M | ND | Anaphylaxis | 4 | - | 0.07325 |
| 8 | 28 | F | 8.5 | Urticaria | 2 | - | 0.07805 |
| 9 | 27 | F | 4 | Urticaria | Unknown | - | 0.0709 |
| 10 | 39 | M | 5.5 | Anaphylaxis | 1 | - | 0.0854 |
| 11 | 18 | M | 8.5 | Urticaria | Unknown | + | 0.09775 |
| 12 | 30 | M | 2.5 | Anaphylaxis | 4 | + | 0.09395 |
| 13 | 26 | M | ND | Urticaria | 5 | + | 0.3405 |
| 14 | 43 | F | ND | Urticaria | 5 | - | 0.0645 |
| 15 | 17 | M | 2 | Urticaria | 0.3 | - | 0.0724 |
SPT, skin prick test; ELISA, enzyme-linked immunosorbent assay.
Allergen preparation
Frozen silkworm pupae were obtained from Anysilk (www.anysilk.com, Boeun, Chungbuk, Korea). It was pulverized in liquid nitrogen and defatted with five times the volume of a 1:1 mixture of ethyl ether and ethyl acetate. Subsequently, proteins were extracted with phosphate buffered saline (PBS), pH 7.4 containing 6 mM 2-mercaptoethanol, 1:1,000 volume of protease inhibitor cocktail set III (Calbiochem, San Diego, CA, USA), and 1 mg/mL of 1-phenyl-3-(2-thiazyl)-2-urea (Sigma-Aldrich, St. Louis, MO, USA) at 4℃. After centrifugation at 10,000 g for 30 min at 4℃, the protein extract was syringe-filtered (0.22 µm, Millipore, Bedford, MS, USA). The protein concentration in the extract was measured by Bradford assay (Bio-Rad, Hercules, CA, USA), and the extract was stored at -70℃ until use.
SDS-PAGE and IgE immunoblotting
To analyze heat-stable proteins, the protein extract was placed in boiling water for 5 min. Denatured or aggregated fraction after heat treatment was regarded as the heat-labile fraction, while the supernatant was considered to contain heat-stable proteins. Aggregated proteins were resuspended in PBS and separated on 10% or 12% SDS-polyacrylamide gels under reducing conditions along with the supernatant. IgE reactive components were probed with pooled serum samples (1:4 diluted). IgE antibodies were detected with alkaline phosphatase-conjugated goat anti-human IgE (1:1,000) (Sigma-Aldrich) for 1 hr. Color development was initiated by adding nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate (Promega, Madison, WI, USA).
Proteomic analysis
Heat-treated extract was desalted with trichloroacetic acid. Proteins were separated on a 9%-17% gradient gel after isoelectric focusing (pH 3-10). Separated proteins were stained with Coomassie Brilliant Blue R250 or electrophoretically transferred onto a polyvinylidenedifluoride (PVDF) membrane (0.45 µm, Millipore). IgE-reactive components were detected with pooled serum (1:4) as described above. To identify proteins, LC-coupled ESI-MS/MS analysis was performed at ProteomeTech (Seoul, Korea).
Production of recombinant protein
The open reading frame of a 27-kDa glycoprotein (NM_001043413) from the silkworm was codon-optimized for expression in Escherichia coli and synthesized by Bioneer (Daegeon, Korea). Subsequently, it was PCR-amplified using oligonucleotide primers (forward: 5'-ATgAtgTggAAgACTgTCTTg-3', reverse: 5'-TTAACggAAAgAAgTCCgACgg-3') and ligated into pEXP5NT/TOPO vector (Invitrogen, Carlsbad, CA, USA). After transformation into E. coli BL21 (DE3), expression of recombinant protein was induced by addition of 1 mM isopropyl-1-thio-β-D-galactopyranoside. Recombinant protein was purified with Ni-NTA agarose (Qiagen, Hercules, CA, USA) under denaturing conditions with 6 M urea. Recombinant silkworm tropomyosin, utilized for the inhibition ELISA, was also expressed using the same expression system and purified by Ni-resin from the soluble fraction.
Specific IgE binding to recombinant protein
IgE reactivity toward the recombinant protein was detected by ELISA. Recombinant protein (2 µg/mL) was coated on a microplate overnight in 0.05 M carbonate buffer, pH 9.6. After blocking with 3% skim milk in PBS-containing 0.05% Tween 20 (PBST), serum samples (diluted 1:4 in PBST containing 1% bovine serum albumin) were added and the plate was incubated for 1 hr. IgE antibodies were detected by adding biotinylated goat anti-human IgE (1:1,000) (Vector, Burlingame, CA, USA) followed by a 1-hr incubation, and then streptavidin-peroxidase conjugate (1:1,000) (Sigma-Aldrich) was added and the plate was incubated for an additional 30 min. Color development was initiated by adding the substrate 3,3'5,5'-tetramethyl-benzidine (Kirkegaard & Perry Laboratories, Gaithersburg, MD, USA). Absorbance at 450 nm was measured after stopping the enzyme reaction by adding 0.5 M H2SO4. The mean absorbance plus 2 standard deviations of the sera from healthy controls was used as a cutoff value.
