Introduction
Primary liver cancer is the sixth most commonly occurring cancer in the world and the second largest contributor to cancer mortality.1 Globally, the most common histology (approximately 80%) is hepatocellular carcinoma (HCC), a tumor of the parenchymal cells of the liver. The second most common histology (approximately 15%) is intrahepatic cholangiocarcinoma (ICC), which arises in the cholangiocytes of the intrahepatic bile duct. Large geographic disparities in incidence and mortality of all types of liver cancer exist.
Incidence and Mortality
The highest incidence rates of liver cancer in the world are in Asia and Africa (Fig. 1).1 Approximately 75% of liver cancer occurs in Asia, with China accounting for over 50% of the world's burden. The country with the single highest incidence rate, however, is Mongolia, with an age-standardized rate (ASR) per 100,000 persons of 78.1.2 In contrast, the lowest incidence rates in the world occur in countries of Northern Europe, the Middle East, Oceania, and North and South America, while countries in Central Europe have intermediate rates. Even within specific geographic regions, however, there is great variability. For example, in Asian countries with cancer registries, the ASR of males range from 2.0 in Bhopal and Dindigul, India, to 77.5 in Qidong City, China.1
Fig. 1.
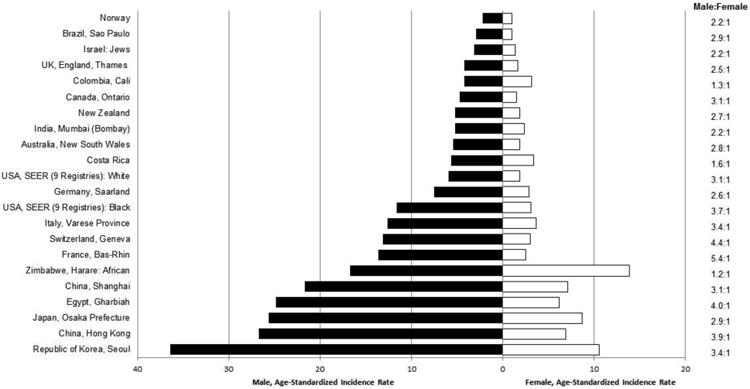
Age-adjusted incidences per 100,000 of liver cancer among men and women by region, 2003-2007. Age-adjusted to world standard. (Data from Ferlay J, Parkin DM, Curado MP, et al. Cancer incidence in five continents, volumes I to X: IARC CANCERBase No. 10 [Internet]. Available at: http://ci5.iarc.fr.)
In the interval between 1983-1987 and 2003-2007, liver cancer incidence increased in many areas of the world, notably in India, Oceania, and North and South America, as well as in most European countries (Figs. 2-3). Recently, however, incidence in the United States has stabilized will little annual change in rate between 2009 and 2011 (Fig. 4).3 In contrast, incidence rates have declined in some Asian countries, Spain, and Italy. The decreasing incidence rates seen China are likely due to programs to reduce aflatoxin B1 (AFB1) exposure and hepatitis B virus (HBV) transmission as well as other public health efforts.4 In Japan, the decreasing incidence of HCC is related to declining rates of hepatitis C virus (HCV) infection in the population.5
Fig. 2.
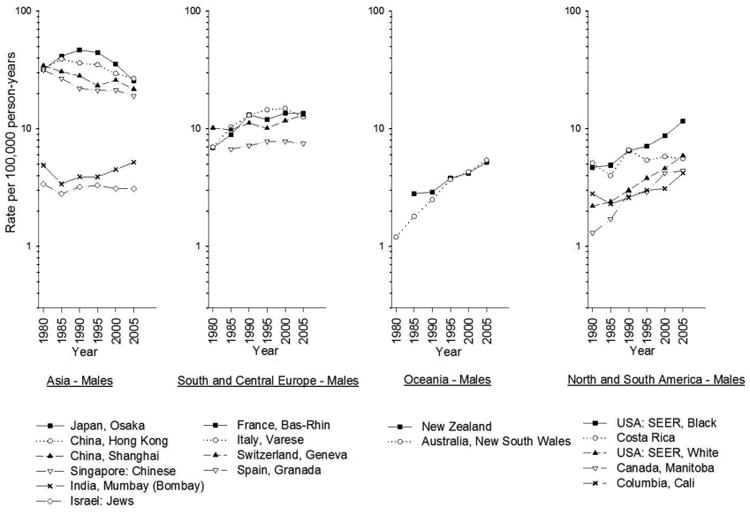
Age-adjusted trends in liver cancer incidence among men by region, 1978-1982 to 2003-2007. Age-adjusted to the world standard. (Data from Ferlay J, Parkin DM, Curado MP, et al. Cancer incidence in five continents, volumes I to X: IARC CANCERBase No. 10 [Internet]. Available at: http://ci5.iarc.fr.)
Fig. 3.
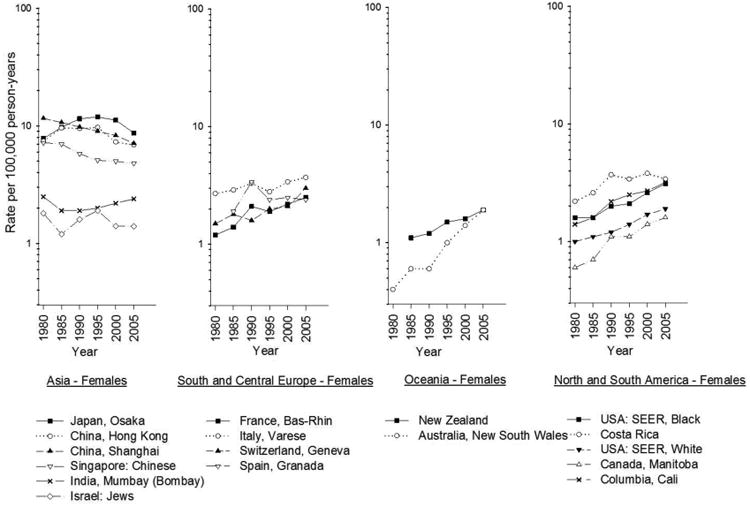
Age-adjusted trends in liver cancer incidence among women by region, 1978-1982 to 2003-2007. Age-adjusted to the world standard. (Data from Ferlay J, Parkin DM, Curado MP, et al. Cancer incidence in five continents, volumes I to X: IARC CANCERBase No. 10 [Internet]. Available at: http://ci5.iarc.fr.)
Fig. 4.
