Abstract
Anemia is common in patients with advanced chronic kidney disease (CKD). Though erythropoiesis-stimulating agents (ESAs) have been strongly endorsed in guidelines, it is of particular financial interest. Recently, the reimbursement of ESAs in non-dialytic patients was started by the Korean National Health Insurance System. Thus, we investigated the impact of the reimbursement of ESAs on the anemia care in non-dialytic CKD patients. Medical records of patients with advanced CKD (estimated GFR <30 mL/min/1.73 m2) were reviewed. Use of ESAs, blood transfusion, and hemoglobin concentrations were analyzed from one year prior to reimbursement to three years following. We used multivariable modified Poisson regression to estimate the utilization prevalence ratio (PRs). A total of 1,791 medical records were analyzed. The proportion of patients receiving ESAs increased from 14.8% before reimbursement to a peak 33.6% in 1 yr after reimbursement; thereafter, ESA use decreased to 22.4% in 3 yr after reimbursement (compared with baseline; PR, 2.19 [95% CI, 1.40-3.42]). In patients with Hb <10 g/dL, the proportion of receiving ESAs increased from 32.1% before reimbursement to 66.7% in 3 yr after reimbursement (compared with baseline; PR, 2.04 [95% CI, 1.25-3.32]). Mean hemoglobin concentrations were 10.06±1.54 g/dL before reimbursement and increased to 10.78±1.51 g/dL in 3 yr after the reimbursement change (P=0.001). However, the requirement of blood transfusion was not changed over time. With the reimbursement of ESAs, the advanced CKD patients were more likely to be treated with ESAs, and the hemoglobin concentrations increased.
Keywords: Anemia; Blood Transfusion; Erythropoietin; Renal Insufficiency, Chronic
Graphical Abstract
INTRODUCTION
Anemia is prevalent in patients with chronic kidney disease (CKD), even before end-stage requiring renal replacement therapy. Although several factors contribute to the pathogenesis of CKD-associated anemia, the reduced erythropoietin production by kidneys is the primary cause (1,2,3). The Third National Health and Nutrition Examination Survey in the USA reported that, 46% of men with advanced CKD had a hemoglobin (Hb) concentration less than 12 g/dL, and 21% of women with advanced CKD had a Hb concentration less than 11 g/dL (4). Because of its remarkable ability to correct anemia and to reduce the need for transfusions (5), erythropoietin was approved for the treatment of anemia associated with chronic renal failure by the US Food and Drug Administration in 1989. Guidelines on the anemia of kidney disease were published by the Kidney Disease Outcomes Quality Initiative (KDOQI) in 1997, in which treatment of anemia using erythropoietin was strongly recommended (6). Since then erythropoiesis-stimulating agents (ESAs) have replaced transfusions as a first-line therapy for anemia of CKD.
However, ESAs have not been frequently utilized in non-dialytic patients for financial reasons. Recent studies have reported that only 20%-30% of patients received ESAs before dialysis (7,8). About 20% of dialytic patients had a Hb concentration less than 11 g/dL, whereas more than 70% of CKD patients had a Hb concentration less than 11 g/dL at the initiation of the dialysis (8,9). This might be related to the limited use of ESAs before dialysis. Although the health care systems differ across the countries, the patients not on dialysis have more barriers than those on dialysis. In Korea, ESAs had not been reimbursed in patients with non-dialytic CKD until October 2010, and thus the cost of ESAs covered by non-dialytic patients had been ten-fold higher than that covered by dialytic patients.
In recent decades, the treatment of anemia in patients with end-stage renal disease has aroused considerable attention, and there have been many clinical trials on the appropriate target Hb concentrations (10,11,12). These clinical studies raised serious concerns about intensive treatment of anemia using ESAs in patients with CKD (10,11,12). The results led to a more critical assessment of target Hb level of ESA therapy in the KDOQI guideline, which recommended the Hb target should be in the range of 11 to 12 g/dL, and the target should not exceed 13 g/dL (13). Recent data on Korean hemodialysis patients also supports this target; a Hb level of 10-11 g/dL was associated with the lowest mortality (14). However, a considerable proportion of advanced CKD patients not on dialysis do not reach even the Hb target of 11 g/dL, despite regular nephrology care (8,9). Also, little is known about trends in the treatment of anemia in patients with CKD not yet requiring dialysis in Korea. In this study, we tried to elucidate the trend of the use of ESAs, blood transfusion, and anemia care in non-dialytic CKD patients before and after the reimbursement of ESAs.