Inhibition analysis
For ELISA inhibition, both natural and heat-treated silkworm pupa extracts (10 µg/mL) were coated microtiter plates and incubated at 4℃ overnight. After blocking with 3% skim milk, the wells were incubated with patient serum (1:4, pooled from two patients positive to recombinant protein), which had been pre-incubated with solutions containing various concentrations of heated extract or recombinant protein, for 2 hr at room temperature. Subsequently, IgE antibodies were detected as described above.
For inhibition immunoblotting, 10 µg of heated extract was run on 12% SDS-PAGE gel under reducing conditions. The separated proteins were electroblotted onto PVDF membrane. After blocking with 3% skim milk in PBST, cut into 4 mm wide strips, and incubated overnight with serum sample (1:4, pooled from two patients positive to recombinant protein), which had been pre-incubated with solutions containing 20 µg of recombinant protein. IgE reactive components on strips were detected as described above.
Ethics statement
Serum samples were collected after obtaining consent from each patient. This study using the collected serum was approved by the institutional review board of Yonsei University Hospital (4-2013-0397).
RESULTS
IgE binding components from silkworm pupa extract
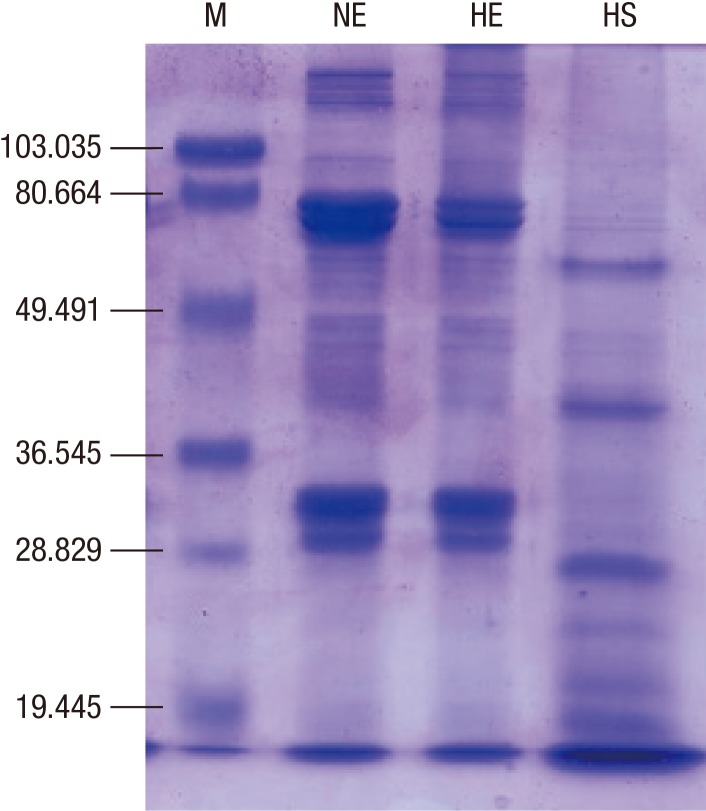
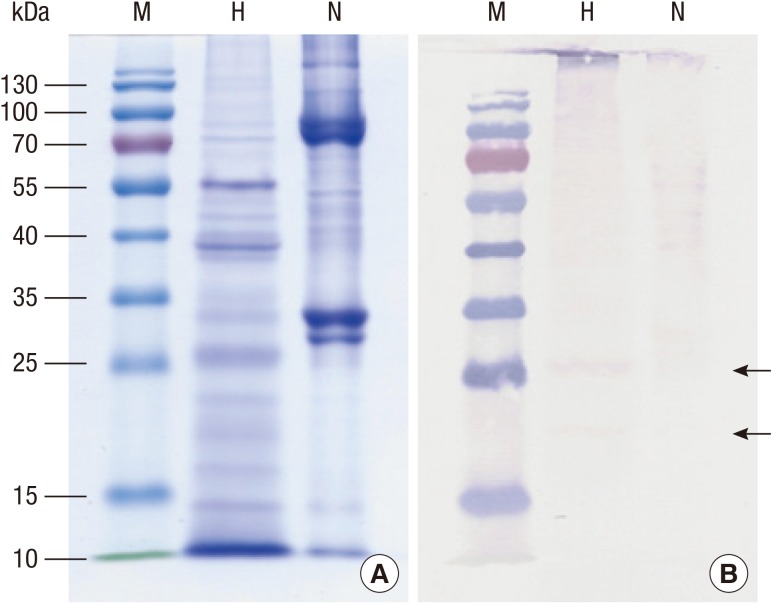
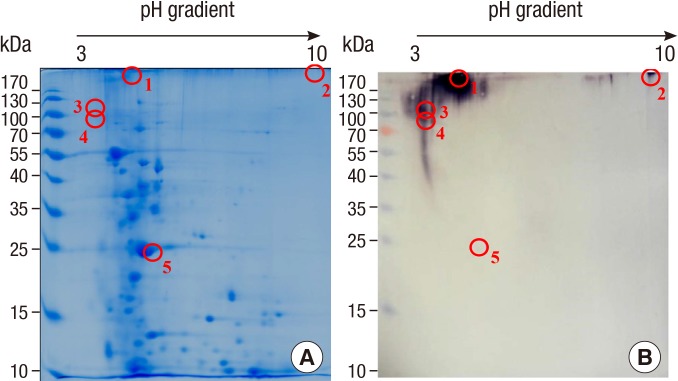
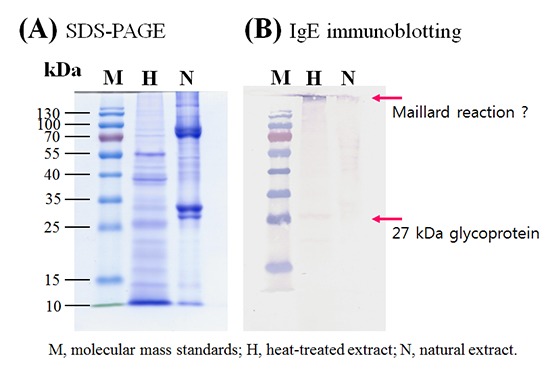
Protein concentration of the extract from the silkworm pupa was 40.05 mg/mL. After heating, 0.39 mg/mL (9.7%) of protein remained in the soluble fraction and 39.66 mg/mL (90.3%) was denatured or aggregated (Fig. 1). Interestingly, IgE reactivity to a 27-kDa protein was increased after heating (Fig. 2), along with IgE reactivity to high molecular weight proteins (above 100 kDa). Proteome analysis revealed that the 27-kDa protein was a 27-kDa hemolymph glycoprotein (Fig. 3). Five spots were selected and subjected to LC-coupled ESI MS/MS analysis (Table 2). All spots showed ion score of 32 to 84, except 27-kDa glycoprotein. Its Mascot score was 341, and its calculated molecular mass and isoelectric point were 24.886 kDa and 5.12, respectively. This 27-kDa glycoprotein shares 52.6%-56.6% sequence identity with 27-kDa glycoproteins in other lepidopterans (moths and butterflies) (Fig. 4). IgE binding to very high molecular weight proteins close to the wells of the gel was also increased after heat treatment.