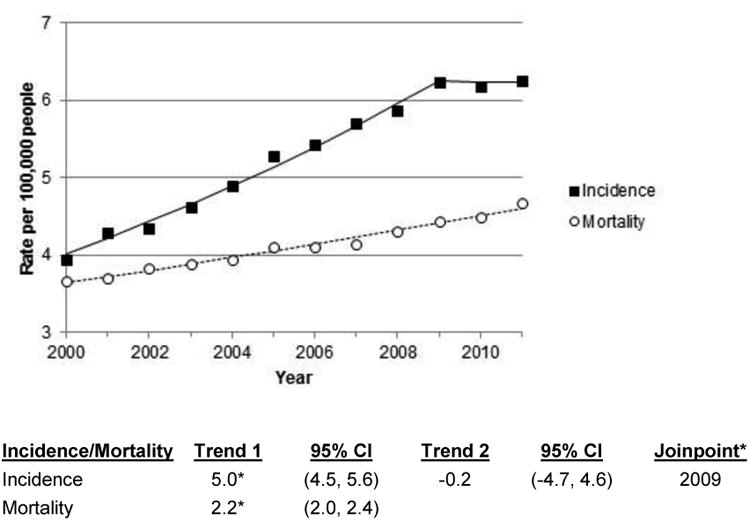
Age-adjusted Surveillance, Epidemiology and End Results (SEER) liver cancer incidence and US liver cancer mortality rates by year; 2000-2011. Age-adjusted to the US standard. CI, confidence interval. Trend indicates annual percent change (APC). Joinpoint regression defines when a trend changes. *Slope of trend differs from zero (p<0.05).
In the United States, the 5-year relative survival of liver cancer is only 14%.6 Prognosis is even poorer in less developed regions, thus incidence and mortality rates are roughly equivalent in all countries. In the U.S., mortality, unlike incidence, has not yet begun to stabilize; the annual percentage increase in rates was approximately 2.2 between 2000 and 2011.3 In some countries, mortality can appear to be even higher than incidence as the liver is a common site for metastases and secondary liver cancer can be mistakenly counted as primary liver cancer.
Gender and racial/ethnic differences in rates
Gender disparity in incidence is notable in almost all countries, with rates among males being two to three-fold higher than rates among females (Fig. 1). High-rate areas do not, however, have greater gender disparity than other areas. In fact, the greatest disparity in incidence occurs in Central European countries where some registries have rates among males four to five-fold higher than rates among females. The gender disparity in rates is not well understood, although most liver cancer risk factors are more prevalent in men than women. It has also been hypothesized that differences in sex steroid hormones, immune responses and epigenetics could be related to the higher rates among men.
In addition to gender differences, racial/ethnic disparity within multiethnic populations is also notable. In the United States between 2006 and 2010, Asians/Pacific Islanders had the highest incidence rate per 100,000 (11.7), followed by Hispanics (9.5), blacks (7.5), and finally, whites (4.2).7 Rates of liver cancer among persons of the same ethnicity also vary by geographic location. For example, liver cancer rates among Chinese populations outside China are lower than the rates reported by Chinese registries. As with gender differences, racial/ethnic differences are likely due to variability in the prevalence of risk factors between racial/ethnic groups and between geographic locations.
Hepatocellular Carcinoma (HCC) Risk Factors and Prevention
The dominant risk factors for HCC vary in high and low-rate regions. In most high-rate countries of Asia and Africa, chronic HBV infection and AFB1 exposure are the major risk factors. In contrast, HCV infection, excessive alcohol consumption, and diabetes/obesity/metabolic syndrome play more important roles in low-rate areas. Exceptions to these patterns are seen in Japan and Egypt where the dominant risk factor is HCV infection. Of the two HCC-related viruses, HBV is responsible for 75 to 80% of virus-associated HCC, while HCV is responsible for 10 to 20%.8 In addition to the major risk factors, certain inherited metabolic disorders such as hemochromatosis, α–1 antitrypsin deficiency, tyrosinemia and several porphyrias also increase risk. The rarity of these disorders, however, results in them contributing little to risk at the population level.
The global pattern of HCC incidence is related to the history of the major HCC risk factors and the length of time the factors have been present in human populations. Evidence suggests that HBV entered human populations about 33,600 years ago,9 while HCV dates back less than 1000 years and only became globally widely dispersed during the twentieth century.10 Alcohol consumption has been a common exposure among humans during all recorded history, while high rates of obesity, diabetes and metabolic syndrome are phenomena of the late twentieth century.
Hepatitis B virus (HBV)
In 1994, the International Agency for Research on Cancer (IARC) classified HBV as carcinogenic to humans.11 Currently, about 5% of the world's population (240-350 million people) is chronically infected.12 The evidence supporting the causal association of HBV with HCC is substantive. Countries with a chronic HBV infection prevalence of greater than 2% have increased incidence and mortality rates of HCC.13 Case-control studies in all regions of the world have shown that chronic HBV infection is significantly more common among HCC cases than controls with odds ratios ranging between 5:1 and 65:1.11 Similarly, prospective studies of HBV carriers have consistently demonstrated high relative risks for HCC, ranging from 5 to 103.11 In high-rate HCC areas where HBV infection is common, approximately 70% of HBV infections are acquired in the perinatal period or in early childhood.13,14 HBV DNA is integrated into the genome of liver tissues in almost all HCC cases who are HBV carriers. Investigators have also detected HBV DNA sequences in 10% to 20% of HCC tumors from patients who were seronegative for hepatitis B surface antigen (HBsAg).15
Among chronic carriers of HBV, risks of HCC vary by several factors, the major one being serum HBV DNA levels (viral load).16,17 Although there is no discrete cut-off level, having >105/ml viral copies confers a 2.5 to 3 fold greater risk over an 8-10 year follow-up period, than does having a lower viral load. Eight major HBV genotypes (A to H) and a number of sub-genotypes have been reported.18 In multiple population-based studies, genotype C has been associated with a higher risk of HCC than genotypes A2, Ba, Bj, and D.19 In studies that controlled for genotype, double mutations in the basal core promoter (BCP) of the HBV genome were independent predictors of increased risk. Mutations in the precore (PC) region of the viral genome have also been associated with risk, although less consistently so.20 The lifetime risk of HCC among HBV carriers is estimated to be 10% to 25%. Estimates suggest that, annually, 780,000 chronically infected people die from HCC and chronic liver disease and, eventually, 35 to 87 million of the 240 - 350 million HBV carriers will die of HCC.21
Prevention of chronic HBV infection via vaccination drastically reduces the risk of HCC,22 although the vaccine is ineffective in 5% of individuals.23 On the population level, it is anticipated that the widespread neonatal vaccination that started in the mid-1980s in most east Asian countries will result in a 70-85% decrease in the incidence of HBV-related HCC.22 In Taiwan, 30 years after the initiation of universal newborn vaccination, HBV carrier rates in persons under age 30 have fallen from 10-17% to 0.7-1.7%24 and rates of HCC have fallen 80%.25 Similar low rates of HBsAg prevalence are being achieved in China26 and Singapore27, thus declines in HCC incidence similar to Taiwan's can be expected. Currently, all countries in Asia and eastern Europe have integrated newborn HBV vaccination into their routine vaccination programs and are delivering 3 immunization doses. Perinatal transmission of HBV in Africa is rare14 and vaccination, without a birth dose, is now routine in >50% of sub-Saharan Africa countries. To eradicate HBV, new therapies for chronic infections need to be devised. With the identification of the cell receptor for HBV29 and new approaches to targeting cccDNA, the mini-chromosome of HBV,30 such drugs will soon become a reality.
