Abstract
Human antinuclear autoantibodies (ANAs) targeting the dense fine speckled (DFS) nuclear protein DFS70, commonly known as lens epithelium derived growth factor p75 (LEDGFp75), present a clinical puzzle since their significance remains elusive. While their frequencies are low in ANA-positive autoimmune rheumatic diseases, they are relatively elevated in clinical laboratory referrals, diverse inflammatory conditions, and ‘apparently’ healthy individuals. We reported previously that DFS70/LEDGFp75 is an autoantigen in prostate cancer that closely interacts with another 70 kD DFS nuclear protein, methyl CpG binding protein 2 (MeCP2). This led us to investigate if anti-DFS sera exclusively target DFS70/LEDGFp75 or also recognize MeCP2. Using several complementary autoantibody detection platforms and cellular/molecular approaches we evaluated 65 human sera producing anti-DFS autoantibodies. Our results show that these antibodies are highly specific for DFS70/LEDGFp75 and do not target MeCP2. Establishing the specificity of anti-DFS autoantibodies has implications for increasing our understanding their biological significance and clinical utility.
Keywords: autoantibodies, autoimmunity, dense fine speckles, DFS70, LEDGFp75, MeCP2
1. Introduction
Circulating antinuclear autoantibodies (ANAs) are an immunological hallmark of systemic autoimmune rheumatic diseases, but can also be found in patients with cancer and other inflammatory and autoimmune conditions [1,2]. These autoantibodies are typically detected by indirect immunofluorescence (IIF) microscopy using the HEp-2-ANA test, which remains the recommended method for ANA screening [3]. Their diagnostic and prognostic value, as well as their utility as probes in molecular cell biology, have been widely investigated [1].
The nuclear dense fine speckled (DFS) pattern is one of the most commonly observed IIF-ANA patterns in patients who are ANA-positive, have no evident diagnosis of ANA-associated rheumatic autoimmune diseases (AARD), and have been referred to clinical laboratories for ANA testing because of non-specific complaints and symptoms [4-6]. This pattern is produced by autoantibodies recognizing uniformly distributed DFS located throughout interphase nuclei, often excluding the nucleoli, and in condensed mitotic chromosomes [6,7]. These anti-DFS autoantibodies are typically of the IgG class (although IgE antibodies have been also detected), and react by immunoblotting with a nuclear protein of approximately 70 kD protein designated DFS70 [4,7,8], most commonly known as lens epithelium-derived growth factor p75 (LEDGF/p75) [9]. Anti-DFS70/LEDGFp75 autoantibodies have been found at varied frequencies (typically ranging from 6% to 20%), and often at high titers (≥1:640), in diverse inflammatory and atopic conditions, prostate cancer, certain non-AARD autoimmune conditions, eye diseases, as well as in apparently healthy individuals [9-12].
In addition to its possible role in autoimmunity, DFS70/LEDGFp75 has emerged in recent years as a key protein that facilitates the integration of human immunodeficiency virus 1 (HIV-1) into host chromatin by interacting with the HIV-integrase and tethering it to transcriptionally active sites [13,14]. This autoantigen has also surfaced as a key stress oncoprotein upregulated in prostate cancer and other cancer types that promotes cell proliferation, resistance to cell death and chemotherapy, DNA repair, clonogenicity, angiogenesis, and tumor growth [15-24]. DFS70/LEDGFp75 normally functions as a transcription coactivator that is upregulated and activated as part of the cellular response to environmental stressors such as oxidative stress, heat, UV radiation, and certain drugs and viruses [15-18,22,25-27]. It is known to interact with specific transcription factors and chromatin associated proteins at transcriptionally active sites to regulate the expression of specific stress, inflammatory, antioxidant, and cancer-associated genes [27-33]. Its cellular interacting partners include pogo transposable element PogZ, c-Myc interacting protein JPO2, Cdc7-activator of S-phase kinase (ASK), leukemia-associated transcription complex Menin-MLL (mixed lineage leukemia), and the methyl CpG binding protein MeCP2 [34-38]. These interacting partners co-localize with DFS70/LEDGFp75 in the nucleus, displaying the characteristic DFS-IIF pattern.
The target antigen specificity of serum autoantibodies producing the typical DFS-IIF pattern remains to be firmly established. An emerging question is whether all patient sera that are positive for the DFS-IIF pattern specifically target DFS70/LEDGFp75, or also contain antibodies against interacting partners of this autoantigen, or other related proteins, that share the same nuclear localization pattern. For instance, Bizzaro and colleagues [39] reported that over 20% of human sera showing the DFS-IIF pattern in HEp-2 substrates also reacted strongly against reticular fibers of the lens and the corneal epithelium. Immunohistochemical analysis of these putative anti-DFS70/LEDGFp75 positive sera in mouse lens tissues revealed different distribution patterns, suggesting that companion autoantibodies targeting other autoantigens may also be present in these sera [39]. These authors suggested that the characteristic DFS-IIF pattern produced by anti-DFS70/LEDGFp75 autoantibodies in HEp-2 cells might also be produced by companion antibodies targeting interacting partners of this autoantigen. They also pointed that it is not easy to recognize the DFS pattern solely by IIF microscopy in HEp-2 cells and that alternative platforms are necessary to accurately detect the anti-DFS70/LEDGFp75 antibodies [40], a conclusion that has been supported recently by other groups [41,42]. Addressing the target antigen specificity of the DFS-IIF autoantibody pattern is therefore important to advance our understanding of its clinical utility and biological significance.
MeCP2 is a cellular interacting partner of DFS70/LEDGFp75 that is mutated in Rett syndrome and functions as a chromatin remodeling protein that contributes to gene activation or repression depending on the cellular and molecular context [43]. Our group demonstrated previously that MeCP2 interacts with DFS70/LEDGFp75 in cancer cells to regulate the promoter activity of heat shock protein 27 (HSP27) gene [38]. MeCP2 displayed a nuclear DFS-IIF pattern strikingly similar to that of DFS70/LEDGFp75, and the two proteins co-localized in the nucleus when targeted endogenously by specific antibodies and when ectopically overexpressed in cells [38]. Furthermore, both proteins are recognized by antibodies in immunoblots around the 70-75 kD region. These observations raised the possibility that MeCP2 could also be a target of the anti-DFS sera. In this study, we determined if human sera presenting the DFS-IIF pattern specifically react with either DFS70/LEDGFp75 or MeCP2, or target both proteins.
2. Materials and Methods
2.1. Cell lines and antibodies
PC3 and DU145 prostate cancer cell lines and U2OS osteoblastoma cells were acquired from the American Type Culture Collection (ATCC). Cell lines were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (Corning), penicillin-streptomycin (Corning), and gentamycin (Gibco, Life Technologies) as recommended by the supplier in a humidified incubator with 5% CO2 at 37°C. The following antibodies were used in this study: mouse monoclonals anti-MeCP2 (Abcam) and anti-β-actin (Sigma-Aldrich); rabbit polyclonals anti-DFS70/LEDGFp75 (Bethyl Laboratories Inc.) and anti-MeCP2 (Proteintech group); and fluorescein isothiocyanate (FITC)-conjugated goat anti-human IgG (Invitrogen), and FITC- and rhodamine-conjugated goat anti-rabbit IgG (Invitrogen) secondary antibodies.
2.2. Human sera and ANA testing
Seventy-one human serum samples with no patient identifiers were obtained from serum banks in Sao Paulo, Brazil (Dr. Luis E. Andrade), Luxembourg (a kind gift from Professor Rene-Louis Humbel), and La Jolla, California, USA (a kind gift from Dr. Eng M. Tan). These sera had been previously determined by the donor laboratories to have a suspected DFS-IIF pattern in HEp-2-ANA substrates, and were obtained from patients with diverse disease conditions [4,7]. They were examined under the approval of the Institutional Review Board of Loma Linda University. Sera were re-evaluated in our laboratory for the presence of ANAs using the NOVA Lite HEp-2-ANA kit/substrate slides (Inova Diagnostics, San Diego, CA) according to the manufacturer's instructions. Briefly, human sera and commercial anti-DFS70/LEDGFp75 or MeCP2 antibodies were added to individual wells of HEp-2-ANA slides. Serum and antibodies were diluted at 1:80 or 1:160 in PBS for initial ANA screening and further diluted in subsequent tests according to the strength of their reactivity. FITC- or rhodamine-conjugated secondary antibodies were used at 1:100 dilution for the detection of primary antibodies. Visualization of ANA patterns and image acquisition were done on a Keyence BZ9000 Biorevo fluorescence microscope.
