Abstract
The presence of fetal cells has been associated with both positive and negative effects on maternal health. These paradoxical effects may be due to the fact that maternal and offspring fitness interests are aligned in certain domains and conflicting in others, which may have led to the evolution of fetal microchimeric phenotypes that can manipulate maternal tissues. We use cooperation and conflict theory to generate testable predictions about domains in which fetal microchimerism may enhance maternal health and those in which it may be detrimental. This framework suggests that fetal cells may function both to contribute to maternal somatic maintenance (e.g. wound healing) and to manipulate maternal physiology to enhance resource transmission to offspring (e.g. enhancing milk production). In this review, we use an evolutionary framework to make testable predictions about the role of fetal microchimerism in lactation, thyroid function, autoimmune disease, cancer and maternal emotional, and psychological health.
Also watch the Video Abstract.
Keywords: attachment, autoimmune disease, cancer, inflammation, lactation, maternal‐fetal conflict, parent‐offspring conflict
Introduction
The function of fetal cells in maternal tissues is unknown
Maternal‐offspring interactions are often viewed as solely cooperative, both parties having an interest in the other's survival and well‐being. However, parent‐offspring interactions are characterized by both cooperation – because shared genes lead to fitness benefits from maternal investment in offspring 1 – and also conflict – because parents and offspring do not share all genes and future maternal reproduction can be negatively impacted by too much maternal investment in current offspring 2. In the context of pregnancy, resource conflict has led to the evolution of fetal manipulation of maternal systems to increase resource transfer via the placenta, and the evolution of maternal countermeasures to limit resource flow 3, 4. This conflict is instantiated through a number of mechanisms including genomic imprinting and placental hormone production 3, 5. The escalation of conflict between fetal and maternal systems over resource allocation has been proposed as an explanation for several pathological conditions of pregnancy including gestational diabetes and preeclampsia 3, 5. Here, we extend the concept of maternal‐offspring conflict beyond the womb to interactions between fetal cells and maternal tissues throughout the body both during and after pregnancy.
During pregnancy, there is a bi‐directional flow of fetal and maternal cells (Fig. 1). The transfer of these cells is asymmetrical, with more fetal cells being transferred to the mother than vice versa 6. Fetal cells increase in frequency in the maternal body with increasing gestational age 7, 8 and have been identified in maternal blood and tissues for decades following birth 9, 10. After parturition, the maternal immune system actively clears some but not all fetal cells from the maternal blood through inducing apoptosis 11. Maternal microchimerism (the transfer of maternal cells to the fetus) is commonly found in fetal tissues as well 12, 13, and also persist for decades after birth 14. Microchimerism is not thought to be limited to just the bi‐directional exchange of mother and fetal cells: cells from older siblings and even maternal grandmother cells might also be transferred to the fetus 15.
Figure 1.
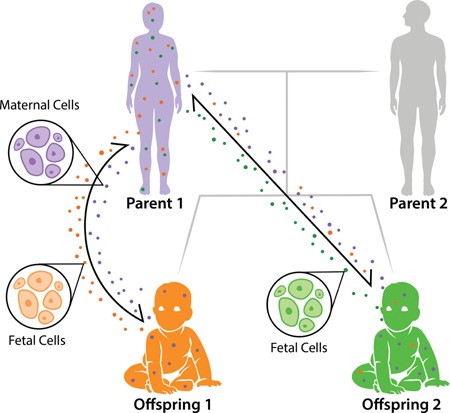
Pedigree of microchimerism. Microchimerism is a bidirectional exchange of fetal and maternal cells during pregnancy. During pregnancy, fetal cells (represented as orange and green circles) traffic into the maternal body, increasing in quantity throughout the gestational period. Likewise, each fetus inherits maternally derived cells (represented as purple circles). It has been predicted that younger siblings could also obtain older siblings' cells 15, as depicted with offspring 1 cells (orange) circulating within the younger sibling's body (offspring 2).
The current state of literature on fetal microchimerism and maternal health presents a paradoxical picture, with some papers concluding that fetal microchimerism contributes to poor health outcomes in certain cases and improved outcomes in others. For example, fetal cells have been proposed to play a role in maternal wound healing 16, 17, but have also been associated with pregnancy complications including, atypical fetal karyotype (e.g. Down Syndrome), pre‐eclampsia, miscarriage, and premature birth 18, 19, 20, 21, 22. Fetal cells have also been identified at multiple tumor sites, including breast, cervical, and thyroid cancers, as well as melanomas (reviewed in 23). Here we review these contradictory findings and describe how the cooperation and conflict framework can help elucidate both the proximate and evolutionary function of fetal cells in maternal tissues (Table 1).
Table 1.
Causes of microchimerism
| Levels of explanation | Definition | Explanations for fetal microchimerism |
|---|---|---|
| Proximate | The immediate cause of the pathology | Placentation allows for the transfer of small numbers of cells between the fetus and the mother |
| Developmental | How the pathology arose as a result of events during an individual's life | Evidence suggests that fetal cell microchimerism begins before the placental is completely formed, likely beginning with the initiation of placentation itself 26, 111 |
| Evolutionary | How natural selection and other mechanisms of evolution (drift, migration) have left the body vulnerable to the pathology | Maternal‐fetal genomic conflict, through genetic imprinting may have allowed for selection of higher proportions of fetal cell microchimerism |
| Phylogenetic | When, in evolutionary history, did the vulnerability to the pathology arise? | Microchimerism has thus far only been detected in eutherian mammals 14, 27, 28, 29, suggesting it arose at least in the common ancestor of eutherian mammals, approximately 93 million years ago 112 |
Here, we describe the proximate, developmental, evolutionary, and phylogenetic explanations of microchimerism.
Microchimerism is common among placental mammals
Considering a broader phylogenetic perspective, microchimerism is special case of chimerism, defined as organism being made up of more than one genetically distinct individual. Although large‐scale chimerism is relatively rare among organisms 24, microchimerism is common in placental mammals. Placentas exhibit a high degree of diversity in physiology and morphology 25, and some of this placental diversity has been proposed to be the evolutionary consequence of ongoing conflict between mother and offspring over resource transfer 4. One important dimension on which placentas vary is the depth of invasion of fetal trophoblast cells. Humans have the most invasive placental type, hemochorial (reviewed in 26). Placental invasiveness has been proposed to enhance both nutrient transfer from mother to fetus and may also allow more exchange of both fetal and maternal cells 26. Microchimerism has been detected in all placental types (hemochorial, epithelial, endothelial), including primates 14, dogs 27, cows 28, and mice 8, 29. Similar to fetal trophoblast cells invading maternal endometrium during placentation, fetal cells circulating in the maternal body may be able to colonize other tissues and persist in the maternal body after parturition.