Hepatitis C Virus (HCV)
Hepatitis C virus (HCV) was identified in 1989.31 Reliable serologic tests for antibody to HCV (anti-HCV) became available in 1990, and in 1994 IARC classified HCV as carcinogenic to humans.11 Phylogenetic analysis of HCV has identified at least six major genotypes (1 to 6) and numerous subtypes (denoted by lowercase letters).32,33 Evidence indicates that HCV existed as a long-term, low-level, endemic virus prior to the 20th century, but spread worldwide, via a number of transmission routes, starting around 1900.34 How HCV was maintained as an endemic infection prior to the twentieth century is uncertain35 The highest rates of chronic HCV infection in the world occur in northern Africa, particularly Egypt, where the rate is estimated at 18%.36 In Asia, the HCV infection rate is highest in Mongolia (10%)36, while rates in Europe (0.5-2.5%) are similar to the U.S. (1.8%), but higher than Canada (0.1-0.8%) which has one of the world's lowest rates.
The population dispersal times of HCV in Japan are consistent with the introduction of anti-schistosomal therapy using intravenous antimony sodium tartrate which began in the 1920s.37,38 Molecular clock studies of HCV in Egypt and have also suggested spread via intravenous anti-schistosomal therapy.39 Anti-schistosomal campaigns likely also spread HBV but the risk of an adult becoming a chronic HBV carrier after infection is low (∼10%), while the risk of an adult developing a chronic HCV infection after exposure is high (∼80%).
Molecular analysis of HCV genotype 1a in the U.S. suggests that the virus first entered the population around 1910 and became more widely disseminated in the 1960s.37 The reason for dissemination of HCV more widely in the 1960s is less clear, but the timing of the dissemination is consistent with the estimates derived from mathematical modeling.40,41 Although several models infer that HCC incidence could hit the very high levels seen earlier in Japan, other studies suggest that the long-term risk of HCC among HCV-infected Americans will be lower.42 As HCV circulated in the US blood supply for fewer years than it did in Japan, and newer, more effective anti-viral agents are being used to treat HCV infection, the long-term effect of HCV on HCC rates is likely to be less dramatic in the US than in Japan.
Until 2014, HCV infection was difficult to eradicate. With the discovery of sofosbuvir43 and other anti-viral drugs, almost all HCV infection could be cured.44 These medications are currently very expensive, but their price will decline as more drugs come on the market and with widespread use, the incidence of HCV-related HCC should rapidly decline.
Aflatoxin
Aflatoxin, a mycotoxin produced by molds of the Aspergillus species (Aspergillus flavus and Aspergillus parasiticus), contaminates maize, groundnuts and tree nuts in warm, humid environments and is a known hepatic carcinogen. Aflatoxin B1 (AFB1), the most potent aflatoxin, has been classified by IARC as a group 1 human carcinogen.45 The regions of the world with the highest levels of AFB1 exposure are sub-Saharan Africa, Southeast Asia and China. Within these areas, higher levels are found among rural than urban populations46, among males than females47,48 and among persons chronically infected with HBV.48 Exposure to AFB1 is associated with a signature DNA mutation in the p53 cancer suppressor gene at the third base of codon 249 (p53 249ser mutation).
There is a synergistic association between AFB1 and HBV in increasing risk of HCC. Compared to persons with neither risk factor, the risk of HCC is reported to be 4-fold greater among persons with elevated levels of AFB1, 7-fold greater among chronic HBV carriers, and 60-fold greater among persons with both factors.49,50 Evidence suggests that there is also a synergistic effect between AFB1 and HCV infection.51 AFB1 exposure, however, is more common in areas where HBV is the dominant virus. A recent examination of the population attributable risk of AFB1 in high rate areas reported that between 8.8% and 21% of liver cancer could be attributed to AFB1.52
In general, in areas of the world where AFB1 exposure is high, chronic HBV infection is highly prevalent. As little can be done to alter the HBV chronic infection state, eradicating AFB1 from the food supply is an important strategy to reduce HCC incidence.53 In areas of the world of the world where AFB1 eradication programs have been implemented, such as China, notable reductions in HCC rates have been documented.54
Alcohol consumption
Although the relationship of alcohol consumption to HCC has been widely studied, deciphering the association has been challenging as heavy consumption frequently leads to chronic liver disease, which in turn results in a cessation of consumption. In general, however, past studies in low-rate populations have found alcohol to increase risk of HCC, while studies from high-rate areas have been less consistent.55 The disparity between low and high-rate regions may have been due to lower mean alcohol consumption in high-rate populations and/or differences in the interaction between alcohol with other risk factors. Evidence suggests that both HBV and HCV, in conjunction with alcohol, have synergistic effects on HCC risk.56-58 Based on the accumulated evidence, IARC concluded in 1988 that alcohol consumption was causally related to liver cancer59 while a 2007 World Cancer Research Fund/American Institute for Cancer Research report concluded that alcohol consumption was a probable cause of liver cancer.60 A recent meta-analysis of nineteen prospective studies estimated a 16% increased risk of liver cancer among consumers of 3 or more drinks per day and a 22% increased risk among consumers of 6 or more drinks per day.61
Between 1960 and 2000, per capita consumption declined in European, North American and African countries. During the same interval, consumption levels increased in Southeast Asia and Western Pacific countries. As excessive alcohol consumption has historically been a more important HCC risk factor in low-rate HCC areas, declines in consumption suggest a favorable effect on HCC rates in those areas. Increasing consumption in Southeast Asia and the Western Pacific countries is a concern, however. For example, high alcohol consumption among men in Mongolia is thought to be contributing to that country's very high HCC rate.62
Obesity, Diabetes Mellitus and Metabolic Syndrome
The related metabolic disorders of obesity, type II diabetes and metabolic syndrome, with its hepatic manifestation of non-alcoholic fatty liver disease, have been increasing in frequency in many countries. Studies of each of the conditions have indicated that they are significantly related to the development of HCC. Meta-analyses of diabetes and HCC have consistently estimated relative risks of 2.0-2.5 and have found the relationship is consistent across various populations and is independent of other risk factors.63-66 A number of studies, as summarized in recent reviews, have reported that obesity is also related to liver cancer.67 In comparing normal weight persons with overweight and obese persons, a meta-analysis of 11 cohort studies found significant liver cancer risks was 1.17 among overweight (OR=1.17) and obese (OR=1.87) persons.68 Similarly, a meta-analysis of four studies of metabolic syndrome and HCC estimated a significant relative risk of 1.81.69
While the relative risks of diabetes, obesity and metabolic syndrome do not approach those of HCV or HBV, they are far more prevalent conditions in developed countries. In developing countries, the prevalence of diabetes is growing much faster than it is in developed countries.70 It has been estimated that there are currently 285 million persons in the world, or 6.4% of the global population, with diabetes.70 Further, the prevalence is projected to increase by 69% in developing countries, and 20% in developed countries, by the year 2030. Similarly, increases in BMI have been documented in many countries since 1980.71 Given the increasing prevalence of these conditions, the proportion of HCC related to obesity, diabetes and metabolic syndrome will likely grow in the future.