MATERIALS AND METHODS
Study population
Medical records of CKD patients were reviewed retrospectively from renal outpatient clinics of Chungbuk National University Hospital, a provincial tertiary referral center, between July 2009 and December 2013. We included adult (age≥18 yr), non-dialytic CKD patients with an estimated glomerular filtration rate (GFR) less than 30 mL/min/1.73 m2. Patients were excluded if they initiated renal replacement therapy or died within 30 days after enrollment, if their renal function changed by >25% within 30 days at the time of enrollment, or if they had a cancer within the previous 5 yr. Also excluded from the analysis were patients who did not have data on demographic characteristics nor on Hb levels. The following information from each patient was collected: age, sex, cause of CKD, the presence of comorbidity such as diabetes mellitus, hypertension, coronary artery disease, cerebrovascular disease, and peripheral vascular disease. We also ascertained their estimated GFR, Hb levels, hematocrit (Hct) concentrations, use of oral iron therapy, ESAs (epoetin alfa, epoetin beta and darbepoetin alfa), and blood transfusions. For ESAs, we defined the doses and number of days of each prescription. For conversion from darbepoetin to erythropoietin, we used a fixed conversion ratio of 1 µg of darbepoetin to 200 units of erythropoietin (15). For blood transfusions, we identified the number, days, and cause of transfusion.
Study design
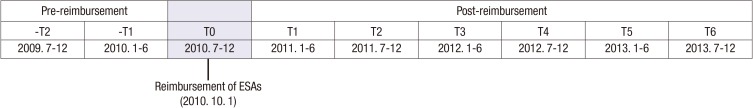
To evaluate the effect of reimbursement on the use of ESAs, we divided the study time frame into 6-month periods (Fig. 1). As the reimbursement criteria applied on October 1st 2010, July 2010 to December 2010 became T0. Therefore, -T2 and -T1 were included in the pre-reimbursement period, and T1 to T6 were included in the post-reimbursement period. In every 6-month period, we enrolled the eligible patients and obtained the clinical and laboratory data retrospectively. If the Hb and/or Hct concentrations were checked multiple times during a 6-month period, we calculated the minimum, mean, and maximum value.
Fig. 1. Schematic representation of 6-month periods, pre-reimbursement (-T2 to -T1) and post-reimbursement (T1 to T6).
Statistical analyses
We described all patient characteristics using means and standard deviations (SD) for continuous variables and percentages for categorical variables by 6-month periods that were categorized into 3 phases: July 2009 to December 2010, January 2011 to June 2012, and July 2012 to December 2013. The ANOVA test was used to compare continuous data among different phases, and the chi-square test was used to compare categorical data. We tabulated and/or plotted use of ESAs, doses of ESAs, and Hb concentrations over time. Using multivariable Poisson regression models with modified variances, we estimated unadjusted and adjusted prevalence ratios and their corresponding 95% confidence intervals (CIs). In the multivariable models, we adjusted for age, sex, cause of renal disease, diabetes, hypertension, cerebrovascular disease, peripheral vascular disease, coronary artery disease, Hb levels, and time. In order to allow for non-linear changes, we treated time as a categorical variable (-T2 to T6), using the earliest term (-T2) as the reference. All statistical tests were two-sided and conducted at the 0.05 level of significance. All analyses were conducted using SPSS for Windows version 17.0 (SPSS Inc., Chicago, IL, USA).
Ethics statement
The study protocol was approved by the institutional review board (IRB) of Chungbuk National University Hospital (approval number: 2015-05-004). The IRB waived the requirement for documentation of written informed consent from patients, as this study was conducted retrospectively.