Considering the evolutionary and phylogenetic origins of fetal cells suggests that they may have a similar function to the placenta. Just as the placenta's physiology is designed to transfer resources from the maternal body to the offspring in the womb 3, 5, the physiology of fetal cells in maternal tissues may enhance resource transfer to the offspring after parturition through, for example enhancing lactation or heat transfer. This resource transfer may be mutually beneficially for both maternal and offspring fitness interests, or fetal manipulation may push maternal tissues beyond the maternal optimum 2 leading to conflict over resource allocation. Fetal cells also may offer important benefits to maternal tissues for wound healing or somatic maintenance due to their stem‐like nature.
In this paper we use an evolutionary approach to identify the domains in which we should expect cooperation or conflict between fetal cells and maternal tissues. We then use this framework to make predictions about the conditions under which the presence of fetal cells should have positive or negative effects on maternal health. We review the current state of the literature on the effects of microchimerism on maternal health, identifying the tissues in which fetal microchimerism has been found and describing the functions of each of these tissues. The evolutionary and functional framework for fetal microchimerism that we present here explains some of the contradictory findings in the literature and identifies gaps in our understanding that can help guide future work.
Cooperation and conflict occur between fetal cells and maternal tissues
There are three main hypotheses about the role of fetal cells in maternal health in the existing literature: (1) fetal cells are deleterious, contributing to an inflammatory response that can cause maternal tissue damage; (2) fetal cells are protective, with fetal progenitor cells helping to repair and maintain maternal tissues or (3) fetal cells are simply bystanders, having no causal effect on maternal health 30. These hypotheses may not be mutually exclusive. The cooperation and conflict approach makes a number of predictions about the conditions under which fetal cells may have positive or negative effects on maternal health.
Cooperation for offspring survival and maternal somatic maintenance
Parental investment is an important part of the human life history strategy 31 for survival and subsequent success of offspring. This begins in the womb and extends long after birth. Humans have very high levels of postpartum parental investment compared to other species, and have clear adaptations for parental investment which include lactation but also extended food sharing 32 both of which are facilitated by emotional attachment and bonding 33. Both parent and offspring benefit from each others' survival, with maternal fitness interests being aligned with those of the offspring because of direct fitness benefits (i.e. enhancing reproductive success of the parent) and offspring interests being aligned with mother's interests because of both indirect and direct benefits 5. We predict that microchimerism should be associated with enhanced maternal health where there is potential for the fetus to enhance maternal health at low or no cost to itself, such as in the transmission of fetal stem cells that might provide a benefit for somatic maintenance (i.e. maternal tissue repair or replenishing stem cell niches).
Conflict arises over optimal allocation of resources to offspring
Offspring interests often favor higher levels of resource transmission than what is optimal for maternal fitness, likely leading to selection on fetal microchimeric cell phenotypes to manipulate maternal tissues to increase the level of resource transfer to offspring. One approach to understanding the function of fetal cells in maternal tissues is to consider parallels with the function of the placenta during pregnancy. During pregnancy, fetal cells may operate as an extension of the placenta, helping to coordinate manipulation of maternal resource transfer to the offspring across maternal tissues. After pregnancy, fetal cells may play important roles in continued maternal investment in the offspring through manipulation of lactation, thermoregulation, and attachment systems. The framework we propose here predicts that fetal cells should be more common in tissues that are the site of resource transfers (e.g. the breast, thyroid, and brain; Fig. 2), and that in these tissues, the presence of fetal cells may be associated with poorer health outcomes for the mother.
Figure 2.
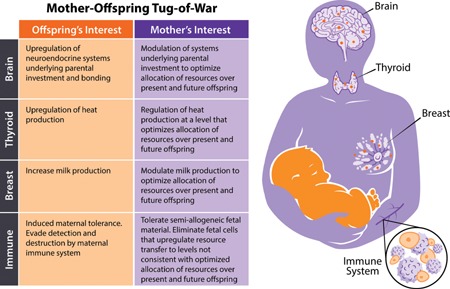
Mother‐offspring tug‐of‐war. Cooperation and conflict theory can elucidate the paradoxical role fetal cell microchimerism plays in maternal health. We predict that tissues involved in resource allocation, such as the brain, thyroid and breast would likely be reservoirs for fetal cells (depicted as orange circles in maternal tissues). Additionally, the maternal immune system is likely to play an active role in fetal‐maternal interactions. Within these tissues, the outcomes of these fetal‐maternal negotiations (mother‐offspring tug‐of‐war) are important in maternal health and wellbeing.
Maternal countermeasure and the escalation of conflict
Maternal‐fetal conflict not only predicts the evolution of fetal adaptations to enhance resource flow to offspring, but also maternal countermeasures to limit that flow 3. Extending this framework beyond the placenta suggests that the maternal systems should have adaptations to limit the capacity of fetal cells to manipulate maternal tissues to the mother's fitness detriment. One likely candidate mechanism for maternal countermeasures is the targeting and elimination of fetal cells by maternal immune cells. In mice, it has been shown that fetal cells can be eliminated by the maternal immune system 34. Limiting fetal cells may lead to a more maternally optimal resource transfer level to the offspring. This leads to the prediction that the capacity of the maternal immune system to limit the abundance of fetal cells may have positive impacts on maternal health. However, if fetal cells have the capacity to alter their phenotype and become more aggressive in response to maternal countermeasures, this could lead to costly escalation of conflict with potentially negative consequences for both parties.
Fetal cells may be able to respond to maternal countermeasures through increasing proliferation rates, producing signaling factors that manipulate maternal tissues more intensely, or even targeting the destruction of maternal immune cells. One study demonstrated that invading trophoblast cells can induce apoptosis in maternal T‐cells 35, suggesting that fetal cells may have sophisticated countermeasures to maternal responses. This presents a critical question: does conflict between maternal tissues and fetal cells escalate, de‐escalate or reach some sort of détente? The maternal‐fetal dyad is a complex and dynamic system and there may be substantial individual differences with regard to this trajectory. The cooperation and conflict framework suggests that it is in the situations of escalating conflict that the greatest likelihood of negative outcomes for maternal health, similar to the negative health outcomes (e.g. gestational diabetes and preeclampsia) that have been attributed to escalating maternal‐fetal conflict during pregnancy 3, 5.
It is important to point out here that cooperation and conflict between maternal and offspring interests does not imply intention on the part of either party, but it is instead a consequence of selection that can be instantiated through a variety of mechanisms. Genomic imprinting is one likely candidate, as it allows for phenotypic plasticity and it has already been implicated in maternal‐fetal conflict in the womb via imprinting of maternal and paternal genes in the placenta 36.
Contradictory effects of fetal cells on maternal health
Fetal cells are associated with wound healing/response to injury
Some fetal cells have stem‐like properties 9, 37, 38 that may allow them to provide maternal benefits. The replenishing of stem cell niches may enhance maternal survival through counteracting some of the negative effects of stem cell loss and damage due to aging 39. It is in the fitness interest of the offspring to enhance maternal survival and contribute to maintenance of the maternal body. This predicts that fetal cells may take over stem cell niches, which may be associated with greater survival and reduced aging of mothers. Additionally, fetal microchimeric phenotypes may be under selective pressure to contribute to maternal health through enhancing wound healing. This predicts that fetal cells should be found at the sites of wounds and that their presence should be associated with better outcomes for maternal health.