2.3. Confocal microscopy
Endogenous DFS70/LEDGFp75 and MeCP2 were visualized in PC3 and U2OS cells using specific antibodies as described previously [38]. Briefly, cells were fixed with 4% formaldehyde and permeabilized with 0.5% Triton X-100. Following fixation/permeabilization, cells were co-incubated with validated human anti-DFS70/LEDGFp75 sera and a rabbit anti-MeCP2 antibody, both used at 1:200 dilution, at room temperature for 30 minutes. After incubation with corresponding secondary antibodies labeled with either FITC or rhodamine, cells were mounted with mounting medium (Vectashield, Vector Labs) containing 4′,6-diamidino-2-phenylindole (DAPI). Antibody reactivity was visualized by confocal microscopy using a Zeiss LSM 710 NLO microscope with 63× oil immersion objective and appropriate filters. Image analysis and acquisition were done using ImageJ software.
2.4. Immunoblotting analysis
Immunoblotting procedures were essentially conducted as previously described [38]. Concisely, SDS-PAGE (NuPAGE 4-12% gels, Life Technologies) was used to separate total cellular proteins followed by transfer to nitrocellulose membranes (GE Healthcare Life Sciences). Membranes were cut into strips and blocked overnight with 5% dry milk solution in TBS-T buffer (20 mM Tris-HCl, pH 7.6, 140 mM NaCl, 0.15% Tween 20) and individual strips were then probed with different patient sera or commercial antibodies for 2 h. After several washes with TBS-T, the individual membrane strips were incubated with appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies. Detection of antibodies bound to proteins was achieved by enhanced chemiluminescence (Thermo Fisher Scientific Pierce).
2.5. QUANTA-Flash DFS70 chemiluminescent immunoassay
Sera that exhibited the DFS-IIF pattern and recognized a 75 kD protein band by immunoblotting were further analyzed by the QUANTA Flash® CIA to confirm the presence of anti-DFS70/LEDGFp75 antibodies as described previously [11]. This assay is hereafter referred to as DFS70-CIA and uses recombinant DFS70/LEDGFp75 antigen coated onto paramagnetic beads. Briefly, the relative light units (RLUs) were proportional to the amount of isoluminol conjugate bound to the anti-human IgG, which in turn was proportional to the amount of serum autoantibodies bound to recombinant DFS70/LEDGFp75 antigen on the beads. Using a standard curve, RLU values were converted into calculated units (CU). Sera with CUs less than 20 were considered negative for DFS70/LEDGFp75 antibodies while those with CUs 20-100, 101-300, and >300 were considered low positive, moderately positive, and high positive, respectively.
2.6. Generation of PC3 cells with stable overexpression and depletion of DFS70/LEDGFp75
PC3 cells stably overexpressing DFS70/LEDGFp75 were generated by transduction using retroviral vectors encoding the full-length protein (pLNC_LEDGF BC-Ires-bsd) as described previously [44]. PC3 cells with stable depletion of DFS70/LEDGFp75 were generated using a miRNA-based short hairpin interfering RNA (shRNA) cloned into pLNC_MCS plasmid (pLNC-2× miRNA_L3 ZeoR) as described previously [44]. A shSCR (scrambled) sequence served as the non-interfering shRNA control. Transduced cells were selected with zeocin (200μg/ml). DFS70/LEDGFp75 overexpression or depletion was assessed by RT-PCR and immunoblotting.
2.7. Immunoadsorption of anti-DFS70/LEDGFp75 antibodies with recombinant antigen
Anti-DFS70/LEDGFp75 autoantibody reactivity in HEp-2 substrates was diminished by immunoadsorption with a recombinant DFS70/LEDGFp75 peptide (component of HEp-2 Select, Inova Diagnostics) as described previously [6]. Briefly, the DNA sequence encoding a DFS70/LEDGFp75 peptide corresponding to the autoepitope (amino acids 349-435) was cloned into pIExBac-3 expression vector and expressed in insect Sf9 cells or E. coli. The purified recombinant DFS70/LEDGFp75 peptide was diluted in TBS-T at final concentration of approximately 0.4 mg/ml and the resulting solution was then used as sample diluent to adsorb the anti-DFS sera. Sera diluted at 1:80 in TBS-T with and without the peptide were then evaluated by the HEp-2-ANA test and by immunoblotting.
3. Results
3.1. Detection of anti-DFS70/LEDGFp75 autoantibodies in human anti-DFS sera
Seventy-one human sera suspected of containing anti-DFS autoantibodies were re-examined by IIF microscopy in HEp-2-ANA test slides. Sixty-five of these sera recognized the characteristic DFS-IIF pattern, defined by interphasic nuclear DFS and strong staining of condensed mitotic chromosomes (Fig. 1A), whereas 6 recognized other ANA patterns. The DFS-IIF pattern was observed at various titers and was the only ANA pattern observed in all but one of the 65 sera. This particular serum contained both anti-DFS and anti-p80 coilin antibodies, which have been found to co-exist in some patients [45]. Sixty-four of the DFS-IIF positive sera were available for further analysis by DFS70-CIA, of which 61 (95.3%) tested positive for the presence of anti-DFS70/LEDGFp75 autoantibodies and 3 (4.7%) tested negative (Figs. 1B,C). The 6 sera mentioned above that recognized other IIF patterns also tested negative for DFS70-CIA. Twenty-four (37.5%) of the 61 sera were considered to be low positive, 21 (32.8%) moderately positive, and 16 (25%) high positive by DFS70-CIA based on CU values (Fig. 1C).
Figure 1. Detection of anti-DFS70/LEDGFp75 autoantibodies in human sera.
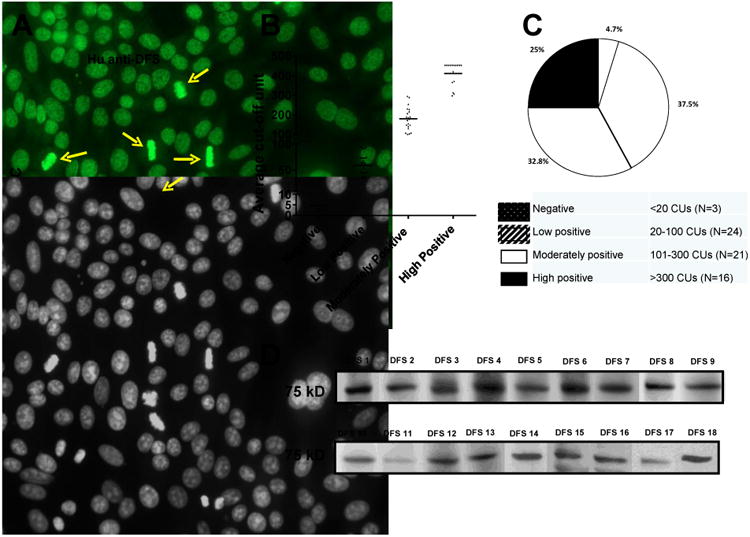
A) Representative human anti-DFS serum displaying the characteristic dense fine speckled nuclear pattern (FITC) in HEp-2-ANA slides. Yellow arrows point to the staining of mitotic chromosomes. Corresponding DAPI staining is shown in black and white for better visualization of chromatin. B) Scatter plot of sera with suspected anti-DFS autoantibodies that were tested using the DFS70-QuantaFlash chemiluminescent immunoassay (DFS70-CIA). C) Pie chart of data from the scatter plot. D) Blots showing immunoreactivity of selected anti-DFS sera against a 75 kD protein in whole PC3 cell lysates.
Next we evaluated the reactivity of the sera by immunoblotting using whole lysates from PC3 prostate cancer cells. We chose this cell line because in previous studies our group demonstrated that DFS70/LEDGFp75 is overexpressed in prostate cancer cells and tumor tissues, and is the target of autoantibodies in certain patients with prostate cancer [15,16,46]. MeCP2 is also expressed at elevated levels in these cell lines [38]. Immunoblotting analysis revealed that the 61 sera that tested positive by DFS70-CIA also tested positive, albeit at varying intensities, for the presence of anti-DFS70/LEDGFp75 autoantibodies (Table 1; also see representative serum immunoreactivities in Fig. 1D). The 3 sera that tested negative by DFS70-CIA showed very weak or negative reactivity against a 75 kD band by immunoblotting (Table 1). Table 1 shows a strong concordance between anti-DFS70/LEDGFp75 antibody specificity by HEp-2-ANA test, DFS70-CIA, and immunoblotting. Thus, using different antibody detection platforms we were able to confirm the presence of these autoantibodies in most human sera with suspected DFS-IIF pattern (64 of 71) that were available to us at the time we initiated this study.