Fig. 1. Protein analysis of silkworm pupa extract. Proteins were run on a 10% polyacrylamide gel containing sodium dodecyl sulfate under reducing conditions. M, molecular mass marker; NE, natural extract; HE, resuspended extract after heat treatment (heat-labile); HS, soluble fraction after heat treatment (heat-stable).
Fig. 2. Identification of heat stable allergens. Proteins (10 µg) before (N) and after (H) heat treatment were separated on a 10% polyacrylamide gel containing sodium dodecyl sulfate under reducing conditions. Proteins were stained with Coomassie Blue (A) or probed with pooled serum from silkworm allergic patients (B). Arrows indicate the heat stable IgE binding proteins.
Fig. 3. Protein profile (A) and IgE reactive components (B) of silkworm pupa extract on two-dimensional gels. Proteins were visualized by Coomassie Blue staining (A) and IgE-reactive components were probed with pooled serum from silkworm-allergic subjects (B). The protein in the circle was excised and subjected to LC-coupled ESI-MS/MS analysis.
Table 2. Identification of IgE-reactive components from heat treated silkworm pupa extract.
| Spot | Putative identity | Accession No. | Molecular weight | Isoelectrical point | Ion score | Coverage (%) |
|---|---|---|---|---|---|---|
| 1 | Xanthin dehydrogenase | gi|13936381 | 147,633 | 6.38 | 36 | 18 |
| 2 | Juvenile hormone epoxy hydrolase | gi|255977192 | 52,356 | 6.08 | 32 | 3 |
| 3 | Cell differentiation protein | gi|290760296 | 32,168 | 7.06 | 40 | 19 |
| 4 | Putative cuticle protein | gi|223671172 | 45,680 | 4.14 | 46 | 3 |
| 5 | 27 kDa glycoprotein | gi|19911074 | 24,886 | 5.12 | 341 | 45 |
| Putative cuticle protein | gi|19911074 | 28,278 | 4.63 | 84 | 20 | |
| Heat shock protein | gi|19911074 | 21,391 | 5.79 | 69 | 29 |
Fig. 4. Amino acid sequence alignment of the 27-kDa glycoprotein from lepidopterans. Bombyx mori, silkworm, Accession No. NP_001036878; Manduca sexta, tobacco hornworm, Q25513; Galleria mellonella, wax moth, P83632; Helicoverpa armigera, cotton bollworm, ABU98620; Papilio polytes, swallowtail, BAM19037; Danaus plexippus, monarch butterfly, EHJ70893. Percent sequence identity is indicated at the end of each sequence. *identical; :highly conserved; .less conserved.