Several studies suggest that fetal cells may play a role in maternal wound healing. Murine injury models have tracked fetal cells actively migrating to the site of injury in the maternal body 17, 40, 41, 42. Two of these injury models report clustering of fetal cells in relation to maternal blood vessels at sites of inflammation, suggesting participation in maternal angiogenesis 17, 40. Additionally, in humans, fetal cells were identified in healed cesarean section scars and expressed markers of cytokeratin and collagen 16, suggesting that fetal cells actively participate in maternal wound healing.
Also, consistent with the hypothesis that fetal cells contribute to ongoing maternal somatic maintenance, fetal cells have been identified in many healthy tissues in human mothers 10, 43, 44, 45 and rodent mothers 41, 42, 46, 47. Further, across tissues in the maternal body (in humans and mice), there is evidence that fetal cells differentiate into multiple cell types, including endothelial cells 16, neurons 47, smooth muscle cells, and cardiomyocytes 10, 48. Mesenchymal cells of fetal origin have consistently been detected in the bone marrow of women who had given birth to at least one son 37, and in healthy women, fetal cells were present in numerous maternal immune cell populations, including T cells, B cells, and natural killer cells 49 as well as T cells and B cells in mouse models 38. These studies suggest that fetal cells are at least passively contributing to increased somatic maintenance in mothers and are likely actively contributing to wound healing.
Fetal cells are found at sites of maternal‐offspring resource allocation
In addition to the positive role that fetal cells may play in maternal wound healing and somatic maintenance, they may also play a negative role (from the perspective of maternal fitness interests) by manipulating resource transfer to offspring above the maternal optimum. Below we expand upon our predictions and then review the current literature regarding the presence of fetal cells in tissues involved in resource allocation and the association of these cells with maternal health or disease. Most human studies that we report below tested for the presence or absence of male DNA/male cells in blood or tissue from female patients who have or have not previously given birth to a son. We summarize these results below and are also shown in Table 2.
Table 2.
Overview of microchimerism in maternal health and disease
| Tissue | Disease/Function | Sample | Species | Fetal cell association with maternal health or disease | Findings |
|---|---|---|---|---|---|
| Brain | Alzheimer's disease | Brain | Human | Health | Fetal cells found less frequently in tissue of patients compared to healthy controls 75 |
| Parkinson's disease | Brain | Mouse | Disease | Fetal cells found more frequently in disease tissue compared to healthy controls initially, but not after long‐term observation 47 | |
| Breast | Cancer | Blood/Breast | Human | Health | Fetal cells less frequent in blood and tissue of patients compared to healthy controls 54, 55, 56, 57 |
| Cancer | Breast | Human | Health | Lower levels of fetal cells in ER/PR‐positive breast cancer tissue compared to healthy controls 53 | |
| Cancer | Breast | Human | Disease | Higher levels of fetal cells in HER‐2 breast cancer tissue compared to healthy controls 53 | |
| Cancer | Breast | Human | Disease | Presence of fetal‐derived cells in tumor stroma 58 | |
| Cancer | Breast | Mouse | Disease | Fetal cells are present in murine breast carcinomas. High‐grade tumors contain more fetal cells 59 | |
| Thyroid | Cancer | Blood/Thyroid | Human | Health | Fetal cells/male DNA found more frequently in the blood of healthy controls compared to patients. Found no significant difference in tissue samples 66 |
| Cancer | Thyroid | Human | Disease | Fetal cells found more frequently in the disease tissue of patients compared to healthy tissue 44, 66 | |
| Hashimoto's thyroiditis | Blood/Thyroid | Human | Disease | Fetal cells/male DNA found more frequently in patients compared to healthy controls 44, 68, 69, 70, 113 | |
| Graves' disease | Blood/Thyroid | Human | Disease | Fetal cells/male DNA found more frequently in patients compared to healthy controls 69, 70, 71 | |
| Thyroiditis | Thyroid | Mouse | Disease | Fetal cells found more frequently in patients compared to healthy controls 67 | |
| Immune system | Systemic sclerosis | Blood/Skin lesion | Human | Disease | Fetal cells/male DNA found more frequently in patients than healthy controls 49, 79, 80 |
| Systemic sclerosis | Blood | Human | No association | No difference in frequency of male DNA between patients and controls 87, 88 | |
| Sjögren syndrome | Blood/Salivary gland | Human | Disease | Male DNA was higher in tissue but not in blood in patients compared to healthy controls 81 | |
| SLE | Blood | Human | Disease | Male DNA/fetal cells higher in patients than healthy controls 82, 83 | |
| SLE | Multiple tissues | Human | Disease | Fetal cells/male DNA found more frequently in damaged tissue compared to healthy tissue 114 | |
| SLE | Blood | Human | No association | No difference in frequency or quantity of male DNA between patients and controls 89 | |
| Rheumatoid arthritis | Nodule | Human | Disease | Male DNA detected in rheumatoid nodules 86 | |
| Rheumatoid arthritis | Blood | Human | Disease | Prevalence of fetal cells/male DNA higher in patients than healthy controls 83, 84 | |
| Rheumatoid arthritis | Blood | Human | No association | No difference in male DNA between patients and controls 90 | |
| Lungs | Cancer | Lung/Thymus | Human | Disease | Fetal cells found more frequent in diseased tissue compared to healthy tissue from same patient 92 |
| Heart | Injury model | Heart | Mouse | Health | Fetal cells home to injured maternal hearts and differentiate into endothelial cells, smooth muscle cells, and cardiomyocytes 48 |
| Cardiomyopathy | Heart | Human | Disease | Fetal cells were found in found in patients, but not healthy controls 10 | |
| Liver/Kidney/Spleen | Injury model | Injured tissue | Mouse | Health | Fetal cells home to injured tissues 41, 42 |
| Reproductive tissues | Cancer | Cervical tissue | Human | Disease | Male fetal cells found more frequently in tissue of patients compared to controls 115 |
| Cancer | Endometrial tissues | Human | No association | Male DNA found in both benign and diseased tissue 116 | |
| Endometriosis | Endometrial tissues | Human | No association | Male DNA were not observed in disease or healthy tissue 117 | |
| Colon | Cancer | Blood | Human | Disease | Fetal cells found more frequently in the blood of patients compared to healthy controls 56 |
| Skin | Caesarean section | Skin | Human | Health | Fetal cells identified in some healed maternal CS scars and expressed cytokeratin, and collagen I, III, and TGF‐β3 in healed maternal scar 16 |
| Injury model | Skin | Mouse | Health | Fetal cells more frequent in maternal inflamed tissues and participate in maternal angiogenesis and inflammation 17, 40 | |
| Melanoma | Skin | Human/Mouse | Disease | Fetal cells detected more frequently in melanoma compared to benign or healthy tissue 118 | |
| PEP | Skin | Human | Disease | Male DNA detected in tissue of patients with no detection in healthy controls 85 |
SLE, systemic lupus erythematosus; PEP, polymorphic eruptions of pregnancy; CS, cesarean section.