Table 1. Detection of anti-DFS70/LEDGFp75 autoantibodies by immunoblotting and DFS70-CIA in sera.
| Serum | Immunoblotting | DFS70-CIA |
|---|---|---|
| DFS 1 | Strong | High Positive |
| DFS 2 | Strong | Positive |
| DFS 3 | Strong | Positive |
| DFS 4 | Strong | High Positive |
| DFS 5 | Strong | Positive |
| DFS 6 | Strong | High Positive |
| DFS 7 | Strong | Positive |
| DFS 8 | Strong | High Positive |
| DFS 9 | Strong | High Positive |
| DFS 10 | Strong | High Positive |
| DFS 11 | Moderate | Low Positive |
| DFS 12 | Strong | Positive |
| DFS 13 | Moderate | Low Positive |
| DFS 14 | Strong | Positive |
| DFS 15 | Weak | Low Positive |
| DFS 16 | Strong | Positive |
| DFS 17 | Strong | Low Positive |
| DFS 18 | Strong | Positive |
| DFS 19 | Strong | High Positive |
| DFS 20 | Moderate | Positive |
| DFS 21 | Strong | High Positive |
| DFS 22 | Moderate | Positive |
| DFS 23 | Weak | Low Positive |
| DFS 24 | Weak | Low Positive |
| DFS 25 | Moderate | Positive |
| DFS 26 | Moderate | High Positive |
| DFS 27 | Moderate | Positive |
| DFS 28 | Moderate | Positive |
| DFS 29 | Moderate | High Positive |
| DFS 30 | Moderate | High Positive |
| DFS 31 | Weak | Low Positive |
| DFS 32 | Moderate | Positive |
| DFS 33 | Weak | Low Positive |
| DFS 34 | Moderate | Positive |
| DFS 35 | Moderate | Positive |
| DFS 36 | Weak | Low Positive |
| DFS 37 | Weak | Low Positive |
| DFS 38 | Moderate | High Positive |
| DFS 39 | Moderate | Positive |
| DFS 40 | Weak | Low Positive |
| DFS 41 | Very weak | Negative |
| DFS 42 | Moderate | Positive |
| DFS 43 | Weak | Low Positive |
| DFS 44 | Weak | Low Positive |
| DFS 45 | Moderate | High Positive |
| DFS 46 | Weak | Low Positive |
| DFS 47 | Weak | Low Positive |
| DFS 48 | Weak | High Positive |
| DFS 49 | Weak | Low Positive |
| DFS 50 | Weak | Low Positive |
| DFS 51 | Weak | Low Positive |
| DFS 52 | Very weak | Negative |
| DFS 53 | Moderate | Positive |
| DFS 54 | Weak | Low Positive |
| DFS 55 | Weak | Low Positive |
| DFS 56 | Very weak | Negative |
| DFS 57 | Weak | Low Positive |
| DFS 58 | Moderate | High Positive |
| DFS 59 | Weak | Low Positive |
| DFS 60 | Strong | High Positive |
| DFS 61 | Weak | Low Positive |
| DFS 62 | Moderate | Positive |
| DFS 63 | Weak | Low Positive |
| DFS 64 | Moderate | Positive |
CIA, chemiluminescence immunoassay; DFS, dense fine speckles; DFS70, dense fine speckled protein of 70 kD; LEDGF/p75, lens epithelium derived growth factor protein of 75 kD
3.2. Similarities in the reactivity of antibodies recognizing DFS70/LEDGFp75 and MeCP2
To determine if the DFS-IIF positive sera also contained antibodies to MeCP2, an interacting partner of DFS70/LEDGFp75 in prostate cancer cells that also migrates around the 70-75 kD region [38], we compared the reactivity of human and commercial antibodies to these two proteins by IIF microscopy and immunoblotting. As expected, the classical DFS-IIF pattern displayed by the anti-DFS70/LEDGFp75 human sera was also reproduced in HEp-2 cells using a commercial rabbit anti-DFS70/LEDGFp75 antibody (Fig. 2A). Interestingly, a rabbit antibody recognizing MeCP2 also showed a very similar DFS-IIF reactivity in HEp-2 cells. Immunoblotting analysis using PC3 and DU145 prostate cancer cell lysates, revealed that two commercial anti-MeCP2 antibodies also recognized a doublet protein around the 75 kD region, similar to the reactivity of the anti-DFS70/LEDGFp75 human and rabbit antibodies (Fig. 2B). One of the anti-MeCP2 antibodies also reacted weakly with a 55 kD band.
Figure 2. Antibodies to DFS70/LEDGFp75 and MeCP2 display similar features in their reactivity.
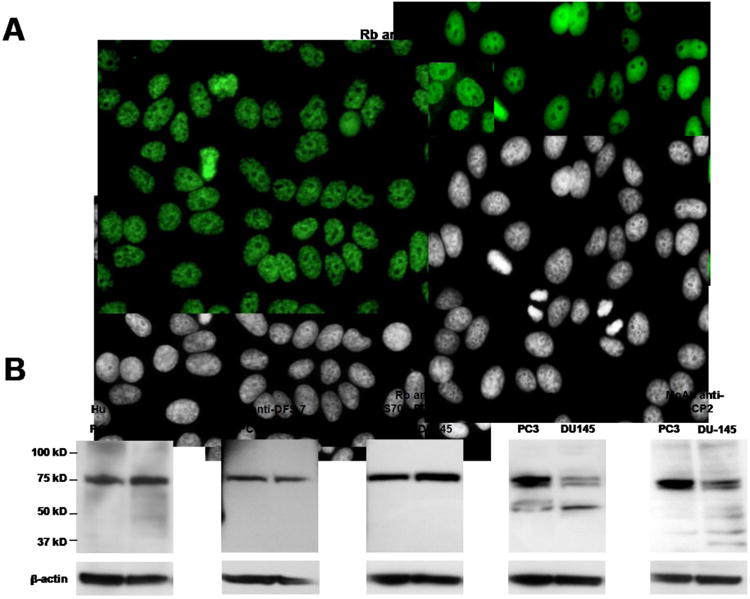
A) DFS-IIF pattern (FITC) in HEp-2-ANA slides produced by human anti-DFS sera (a representative serum is shown), rabbit antibody to DFS70/LEDGFp75, and rabbit antibody to MeCP2. Corresponding DAPI images are shown in black and white for better visualization of chromatin. B) Immunoblots showing the reactivity of human anti-DFS sera (two representative sera are shown), rabbit antibody to DFS70/LEDGFp75, rabbit antibody to MeCP2, and mouse monoclonal antibody to MeCP2, against whole protein lysates from PC3 and DU145 cells. Beta-actin was used as loading control.
To further explore the similarities in IIF reactivity between antibodies to DFS70/LEDGFp75 and MeCP2, we co-incubated a prototype human anti-DFS70/LEDGFp75 serum (positive by HEp-2-ANA test, DFS70-CIA, and immunoblotting) with the rabbit anti-MeCP2 antibody in HEp-2 ANA slides. We observed that the two antibodies produced a strikingly similar DFS-IIF pattern with their target antigens co-localizing in HEp-2 cells (Fig. 3A). We confirmed this co-localization using several prototype anti-DFS human sera (data not shown). Control experiments with single antibody incubation revealed that each antibody produced the DFS-IIF pattern independently, without crossreactivity or bleed-through when the FITC or rhodamine signals were imaged separately (Fig. 3B,C). Confocal microscopy analysis of PC3 and U2OS cells co-incubated with the two antibodies (human anti-DFS serum and rabbit anti-MeCP2) also showed strong co-localization in nuclear DFS and metaphase chromosomes (Fig. 3D,E). This is consistent with our previous observation that DFS70/LEDGFp75 and MeCP2 co-localize in cancer cells and together regulate HSP27 promoter activity [38].
Figure 3. Nuclear colocalization of DFS70/LEDGFp75 and MeCP2.