Expression and purification of recombinant proteins
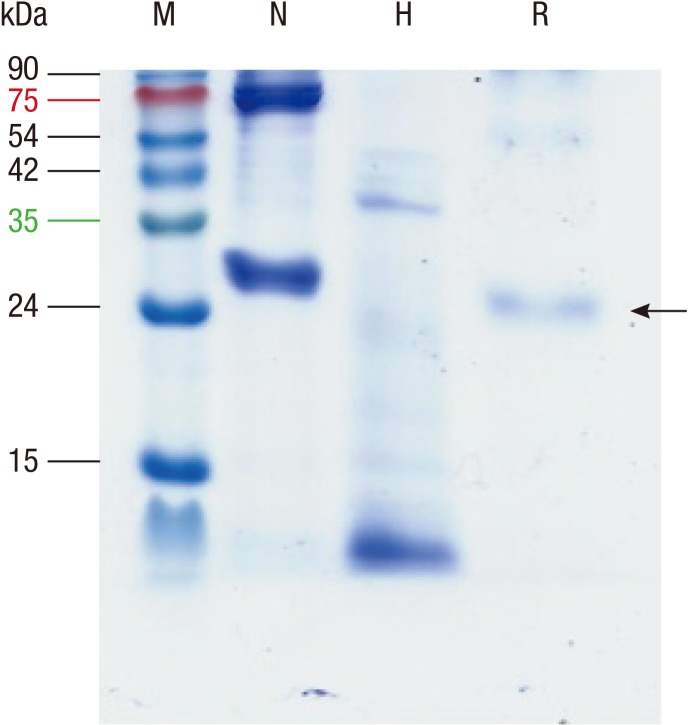
A total of 79 nucleotides (5 T to G at positions 14, 51, 78, 123, and 432; 11 T to C at positions 18, 45, 60, 150, 178, 279, 475, 480, 520, 666, and 685; 5 G to T at positions 47, 49, 81, 291, and 381; 9 A to G at positions 72, 90, 180, 273, 351, 366, 477, 534, and 585; 5 T to A at positions 129, 309, 549, 577, and 693; 3 A to T at positions 138, 168, and 490; 21 C to T at positions 156, 159, 240, 249, 255, 282, 336, 354, 393, 399, 414, 438, 441, 462, 498, 510, 519, 636, 660, 681, and 705; 7 C to G at positions 162, 318, 360, 426, 578, 582, and 606; 9 G to A at positions 213, 246, 390, 411, 444, 450, 456, 633, and 675; 1 G to C at position 402; 3 C to A at positions 417, 564, and 691) were changed for expression in E. coli without change of amino acids. Codon-optimized protein was successfully expressed in E. coli and purified using a Ni-column (Fig. 5).
Fig. 5. Production of recombinant protein. Proteins (10 µg) were run on a 12% polyacrylamide gel containing sodium dodecyl sulfate under reducing conditions. N, natural extract; H, heat-treated extract; R, recombinant protein.
IgE binding reactivity of the recombinant protein
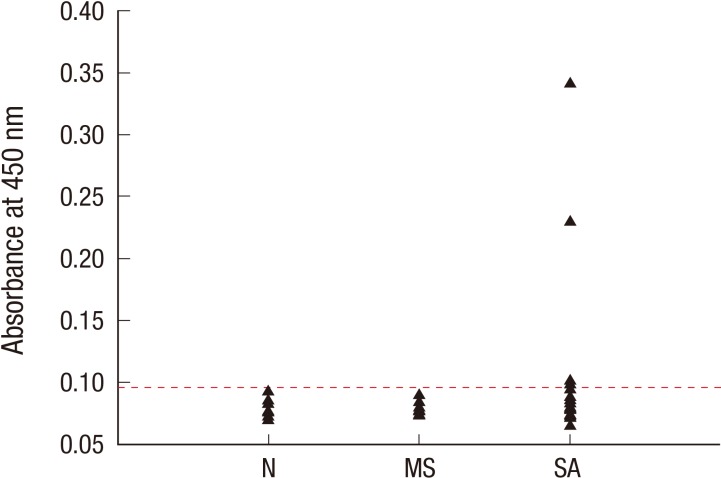
The purified recombinant protein was recognized by IgE antibodies from the serum sample of five of 15 (33.3%) silkworm-allergic patients (Fig. 6), when mean absorbance level (0.078525, range 0.0727 to 0.0894) from the control plus 2 standard deviations was used as cutoff value (0.089701). No IgE reactivity to the recombinant protein was observed from the dust mite sensitized subjects' sera.