RESULTS
We identified 2,384 medical records of adult patients with advanced CKD (estimated GFR <30 mL/min/1.73 m2) not yet requiring renal replacement therapy between July 2009 and December 2013. Patients were excluded if they initiated renal replacement therapy or died within 30 days after enrollment (226 patients), if their renal function changed by >25% within 30 days at the time of enrollment (36 patients), if they had an active cancer within the previous 5 yr (94 patients), or if they had insufficient data on demographic characteristics or Hb levels (237 patients). Finally, 1,791 patients were included as the study population. We summarized the baseline characteristics into 3 phases: July 2009 to December 2010, January 2011 to June 2012, and July 2012 to December 2013 (Table 1). Patients in more recent phases were older (P=0.001), and used less oral iron therapy (P=0.001). However, there were no significant differences in cause of renal disease or comorbidities.
Table 1. Baseline demographic characteristics and comorbid conditions of patients with non-dialytic chronic kidney disease by 3 phases.
| Parameters | All n = 1,791 |
-T2, -T1, T0 2009.7-2010.12 n = 467 |
T1, T2, T3 2011.1-2012.6 n = 540 |
T4, T5, T6 2012.7-2013.12 n = 784 |
P |
|---|---|---|---|---|---|
| Age (yr) | 60.7±14.7 | 59.2±14.6 | 60.0±14.5 | 62.1±14.7 | 0.001 |
| Age group (%) | 0.011 | ||||
| 18-44 | 15.1 | 15.6 | 15.6 | 14.4 | |
| 45-64 | 39.8 | 41.8 | 41.3 | 37.6 | |
| 65-74 | 25.9 | 27.4 | 26.7 | 24.5 | |
| 75- | 19.2 | 15.2 | 16.5 | 23.5 | |
| Male (%) | 44.9 | 48.6 | 44.3 | 43.2 | 0.170 |
| GFR (mL/min/1.73 m2) | 20.32±6.85 | 19.98±6.73 | 20.70±6.71 | 20.25±7.01 | 0.510 |
| CKD stage (%) | 0.894 | ||||
| Stage 4 | 73.7 | 74.5 | 73.5 | 73.3 | |
| Stage 5 | 26.3 | 25.5 | 26.5 | 26.7 | |
| Cause of CKD (%) | 0.246 | ||||
| Diabetes | 40.4 | 42.4 | 39.4 | 39.8 | |
| Hypertension | 34.3 | 35.5 | 34.1 | 33.8 | |
| Glomerulonephritis | 20.2 | 15.8 | 21.9 | 21.7 | |
| Other | 3.4 | 3.6 | 3.0 | 3.4 | |
| Missing | 1.7 | 2.6 | 1.7 | 1.3 | |
| Comorbidities (%) | |||||
| Diabetes | 44.2 | 47.5 | 43.7 | 42.5 | 0.212 |
| Hypertension | 95.1 | 94.4 | 95.7 | 95.2 | 0.629 |
| Coronary artery disease | 19.4 | 22.9 | 18.9 | 17.7 | 0.080 |
| Cerebrovascular disease | 17.4 | 20.6 | 15.4 | 16.8 | 0.088 |
| Peripheral vascular disease | 5.9 | 6.4 | 6.1 | 5.4 | 0.709 |
| Use of oral iron (%) | 38.8 | 44.5 | 40.3 | 34.1 | 0.001 |
CKD, chronic kidney disease; GFR, glomerular filtration rate.