Tobacco
The effect of cigarette smoking on risk of HCC has been widely examined in studies from both high and low-rate countries.55 Inconsistent findings in studies of the same populations, and the correlation of smoking with other risk factors such as alcohol, have made the relationship between tobacco and HCC difficult to define. In 2004, however, IARC concluded that there was sufficient evidence that tobacco smoking caused liver cancer.72 This same position was adopted by the 2014 U.S. Surgeon General's report.73 A recent meta-analysis estimated that there was a 1.5 fold increased risk of HCC among current smokers, a risk similar that imposed by obesity.74
Coffee and tea
Recent meta-analyses have examined the association between both coffee75 and tea76 and risk of HCC. Including 16 studies, the coffee meta-analysis found a significant 40% reduced risk of HCC among consumers.75 Based on 12 studies, tea was associated with a non-significant 23% reduced risk.76 Since the publication of these results, an additional study from Europe reported 72% and 59% significantly reduced risks associated with coffee and tea, respectively.77 The sole study of coffee from the US, thus far, reported a significant 41% decreased risk of HCC.78
Compounds in coffee that potentially have chemopreventive effects include diterpenes (i.e., cafestol and kahweol), chlorogenic acid, and caffeine.79 Diterpenes are lipids that inhibit enzyme expression and enzymatic activity, induce detoxifying enzymes, and regulate signaling pathways.80 Chlorogenic acid is a polyphenol that increases activity of detoxifying enzymes.81 Caffeine has antioxidant properties and increases metabolic rate and energy expenditure, which could potentially regulate weight and reduce the risk of developing metabolic syndrome.82 Similarly, tea contains bioactive compounds, including caffeine and polyphenolic compounds. One specific polyphenol, (–)-epigallocatechin-3-gallate, has shown promise as a chemopreventive by inhibiting enzymatic activities, cell invasion, angiogenesis, and metastasis.83
Chemoprevention
Statins
Statins (3-hydroxy-3-methylglutaryl coenzyme A (HMG-Co-A) reductase inhibitors) are commonly prescribed cholesterol lowering medications used for the prevention of cardiovascular disease.84 Statins may also have anti-carcinogenic effects 85 related to inhibited angiogenesis, enhanced apoptosis and metastasis inhibition.86 Promising evidence that statins may decrease risk of HCC has been reported in observational studies in Taiwan.87-91 Two general population studies reported significant inverse associations between statins and HCC with odds ratios of 0.5387 and 0.44.89 In addition, significant inverse associations were reported in studies of HBV(+) persons91 and HCV(+) persons90 and in association with individual statins.88 In contrast, early results from low-rate HCC areas reported null associations.92,93 Subsequent studies from these areas, however, have provided support for an inverse association. Studies from the U.S. Veterans Affairs' population have found inverse associations among men with diabetes94 and HCV infection95, while studies conducted among members of U.S. health maintenance organizations 96,97 and a large Swedish record-linkage study also reported significant inverse associations.98 Meta-analyses of these results concluded that an inverse relationship exists.99,100 Collectively, the evidence suggests that statin use could contribute to a decline in HCC incidence.
Anti-diabetic medications
The relationship between diabetes and HCC has suggested that anti-diabetic medication use could modify HCC risk. Metformin, a widely prescribed anti-diabetic drug which reduces levels of circulating glucose and insulin, is frequently prescribed as a first line treatment. As diabetes progresses, individuals often transition to use of other oral hypoglycemic drugs and ultimately, to insulin.
Three recent meta-analyses of metformin and HCC have evaluated the results of published studies and have reported significantly decreased risks with odds ratios between 0.24 and 0.50.101-103 All studies included in the meta-analyses, however, compared use of metformin to the use of other anti-diabetic drugs. The close correlation of the drugs with duration and severity of disease, however, has made the interpretation of these results unclear. Such comparisons are likely to overestimate the protective effect of metformin, an early disease treatment, and overestimate the risk imposed by insulin, a treatment of last resort. In a study published after the meta-analyses were conducted, data from the UK were used to compare metformin use with use of no medication and the results found no association between metformin and risk of HCC.104 Similarly, there was no increased risk with use of insulin. These results suggest that metformin may not be a good candidate for chemoprevention of HCC.
Aspirin
NSAIDs, including aspirin and non-aspirin NSAIDs, are widely used as analgesic drugs, and low-dose aspirin is commonly used in the chemoprevention of cardiovascular and cerebrovascular disease.105 In-vitro studies and animal experiments suggest that NSAIDs also have chemopreventive and therapeutic benefit for HCC.106-108 However, results of early human studies of NSAID use and HCC109,110 were inconsistent. More recently, a U.S. cohort study reported a 37% reduced risk of HCC in association with NSAID use.111 Aspirin-only users had a 49% reduced risk of HCC, while there was no effect of non-aspirin NSAIDs. The protective effect of aspirin was consistent across frequency (daily, monthly, and weekly) of use. As daily aspirin use was likely to involve a low-dose formulation, these data may suggest that the same low-dose may be associated with a lower risk of HCC. Experimental and in-vivo evidence for a protective effect of NSAIDs against liver cancer offer biological plausibility for this association. NSAIDs modulate the risk of inflammation by inhibiting the COX enzymatic pathways necessary for synthesis of prostaglandins.112 This inhibition, as well as decreases in epithelial proliferation and angiogenesis, coupled with increased apoptosis, results in the reduction of the inflammatory response, which has implications for prevention.113 It has also been suggested that aspirin and NSAIDs in general might play a protective role in hepatic carcinogenesis through other non-COX inhibitory pathways108,114 and down regulation of pro-inflammatory cytokines.115
Summary
As the second largest contributor to cancer mortality in the world, liver cancer is a huge contributor to the world's cancer burden, but it is a preventable disease. Vaccination against HBV will have a dramatic effect on HCC incidence in coming generations. New infections with HCV have declined in most countries since the early 1990s and new curative treatments for HCV infection should have a major impact when their use becomes more widespread. Aflatoxin eradication programs and decreasing levels of alcohol consumption in some populations may also have favorable effects on HCC rates. The epidemic of obesity, diabetes and metabolic syndrome in many areas, however, may prevent very steep declines in HCC rates that would otherwise be possible.