Fig. 6. IgE reactivities of a recombinant 27-kDa glycoprotein. Absorbance ranges of subjects who were non-allergic (N), sensitized to house dust mite not silkworm (MS), and allergic to silkworm (SA) are plotted. Dotted line indicates the cutoff value.
Inhibition analysis
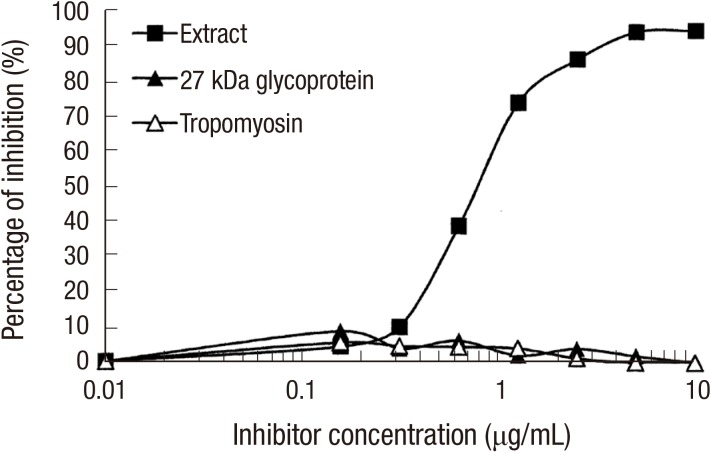
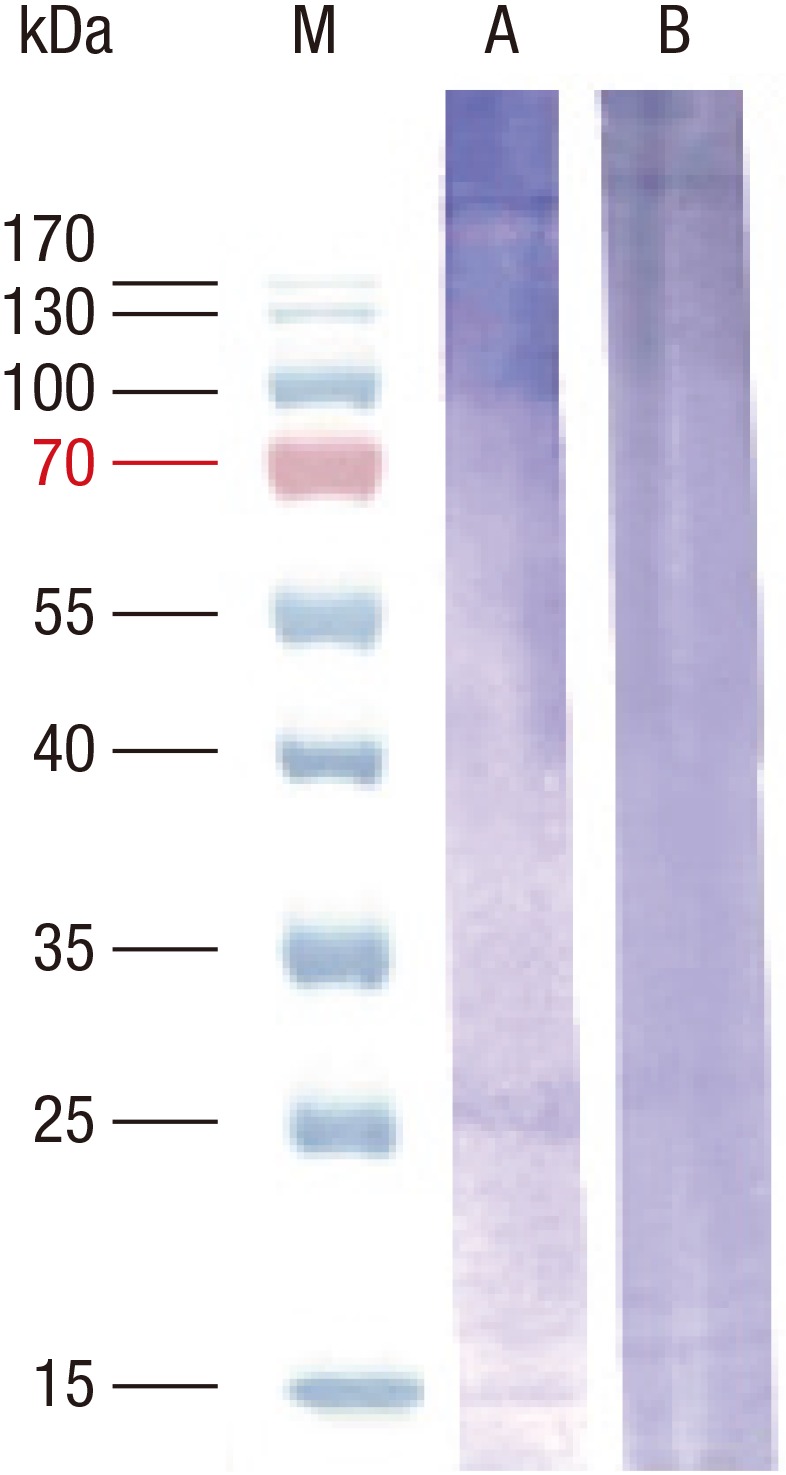
Inhibition analysis was performed in order to evaluate the role of 27 kDa glycoprotein in silkworm allergy. For the ELISA inhibition, heated allergen extract (10 µg/mL) was coated and a pooled serum, from two subjects who showed strong IgE reactivity to recombinant 27-kDa glycoprotein, which were pre-incubated with various concentrations of the extract or recombinant proteins were incubated as inhibitors. Heat-treated allergen extract was able to inhibit the maximum of 94.3% of IgE reactivity, whereas recombinant proteins (27-kDa glycoprotein and tropomyosin) inhibited less than 10% of IgE reactivity (Fig. 7). Attenuation of IgE binding to 27-kDa glycoprotein was observed by inhibition immunoblotting (Fig. 8). No inhibition was shown by recombinant tropomyosin.
Fig. 7. ELISA inhibition analysis. IgE binding to heated extract was inhibited with heated extract, recombinant 27 kDa glycoprotein, and recombinant silkworm tropomyosin.
Fig. 8. Inhibition immunoblotting analysis of silkworm allergens. Heat treated allergen extracts were separated on 12% SDS-PAGE and IgE reactive components were probed with patients' sera. M, molecular mass standard; A, IgE reactive components probed with sera without inhibitor; B, IgE reactive components probed with sera pre-incubated with recombinant 27-kDa glycoprotein.
DISCUSSION
A precursor of a 27-kDa hemolymph glycoprotein was first described in Manduca sexta (17). The protein is synthesized in the fat body and is present at all stages of development in both sexes. However, its function is still unknown. A 27-kDa hemolymph protein from the wax moth, Galleria mellonella, was also reported as an inhalant allergen in a patient with rhinoconjunctivitis (18). It shares 54.9% amino acid sequence identity with silkworm 27-kDa glycoprotein. This report suggests the possibility of the different sensitization route for 27 kDa insect hemolymph allergen other than oral intake.