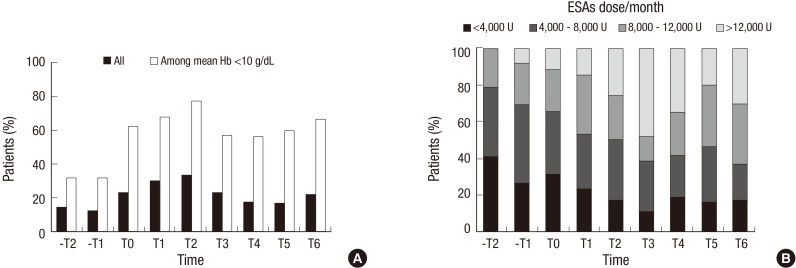
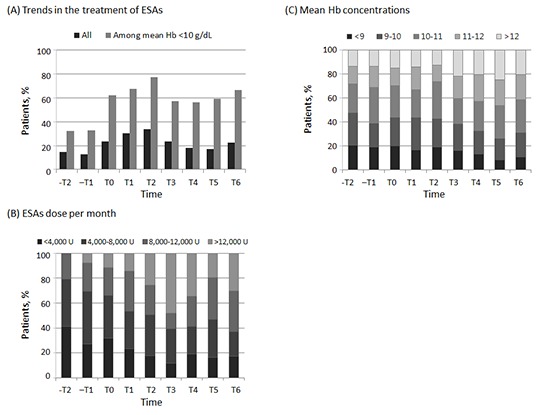
The proportion of patients who received ESAs increased from 14.8% in -T2 to 33.6% in T2; thereafter, the proportion decreased to 22.4% in T6 (Fig. 2A). Among patients whose mean Hb concentration was less than 10 g/dL, the proportion of patients who received ESAs increased from 32.1% in -T2 to a peak of 77.3% in T2, and then decreased slightly to 66.7% in T6. Among patients who received ESAs, the median dose of ESAs increased considerably from 4,333 U/month in -T2 to 9,667 U/month in T6. These findings were consistent with the distribution of the monthly dose of ESAs (Fig. 2B). When temporal trends of the use of ESAs in pre-dialytic CKD were modeled (Table 2), compared with patients in pre-reimbursement period (-T2 as referent), patients in post-reimbursement period were not different in receiving ESAs (PR of T6, 1.45 [95% CI, 0.94-2.24]). However, after adjustment for demographic characteristics, cause of renal disease, comorbidities, and mean Hb levels, patients in the post-reimbursement period were 2 times more likely to have used ESAs (PR of T2, 2.55 [95% CI, 1.63-3.98]; PR of T6, 2.19 [95% CI, 1.40-3.42]). Among patients whose Hb concentration was less than 10 g/dL, patients in post-reimbursement period received ESAs 2 times more than those in the pre-reimbursement period. These findings were consistent after adjustment.
Fig. 2. Trends in the treatment of erythropoiesis-stimulating agents (ESAs) in patients with non-dialytic chronic kidney disease over time. Proportion of patients received ESAs according to (A) hemoglobin level and, (B) ESAs dose per month from -T2 to T6. Hb, hemoglobin.
Table 2. Unadjusted and adjusted HRs of prevalence of erythropoiesis-stimulating agents.
| Term | All | Patients with mean Hb < 10 g/dL | ||
|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| -T2 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| -T1 | 0.88 (0.52-1.49) | 0.91 (0.53-1.54) | 1.02 (0.57-1.83) | 1.02 (0.57-1.82) |
| T0 | 1.66 (1.04-2.66) | 1.96 (1.22-3.14) | 1.94 (1.18-3.19) | 1.92 (1.16-3.17) |
| T1 | 1.94 (1.24-3.04) | 2.34 (1.49-3.68) | 2.11 (1.30-3.42) | 2.07 (1.27-3.38) |
| T2 | 2.21 (1.43-3.44) | 2.55 (1.63-3.98) | 2.41 (1.49-3.89) | 2.38 (1.46-3.86) |
| T3 | 1.67 (1.09-2.58) | 2.25 (1.45-3.49) | 1.79 (1.10-2.91) | 1.80 (1.11-2.94) |
| T4 | 1.32 (0.85-2.06) | 2.13 (1.35-3.36) | 1.76 (1.07-2.91) | 1.74 (1.04-2.90) |
| T5 | 1.22 (0.78-1.89) | 2.08 (1.32-3.27) | 1.86 (1.14-3.03) | 1.84 (1.12-3.04) |
| T6 | 1.45 (0.94-2.24) | 2.19 (1.40-3.42) | 2.08 (1.30-3.34) | 2.04 (1.25-3.32) |
Adjusted HRs are from multivariate modified Poisson regressions, adjusted for age, sex, cause of renal disease, diabetes mellitus, hypertension, cerebrovascular disease, peripheral vascular disease, coronary artery disease, and mean hemoglobin level.