Acknowledgments
This work was supported by funding of the National Institutes of Health Intramural Research Program.
Footnotes
The authors have no conflicts to disclose.
References
- 1.Ferlay J, Parkin DM, Curado MP, et al. Cancer incidence in five continents, volumes I to X: IARC CANCERBase No. 10. [Accessed November 25, 2014];2014 [Internet]. Available at: http://ci5.iarc.fr.
- 2.Ferlay J, Soerjomataram I, Ervik M, et al. Lyon, France: International Agency for Research on Cancer; 2013. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. [Internet] Available from: http://globocan.iarc.fr. 2014. [Google Scholar]
- 3.Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2014;109(4):542–553. doi: 10.1038/ajg.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao S, Yang WS, Bray F, et al. Declining rates of hepatocellular carcinoma in urban Shanghai: incidence trends in 1976-2005. European journal of epidemiology. 2012;27(1):39–46. doi: 10.1007/s10654-011-9636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka H, Imai Y, Hiramatsu N, et al. Declining incidence of hepatocellular carcinoma in Osaka, Japan, from 1990 to 2003. Ann Intern Med. 2008;148(11):820–826. doi: 10.7326/0003-4819-148-11-200806030-00004. [DOI] [PubMed] [Google Scholar]
- 6.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2013 Sub (1973-2011) Total U.S., National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, based on the November 2013 submission. 2014.
- 7.Surveillance, Epidemiology and End Results (SEER) Program. SEER*Stat Database: North American Association of Central Cancer Registries (NAACCR) Incidence-CiNA Analytic File, 1995-2009. Bethesda, MD: National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program, Surveillance Systems Branch; 2012. [Google Scholar]
- 8.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45(4):529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Paraskevis D, Magiorkinis G, Magiorkinis E, et al. Dating the origin and dispersal of hepatitis B virus infection in humans and primates. Hepatology. 2013;57(3):908–916. doi: 10.1002/hep.26079. [DOI] [PubMed] [Google Scholar]
- 10.Simmonds P. Reconstructing the origins of human hepatitis viruses. Philos Trans R Soc Lond B Biol Sci. 2001;356(1411):1013–1026. doi: 10.1098/rstb.2001.0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IARC. Hepatitis viruses. Lyon, France: International Agency for Research on Cancer; 1994. [Google Scholar]
- 12.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30(12):2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 13.CDC. Hepatitis B. 2010 http://wwwnc.cdc.gov/travel/yellowbook/2010/chapter-2/hepatitis-b.aspx.
- 14.Marinier E, Barrois V, Larouze B, et al. Lack of perinatal transmission of hepatitis B virus infection in Senegal, West Africa. Journal of Pediatrics. 1985;106(5):843–849. doi: 10.1016/s0022-3476(85)80371-1. [DOI] [PubMed] [Google Scholar]
- 15.Ming L, Thorgeirsson SS, Gail MH, et al. Dominant role of hepatitis B virus and cofactor role of aflatoxin in hepatocarcinogenesis in Qidong, China. Hepatology. 2002;36(5):1214–1220. doi: 10.1053/jhep.2002.36366. [DOI] [PubMed] [Google Scholar]
- 16.Chen G, Lin W, Shen F, Iloeje UH, London WT, Evans AA. Past HBV viral load as predictor of mortality and morbidity from HCC and chronic liver disease in a prospective study. Am J Gastroenterol. 2006;101(8):1797–1803. doi: 10.1111/j.1572-0241.2006.00647.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen CJ, Yang HI, Iloeje UH. Hepatitis B virus DNA levels and outcomes in chronic hepatitis B. Hepatology. 2009;49(5 Suppl):S72–84. doi: 10.1002/hep.22884. [DOI] [PubMed] [Google Scholar]
- 18.McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49(5 Suppl):S45–55. doi: 10.1002/hep.22898. [DOI] [PubMed] [Google Scholar]
- 19.McMahon BJ. Natural history of chronic hepatitis B. Clin Liver Dis. 2010;14(3):381–396. doi: 10.1016/j.cld.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Sumi H, Yokosuka O, Seki N, et al. Influence of hepatitis B virus genotypes on the progression of chronic type B liver disease. Hepatology. 2003;37(1):19–26. doi: 10.1053/jhep.2003.50036. [DOI] [PubMed] [Google Scholar]
- 21.WHO. Hepatitis B. 2010 http://www.who.int/immunization/topics/hepatits_b/en/index.html, 2010.
- 22.Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005;34(6):1329–1339. doi: 10.1093/ije/dyi206. [DOI] [PubMed] [Google Scholar]
- 23.WHO. Hepatitis B Fact Sheet No. 204. 2014 http://www.who.int/mediacentre/factsheets/fs204/en/, 2014.