In this study, we identified a 27-kDa glycoprotein from a silkworm pupa as a heat stable IgE binding component. However, its IgE reactivity was low (Fig. 6). It might be cross-reactive to IgE antibodies that recognize similar proteins in insects. The 27-kDa protein we identified shared 52.6%-56.6% amino acid identity to a 27-kDa protein in other lepidopteran insects (Fig. 4). IgE reactivity to the 27-kDa glycoprotein is not likely to be dependent on carbohydrate epitopes, because post-translational modifications do not occur in bacterial expression systems.
Specific IgE to recombinant 27-kDa glycoprotein was detected from one third (5/15) of silkworm allergy subjects tested. However, recombinant protein was able to inhibit less than 10% of IgE binding to the heat treated total extract (Fig. 7). In inhibition immunoblotting, only partial inhibition of IgE binding to the 27-kDa allergen was observed. The possibility of the presence of another allergen of similar molecular weight in the extract may explain the incomplete inhibition.
Pan-allergens in the extract may lead to false-positive reaction due to cross-reactivity. Arginine kinase, which is known as an invertebrate pan-allergen (15), is the only one allergen identified from silkworm allergen (14). Mite group 20 allergens are arginine kinase, however, little IgE reactivity (less than 10% of IgE binding frequency) was described to date (19,20). In this study, we included the sera from house dust mite sensitized patients as a control for the evaluation of IgE reactivity to the 27-kDa glycoprotein from silkworm (Fig. 6). No IgE reactivity was detected from house dust mite sensitized patients, indicating that IgE reactivity to recombinant protein is not a cross reaction.