A summary of the current literature on fetal cell microchimerism and the proposed association with maternal health and disease.
Fetal cells are found in the breast
Mother's milk provides calories, nutrients, and immunological protection for offspring 50, 51; however, lactation is costly for the mother. This means that there can be conflict over maternal milk supply, with offspring interests favoring higher milk supply than what is optimal for the mother 5. If fetal cells are able to migrate to the breast and up‐regulate milk production either through producing factors that manipulate maternal mammary glands or by differentiating into mammary gland themselves, this could benefit offspring fitness. This predicts that the presence of fetal cells in the breast should be associated with higher levels of milk production or enhanced quality of mother's milk and could be associated with negative health outcomes for the mother in some cases 52.
Fetal cells are found frequently in normal breast tissue of women postpartum. In healthy women, male DNA was detected in the mammary glands of more than half of women sampled 53. However, existing research presents a complicated picture of fetal cells role in diseases of the breast. Fetal cells have been found less frequently in the blood and tissue of women with breast cancer compared to healthy controls 54, 55, 56, 57, suggesting that more fetal cells may actually be associated with better health of the mother. However, a murine model of breast cancer found that high‐grade tumors harbored significantly more fetal cells than low‐grade tumors 58 and fetal cells have also been found in the tumor stroma of human females 59. Additionally, the association of fetal cells with breast cancer may be subtype‐specific, as higher levels of fetal cells were reported in the tissue of women diagnosed with HER‐2 subtype of breast cancer, while lower levels of fetal cells were associated with estrogen receptor/progesterone receptor positive tissue 53.
It is also possible that fetal microchimerism, through its long co‐evolutionary history with the maternal body (Table 1), may now play a role in normal breast physiology. The maternal mammary gland harbors a population of stem cells that contribute to normal breast development and can be transferred to the fetus during lactation 60. Fetal cells with stem‐like properties located in the mammary gland could be responding to the same maternal signals. Mouse fetal fibroblast cells have been shown to differentiate into mammary epithelioid cells when exposed to lactation hormones (insulin, progesterone, and oxytocin) in vitro 61 and a functional mammary gland has been generated from a single stem cell in an pregnancy mouse model 62. This suggests that fetal progenitor cells can play a role in maternal milk supply. Interestingly, women who reported to have an oversupply of milk during lactation were more likely to develop breast cancer 52, while reports are inconsistent between insufficient milk supply and breast cancer 63. Together these findings suggest that fetal microchimeric cells may play a role in breast physiology and milk supply, but the effects on maternal health are not yet clear.
Fetal cells are found in the thyroid
The thyroid is important for thermoregulation and metabolism 64. Production of heat is metabolically costly for both the mother and fetus, meaning that the interests of the mother and offspring are not always aligned. Offspring benefit from maternal heat generation and transmission 65. Fetal cells in the maternal thyroid could enhance offspring fitness by manipulating maternal thermoregulation after birth. This predicts that fetal cell presence in the thyroid should be associated with higher maternal body temperature. Presence of fetal cells in the thyroid might also contribute to thyroid cancer risk or susceptibility to other thyroid diseases if these cells are proliferating or producing factors that may induce abnormal thyroid physiology.
While fetal cells are found in healthy thyroid tissue postpartum 66, current research also suggests an association with thyroid diseases. Murine models of thyroiditis find a higher frequency of fetal cell microchimerism in the thyroid during and after pregnancy 67. Fetal cells have been found more frequently in the blood and thyroid tissue of women with Hashimoto's thyroiditis 44, 68, 69, 70, Graves' disease 68, 69, 70, 71, and cancer 44, 66, compared to healthy controls. These results suggest that fetal cell presence in the thyroid is associated with maternal disease rather that health.
Fetal cells are found in the brain
Maternal attachment and bonding are important for the health of the infant 72. “Maternal hormones” such as oxytocin and prolactin are released in the brain and play important roles in “letdown” contractions in breastfeeding 73, maternal milk supply, as well as maternal calm and interest in the offspring 74. The present framework suggests that selection may have favored fetal microchimeric phenotypes that can manipulate maternal brain function to enhance maternal resource transfer and attachment to offspring.
Fetal cells in the mouse maternal brain have been found to be able to integrate into to maternal brain circuitry and express appropriate immunocytochemical markers for brain tissue 47. More generally, the totipotent nature of fetal cells 9, 37, 43 suggest that they have the capacity to differentiate into cells that could participate in the neural circuitry and chemical communication taking place in the brain, possibilities that should be tested in future research. At the moment, several studies have found male DNA in the human and mouse maternal brain 47, 75, but the function of these cells, if any, is unknown. However, one of these studies found fetal cell DNA in multiple regions of the maternal brain, and in one case, decades after the woman had given birth to a son 75, suggesting that fetal microchimerism in the human maternal brain may be pervasive and long‐lasting.
Fetal microchimerism in the brain is not clearly associated with either maternal health or disease. Male DNA has been detected more frequently in disease tissues compared to healthy controls in a mouse model of Parkinson's disease 47. However, fetal cells were found to be less common in the brains of women diagnosed with Alzheimer's disease compared to healthy controls 75. These finding suggest that fetal cells might have important effects on brain function, though the underlying mechanisms are not yet understood.
If fetal cell manipulation of the maternal brain induces maternally suboptimal levels of resource transmission to offspring, maternal countermeasures such as immune targeting of fetal cells in the brain might also be expected, which could raise levels of inflammation in the brain. This raises the intriguing possibility that conflict between fetal cells in the brain and maternal immune cells counteracting those effects could be a partial explanation for several empirical findings, including the observation that post‐partum depression 76 is associated with inflammation, and also with higher parity and shorter interbirth interval 77, However, there are many other potential explanations for these associations and future research is needed to determine whether fetal microchimerism plays a role in maternal emotional health. The possibility the fetal microchimerism may play a role in maternal mental health has elsewhere been suggested 78, though not within the evolutionary framework proposed here.
Fetal cells are found at autoimmune disease sites
The prospect of an escalating conflict between fetal cells and maternal tissues, especially in the context of maternal immune targeting of fetal cells, may contribute to maternal disease after pregnancy. In situations where maternal‐fetal cell conflict escalates, we predict co‐localization of fetal cells and maternal immune cells, but co‐localization could also be the result of cooperation between maternal immune cells and fetal cells for maternal wound healing. This makes it important to disentangle the predictions and effects from each of these potential roles of fetal cells. If fetal cells enhance maternal wound healing, co‐localization of immune cells and fetal cells would simply be a byproduct of the fact that both may be recruited to sites of injury. A characterization of the functional effects of fetal cells and immune cells on each other and on the maternal tissue would allow for the testing of the specific hypotheses that derive from the conflict escalation hypothesis.