Despite frequent use of ESAs in the post-reimbursement period, the proportion of patients receiving at least one transfusion was not changed over time (P=0.967); 3.8% in -T2, 4.9% in -T1, 5.5% in T0, 4.4% in T1, 3.8% in T2, 3.5% in T3, 2.9% in T4, 3.9% in T5, and 3.4% in T6. Most patients (72.9%) needed transfusions for symptomatic relief and the remaining patients received blood transfusions for perioperative care. Confining transfusion for symptomatic relief, the use of blood transfusion was not changed over time (P=0.468). The median number of pints of blood transfusion was 2 (interquartile range, 1-4). The number of pints per patient-year also was not changed before and after reimbursement of ESAs (P=0.815); 0.33 pint/patient-year in -T2, 0.37 in -T1, 0.25 in T0, 0.28 in T1, 0.36 in T2, 0.34 in T3, 0.13 in T4, 0.34 in T5, and 0.33 in T6.
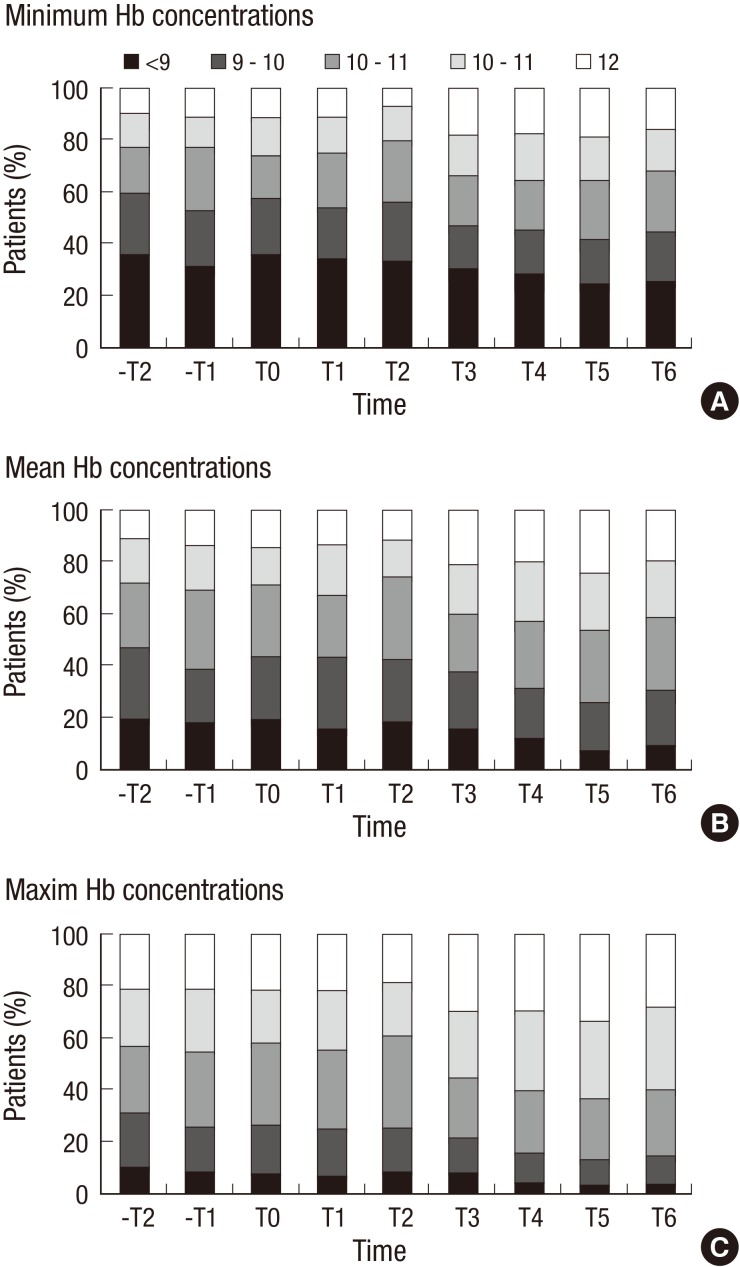
During the period when these changes in the use of ESAs were taking place, mean Hb concentrations were 10.06±1.54 g/dL in -T2, and they increased steadily, reaching 10.78±1.51 g/dL in T6 (Table 3, P for linear trend <0.001). Minimum and maximum Hb concentrations showed similar patterns. The corresponding fractions of patients with mean Hb concentrations over 11 g/dL (the target value suggested by KDOQI guideline) were 28.1% in -T2, and increased to 41.4% in T6 (Fig. 3).