- 24.Chang MH, You SL, Chen CJ, et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst. 2009;101(19):1348–1355. doi: 10.1093/jnci/djp288. [DOI] [PubMed] [Google Scholar]
- 25.Chiang CJ, Yang YW, You SL, Lai MS, Chen CJ. Thirty-year outcomes of the national hepatitis B immunization program in Taiwan. JAMA. 2013;310(9):974–976. doi: 10.1001/jama.2013.276701. [DOI] [PubMed] [Google Scholar]
- 26.Liang X, Bi S, Yang W, et al. Evaluation of the impact of hepatitis B vaccination among children born during 1992-2005 in China. J Infect Dis. 2009;200(1):39–47. doi: 10.1086/599332. [DOI] [PubMed] [Google Scholar]
- 27.Ang LW, Cutter J, James L, Goh KT. Seroepidemiology of hepatitis B virus infection among adults in Singapore: a 12-year review. Vaccine. 2013;32(1):103–110. doi: 10.1016/j.vaccine.2013.10.057. [DOI] [PubMed] [Google Scholar]
- 28.Organization WH. World Health Organization. Fact Sheet no. 204, Hepatitis B. 2014 http://www.who.int/mediacentre/factsheets/fs204/en/
- 29.Yan H, Peng B, Liu Y, et al. Viral entry of hepatitis B and D viruses and bile salts transportation share common molecular determinants on sodium taurocholate cotransporting polypeptide. J Virol. 2014;88(6):3273–3284. doi: 10.1128/JVI.03478-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding S, Robek MD. Cytidine deamination and cccDNA degradation: A new approach for curing HBV? Hepatology. 2014;60(6):2118–2121. doi: 10.1002/hep.27386. [DOI] [PubMed] [Google Scholar]
- 31.Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 32.Simmonds P. Genetic diversity and evolution of hepatitis C virus--15 years on. J Gen Virol. 2004;85(Pt 11):3173–3188. doi: 10.1099/vir.0.80401-0. [DOI] [PubMed] [Google Scholar]
- 33.Simmonds P, Holmes EC, Cha TA, et al. Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J Gen Virol. 1993;74(Pt 11):2391–2399. doi: 10.1099/0022-1317-74-11-2391. [DOI] [PubMed] [Google Scholar]
- 34.Pybus OG, Barnes E, Taggart R, et al. Genetic history of hepatitis C virus in East Asia. J Virol. 2009;83(2):1071–1082. doi: 10.1128/JVI.01501-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pybus OG, Markov PV, Wu A, Tatem AJ. Investigating the endemic transmission of the hepatitis C virus. Int J Parasitol. 2007;37(8-9):839–849. doi: 10.1016/j.ijpara.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Bostan N, Mahmood T. An overview about hepatitis C: a devastating virus. Crit Rev Microbiol. 2010;36(2):91–133. doi: 10.3109/10408410903357455. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka Y, Hanada K, Mizokami M, et al. Inaugural Article: A comparison of the molecular clock of hepatitis C virus in the United States and Japan predicts that hepatocellular carcinoma incidence in the United States will increase over the next two decades. Proc Natl Acad Sci U S A. 2002;99(24):15584–15589. doi: 10.1073/pnas.242608099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iida F, Iida R, Kamijo H, et al. Chronic Japanese schistosomiasis and hepatocellular carcinoma: ten years of follow-up in Yamanashi Prefecture, Japan. Bull World Health Organ. 1999;77(7):573–581. [PMC free article] [PubMed] [Google Scholar]
- 39.Frank C, Mohamed MK, Strickland GT, et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355(9207):887–891. doi: 10.1016/s0140-6736(99)06527-7. [DOI] [PubMed] [Google Scholar]
- 40.Armstrong GL, Alter MJ, McQuillan GM, Margolis HS. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology. 2000;31(3):777–782. doi: 10.1002/hep.510310332. [DOI] [PubMed] [Google Scholar]
- 41.Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Empirically calibrated model of hepatitis C virus infection in the United States. Am J Epidemiol. 2002;156(8):761–773. doi: 10.1093/aje/kwf100. [DOI] [PubMed] [Google Scholar]
- 42.Seeff LB, Miller RN, Rabkin CS, et al. 45-year follow-up of hepatitis C virus infection in healthy young adults. Ann Intern Med. 2000;132(2):105–111. doi: 10.7326/0003-4819-132-2-200001180-00003. [DOI] [PubMed] [Google Scholar]
- 43.Sofia MJ, Bao D, Chang W, et al. Discovery of a beta-d-2′-deoxy-2′-alpha-fluoro-2′-beta-C-methyluridine nucleotide prodrug (PSI-7977) for the treatment of hepatitis C virus. Journal of medicinal chemistry. 2010;53(19):7202–7218. doi: 10.1021/jm100863x. [DOI] [PubMed] [Google Scholar]
- 44.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(3):211–221. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 45.IARC. Overall evaluations of carcinogenicity: an updating of IARC monographs volumes 1 to 42. 7. Vol. 7. Lyon, France: International Agency for Research on Cancer; 1987. [PubMed] [Google Scholar]
- 46.Wild CP, Hall AJ. Primary prevention of hepatocellular carcinoma in developing countries. Mutat Res. 2000;462(2-3):381–393. doi: 10.1016/s1383-5742(00)00027-2. [DOI] [PubMed] [Google Scholar]
- 47.Plymoth A, Viviani S, Hainaut P. Control of hepatocellular carcinoma through hepatitis B vaccination in areas of high endemicity: perspectives for global liver cancer prevention. Cancer Lett. 2009;286(1):15–21. doi: 10.1016/j.canlet.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 48.Sun CA, Wu DM, Wang LY, Chen CJ, You SL, Santella RM. Determinants of formation of aflatoxin-albumin adducts: a seven-township study in Taiwan. Br J Cancer. 2002;87(9):966–970. doi: 10.1038/sj.bjc.6600584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qian GS, Ross RK, Yu MC, et al. A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People's Republic of China. Cancer Epidemiol Biomarkers Prev. 1994;3(1):3–10. [PubMed] [Google Scholar]
- 50.Ross RK, Yuan JM, Yu MC, et al. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet. 1992;339(8799):943–946. doi: 10.1016/0140-6736(92)91528-g. [DOI] [PubMed] [Google Scholar]
- 51.Kuang SY, Lekawanvijit S, Maneekarn N, et al. Hepatitis B 1762T/1764A mutations, hepatitis C infection, and codon 249 p53 mutations in hepatocellular carcinomas from Thailand. Cancer Epidemiol Biomarkers Prev. 2005;14(2):380–384. doi: 10.1158/1055-9965.EPI-04-0380. [DOI] [PubMed] [Google Scholar]
- 52.Liu Y, Wu F. Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ Health Perspect. 2010;118(6):818–824. doi: 10.1289/ehp.0901388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wild CP, Gong YY. Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis. 2010;31(1):71–82. doi: 10.1093/carcin/bgp264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen JG, Egner PA, Ng D, et al. Reduced aflatoxin exposure presages decline in liver cancer mortality in an endemic region of China. Cancer Prev Res (Phila) 2013;6(10):1038–1045. doi: 10.1158/1940-6207.CAPR-13-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.London WT, McGlynn KA. Liver Cancer. In: Schottenfeld D, Fraumeni JF, editors. Cancer Epidemiology and Prevention. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 56.Donato F, Tagger A, Gelatti U, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155(4):323–331. doi: 10.1093/aje/155.4.323. [DOI] [PubMed] [Google Scholar]