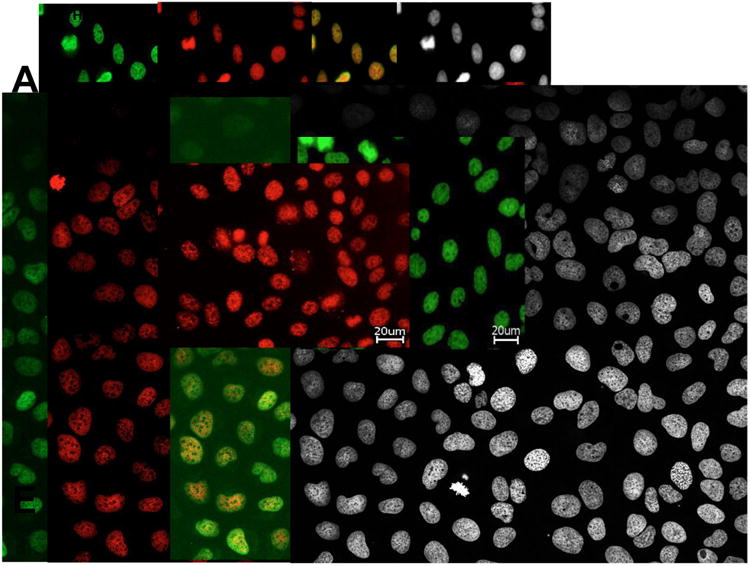
A) A representative human anti-DFS serum displays the characteristic DFS-IIF pattern in HEp-2-ANA slides, detected with a FITC-labeled secondary anti-human antibody (green). This pattern was also produced by a rabbit anti-MeCP2 antibody co-incubated with human anti-DFS serum in the same cells, and detected with rhodamine-labeled secondary anti-rabbit antibody (red). Merged images show the yellow-orange staining typical of colocalization. B) Staining of HEp-2 cells with only human anti-DFS serum. C) Staining of HEp-2 cells with only rabbit anti-MeCP2 antibody. D) Colocalization of DFS70/LEDGFp75 and MeCP2 in PC3 cells (D) and U2OS cells (E), each simultaneously incubated with human anti-DFS serum (green) and anti-rabbit MeCP2 antibody (red). Merged images show the yellow-orange staining typical of colocalization. Corresponding DAPI images are shown in black and white for better visualization of chromatin.
3.3. Immunoblotting analysis of anti-DFS serum reactivity against proteins from cells with stable overexpression or depletion of DFS70/LEDGF/p75
In order to determine if human sera that were immunoreactive with DFS70/LEDGFp75 also contained antibodies to MeCP2, we selected a sub-group of 18 representative sera that produced the DFS-IIF pattern in HEp-2-ANA test slides, reacted with a 75 kD protein band by immunoblotting (Fig. 1D), and tested positive by DFS70-CIA. To confirm the specificity of these sera for anti-DFS70/LEDGFp75 autoantibodies, we first tested them by immunoblotting against lysates from PC3 cells with stable overexpression or depletion of DFS70/LEDGF/p75 protein. We hypothesized that if the sera reacted specifically with DFS70/LEDGFp75, we would then observe increased reactivity against the 75 kD protein band in lysates from cells overexpressing this protein, concomitant with decreased or no reactivity in lysates from cells with DFS70/LEDGFp75 depletion. However, if the human sera also contained autoantibodies to MeCP2, we would then be able to observe reactivity against a 75 kD protein in PC3 lysates with DFS70/LEDGFp75 depletion.
All the selected sera displayed elevated reactivity against the 75 kD protein band in PC3 cells overexpressing DFS70/LEDGFp75 (OE) compared to PC3 cells transfected with empty control vector (Vec) (representative sera shown in Fig. 4). This was consistent with the observation that these sera had tested positive for autoantibodies to this protein using the different detection platforms described above. By contrast, the two commercial antibodies against MeCP2 reacted with similar intensity with the 75 kD protein band in both PC3-Vec and PC3-OE lysates, consistent with their specificity for MeCP2 (Fig. 4).
Figure 4. Immunoreactivity of human anti-DFS sera against cells with and without DFS70/LEDGFp75 overexpression.
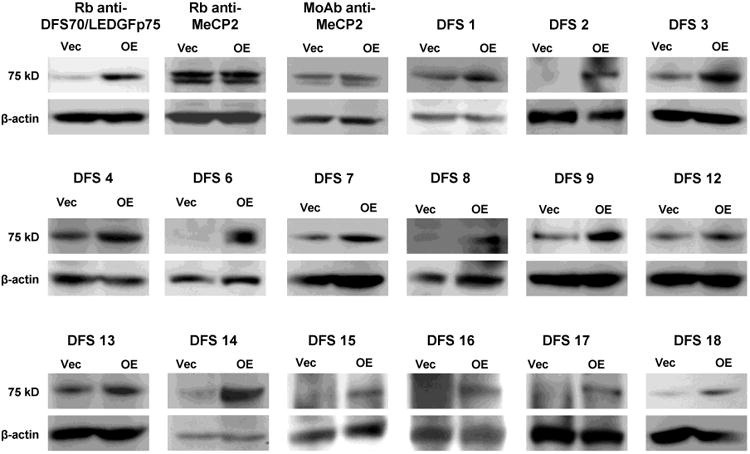
Immunoblots showing the reactivity of representative human anti-DFS sera against DFS70/LEDGFp75 in whole lysates from PC3 cells stably overexpressing this protein (OE) and stably transfected with control empty vector (Vec). Note increased intensity of the 75 kD protein band in cells overexpressing DFS70/LEDGFp75. Beta-actin was used as loading control.
We then probed the sera against lysates from PC3 cells with stable depletion of DFS70/LEDGFp75 (shRNA) and scrambled control shRNA (SCR). We observed that all the selected human anti-DFS sera reacted specifically with DFS70/LEDGFp75. This was evident from the absence of, or limited, reactivity of the sera with the 75 kD protein band in the PC3-shRNA cells (representative sera shown in Fig. 5). By contrast, depletion of DFS70/LEDGFp75 did not affect the reactivity of the commercial anti-MeCP2 antibodies against the 75 kD protein in PC3-SCR or PC3-shRNA lysates. These results suggest that the selected group of human sera, which were confirmed to contain anti-DFS70/LEDGFp75 autoantibodies using different detection platforms, do not react against MeCP2.
Figure 5. Immunoreactivity of human anti-DFS sera against cells with and without DFS70/LEDGFp75 depletion.
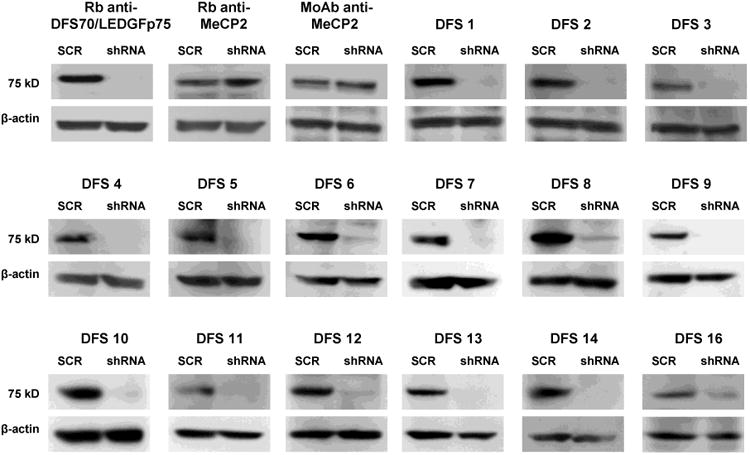
Immunoblots showing the reactivity of representative human anti-DFS sera against DFS70/LEDGFp75 in whole lysates from PC3 cells with stable shRNA-mediated depletion of this protein and stably transfected with scrambled shRNA control (SCR). Note the disappearance of the 75 kD protein band in cells with DFS70/LEDGFp75 depletion (shRNA). Beta-actin was used as loading control.