Fetal cells have been found at the sites of inflammation and are associated with many autoimmune diseases. Fetal cells have been found more frequently in blood and/or tissue samples in women with systemic sclerosis 49, 79, 80, Sjögren's syndrome 81, systemic lupus erythematosus (SLE) 82, 83, and rheumatoid arthritis (RA) 83, 84. Further, fetal cells have been detected at sites of inflammation in the maternal body, including skin lesions of women with polymorphic eruptions of pregnancy (PEP) 85, rheumatoid nodules of patients with RA 86, and in the goiter of a patient with thyroid disease 68. In contrast, a few studies report no association between fetal cells and the diseases systemic sclerosis 87, 88, SLE 89, and RA 90, when compared to healthy controls. It is unknown whether these findings are due to active fetal cell migration from the blood into diseased tissue or whether the presence of fetal cells contributes to the disease. It is also not known whether the co‐localization of fetal and maternal immune cells contributes to autoimmune disease.
Fetal cells are also found in maternal tissues not associated with resource conflict
During pregnancy, fetal cells enter the maternal body through the placenta and travel through the maternal vessels, being found frequently in the blood, and maternal lung 8, 29. It is generally accepted that the presence of fetal cells in the lung is due to high fetal cell traffic, as the blood from the placenta must first pass through the mother's pulmonary circulation 30, 91. However, despite the fact that fetal cells in the lung are likely just a byproduct of circulatory physiology, the presence of fetal cells in the lung does show an association with cancer: one study reported significantly more fetal cells in the lung and thymus tissue compared to the bone marrow and high levels of male DNA in the diseased lung compared to healthy bone marrow from the same individual 92.
Pregnancy is associated with changes in autoimmune disease presence and susceptibility
Pregnancy is associated with onset of some autoimmune diseases and cancer
Fetal cells enter the maternal tissues during pregnancy, possibly enhancing susceptibility to some diseases such as autoimmune diseases and cancer. Many studies have found an association of pregnancy with diseases of the breast and thyroid. While pregnancy is protective against long‐term breast cancer risk, there is a transient increased risk of breast cancer in years immediately following pregnancy 93, suggesting a possible role for fetal cells. Thyroid disease in common in pregnancy, affecting 2–3% of women 94 and there is an increased incidence of Hashimoto's thyroiditis or Graves' disease postpartum 95. Women are at a higher risk than men for developing autoimmune diseases 96 and the risk of autoimmune disease in parous women is significantly higher after the first year postpartum 97. However, these findings are inconsistent, as some studies report no link between parity and subsequent autoimmune disease 98. Several pregnancy complications are associated with higher detectable fetal cells circulating in the maternal blood, including preeclampsia, abnormal karyotype and miscarriages 19, 20, 21, 22.
Pregnancy alters symptoms of autoimmune diseases
Little research has focused whether fetal cells may contribute to pathogenesis or amelioration of symptoms of existing autoimmune diseases during pregnancy. During gestation, the maternal immune system develops a tolerance to the fetus, and suppression of the maternal response is lifted postpartum 99. Tolerance to the fetus may help explain why some autoimmune disease symptoms are lessened during pregnancy in some women. For example, between 43 and 75% of patients with RA exhibit amelioration of some or all of their RA symptoms during pregnancy (reviewed in 100). Further, in RA patients, symptoms tend to only show improvement during pregnancy, but exacerbation of symptoms postpartum 100. Similarly, the rate of relapse declines with pregnancy in women diagnosed with multiple sclerosis (MS), with the lowest rate in the third trimester. Relapse rates return to prepregnancy levels postpartum 101. However, in other autoimmune diseases, pregnancy is associated with no change symptoms or an exacerbation of symptoms 102, 103. The present framework suggests that one contributor to autoimmune disease may be the maternal immune response to the presence and action of fetal cells that manipulate maternal tissues. Given that maternal tolerance of the fetus (and, therefore, also fetal cells) is lifted postpartum, it may be that the presence of high quantities of fetal cells (or fetal cells that are actively manipulating maternal tissues) leads to an intense maternal immune response, possibility increasing autoimmune disease symptoms. Together this suggests that fetal cells could be involved in autoimmune disease symptom changes, and should be incorporated into future studies of the role of pregnancy on autoimmune disease pathology.
Future research should use more sensitive methods for detecting fetal cells and determining their function
Major challenges for future work on fetal microchimerism and maternal health include: (1) distinguishing fetal alleles from maternal alleles; (2) detecting extremely low levels of fetal cells; (3) identifying the functional role of fetal cells in maternal tissues; and (4) measuring the interactions between fetal cells and maternal immune cells. Initial studies of fetal microchimerism relied on detecting the presence or absence of Y‐linked alleles in tissue samples from the mother, specifically evidence of the sex‐determining region of the Y (SRY). Another common technique is to detect variable tandem repeat numbers in mothers versus offspring in microsatellite loci (short tandem repeats) within the Human Leukocute Antigen (HLA) A and B 30, or detecting differences in unshared HLA‐DRB1 alleles 104. Common methods for distinguishing fetal from maternal alleles at a single locus include flow cytometry, fluorescence immunoprecipitation of the gene or through classical or quantitative PCR 30, 105. PCR is more accurate than immunofluorescence for detecting microchimeric cells 42, but is not as sensitive as flow cytometry, with PCR detecting microchimeric events at about 0.2% frequency (∼198 cells out of 1 × 105 host cells), while flow cytometry could detect microchimeric events at 0.05% frequency (∼48 cells out of 1 × 105 host cells) 105. Detection of low‐frequency microchimeric alleles may also suffer from false positives due to contamination of samples with extraneous DNA, as may be common in clinical laboratory equipment 106.
To increase detection of fetal alleles, in each new pregnancy, the genotype of the mother and father could be used to develop multiple sensitive genomic loci for detection, either using traditional assays, or next‐generation whole genome sequencing. This may also avoid confounding contemporary microchimerism with events from previous pregnancies. Testing multiple loci will likely also improve the ability to detect extremely low levels of fetal cell microchimerism. Low coverage whole genome sequencing of maternal plasma DNA is already used for sensitive non‐invasive prenatal testing 107, and could additionally be utilized to detect fetal cell microchimerism both during and after pregnancy. Methods for detecting fetal cell microchimerism should also include testing of multiple tissues. Although blood is the most commonly and easily assayed tissue, and cell free fetal DNA is routinely detected in maternal blood during pregnancy 107, most fetal microchimerism in the blood is removed postpartum 11, and clinically relevant fetal microchimeric events may lie in the tissue related to the pathology of interest. Detecting microchimerim cells is one hurdle, but detection alone is not enough. Future studies will also need to consider the function of these cells, perhaps by flow sorting microchimeric cells (e.g. 108) from tissues and assaying the transcriptome and proteome. Perhaps more important that understanding the function of the microchimeric cells alone will be how they interact with maternal tissues. For example, initial studies that have identified that cells of fetal origin (specifically extravillous trophoblasts) do modulate the maternal immune system by interacting with maternal leukocytes 109, 110, could be replicated in the breast, thyroid, and brain.