In addition to the 27-kDa allergen, high molecular weight allergens were found by the immunoblotting assay. The IgE reactivity to high molecular weight proteins was shown to be increased after heating the protein extract (Fig. 2), even though more than 90% of proteins were degraded or aggregated after the heating (Fig. 1). We attempted to identify these high molecular weight proteins by proteome analysis. However, we were unsuccessful. Poor ion scores of the proteins analyzed may imply the structural modification (Table 2). It is tempting to speculate that aggregation of the proteins may create new IgE binding epitopes. Glycation by the Maillard reaction may at least partially explain the production of high molecular weight proteins (21). Various modifications caused by food processing can also affect allergenicity (22). Therefore, the allergenicity of silkworm pupa could also be affected by various cooking methods, making the characterization of the allergens more difficult. Likewise, food processing may increase the allergenicity of 27-kDa glycoprotein by chemical modification and/or structural change of the protein.
Recently, silkworm pupa has been reported to be cross-reactive with vegetable worm (Cordylceps sinensis), which is utilized in Chinese traditional medicine to enhance immunity, improve kidney function, and protect the respiratory system (23). Investigation of vegetable worm allergens is therefore important. IgE immunoblotting studies of other investigators are not always consistent to ours. Liu et al. (14) reported the 42-kDa arginine kinase as major allergen of the silkworm pupa, and in their immunoblotting, but we could not identify the 42-kDa IgE binding band. We do not know the causes of the discrepancy, but different method of allergen extraction, different stage of silkworm when extracted, and sensitivity of IgE immunoblotting may contribute to the differences. Liu et al. (24) reported 30-kDa allergen as a major allergen of the pupa through IgE immunoblotting, however more detail information about the molecule was not provided.
In conclusion, we investigated the presence of allergens in a protein extract of a silkworm pupa. IgE reactivity to high molecular weight proteins was increased after heat treatment. A 27-kDa hemolymph glycoprotein was identified as one of the heat stable IgE binding components. However, IgE reactivity to this recombinant protein was detected in five of 15 (33.3%) patients allergic to silkworm. Further studies on a larger scale are necessary to examine the usefulness of this recombinant protein and high molecular weight proteins produced after heating of silkworm pupa extract.
Footnotes
Funding: This study was supported by a faculty research grant from Yonsei University College of Medicine for 2010 (6-2010-0043).
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study design: Jeong KY, Park JW. Data collection: Jeong KY, Son M, Lee JY, Park KH, Lee JH. Data analysis and writing: Jeong KY, Park JW. Revision: Park JW. Agreement of final manuscript and submission: all authors.
References
- 1.Harindranath N, Prakash O, Subba Rao PV. Prevalence of occupational asthma in silk filatures. Ann Allergy. 1985;55:511–515. [PubMed] [Google Scholar]
- 2.Johansson SG, Wüthrich B, Zortea-Caflisch C. Nightly asthma caused by allergens in silk-filled bed quilts: clinical and immunologic studies. J Allergy Clin Immunol. 1985;75:452–459. doi: 10.1016/s0091-6749(85)80017-8. [DOI] [PubMed] [Google Scholar]
- 3.Celedón JC, Palmer LJ, Xu X, Wang B, Fang Z, Weiss ST. Sensitization to silk and childhood asthma in rural China. Pediatrics. 2001;107:E80. doi: 10.1542/peds.107.5.e80. [DOI] [PubMed] [Google Scholar]
- 4.Wen CM, Ye ST, Zhou LX, Yu Y. Silk-induced asthma in children: a report of 64 cases. Ann Allergy. 1990;65:375–378. [PubMed] [Google Scholar]
- 5.Suzuki M, Itoh H, Sugiyama K, Takagi I, Nishimura J, Kato K, Mamiya S, Baba S, Ohya Y, Itoh H, et al. Causative allergens of allergic rhinitis in Japan with special reference to silkworm moth allergen. Allergy. 1995;50:23–27. doi: 10.1111/j.1398-9995.1995.tb02479.x. [DOI] [PubMed] [Google Scholar]
- 6.Dewair M, Baur X, Ziegler K. Use of immunoblot technique for detection of human IgE and IgG antibodies to individual silk proteins. J Allergy Clin Immunol. 1985;76:537–542. doi: 10.1016/0091-6749(85)90772-9. [DOI] [PubMed] [Google Scholar]