3.4. Confirmation of anti-DFS70/LEDGFp75 antibody specificity in anti-DFS sera by preadsorption with autoepitope peptide
To further confirm that human sera producing the classical DFS-IIF pattern preferentially react with DFS70/LEDGFp75 and not with MeCP2, we preadsorbed the selected 18 anti-DFS human sera with a recombinant DFS70/LEDGFp75 antigen corresponding to the autoepitope (amino acids 349-435) recognized by anti-DFS autoantibodies [47]. We hypothesized that if the pre-adsorption abolished the reactivity of the 18 sera against DFS70/LEDGFp75 by immunoblotting and HEp-2-IIF analyses, then the sera would be specific for this protein and would not contain antibodies to MeCP2. We observed that the recombinant autoepitope peptide abolished the reactivity of all the 18 anti-DFS sera, strongly suggesting their specificity for DFS70/LEDGFp75 (representative sera shown in Fig. 6A). As expected, the autoepitope peptide did not preadsorb the reactivity of the commercial anti-MeCP2 antibodies, as shown by immunoblotting (Fig. 6A). It should be noted that the autoepitope peptide did not preadsorb the reactivity of the commercial rabbit antibody to DFS70/LEDGFp75, which was raised against a C-terminal region of this autoantigen that fell outside the autoepitope (amino acids 480-530). IIF analysis revealed that preadsorption of human anti-DFS sera with the autoepitope peptide abrogated their nuclear reactivity in HEp-2 cells but did not affect the nuclear reactivity of rabbit anti-MeCP2 antibodies (Fig. 6B). These results further confirmed the specificity of these sera for DFS70/LEDGFp75.
Figure 6. Immunoadsorption of anti-DFS sera with autoepitope-containing peptide.
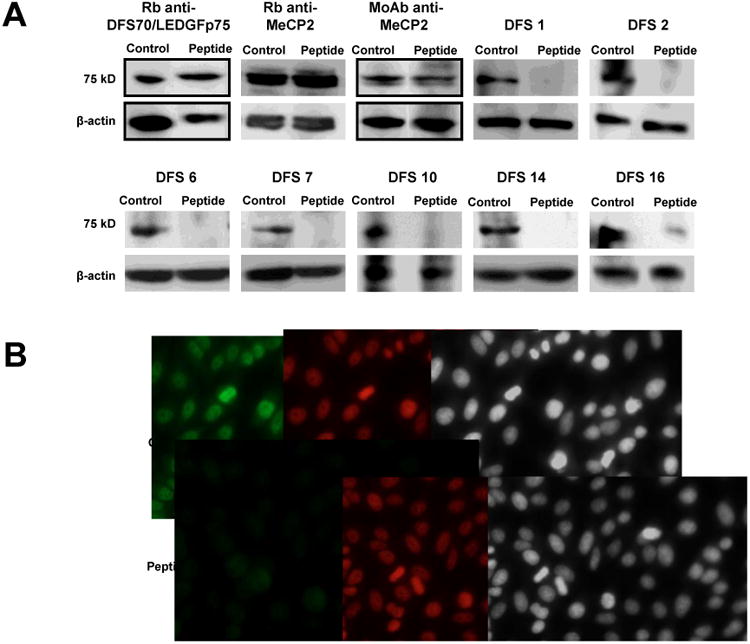
A) Immunoblots showing the abolition of anti-DFS/LEDGFp75 reactivity against PC3 lysates in human anti-DFS sera preadsorbed with a peptide corresponding to the C-terminal autoepitope region of DFS70/LEDGFp75 (representative sera shown). Preadsorption of anti-MeCP2 antibodies with the autoepitope-containing peptide did not abrogate their immunoreactivity. Similarly, preadsorption of the rabbit anti-DFS70/LEDGFp75 antibody, which targets a region outside the autoepitope, did not abrogate its immunoreactivity. Beta-actin was used as loading control. B) Preadsorption of representative human anti-DFS serum with the autoepitope-containing peptide abolished its immunoreactivity (green) in HEp-2-ANA slides. However, preadsorption of rabbit anti-MeCP2 antibody did not abolish its immunoreactivity (red) in HEp-2-ANA slides. Corresponding DAPI images are shown in black and white for better visualization of chromatin.
4. Discussion
During the past decade several groups have documented the presence of high titer, anti-DFS70/LEDGFp75 IgG in apparently healthy individuals and in a wide variety of non-AARD inflammatory conditions, including interstitial cystitis, atopic dermatitis, alopecia areata, cataracts and other eye diseases, chronic fatigue syndrome in children, and prostate cancer [4-12,46]. There is growing consensus that these autoantibodies are present at low prevalence in patients with AARD, and when found in these patients are usually accompanied by other AARD-marker autoantibodies such as anti-DNA and anti-Sm [4-12,41,48,49]. This has led to the hypothesis that when they are the sole ANA specificity in the patient's serum, these autoantibodies could be used as exclusion biomarkers of AARD diagnosis in ANA-positive individuals [5,6,11]. This was illustrated in a recent case report of a child with respiratory distress who presented high-titer anti-DFS70/LEDGFp75 autoantibodies as the sole ANA specificity and with no clinical evidence of SARD [50]. In that particular case, these antibodies were used to rule out suspected autoimmune disease, guiding the clinicians to the proper therapeutic intervention [50].
A challenge facing clinicians that refer patients with non-specific complaints to clinical diagnostic laboratories for ANA screening is how to interpret adequately a positive anti-DFS antibody result. Such result, if interpreted out of the context of our current knowledge of this autoantigen-autoantibody system, might cause undue concern and anxiety among patients and their families, and even lead to unnecessary therapeutic interventions [41]. Likewise, a challenge facing routine diagnostic laboratories is how to accurately identify the characteristic DFS-IIF pattern and the presence of anti-DFS70/LEDGFp75 autoantibodies in patient serum, given the similarity of this pattern, particularly in interphasic nuclei, to other fine speckled ANA patterns [40-42]. For instance, clinical laboratory personnel who are not familiar with the DFS-IIF pattern may confuse it, especially when present at low titers, with the homogeneous or the ‘quasi-homogeneous’ ANA patterns or with other nuclear DFS-IIF patterns lacking mitotic chromatin staining, leading to false positive tests for the presence of AARD-associated ANAs, with their unintended consequences [41].
In this study we used a combination of complementary antibody detection platforms and molecular/cellular approaches to accurately and unambiguously establish the monospecificity of anti-DFS70/LEDGFp75 autoantibodies in sera that produce the characteristic DFS-IIF pattern inHEp-2-ANA substrates. The high concordance of our results in the three detection platforms (HEp-2-ANA test, DFS70-CIA, and immunoblotting) reinforced the notion that accurate detection of anti-DFS70/LEDGFp75 autoantibodies may require, in addition to HEp-2-ANA, a second confirmatory detection method. The use of cell lysates with DFS70/LEDGFp75depletion and a recombinant peptide encompassing the autoepitope region for anti-DFS autoantibody immunoadsorption were critical to validate our results. These results clearly confirmed that the major autoepitope is recognized by the majority, if not by all the anti-DFS70/LEDGFp75 positive patients and that the CIA reliably detects the antibodies. Our results also support the argument that inter-laboratory discrepancies regarding detection of anti-DFS70/LEDGFp75 autoantibodies during routine HEp-2-ANA screening could be rendered moot by the availability of a second, validated test that complements the ANA results [42]. It should be emphasized, however, that immunoblotting procedures for anti-DFS autoantibody detection might not be the best practice for routine diagnostics given their complexity and labor intense nature. A single, simple, and well-characterized assay that has been widely validated in various international laboratories would be ideal for this purpose.
Another important issue that should be considered in the identification of the DFS-IIF pattern in routine clinical laboratory ANA screening is the possibility that autoantibodies to nuclear autoantigens other than DFS70/LEDGFp75 may also produce this pattern. We explored this possibility by focusing on MeCP2, a close interacting partner of DFS70/LEDGFp75 with similar nuclear localization and molecular weight [38]. As mentioned earlier, DFS70/LEDGFp75 is a component of chromatin-associated protein complexes that participate in the regulation of gene expression, and some of its interacting partners, including MeCP2, co-localize with this protein, producing an identical DFS-IIF pattern [34-38]. Our combined results demonstrated that human sera producing the characteristic DFS-IIF pattern preferentially target DFS70/LEDGFp75 and not MeCP2. We cannot rule out the possibility that MeCP2 could be the target of human autoantibodies in specific disease contexts given that we did not examine hundreds of anti-DFS sera for the presence of these autoantibodies. However, the fact that none of the 18 sera selected for their moderate to strong anti-DFS immunoreactivity by HEp-2-ANA, DFS70-CIA, and immunoblotting showed reactivity against MeCP2, or any other common antigen band in immunoblots, provides compelling evidence that when present as the sole ANA pattern in serum, the DFS-IIF pattern is monospecific for DFS70/LEDGFp75. This suggests that this autoantigen by itself, and not a protein complex in which it may be involved, is the immunogen inciting this antibody response in susceptible individuals.