Conclusions and outlook
Microchimerism is common in humans and has been found across eutherian mammals of all placental types. Fetal cells have many parallels with the placenta and can perhaps be considered a far‐reaching extension of the placenta “beyond the womb,” into maternal tissues during pregnancy and lasting long after parturition. The cooperation and conflict framework that we propose here can help to organize and explain some of the contradictory findings in the literature regarding the effects of fetal cells on maternal health. However, many important questions remain. A casual role of fetal cells (and maternal immune response to fetal cells) in maternal health or disease has not yet been definitely shown. Improved methods for distinguishing maternal and fetal alleles and detecting low levels of microchimerism in the blood and maternal tissues will help to advance our understanding of the role of fetal microchimerism in maternal health and disease. Our framework makes many predictions (Fig. 2 and Table 3) that can be tested in future work, with potentially important implications for our understanding of health and disease pathology, including lactation science, thyroid diseases, autoimmune diseases, cancer and even maternal emotional, and psychological health postpartum.
Table 3.
Testable predictions about microchimerism
| Predictions | Rationale | Testing |
|---|---|---|
| Fetal microchimeric cells in the breast increases maternal milk production and quality | It is in the offspring's fitness interest to increase maternal milk production and quality | Assay microchimerism in maternal blood and breast milk and quantify the amount of milk produced at routine time points (e.g. 1, 2, 4, and 10 weeks postpartum) |
| Fetal cell microchimerism in the thyroid increases maternal body temperature | Offspring benefit from increased maternal heat production as they do not have to engage in costly heat production 65 | Quantify fetal cell abundance in blood and measure maternal body temperature prepartum, once each trimester, and postpartum |
| Attachment and bonding mechanism are enhanced by fetal cells | Offspring are likely to receive more resources as a result of increased maternal attachment and bonding with positive fitness consequences 73 | Measure abundance of circulating fetal cells and oxytocin prepartum (control), third trimester, and for three month intervals postpartum. Flow sort fetal cells and assay transcript abundance of genes regulating oxytocin and prolactin production |
| Microchimeric fetal cells contribute to the development and/or progression of cancers | Fetal microchimeric cells have some characteristics of cancer 119, including evading the maternal immune system 11 and inducing angiogenesis 40. These cancer‐like properties could lead to greater cancer vulnerabilities, specifically at the initial stages of tumor development, when escaping destruction from the immune system and growth may be most important | Investigate transcription of clones within the tumor and possible microchimeric tumor cells using single‐cell sequencing from multiple positions in the tumor, including the center, and surrounding healthy tissue and compare across patients to distinguish between fetal and other microchimeric events (men, women who have never had a pregnancy, and women who have had at least one pregnancy) |
| Fetal microchimeric cells in the immune system increase autoimmune disease susceptibility | It would be in the fetal interest to evade detection and destruction by the maternal immune system, which may have variable response by HLA haplotypes 7. An over‐reactive response from the maternal immune system to small numbers of fetal cells, or a comparable response to over‐proliferating fetal cells, may increase autoimmune disease susceptibility, or symptoms of an existing autoimmune disease. Alternatively, a reduced maternal response is expected to lead to amelioration of autoimmune disease symptoms | In patients with preexisting autoimmune disease, a family history of autoimmune disease, and no susceptibility to autoimmune disease, measure abundance of fetal cells and measure autoimmune disease symptoms, C‐reactive protein levels, and abundance of T regulatory cells pre‐partum, each trimester, and postpartum. Assay HLA profiles of maternal and fetal cells by sequencing the cell free DNA. Characterize antigen expressed on fetal cells |
| Fetal cell quantity in the maternal body contributes to signaling pregnancy success, completion, and/or termination | (1) Fetal cells may be important in conception and retention of the fetus. The maternal body must suppress immune function for a successful placentation and gestation. Fetal cells could play a role in tolerance for successful pregnancy by homing to thymus prior to placentation and stimulation T regulatory cells (2) Fetal cells increase in maternal blood until onset of labor 8, 18, this could be one component important for triggering labor, even pre‐term labor, either through maternal immune response, or oxytocin signaling | Prospectively collect a cohort planning a pregnancy, without pre‐existing diseases. Measure abundance of T‐regulatory cells for 3 month intervals until conception, then continue to measure T‐regulatory abundance as well as assay abundance and antigen composition of microchimeric cells in the blood at 6, 12, 20, 28, 36, 40 weeks, and 3 months postpartum |
Based on our cooperation and conflict framework, we propose testable predictions, rationale, and methods for testing predictions for the potential role of fell cells in maternal health.
The authors have declared no conflicts of interest.
Acknowledgments
We thank the reviewers for helpful comments. This work was made possible by support by grants from the US National Institutes of Health (NIH) R01 CA170595, R01 CA185138, NIH/NCI R01 PQC3 “Genomic and Microenvironmental Diversity as Drivers of Progression in DCIS,” the John Templeton Foundation grant “Generous by nature: Need‐based transfers and the origins of human cooperation (CAA),” and startup funding from the School of Life Sciences, the Biodesign Institute (MAWS), and the Department of Psychology (CAA) at Arizona State University. Any opinions, findings, and conclusions or recommendation expressed in this material are those of the author(s) and do not necessarily reflect the views of the John Templeton Foundation or National Institute of Health (NIH).