- 57.Kuper H, Tzonou A, Kaklamani E, et al. Tobacco smoking, alcohol consumption and their interaction in the causation of hepatocellular carcinoma. Int J Cancer. 2000;85(4):498–502. [PubMed] [Google Scholar]
- 58.Yuan JM, Govindarajan S, Arakawa K, Yu MC. Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the U.S. Cancer. 2004;101(5):1009–1017. doi: 10.1002/cncr.20427. [DOI] [PubMed] [Google Scholar]
- 59.IARC. Alcohol Drinking. Lyon, France: International Agency for Research on Cancer; 1988. [Google Scholar]
- 60.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, D.C.: American Institute for Cancer Research; 2007. [Google Scholar]
- 61.Turati F, Galeone C, Rota M, et al. Alcohol and liver cancer: a systematic review and meta-analysis of prospective studies. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2014;25(8):1526–1535. doi: 10.1093/annonc/mdu020. [DOI] [PubMed] [Google Scholar]
- 62.Alcorn T. Mongolia's struggle with liver cancer. Lancet. 2011;377(9772):1139–1140. doi: 10.1016/s0140-6736(11)60448-0. [DOI] [PubMed] [Google Scholar]
- 63.Chen J, Han Y, Xu C, Xiao T, Wang B. Effect of type 2 diabetes mellitus on the risk for hepatocellular carcinoma in chronic liver diseases: a meta-analysis of cohort studies. Eur J Cancer Prev. 2014 doi: 10.1097/CEJ.0000000000000038. [DOI] [PubMed] [Google Scholar]
- 64.El-Serag HB, Richardson PA, Everhart JE. The role of diabetes in hepatocellular carcinoma: a case-control study among United States Veterans. Am J Gastroenterol. 2001;96(8):2462–2467. doi: 10.1111/j.1572-0241.2001.04054.x. [DOI] [PubMed] [Google Scholar]
- 65.Wang C, Wang X, Gong G, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130(7):1639–1648. doi: 10.1002/ijc.26165. [DOI] [PubMed] [Google Scholar]
- 66.Wang P, Kang D, Cao W, Wang Y, Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes/metabolism research and reviews. 2012;28(2):109–122. doi: 10.1002/dmrr.1291. [DOI] [PubMed] [Google Scholar]
- 67.Saunders D, Seidel D, Allison M, Lyratzopoulos G. Systematic review: the association between obesity and hepatocellular carcinoma - epidemiological evidence. Aliment Pharmacol Ther. 2010;31(10):1051–1063. doi: 10.1111/j.1365-2036.2010.04271.x. [DOI] [PubMed] [Google Scholar]
- 68.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97(7):1005–1008. doi: 10.1038/sj.bjc.6603932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jinjuvadia R, Patel S, Liangpunsakul S. The association between metabolic syndrome and hepatocellular carcinoma: systemic review and meta-analysis. J Clin Gastroenterol. 2014;48(2):172–177. doi: 10.1097/MCG.0b013e3182a030c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 71.James WP. The epidemiology of obesity: the size of the problem. J Intern Med. 2008;263(4):336–352. doi: 10.1111/j.1365-2796.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- 72.IARC. Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinogen risks Humans. 2004;83:1–1438. [PMC free article] [PubMed] [Google Scholar]
- 73.The Health Consequences of Smoking - 50 Years of Progress: A Report of the Sugeon General. 2014 http://www.surgeongeneral.gov/library/reports/50-years-of-progress/
- 74.Lee YC, Cohet C, Yang YC, Stayner L, Hashibe M, Straif K. Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. Int J Epidemiol. 2009;38(6):1497–1511. doi: 10.1093/ije/dyp280. [DOI] [PubMed] [Google Scholar]
- 75.Bravi F, Bosetti C, Tavani A, Gallus S, La Vecchia C. Coffee reduces risk for hepatocellular carcinoma: an updated meta-analysis. Clin Gastroenterol Hepatol. 2013;11(11):1413–1421 e1411. doi: 10.1016/j.cgh.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 76.Fon Sing M, Yang WS, Gao S, Gao J, Xiang YB. Epidemiological studies of the association between tea drinking and primary liver cancer: a meta-analysis. Eur J Cancer Prev. 2011;20(3):157–165. doi: 10.1097/CEJ.0b013e3283447497. [DOI] [PubMed] [Google Scholar]
- 77.Bamia C, Lagiou P, Jenab M, et al. Coffee, tea and decaffeinated coffee in relation to hepatocellular carcinoma in a European population: Multicentre, prospective cohort study. Int J Cancer. 2014 doi: 10.1002/ijc.29214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Setiawan VW, Wilkens LR, Lu SC, Hernandez BY, Le Marchand L, Henderson BE. Association of Coffee Intake With Reduced Incidence of Liver Cancer and Death From Chronic Liver Disease in the US Multiethnic Cohort. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cavin C, Holzhaeuser D, Scharf G, Constable A, Huber WW, Schilter B. Cafestol and kahweol, two coffee specific diterpenes with anticarcinogenic activity. Food Chem Toxicol. 2002;40(8):1155–1163. doi: 10.1016/s0278-6915(02)00029-7. [DOI] [PubMed] [Google Scholar]
- 80.Muriel P, Arauz J. Coffee and liver diseases. Fitoterapia. 2010;81(5):297–305. doi: 10.1016/j.fitote.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 81.Boettler U, Sommerfeld K, Volz N, et al. Coffee constituents as modulators of Nrf2 nuclear translocation and ARE (EpRE)-dependent gene expression. The Journal of nutritional biochemistry. 2011;22(5):426–440. doi: 10.1016/j.jnutbio.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 82.Ludwig IA, Clifford MN, Lean ME, Ashihara H, Crozier A. Coffee: biochemistry and potential impact on health. Food & function. 2014;5(8):1695–1717. doi: 10.1039/c4fo00042k. [DOI] [PubMed] [Google Scholar]
- 83.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9(6):429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res. 2003;9(1):10–19. [PubMed] [Google Scholar]
- 86.Gazzerro P, Proto MC, Gangemi G, et al. Pharmacological actions of statins: a critical appraisal in the management of cancer. Pharmacological reviews. 2012;64(1):102–146. doi: 10.1124/pr.111.004994. [DOI] [PubMed] [Google Scholar]
- 87.Chiu HF, Ho SC, Chen CC, Yang CY. Statin use and the risk of liver cancer: a population-based case-control study. Am J Gastroenterol. 2011;106(5):894–898. doi: 10.1038/ajg.2010.475. [DOI] [PubMed] [Google Scholar]
- 88.Lai SW, Liao KF, Lai HC, Muo CH, Sung FC, Chen PC. Statin use and risk of hepatocellular carcinoma. European journal of epidemiology. 2013;28(6):485–492. doi: 10.1007/s10654-013-9806-y. [DOI] [PubMed] [Google Scholar]