- 7.Celedón JC, Palmer LJ, Weiss ST, Wang B, Fang Z, Xu X. Asthma, rhinitis, and skin test reactivity to aeroallergens in families of asthmatic subjects in Anqing, China. Am J Respir Crit Care Med. 2001;163:1108–1112. doi: 10.1164/ajrccm.163.5.2005086. [DOI] [PubMed] [Google Scholar]
- 8.Kim SA, Kim KM, Oh BJ. Current status and perspective of the insect industry in Korea. Entomol Res. 2008;38:S79–S85. [Google Scholar]
- 9.MacEvilly C. Bugs in the system. Nutr Bull. 2000;25:267–268. [Google Scholar]
- 10.Yang Y, Tang L, Tong L, Liu H. Silkworms culture as a source of protein for humans in space. Adv Space Res. 2009;43:1236–1242. [Google Scholar]
- 11.Ji KM, Zhan ZK, Chen JJ, Liu ZG. Anaphylactic shock caused by silkworm pupa consumption in China. Allergy. 2008;63:1407–1408. doi: 10.1111/j.1398-9995.2008.01838.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim SH, Kang HR, Kim KM, Kim TB, Kim SS, Chang YS, Kim CW, Bahn JW, Kim YK, Cho SH, et al. The sensitization rates of food allergens in a Korean population: a multi-center study. J Asthma Allergy Clin Immunol. 2003;23:502–514. [Google Scholar]
- 13.van der Poel L, Chen J. Food allergy epidemic - is it only a Western phenomenon? Curr Allergy Clin Immunol. 2009;22:121–126. [Google Scholar]
- 14.Liu Z, Xia L, Wu Y, Xia Q, Chen J, Roux KH. Identification and characterization of an arginine kinase as a major allergen from silkworm (Bombyx mori) larvae. Int Arch Allergy Immunol. 2009;150:8–14. doi: 10.1159/000210375. [DOI] [PubMed] [Google Scholar]
- 15.Binder M, Mahler V, Hayek B, Sperr WR, Schöller M, Prozell S, Wiedermann G, Valent P, Valenta R, Duchêne M. Molecular and immunological characterization of arginine kinase from the Indianmeal moth, Plodia interpunctella, a novel cross-reactive invertebrate pan-allergen. J Immunol. 2001;167:5470–5477. doi: 10.4049/jimmunol.167.9.5470. [DOI] [PubMed] [Google Scholar]
- 16.Bannon GA. What makes a food protein an allergen? Curr Allergy Asthma Rep. 2004;4:43–46. doi: 10.1007/s11882-004-0042-0. [DOI] [PubMed] [Google Scholar]
- 17.Samaraweera P, Law JH. Isolation, cloning and deduced amino acid sequence of a novel glycoprotein from the haemolymph of the hawkmoth Manduca sexta. Insect Mol Biol. 1995;4:7–13. doi: 10.1111/j.1365-2583.1995.tb00002.x. [DOI] [PubMed] [Google Scholar]
- 18.Madero MF, Enríquez-Matas A, Fernández-Nieto M, Sastre B, Del Pozo V, Pastor C, Quirce S, Sastre J. Characterization of allergens from the fish bait Galleria mellonella. J Allergy Clin Immunol. 2007;119:1021–1022. doi: 10.1016/j.jaci.2006.12.644. [DOI] [PubMed] [Google Scholar]
- 19.Hales BJ, Laing IA, Pearce LJ, Hazell LA, Mills KL, Chua KY, Thornton RB, Richmond P, Musk AW, James AL, et al. Distinctive immunoglobulin E anti-house dust allergen-binding specificities in a tropical Australian Aboriginal community. Clin Exp Allergy. 2007;37:1357–1363. doi: 10.1111/j.1365-2222.2007.02786.x. [DOI] [PubMed] [Google Scholar]
- 20.Jeong KY, Lee JY, Son M, Yi MH, Yong TS, Shin JU, Lee KH, Kim YJ, Park KH, Park HJ, et al. Profiles of IgE sensitization to Der f 1, Der f 2, Der f 6, Der f 8, Der f 10, and Der f 20 in Korean house dust mite allergy patients. Allergy Asthma Immunol Res. 2015;7:483–488. doi: 10.4168/aair.2015.7.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poulsen MW, Hedegaard RV, Andersen JM, de Courten B, Bügel S, Nielsen J, Skibsted LH, Dragsted LO. Advanced glycation endproducts in food and their effects on health. Food Chem Toxicol. 2013;60:10–37. doi: 10.1016/j.fct.2013.06.052. [DOI] [PubMed] [Google Scholar]
- 22.Jiménez-Saiz R, Benedé S, Molina E, López-Expósito I. Effect of processing technologies on the allergenicity of food products. Crit Rev Food Sci Nutr. 2015;55:1902–1917. doi: 10.1080/10408398.2012.736435. [DOI] [PubMed] [Google Scholar]
- 23.Choi GS, Shin YS, Kim JE, Ye YM, Park HS. Five cases of food allergy to vegetable worm (Cordyceps sinensis) showing cross-reactivity with silkworm pupae. Allergy. 2010;65:1196–1197. doi: 10.1111/j.1398-9995.2009.02300.x. [DOI] [PubMed] [Google Scholar]
- 24.Liu ZG, Zhang J, Lin G. Analysis, purification and identification of the 30 kD specific allergen of Bombyx mori using mass spectrometry. Acta Entomol Sin. 2007;50:101–105. [Google Scholar]