References
- 1. Hamilton WD. 1964. The genetical evolution of social behaviour. II. J Theor Biol 7: 17–52. [DOI] [PubMed] [Google Scholar]
- 2. Trivers RL. 1974. Parent‐offspring conflict. Am Zool 14: 249–64. [Google Scholar]
- 3. Haig D. 1993. Genetic conflicts in human pregnancy. Q Rev Biol 68: 495–532. [DOI] [PubMed] [Google Scholar]
- 4. Crespi B, Semeniuk C. 2004. Parent‐offspring conflict in the evolution of vertebrate reproductive mode. Am Nat 163: 635–53. [DOI] [PubMed] [Google Scholar]
- 5. Haig D. 2014. Interbirth intervals Intrafamilial, intragenomic and intrasomatic conflict. Evol Med Public Health 2014: 12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lo YM, Lau TK, Chan LY, Leung TN, et al. 2000. Quantitative analysis of the bidirectional fetomaternal transfer of nucleated cells and plasma DNA. Clin Chem 46: 1301–9. [PubMed] [Google Scholar]
- 7. Adams Waldorf KM, Gammill HS, Lucas J, Aydelotte TM, et al. 2010. Dynamic changes in fetal microchimerism in maternal peripheral blood mononuclear cells, CD4+ and CD8+ cells in normal pregnancy. Placenta 31: 589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fujiki Y, Johnson KL, Tighiouart H, Peter I, et al. 2008. Fetomaternal trafficking in the mouse increases as delivery approaches and is highest in the maternal lung. Biol Reprod 79: 841–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, et al. 1996. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci USA 93: 705–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bayes‐Genis A, Bellosillo B, de La Calle O, Salido M, et al. 2005. Identification of male cardiomyocytes of extracardiac origin in the hearts of women with male progeny: male fetal cell microchimerism of the heart. J Heart Lung Transplant 24: 2179–83. [DOI] [PubMed] [Google Scholar]
- 11. Kolialexi A, Tsangaris GT, Antsaklis A, Mavroua A. 2004. Rapid clearance of fetal cells from maternal circulation after delivery. Ann NY Acad Sci 1022: 113–8. [DOI] [PubMed] [Google Scholar]
- 12. Stevens AM, Hermes HM, Kiefer MM, Rutledge JC, et al. 2009. Chimeric maternal cells with tissue‐specific antigen expression and morphology are common in infant tissues. Pediatr Dev Pathol 12: 337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Srivatsa B, Srivatsa S, Johnson KL, Bianchi DW. 2003. Maternal cell microchimerism in newborn tissues. J Pediatr 142: 31–5. [DOI] [PubMed] [Google Scholar]
- 14. Bakkour S, Baker CA, Tarantal AF, Wen L, et al. 2014. Analysis of maternal microchimerism in rhesus monkeys (Macaca mulatta) using real‐time quantitative PCR amplification of MHC polymorphisms. Chimerism 5: 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guettier C, Sebagh M, Buard J, Feneux D, et al. 2005. Male cell microchimerism in normal and diseased female livers from fetal life to adulthood. Hepatol Baltim Md 42: 35–43. [DOI] [PubMed] [Google Scholar]
- 16. Mahmood U, O'Donoghue K. 2014. Microchimeric fetal cells play a role in maternal wound healing after pregnancy. Chimerism 5: 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nassar D, Droitcourt C, Mathieu‐d'Argent E, Kim MJ, et al. 2012. Fetal progenitor cells naturally transferred through pregnancy participate in inflammation and angiogenesis during wound healing. FASEB J 26: 149–57. [DOI] [PubMed] [Google Scholar]
- 18. Bianchi DW, Farina A, Weber W, Delli‐Bovi LC, et al. 2001. Significant fetal‐maternal hemorrhage after termination of pregnancy: implications for development of fetal cell microchimerism. Am J Obstet Gynecol 184: 703–6. [DOI] [PubMed] [Google Scholar]
- 19. Gammill HS, Aydelotte TM, Guthrie KA, Nkwopara EC, et al. 2013. Cellular fetal microchimerism in preeclampsia. Hypertension 62: 1062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gammill HS, Stephenson MD, Aydelotte TM, Nelson JL. 2014. Microchimerism in recurrent miscarriage. Cell Mol Immunol 11.6: 589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peterson SE, Nelson JL, Gadi VK, Gammill HS. 2013. Fetal cellular microchimerism in miscarriage and pregnancy termination. Chimerism 4: 136–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lo YMD, Lau TK, Zhang J, Leung TN, et al. 1999. Increased fetal DNA concentrations in the plasma of pregnant women carrying fetuses with trisomy 21. Clin Chem 45: 1747–51. [PubMed] [Google Scholar]
- 23. Kallenbach LR, Johnson KL, Bianchi DW. 2011. Fetal cell microchimerism and cancer: a nexus of reproduction, immunology and tumor biology. Cancer Res 71: 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pineda‐Krch M, Lehtilä K. 2004. Costs and benefits of genetic heterogeneity within organisms. J Evol Biol 17: 1167–77. [DOI] [PubMed] [Google Scholar]
- 25. Wildman DE. 2011. Review: toward an integrated evolutionary understanding of the mammalian placenta. Placenta 32: S142–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moffett A, Loke C. 2006. Immunology of placentation in eutherian mammals. Nat Rev Immunol 6: 584–94. [DOI] [PubMed] [Google Scholar]
- 27. Axiak‐Bechtel SM, Kumar SR, Hansen SA, Bryan JN. 2013. Y‐chromosome DNA is present in the blood of female dogs suggesting the presence of fetal microchimerism. PLoS One 8: e68114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Turin L, Invernizzi P, Woodcock M, Grati FR, et al. 2007. Bovine fetal microchimerism in normal and embryo transfer pregnancies and its implications for biotechnology applications in cattle. Biotechnol J 2: 486–91. [DOI] [PubMed] [Google Scholar]
- 29. Khosrotehrani K, Johnson KL, Guégan S, Stroh H, et al. 2005. Natural history of fetal cell microchimerism during and following murine pregnancy. J Reprod Immunol 66: 1–2. [DOI] [PubMed] [Google Scholar]
- 30. Fugazzola L, Cirello V, Beck‐Peccoz P. 2011. Fetal microchimerism as an explanation of disease. Nat Rev Endocrinol 7: 89–97. [DOI] [PubMed] [Google Scholar]
- 31. Reiches MW, Ellison PT, Lipson SF, Sharrock KC, et al. 2009. Pooled energy budget and human life history. Am J Hum Biol 21: 421–9. [DOI] [PubMed] [Google Scholar]
- 32. Hillard Kaplan S, Jane Lancaster B. 2003. An evolutionary and ecological analysis of human fertility, mating patterns, and parental investment In Wachter KW, Bulatao RA, ed; Offspring: Human Fertility Behavior in Biodemographic Perspective. Washington, DC: The National Academies Press, p. 170–223. [PubMed] [Google Scholar]
- 33. Geary DC, Flinn MV. 2001. Evolution of human parental behavior and the human family. Parenting 1: 5–61. [Google Scholar]
- 34. Bonney EA, Matzinger P. 1997. The maternal immune system's interaction with circulating fetal cells. J Immunol Baltim Md 950 158: 40–7. [PubMed] [Google Scholar]