While we do not know what makes DFS70/LEDGF/p75 immunogenic, it is interesting to note that the autoepitope region recognized by its cognate autoantibodies (residues 349-435) lies within a highly conserved, functional domain called the integrase binding domain (IBD) [51]. This domain is recognized by the HIV-1 integrase (residues 347-429) to facilitate viral integration into the host genome, and is very important for DFS70/LEDGFp75 stress survival activities and interactions with chromatin-associated proteins, with the exception of MeCp2, which binds to its N-terminal region [34,38,51-53]. The autoimmune targeting of the IBD of DFS70/LEDGFp75 is consistent with the notion that autoepitope regions are located within highly conserved and functional domains of nuclear autoantigens [1].
Our group demonstrated previously that the extreme N-terminal (residues 1-85) and C-terminal domains of DFS70/LEDGFp75 (residues 485-530) are removed by caspases during apoptosis, generating truncated proteins with altered function that still contain an intact autoepitope/IBD region [54]. These cleaved forms of DFS70/LEDGFp75 could have altered immunogenicity if extracellularly released under a pro-inflammatory environment [9]. Recently, we highlighted that DFS70/LEDGFp75 cleavage during apoptosis does not appear to abolish its localization to apoptotic blebs, suggesting that its cleaved forms may be released to the extracellular environment in these particles [9]. We cannot rule out, however, that DFS70/LEDGFp75 might also be released from both apoptotic and non-apoptotic cells in other types of cell-derived particles such as small membrane-based microparticles (MP) or exosomes. These cell-derived microvesicles are known nanocarriers of intracellular antigens that could have altered immunogenicity and participate in antigen-autoantibody complexes [55,56]. Although there is no evidence at the present time for the presence of DFS70/LEDGFp75 in cell-derived microvesicles, there is some circumstantial evidence for its extracellular release as part of the cellular response to stress, and that its interaction with its cognate autoantibodies in the extracellular microenvironment could have pathogenic consequences [reviewed in 9].
The characterization of the antigenic specificity of the DFS-IIF pattern is essential for developing highly accurate assays to detect the presence of anti-DFS70/LEDGFp75 autoantibodies and to evaluate their clinical significance. Although recent advances on the functional analysis of DFS70/LEDGFp75 have shed some light into its multiple molecular and cellular roles, the clinical and biological significance of its cognate autoantibodies still remains an enigma [9,41,57]. Whereas most cell-derived autoantigens are targeted preferentially by autoantibodies associated with specific organ-specific or systemic autoimmune diseases [1,58-60], and even in certain cancer types [61], to date DFS70/LEDGFp75 remains as an autoantigen without a specific clinical association [9].
What then are the DFS70/LEDGFp75 autoantibodies trying to tell us? While we do not have yet the answer to this question, it is likely that these autoantibodies may not be the result of pathogenic events associated with a particular clinical condition. It is conceivable, however, based on our current knowledge of this autoantigen-autoantibody system, that these antibodies could be biosensors of molecular events, not necessarily specific to a particular disease, that involve altered DFS70/LEDGFp75 expression in a microenvironment characterized by inflammation and tissue damage [9]. This would be consistent with the observed cellular and tissue overexpression of DFS70/LEDGFp75 in response to environmental stressors such as oxidative stress, irradiation, starvation, chemotherapeutic drugs, and certain viruses such as the human papilloma virus (HPV) [15-18,22,33]. Combined with its proteolytic cleavage or other structural modifications, DFS70/LEDGFp75 overexpression under a stressful, pro-inflammatory microenvironment could break immune tolerance, resulting in a specific autoantibody response to this protein that bypasses its interacting partners. Depending on the clinical context in which they arise, these autoantibodies could then play protective or pathogenic roles, or just simply be an epiphenomenon unrelated to the spectrum of clinical conditions in which they are detected, and coincident with yet to be defined environmental, immunological, or genetic factors [9].
Finally, although our data strongly support single antigenic specificity for the anti-DFS response, we cannot categorically conclude that the DFS-IIF pattern is exclusively restricted to autoantibodies to DFS70/LEDGFp75. Occasionally, we have encountered sera presenting what looks like the classical DFS-IIF pattern in HEp-2-ANA test slides but without recognition of a 70-75 kD band in immunoblots or positive reactivity by DFS70-CIA [unpublished observations]. Immunoblotting analyses of these sera have failed to reveal any prominent protein bands in the 15 to 200 kD range (unpublished observations). Careful analysis of such sera will help determine if they contain autoantibodies that target a conformational epitope of DFS70/LEDGFp75 that is lost in the detection assays, or other yet to be identified proteins associated with nuclear complexes in which this autoantigen is naturally found. Given that the clinical associations of anti-DFS70/LEDGFp75 autoantibodies are multiple and involve autoimmune, inflammatory, and malignant conditions [9], it would be also important to distinguish them, using different approaches, from other autoantibodies that specifically target antigens of 75 kD such as those targeting autoimmune enteropathy-related 75 kD antigen (AIE-75) in the immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) [58]. We anticipate that as our basic understanding of the anti-DFS70/LEDGFp75 autoantibody response increases, its clinico-biological significance will unravel, leading to translational applications.
Highlights.
We used several approaches to accurately detect DFS70/LEDGFp75 antibodies in DFS sera
We established that DFS sera specifically target DFS70/LEDGFp75
MeCP2, the 70 kD partner of DFS70/LEDGFp75 is not targeted by DFS sera
DFS70/LEDGFp75 itself appears to be the inciting antigen of anti-DFS autoantibodies
The monospecificity of anti-DFS autoantibodies gives insights into their significance
Acknowledgments
This work was supported in part by NIH grant P20MD006988-Project 2 (CAC). LRC was supported by NIH grant R25GM060507. We are indebted to Zeger Debyser and Rik Gijsbers (Katholieke Universiteit Leuven) for their technical assistance in the preparation of PC3 cell lines with stable DFS70/LEDGFp75 overexpression or depletion. Our most sincere thanks to Rene-Louis Humbel (Luxembourg) and Eng M. Tan (Scripps Research Institute, La Jolla) for kindly providing human DFS serum samples. We thank the members of the Casiano laboratory for critically reading and editing this manuscript.
Footnotes
Contributors: AB and CAC designed the study and prepared the manuscript. AB, LWB, GO, LRC, JF, and RA performed experiments, and acquired and analyzed data. LEA, RA, and MM provided valuable reagents and kits for the study and edited the manuscript.