- 89.Leung HW, Chan AL, Lo D, Leung JH, Chen HL. Common cancer risk and statins: a population-based case-control study in a Chinese population. Expert opinion on drug safety. 2013;12(1):19–27. doi: 10.1517/14740338.2013.744392. [DOI] [PubMed] [Google Scholar]
- 90.Tsan YT, Lee CH, Ho WC, Lin MH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J Clin Oncol. 2013;31(12):1514–1521. doi: 10.1200/JCO.2012.44.6831. [DOI] [PubMed] [Google Scholar]
- 91.Tsan YT, Lee CH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol. 2012;30(6):623–630. doi: 10.1200/JCO.2011.36.0917. [DOI] [PubMed] [Google Scholar]
- 92.Friis S, Poulsen AH, Johnsen SP, et al. Cancer risk among statin users: a population-based cohort study. Int J Cancer. 2005;114(4):643–647. doi: 10.1002/ijc.20758. [DOI] [PubMed] [Google Scholar]
- 93.Marelli C, Gunnarsson C, Ross S, et al. Statins and risk of cancer: a retrospective cohort analysis of 45,857 matched pairs from an electronic medical records database of 11 million adult Americans. Journal of the American College of Cardiology. 2011;58(5):530–537. doi: 10.1016/j.jacc.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 94.El-Serag HB, Johnson ML, Hachem C, Morgana RO. Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes. Gastroenterology. 2009;136(5):1601–1608. doi: 10.1053/j.gastro.2009.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khurana V, Saluja A, Caldito G, Fort C, Schiff ER. Statins are protective against hepatocellular cancer in patients with hepatitis C virus infection: half a million U.S. veterans' study. Gastroenterology. 2005;128:A714. [Google Scholar]
- 96.Friedman GD, Flick ED, Udaltsova N, Chan J, Quesenberry CP, Jr, Habel LA. Screening statins for possible carcinogenic risk: up to 9 years of follow-up of 361,859 recipients. Pharmacoepidemiology and drug safety. 2008;17(1):27–36. doi: 10.1002/pds.1507. [DOI] [PubMed] [Google Scholar]
- 97.McGlynn KA, Divine GW, Sahasrabuddhe VV, et al. Statin use and risk of hepatocellular carcinoma in a U.S. population. Cancer epidemiology. 2014 doi: 10.1016/j.canep.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bjorkhem-Bergman L, Backheden M, Soderberg Lofdal K. Statin treatment reduces the risk of hepatocellular carcinoma but not colon cancer-results from a nationwide case-control study in Sweden. Pharmacoepidemiology and drug safety. 2014;23(10):1101–1106. doi: 10.1002/pds.3685. [DOI] [PubMed] [Google Scholar]
- 99.Pradelli D, Soranna D, Scotti L, et al. Statins and primary liver cancer: a meta-analysis of observational studies. Eur J Cancer Prev. 2012 doi: 10.1097/CEJ.0b013e328358761a. [DOI] [PubMed] [Google Scholar]
- 100.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins Are Associated With a Reduced Risk of Hepatocellular Cancer: A Systematic Review and Meta-analysis. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 101.Franciosi M, Lucisano G, Lapice E, Strippoli GF, Pellegrini F, Nicolucci A. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PloS one. 2013;8(8):e71583. doi: 10.1371/journal.pone.0071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Singh S, Singh PP, Singh AG, Murad MH, McWilliams RR, Chari ST. Anti-diabetic medications and risk of pancreatic cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108(4):510–519. doi: 10.1038/ajg.2013.7. quiz 520. [DOI] [PubMed] [Google Scholar]
- 103.Zhang H, Gao C, Fang L, Zhao HC, Yao SK. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients: a meta-analysis. Scand J Gastroenterol. 2013;48(1):78–87. doi: 10.3109/00365521.2012.719926. [DOI] [PubMed] [Google Scholar]
- 104.Hagberg KW, McGlynn KA, Sahasrabuddhe VV, Jick S. Anti-diabetic medications and risk of primary liver cancer in persons with type II diabetes. Br J Cancer. 2014;111(9):1710–1717. doi: 10.1038/bjc.2014.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Conaghan PG. A turbulent decade for NSAIDs: update on current concepts of classification, epidemiology, comparative efficacy, and toxicity. Rheumatology international. 2012;32(6):1491–1502. doi: 10.1007/s00296-011-2263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cervello M, Foderaa D, Florena AM, et al. Correlation between expression of cyclooxygenase-2 and the presence of inflammatory cells in human primary hepatocellular carcinoma: possible role in tumor promotion and angiogenesis. World journal of gastroenterology : WJG. 2005;11(30):4638–4643. doi: 10.3748/wjg.v11.i30.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fodera D, D'Alessandro N, Cusimano A, et al. Induction of apoptosis and inhibition of cell growth in human hepatocellular carcinoma cells by COX-2 inhibitors. Annals of the New York Academy of Sciences. 2004;1028:440–449. doi: 10.1196/annals.1322.052. [DOI] [PubMed] [Google Scholar]
- 108.Leng J, Han C, Demetris AJ, Michalopoulos GK, Wu T. Cyclooxygenase-2 promotes hepatocellular carcinoma cell growth through Akt activation: evidence for Akt inhibition in celecoxib-induced apoptosis. Hepatology. 2003;38(3):756–768. doi: 10.1053/jhep.2003.50380. [DOI] [PubMed] [Google Scholar]
- 109.Cibere J, Sibley J, Haga M. Rheumatoid arthritis and the risk of malignancy. Arthritis and rheumatism. 1997;40(9):1580–1586. doi: 10.1002/art.1780400906. [DOI] [PubMed] [Google Scholar]
- 110.Coogan PF, Rosenberg L, Palmer JR, et al. Nonsteroidal anti-inflammatory drugs and risk of digestive cancers at sites other than the large bowel. Cancer Epidemiol Biomarkers Prev. 2000;9(1):119–123. [PubMed] [Google Scholar]
- 111.Sahasrabuddhe VV, Gunja MZ, Graubard BI, et al. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst. 2012;104(23):1808–1814. doi: 10.1093/jnci/djs452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Knights KM, Mangoni AA, Miners JO. Defining the COX inhibitor selectivity of NSAIDs: implications for understanding toxicity. Expert review of clinical pharmacology. 2010;3(6):769–776. doi: 10.1586/ecp.10.120. [DOI] [PubMed] [Google Scholar]
- 113.Jankowska H, Hooper P, Jankowski JA. Aspirin chemoprevention of gastrointestinal cancer in the next decade. A review of the evidence. Polskie Archiwum Medycyny Wewnetrznej. 2010;120(10):407–412. [PubMed] [Google Scholar]
- 114.Kern MA, Schubert D, Sahi D, et al. Proapoptotic and antiproliferative potential of selective cyclooxygenase-2 inhibitors in human liver tumor cells. Hepatology. 2002;36(4 Pt 1):885–894. doi: 10.1053/jhep.2002.36125. [DOI] [PubMed] [Google Scholar]
- 115.Imaeda AB, Watanabe A, Sohail MA, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. The Journal of clinical investigation. 2009;119(2):305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]