Table 3. Hemoglobin concentration and hematocrit level over time.
| Parameters | -T2 2009.7-12 |
-T1 2010.1-6 |
T0 2010.7-12 |
T1 2011.1-6 |
T2 2011.7-12 |
T3 2012.1-6 |
T4 2012.7-12 |
T5 2013.1-6 |
T6 2013.7-12 |
P* |
|---|---|---|---|---|---|---|---|---|---|---|
| Hb (g/dL) | ||||||||||
| Minimum | 9.47±1.73 | 9.67±1.82 | 9.66±1.73 | 9.79±1.69 | 9.67±1.60 | 10.17±1.91 | 10.29±1.86 | 10.37±1.92 | 10.21±1.79 | < 0.001 |
| Mean | 10.06±1.54 | 10.23±1.56 | 10.24±1.48 | 10.35±1.46 | 10.20±1.39 | 10.68±1.66 | 10.87±1.55 | 10.96±1.60 | 10.78±1.51 | < 0.001 |
| Maximum | 10.69±1.53 | 10.81±1.42 | 10.84±1.36 | 10.94±1.41 | 10.77±1.35 | 11.24±1.57 | 11.43±1.44 | 11.53±1.51 | 11.35±1.40 | < 0.001 |
| Hct (%) | ||||||||||
| Minimum | 28.29±5.21 | 28.46±5.42 | 28.24±5.18 | 29.07±5.09 | 28.71±4.70 | 30.32±5.52 | 30.61±5.32 | 30.81±5.51 | 30.49±5.18 | < 0.001 |
| Mean | 30.13±4.65 | 30.18±4.57 | 30.03±4.37 | 30.77±4.39 | 30.32±4.10 | 31.87±4.70 | 32.33±4.33 | 32.55±4.50 | 32.18±4.26 | < 0.001 |
| Maximum | 32.14±4.65 | 32.07±4.18 | 31.90±3.97 | 32.64±4.24 | 32.07±3.97 | 33.54±4.41 | 34.07±3.98 | 34.22±4.20 | 33.96±3.99 | < 0.001 |
*P for linear trend. Hb, hemoglobin; Hct, hematocrit.
Fig. 3. Proportion of patients in each hemoglobin group among patients with non-dialytic chronic kidney disease over time. (A) minimum hemoglobin concentrations during period, (B) mean hemoglobin concentrations during period, and (C) maximum hemoglobin concentrations during period.
DISCUSSION
We studied the use of ESAs and the treatment pattern of anemia in CKD patients not on dialysis before and after reimbursement change of ESAs. We found that the use and dose of ESA treatment before dialysis increased considerably after the reimbursement of ESAs, which contributed to significantly higher Hb concentrations in CKD patients not on dialysis. However, the requirement of blood transfusion was not changed over time, even when accounting for the cause of blood transfusion.
With the reimbursement of ESAs, the cost of ESAs covered by non-dialytic CKD patients has declined to half as many as before, though it is still five-fold higher than that by dialytic patients. This reduction of cost has enabled physicians to use ESAs intensively for the treatment of anemia. Considering the patients with advanced CKD are usually old and suffering from several comorbidities, this financial benefit might play a critical role in using ESAs for anemia control. The proportion of receiving ESAs doubled between -T2 and T6. However, 30%-40% of CKD patients with Hb less than 10 g/dL did not receive the ESA therapy even in the post-reimbursement period, and the use of ESAs decreased from 77.3% in T2 to 66.7% in T6. Although we could not determine the reason not to use ESAs in those people, it assumed that the patients refused to maintain ESAs rather than that the physicians did not recommend ESA-based therapy. The population in Chungbuk province is mainly rural and social and financial factors associated with this characteristic might contribute to the low use of ESAs. Nonetheless the reimbursement has increased the use of ESAs, some patients do not use the ESAs for the treatment of anemia. The dose of ESAs in non-dialytic CKD patients has increased over time, but it is still lower than that in dialytic patients in Korea, whose median ESA dose was 4,000-6,000 U/week (16). It is likely due to more conserved renal function in non-dialytic patients as well as economical consideration.