Conflicts of interest: RA and MM work at Inova Diagnostics Inc, San Diego, a company that specializes in immunodiagnostics and that develops platforms and reagents for detection of ANAs, including anti-DFS70/LEDGFp75 autoantibodies. AB, LWB, GO, LRC, JF and CAC have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tan EM. Autoantibodies and autoimmunity: a three-decade perspective. A tribute to Henry G. Kunkel. Ann N Y Acad Sci. 1997;815:1–14. doi: 10.1111/j.1749-6632.1997.tb52040.x. [DOI] [PubMed] [Google Scholar]
- 2.Mahler M, Pierangeli S, Meroni PL, Fritzler MJ. Autoantibodies in systemic autoimmune disorders. J Immunol Res. 2014;2014:263091. doi: 10.1155/2014/263091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agmon-Levin N, Damoiseaux J, Kallenberg C, Sack U, Witte T, Herold M, et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann Rheum Dis. 2014;73:17–23. doi: 10.1136/annrheumdis-2013-203863. [DOI] [PubMed] [Google Scholar]
- 4.Dellavance A, Viana VS, Leon EP, Bonfa ES, Andrade LE, Leser PG. The clinical spectrum of antinuclear antibodies associated with the nuclear dense fine speckled immunofluorescence pattern. J Rheumatol. 2005;32:2144–2149. [PubMed] [Google Scholar]
- 5.Mahler M, Fritzler MJ. The clinical significance of the dense fine speckled immunofluorescence pattern on HEp-2 cells for the diagnosis of systemic autoimmune diseases. Clin Dev Immunol. 2012;2012:494356. doi: 10.1155/2012/494356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahler M, Hanly JG, Fritzler MJ. Importance of the dense fine speckled pattern on HEp-2 cells and anti-DFS70 antibodies for the diagnosis of systemic autoimmune diseases. Autoimmun Rev. 2012;11:642–5. doi: 10.1016/j.autrev.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Ochs RL, Muro Y, Si Y, Ge H, Chan EK, Tan EM. Autoantibodies to DFS 70 kd/transcription coactivator p75 in atopic dermatitis and other conditions. J Allergy Clin Immunol. 2000;105:1211–1220. doi: 10.1067/mai.2000.107039. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe K, Muro Y, Sugiura K, Tomita Y. IgE and IgG(4) autoantibodies against DFS70/LEDGF in atopic dermatitis. Autoimmunity. 2011;44:511–9. doi: 10.3109/08916934.2010.549157. [DOI] [PubMed] [Google Scholar]
- 9.Ochs RL, Mahler M, Basu A, Rios-Colon L, Sanchez TW, Andrade LE, et al. The significance of autoantibodies to DFS70/LEDGFp75 in health and disease: integrating basic science with clinical understanding. Clin Exp Med. 2015 doi: 10.1007/s10238-015-0367-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyara M, Albesa R, Charuel JL, El Amri M, Fritzler MJ, Ghillani-Dalbin P, et al. Clinical phenotypes of patients with anti-DFS70/LEDGF antibodies in a routine ANA referral cohort. Clin Dev Immunol. 2013;2013:703759. doi: 10.1155/2013/703759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahler M, Parker T, Peebles CL, Andrade LE, Swart A, Carbone Y, et al. Anti-DFS70/LEDGF antibodies are more prevalent in healthy individuals compared to patients with systemic autoimmune rheumatic diseases. J Rheumatol. 2012;39:2104–10. doi: 10.3899/jrheum.120598. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe A, Kodera M, Sugiura K, Usuda T, Tan EM, Takasaki Y, et al. Anti-DFS70 antibodies in 597 healthy hospital workers. Arthritis Rheum. 2004;50:892–900. doi: 10.1002/art.20096. [DOI] [PubMed] [Google Scholar]
- 13.Debyser Z, Christ F, De Rijck J, Gijsbers R. Host factors for retroviral integration site selection. Trends Biochem Sci. 2014 doi: 10.1016/j.tibs.2014.12.001. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Llano M, Morrison J, Poeschla EM. Virological and cellular roles of the transcriptional coactivator LEDGF/p75. Curr Top Microbiol Immunol. 2009;339:125–46. doi: 10.1007/978-3-642-02175-6_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basu A, Rojas H, Banerjee H, Cabrera IB, Perez KY, De León M, et al. Expression of the stress response oncoprotein LEDGF/p75 in human cancer: a study of 21 tumor types. PLoS One. 2012;7:e30132. doi: 10.1371/journal.pone.0030132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mediavilla-Varela M, Pacheco FJ, Almaguel F, Perez J, Sahakian E, Daniels TR, et al. Docetaxel-induced PCa cell death involves concomitant activation of caspase and lysosomal pathways and is attenuated by LEDGF/p75. Mol Cancer. 2009;8:68. doi: 10.1186/1476-4598-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang TS, Myklebust LM, Kjarland E, Gjertsen BT, Pendino F, Bruserud O, et al. LEDGF/p75 has increased expression in blasts from chemotherapy-resistant human acute myelogenic leukemia patients and protects leukemia cells from apoptosis in vitro. Mol Cancer. 2007;6:31. doi: 10.1186/1476-4598-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daugaard M, Kirkegaard-Sorensen T, Ostenfeld MS, Aaboe M, Hoyer-Hansen M, Orntoft TF, et al. Lens epithelium-derived growth factor is an Hsp70-2 regulated guardian of lysosomal stability in human cancer. Cancer Res. 2007;67:2559–2567. doi: 10.1158/0008-5472.CAN-06-4121. [DOI] [PubMed] [Google Scholar]
- 19.Yokoyama A, Cleary ML. Menin critically links MLL proteins with LEDGF on cancer-associated target genes. Cancer Cell. 2008;14:36–46. doi: 10.1016/j.ccr.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Méreau H, De Rijck J, Cermáková K, Kutz A, Juge S, Demeulemeester J, et al. Impairing MLL-fusion gene-mediated transformation by dissecting critical interactions with the lens epithelium-derived growth factor (LEDGF/p75) Leukemia. 2013;27:1245–53. doi: 10.1038/leu.2013.10. [DOI] [PubMed] [Google Scholar]
- 21.Sapoznik S, Cohen B, Tzuman Y, Meir G, Ben-Dor S, Harmelin A. Gonadotropin-regulated lymphangiogenesis in ovarian cancer is mediated by LEDGF-induced expression of VEGF-C. Cancer Res. 2009;69:9306–9314. doi: 10.1158/0008-5472.CAN-09-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leitz J, Reuschenbach M, Lohrey C, Honegger A, Accardi R, Tommasino M, et al. Oncogenic human papillomaviruses activate the tumor-associated lens epithelial-derived growth factor (LEDGF) gene. PLoS Pathog. 2014;10:e1003957. doi: 10.1371/journal.ppat.1003957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhargavan B, Fatma N, Chhunchha B, Singh V, Kubo E, Singh DP. LEDGF gene silencing impairs the tumorigenicity of PCa DU145 cells by abating the expression of Hsp27 and activation of the Akt/ERK signaling pathway. Cell Death Dis. 2012;3:e316. doi: 10.1038/cddis.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daugaard M, Baude A, Fugger K, Povlsen LK, Beck H, Sørensen CS, et al. LEDGF (p75) promotes DNA-end resection and homologous recombination. Nat Struct Mol Biol. 2012;19:803–10. doi: 10.1038/nsmb.2314. [DOI] [PubMed] [Google Scholar]
- 25.Ge H, Si Y, Roeder RG. Isolation of cDNAs encoding novel transcription coactivators p52 and p75 reveals an alternate regulatory mechanism of transcriptional activation. EMBO J. 1998;17:6723–6729. doi: 10.1093/emboj/17.22.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh DP, Ohguro N, Chylack LT, Jr, Shinohara T. Lens epithelium-derived growth factor: increased resistance to thermal and oxidative stresses. Invest Ophthalmol Vis Sci. 1999;40:1444–1451. [PubMed] [Google Scholar]
- 27.Sharma P, Singh DP, Fatma LT, Chylack LT, Jr, Shinohara T. Activation of LEDGF gene by thermal- and oxidative-stresses. Biochem Biophys Res Commun. 2000;276:1320–1324. doi: 10.1006/bbrc.2000.3606. [DOI] [PubMed] [Google Scholar]
- 28.Singh DP, Fatma N, Kimura A, Chylack LT, Jr, Shinohara T. LEDGF binds to heat shock and stress-related element to activate the expression of stress-related genes. Biochem Biophys Res Commun. 2001;283:943–55. doi: 10.1006/bbrc.2001.4887. [DOI] [PubMed] [Google Scholar]
- 29.Fatma N, Singh DP, Shinohara T, Chrylack LT., Jr Transcriptional regulation of the AOP2 gene, a thiol-specific antioxidant, by LEDGF to protect cells from oxidative stress. J Biol Chem. 2001;276:48899–907. doi: 10.1074/jbc.M100733200. [DOI] [PubMed] [Google Scholar]
- 30.Shin JH, Piao CS, Lim CM, Lee JK. LEDGF binding to stress response element increases alphaB-crystallin expression in astrocytes with oxidative stress. Neurosci Lett. 2008;435:131–136. doi: 10.1016/j.neulet.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 31.Cohen B, Addadi Y, Sapoznik S, Meir G, Kalchenko V, Harmelin A, et al. Transcriptional regulation of vascular endothelial growth factor C by oxidative and thermal stress is mediated by lens epithelium-derived growth factor/p75. Neoplasia. 2009;11:921–33. doi: 10.1593/neo.09636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeichi T, Sugiura K, Muro Y, Ogawa Y, Akiyama M. LEDGF/DFS70 activates the MK2/IL6/STAT3 pathway in HaCaT. J Dermatol Sci. 2011;63:203–5. doi: 10.1016/j.jdermsci.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Basu A, Drame A, Muñoz R, Gijsbers R, Debyser Z, De Leon M, et al. Pathway specific gene expression profiling reveals oxidative stress genes potentially regulated by transcription co-activator LEDGF/p75 in PCa cells. Prostate. 2012;72:597–611. doi: 10.1002/pros.21463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartholomeeusen K, Christ F, Hendrix J, Rain JC, Emiliani S, Benarous R, Debyser Z, Gijsbers R, De Rijck J. Lens epithelium-derived growth factor/p75 interacts with the transposase-derived DDE domain of PogZ. J Biol Chem. 2009;284:11467–77. doi: 10.1074/jbc.M807781200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maertens GN, Cherepanov P, Engelman A. Transcriptional co-activator p75 binds and tethers the Myc-interacting protein JPO2 to chromatin. J Cell Sci. 2006;119:2563–71. doi: 10.1242/jcs.02995. [DOI] [PubMed] [Google Scholar]
- 36.Bartholomeeusen K, De Rijck J, Busschots K, Desender L, Gijsbers R, Emiliani S, et al. Differential interaction of HIV-1 integrase and JPO2 with the C terminus of LEDGF/p75. J Mol Biol. 2007;372:407–421. doi: 10.1016/j.jmb.2007.06.090. [DOI] [PubMed] [Google Scholar]
- 37.Hughes S, Jenkins V, Dar MJ, Engelman A, Cherepanov P. Transcription co-activator LEDGF interacts with the S-phase kinase Cdc7:ASK and stimulates its enzymatic activity. J Biol Chem. 2010;285:541–54. doi: 10.1074/jbc.M109.036491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leoh LS, van Heertum B, De Rijck J, Filippova M, Rios-Colon L, Basu A, et al. The stress oncoprotein LEDGF/p75 interacts with the methyl CpG binding protein MeCP2 and influences its transcriptional activity. Mol Cancer Res. 2012;10:378–91. doi: 10.1158/1541-7786.MCR-11-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bizzaro N, Tonutti E, Visentini D, Alessio MG, Platzgummer S, Morozzi G, et al. Antibodies to the lens and cornea in anti-DFS70-positive subjects. Ann N Y Acad Sci. 2007;1107:174–83. doi: 10.1196/annals.1381.019. [DOI] [PubMed] [Google Scholar]
- 40.Bizzaro N, Tonutti E, Villalta D. Recognizing the dense fine speckled/lens epithelium- derived growth factor/p75 pattern on HEP-2 cells: not an easy task! Arthritis Rheum. 2011;63:4036–7. doi: 10.1002/art.30621. Comment on the article by Mariz et al. [DOI] [PubMed] [Google Scholar]
- 41.Mariz HA, Sato EI, Barbosa SH, Rodrigues SH, Dellavance A, Andrade LE. Pattern on the antinuclear antibody-HEp-2 test is a critical parameter for discriminating antinuclear antibody-positive healthy individuals and patients with autoimmune rheumatic diseases. Arthritis Rheum. 2011;63:191–200. doi: 10.1002/art.30084. [DOI] [PubMed] [Google Scholar]
- 42.Fritzler MJ. The antinuclear antibody test: last or lasting gasp? Arthritis Rheum. 2011;63:19–22. doi: 10.1002/art.30078. [DOI] [PubMed] [Google Scholar]
- 43.Cheng TL, Qiu Z. MeCP2: multifaceted roles in gene regulation and neural development. Neurosci Bull. 2014;30:601–9. doi: 10.1007/s12264-014-1452-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gijsbers R, Ronen K, Vets S, Malani N, De Rijck J, McNeely M, et al. LEDGF hybrids efficiently retarget lentiviral integration into heterochromatin. Mol Ther. 2010;18:552–60. doi: 10.1038/mt.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goto N, Sugiura K, Ogawa Y, Watanabe A, Onouchi H, Tomita Y, et al. Anti-p80 coilin autoantibodies react with a conserved epitope and are associated with anti-DFS70/LEDGF autoantibodies. J Autoimmun. 2006;26:42–51. doi: 10.1016/j.jaut.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Daniels T, Zhang J, Gutierrez I, Elliot ML, Yamada B, Heeb MJ, et al. Antinuclear autoantibodies in PCa: immunity to LEDGF/p75, a survival protein highly expressed in prostate tumors and cleaved during apoptosis. Prostate. 2005;62:14–26. doi: 10.1002/pros.20112. [DOI] [PubMed] [Google Scholar]
- 47.Ogawa Y, Sugiura K, Watanabe A, Kunimatsu M, Mishima M, Tomita Y, et al. Autoantigenicity of DFS70 is restricted to the conformational epitope of C-terminal alpha-helical domain. J Autoimmun. 2004;23:221–231. doi: 10.1016/j.jaut.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Muro Y, Sugiura K, Morita Y, Tomita Y. High concomitance of disease marker autoantibodies in anti-DFS70/LEDGF autoantibody-positive patients with autoimmune rheumatic disease. Lupus. 2008;17:171–6. doi: 10.1177/0961203307086311. [DOI] [PubMed] [Google Scholar]
- 49.Fitch-Rogalsky C, Steber W, Mahler M, Lupton T, Martin L, Barr SG, et al. Clinical and serological features of patients referred through a rheumatology triage system because of positive antinuclear antibodies. PLoS One. 2014;9:e93812. doi: 10.1371/journal.pone.0093812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fabris M, Zago S, Tosolini R, Melli P, Bizzaro N, Tonutti E. Anti-DFS70 Antibodies: A Useful Biomarker in a Pediatric Case With Suspected Autoimmune Disease. Pediatrics. 2014;134:e1706–8. doi: 10.1542/peds.2013-3914. [DOI] [PubMed] [Google Scholar]
- 51.Cherepanov P, Devroe E, Silver PA, Engelman A. Identification of an evolutionarily conserved domain in human lens epithelium-derived growth factor/transcriptional co-activator p75 (LEDGF/p75) that binds HIV-1 integrase. J Biol Chem. 2004;279:48883–48892. doi: 10.1074/jbc.M406307200. [DOI] [PubMed] [Google Scholar]
- 52.Cherepanov P, Maertens G, Proost P, Devreese B, Van Beeumen J, Engelborghs Y, et al. HIV-1 integrase forms stable tetramers and associates with LEDGF/p75 protein in human cells. J Biol Chem. 2003;278:372–81. doi: 10.1074/jbc.M209278200. [DOI] [PubMed] [Google Scholar]
- 53.Llano M, Saenz DT, Meehan A, Wongthida P, Peretz M, Walker WH, et al. An essential role for LEDGF/p75 in HIV integration. Science. 2006;314:461–464. doi: 10.1126/science.1132319. [DOI] [PubMed] [Google Scholar]
- 54.Wu X, Daniels T, Molinaro C, Lilly MB, Casiano CA. Caspase cleavage of the nuclear autoantigen LEDGF/p75 abrogates its pro-survival function: implications for autoimmunity in atopic disorders. Cell Death Differ. 2002;9:915–925. doi: 10.1038/sj.cdd.4401063. [DOI] [PubMed] [Google Scholar]
- 55.Ullal AJ, Pisetsky DS. The role of microparticles in the generation of immune complexes in murine lupus. Clin Immunol. 2013;146:1–9. doi: 10.1016/j.clim.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tran TH, Mattheolabakis G, Aldawsari H, Amiji M. Exosomes as nanocarriers for immunotherapy of cancer and inflammatory diseases. Clin Immunol. 2015 doi: 10.1016/j.clim.2015.03.021. in press. [DOI] [PubMed] [Google Scholar]
- 57.Abeles AM, Abeles M. The clinical utility of a positive antinuclear antibody test result. Am J Med. 2013;126:342–348. doi: 10.1016/j.amjmed.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 58.Chida N, Kobayashi I, Takezaki S, Ueki M, Yamazaki Y, Garelli S, et al. Disease specificity of anti-tryptophan hydroxylase-1 and anti-AIE-75 autoantibodies in APECED and IPEX syndrome. Clin Immunol. 2015;156:36–42. doi: 10.1016/j.clim.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 59.Shen L, Suresh L, Lindemann M, Xuan J, Kowal P, Malyavantham K, Ambrus JL., Jr Novel autoantibodies in Sjogren's syndrome. Clin Immunol. 2012;145:251–5. doi: 10.1016/j.clim.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 60.D'Angelo S, Mignone F, Deantonio C, Di Niro R, Bordoni R, Marzari R, et al. Profiling celiac disease antibody repertoire. Clin Immunol. 2013;148:99–109. doi: 10.1016/j.clim.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 61.Dai L, Ren P, Liu M, Imai H, Tan EM, Zhang JY. Using immunomic approach to enhance tumor-associated autoantibody detection in diagnosis of hepatocellular carcinoma. Clin Immunol. 2014;152:127–39. doi: 10.1016/j.clim.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