In this study, the need of blood transfusion did not decline after reimbursement of ESAs, thus the main objective of ESA therapy, transfusion avoidance, was not achieved. These findings are in contrast with declining transfusion trends in dialytic patients (17), as well as non-dialytic CKD patients, in whom transfusion rates declined between 1992 and 2004 (18). However, the study on elderly patients not on dialysis showed that the proportion of patients who received blood transfusion increased between 1995 and 2010 (19), and a study of younger CKD patients not requiring dialysis reported that the transfusion rate increased substantially between 2002 and 2008 (20). The constant use of blood transfusion in recent 10 yr may be a consequence consequence of clinical trials which gave warnings on more intensive treatment using ESAs (10,11,12), or of the clinician's enthusiasm to reach the Hb target and to relieve patients' reported symptoms. The transfusion rate in present study is lower than previously reported (19), because we used the hospital-based database, not the nation-wide database. Thus, the information on transfusion out of our hospital could not be collected. Moreover, we excluded patients starting renal replacement therapy within 30 days, who were more likely to require blood transfusion. Considering these factors, the constant requirement of blood transfusion in this study can be explained.
The mean Hb concentrations increased by 0.72 g/dL after reimbursement. The proportion of patients with maximum Hb level less than 9 g/dL decreased from 11.2% in -T2 to 4.3% in T6, which implies that the patients whose Hb concentrations were always below 9 g/dL declined. Along with these changes, the proportion of patients with minimum Hb concentrations more than 13 g/dL (the upper limit of Hb using ESA therapy suggested in guidelines) increased from 2.5% in -T2 to 7.2% in T6. However, these patients did not receive any ESAs, so it does not seem to be related to ESA therapy. It might be postulated that the baseline Hb concentration was lower at baseline than that of previous studies (10,11,12).
Our study has several limitations. Considering that this is a single center-based study and that the main population is rural, the result cannot be extrapolated generally. This population could be more susceptible to financial barriers compared with those in big cities. So, this study might represent the trend of the use of ESAs according to the reimbursement. Second, we did not have information on the patient's financial and social status. Therefore we did not analyze the association of those financial factors and ESAs. Also, this study was performed retrospectively, and thus some important factors could not be acquired. Finally, we did not have data on the use of intravenous iron supplementation and the patient's iron status. Since ESAs activates erythropoiesis and iron consumption, thus, even iron-repleted patients can be short of iron. Therefore, the data on iron status could add more information.
In conclusion, the reimbursement of ESAs is associated with the increment of the prescription rate of ESAs and Hb concentration in non-dialytic CKD population. Also, this reimbursement change did not arouse over-treated anemia. However, considerable numbers of patients did not use the ESAs even in the post-reimbursement period. In light of the costs of anemia treatments and the safety concerns of blood transfusions, the anemia treatment strategy in patients with CKD and anemia needs to include financial factors.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study concept and design: Kim SM, Kim HY. Data acquisition: Kim SM, Kim KM. Data review and analysis: Kim SM, Kwon SK. Manuscript preparation: Kim SM, Kim KM. Manuscript revision: Kwon SK, Kim HY. Manuscript approval: all authors.
References
- 1.Eschbach JW, Adamson JW. Anemia of end-stage renal disease (ESRD) Kidney Int. 1985;28:1–5. doi: 10.1038/ki.1985.109. [DOI] [PubMed] [Google Scholar]
- 2.Lim CS. Medical therapy in patients with chronic kidney disease. J Korean Med Assoc. 2012;55:381–389. [Google Scholar]
- 3.Oak CY, Kim NH. Anemia and nutrition in end stage renal disease patient. J Korean Med Assoc. 2013;56:592–599. [Google Scholar]
- 4.Hsu CY, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2002;13:504–510. doi: 10.1681/ASN.V132504. [DOI] [PubMed] [Google Scholar]
- 5.Eschbach JW, Abdulhadi MH, Browne JK, Delano BG, Downing MR, Egrie JC, Evans RW, Friedman EA, Graber SE, Haley NR, et al. Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med. 1989;111:992–1000. doi: 10.7326/0003-4819-111-12-992. [DOI] [PubMed] [Google Scholar]
- 6.NKF-DOQI clinical practice guidelines for the treatment of anemia of chronic renal failure. National Kidney Foundation-Dialysis Outcomes Quality Initiative. Am J Kidney Dis. 1997;30(Suppl 3):S192–S240. [PubMed] [Google Scholar]
- 7.Horl WH, Macdougall IC, Rossert J, Rutkowski B, Wauters JP, Valderrabano F. Predialysis survey on anemia management: Patient referral. Am J Kidney Dis. 2003;41:49–61. doi: 10.1053/ajkd.2003.50018. [DOI] [PubMed] [Google Scholar]
- 8.Valderrabano F, Horl WH, Macdougall IC, Rossert J, Rutkowski B, Wauters JP. PRE-dialysis survey on anaemia management. Nephrol Dial Transplant. 2003;18:89–100. doi: 10.1093/ndt/18.1.89. [DOI] [PubMed] [Google Scholar]
- 9.Pisoni RL, Bragg-Gresham JL, Young EW, Akizawa T, Asano Y, Locatelli F, Bommer J, Cruz JM, Kerr PG, Mendelssohn DC, et al. Anemia management and outcomes from 12 countries in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2004;44:94–111. doi: 10.1053/j.ajkd.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Drueke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 11.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 12.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 13.KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007;50:471–530. doi: 10.1053/j.ajkd.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Jung MY, Hwang SY, Hong YA, Oh SY, Seo JH, Lee YM, Park SW, Kim JS, Wang JK, Kim JY, et al. Optimal hemoglobin level for anemia treatment in a cohort of hemodialysis patients. Kidney Res Clin Pract. 2015;34:20–27. doi: 10.1016/j.krcp.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott SD. Dose conversion from recombinant human erythropoietin to darbepoetin alfa: recommendations from clinical studies. Pharmacotherapy. 2002;22:160S–165S. doi: 10.1592/phco.22.14.160s.33398. [DOI] [PubMed] [Google Scholar]
- 16.ESRD Registry Committee; Korean Society of Nephrology. Current Renal Replacement Therapy in Korea - Insan Memorial Dialysis Registry, 2013; Proceedings of the 34th Annual Spring Meeting of the Korean Society of Nephrology; 2014. pp. 134–154. [Google Scholar]
- 17.Ibrahim HN, Ishani A, Foley RN, Guo H, Liu J, Collins AJ. Temporal trends in red blood transfusion among US dialysis patients, 1992-2005. Am J Kidney Dis. 2008;52:1115–1121. doi: 10.1053/j.ajkd.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 18.Ibrahim HN, Ishani A, Guo H, Gilbertson DT. Blood transfusion use in non-dialysis-dependent chronic kidney disease patients aged 65 years and older. Nephrol Dial Transplant. 2009;24:3138–3143. doi: 10.1093/ndt/gfp213. [DOI] [PubMed] [Google Scholar]
- 19.Winkelmayer WC, Mitani AA, Goldstein BA, Brookhart MA, Chertow GM. Trends in anemia care in older patients approaching end-stage renal disease in the United States (1995-2010) JAMA Intern Med. 2014;174:699–707. doi: 10.1001/jamainternmed.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill KS, Muntner P, Lafayette RA, Petersen J, Fink JC, Gilbertson DT, Bradbury BD. Red blood cell transfusion use in patients with chronic kidney disease. Nephrol Dial Transplant. 2013;28:1504–1515. doi: 10.1093/ndt/gfs580. [DOI] [PubMed] [Google Scholar]