- 35. Abrahams VM, Straszewski‐Chavez SL, Guller S, Mor G. 2004. First trimester trophoblast cells secrete Fas ligand which induces immune cell apoptosis. Mol Hum Reprod 10: 55–63. [DOI] [PubMed] [Google Scholar]
- 36. Reik W, Walter J. 2001. Genomic imprinting: parental influence on the genome. Nat Rev Genet 2: 21–32. [DOI] [PubMed] [Google Scholar]
- 37. O'Donoghue K, Chan J, de la Fuente J, Kennea N, et al. 2004. Microchimerism in female bone marrow and bone decades after fetal mesenchymal stem‐cell trafficking in pregnancy. Lancet 364: 179–82. [DOI] [PubMed] [Google Scholar]
- 38. Khosrotehrani K, Leduc M, Bachy V, Nguyen Huu S, et al. 2008. Pregnancy allows the transfer and differentiation of fetal lymphoid progenitors into functional T and B cells in mothers. J Immunol Baltim Md 1950 180: 889–97. [DOI] [PubMed] [Google Scholar]
- 39. Sharpless NE, DePinho RA. 2007. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol 8: 703–13. [DOI] [PubMed] [Google Scholar]
- 40. Nguyen Huu S, Oster M, Uzan S, Chareyre F, et al. 2007. Maternal neoangiogenesis during pregnancy partly derives from fetal endothelial progenitor cells. Proc Natl Acad Sci USA 104: 1871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang Y, Iwatani H, Ito T, Horimoto N, et al. 2004. Fetal cells in mother rats contribute to the remodeling of liver and kidney after injury. Biochem Biophys Res Commun 325: 961–7. [DOI] [PubMed] [Google Scholar]
- 42. Khosrotehrani K, Reyes RR, Johnson KL, Freeman RB, et al. 2007. Fetal cells participate over time in the response to specific types of murine maternal hepatic injury. Hum Reprod Oxf Engl 22: 654–61. [DOI] [PubMed] [Google Scholar]
- 43. Khosrotehrani K, Johnson KL, Cha D, Salomon RN, et al. 2004. Transfer of fetal cells with multilineage potential to maternal tissue. JAMA 292: 75–80. [DOI] [PubMed] [Google Scholar]
- 44. Srivatsa B, Srivatsa S, Johnson KL, Samura O, et al. 2001. Microchimerism of presumed fetal origin in thyroid specimens from women: a case‐control study. Lancet 358: 2034–8. [DOI] [PubMed] [Google Scholar]
- 45. Nelson JL. 2012. The otherness of self: microchimerism in health and disease. Trends Immunol 33: 421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tan X‐W, Liao H, Sun L, Okabe M, et al. 2005. Fetal microchimerism in the maternal mouse brain: a novel population of fetal progenitor or stem cells able to cross the blood‐brain barrier? Stem Cells Dayt Ohio 23: 1443–52. [DOI] [PubMed] [Google Scholar]
- 47. Zeng XX, Tan KH, Yeo A, Sasajala P, et al. 2010. Pregnancy‐associated progenitor cells differentiate and mature into neurons in the maternal brain. Stem Cells Dev 19: 1819–30. [DOI] [PubMed] [Google Scholar]
- 48. Kara RJ, Bolli P, Karakikes I, Matsunaga I, et al. 2011. Fetal cells traffic to injured maternal myocardium and undergo cardiac differentiation. Circ Res 111: 249037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Evans PC, Lambert N, Maloney S, Furst DE, et al. 1999. Long‐term fetal microchimerism in peripheral blood mononuclear cell subsets in healthy women and women with scleroderma. Blood 93: 2033–7. [PubMed] [Google Scholar]
- 50. Hinde K, Skibiel AL, Foster AB, Rosso LD, et al. 2015. Cortisol in mother's milk across lactation reflects maternal life history and predicts infant temperament. Behav Ecol 26: 269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhou L, Yoshimura Y, Huang Y, Suzuki R, et al. 2000. Two independent pathways of maternal cell transmission to offspring: through placenta during pregnancy and by breast‐feeding after birth. Immunology 101: 570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gustbée E, Anesten C, Markkula A, Simonsson M, et al. 2013. Excessive milk production during breast‐feeding prior to breast cancer diagnosis is associated with increased risk for early events. SpringerPlus 2: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dhimolea E, Denes V, Lakk M, Al‐Bazzaz S, et al. 2013. High male chimerism in the female breast shows quantitative links with cancer. Int J Cancer 133: 835–42. [DOI] [PubMed] [Google Scholar]
- 54. Gadi VK, Nelson JL. 2007. Fetal microchimerism in women with breast cancer. Cancer Res . 67: 9035–8. [DOI] [PubMed] [Google Scholar]
- 55. Gadi VK, Malone KE, Guthrie KA, Porter PL, et al. 2008. Case‐control study of fetal microchimerism and breast cancer. PLoS One 3: e1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kamper‐Jørgensen M, Biggar RJ, Tjønneland A, Hjalgrim H, et al. 2012. Opposite effects of microchimerism on breast and colon cancer. Eur J Cancer Oxf Engl 1990 48: 2227–35. [DOI] [PubMed] [Google Scholar]
- 57. Eun JK, Guthrie KA, Zirpoli G, Gadi VK. 2013. In situ breast cancer and microchimerism. Sci Rep 3: 2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dubernard G, Aractingi S, Oster M, Rouzier R, et al. 2008. Breast cancer stroma frequently recruits fetal derived cells during pregnancy. Breast Cancer Res 10: R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dubernard G, Oster M, Chareyre F, Antoine M, et al. 2009. Increased fetal cell microchimerism in high grade breast carcinomas occurring during pregnancy. Int J Cancer 124: 1054–9. [DOI] [PubMed] [Google Scholar]
- 60. Hassiotou F, Beltran A, Chetwynd E, Stuebe AM, et al. 2012. Breastmilk is a novel source of stem cells with multilineage differentiation potential. Stem Cells Dayt Ohio 30: 2164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang S, Zhang J, Geng Y, Zhu S. 2003. Plasticity of the response of fetal mouse fibroblast to lactation hormones. Cell Biol Int 27: 755–60. [DOI] [PubMed] [Google Scholar]
- 62. Shackleton M, Vaillant F, Simpson KJ, Stingl J, et al. 2006. Generation of a functional mammary gland from a single stem cell. Nature 439: 84–8. [DOI] [PubMed] [Google Scholar]
- 63. Cohen JM, Hutcheon JA, Julien SG, Tremblay ML, et al. 2009. Insufficient milk supply and breast cancer risk: a systematic review. PLoS One 4: e8237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Arthur JR, Beckett GJ. 1999. Thyroid function. Br Med Bull 55: 658–68. [DOI] [PubMed] [Google Scholar]
- 65. Winberg J. 2005. Mother and newborn baby: mutual regulation of physiology and behavior‐a selective review. Dev Psychobiol 47: 217–29. [DOI] [PubMed] [Google Scholar]
- 66. Cirello V, Recalcati MP, Muzza M, Rossi S, et al. 2008. Fetal cell microchimerism in papillary thyroid cancer: a possible role in tumor damage and tissue repair. Cancer Res 68: 8482–8. [DOI] [PubMed] [Google Scholar]
- 67. Imaizumi M, Pritsker A, Unger P, Davies TF. 2002. Intrathyroidal fetal microchimerism in pregnancy and postpartum. Endocrinology 143: 247–53. [DOI] [PubMed] [Google Scholar]
- 68. Klintschar M, Immel U‐D, Kehlen A, Schwaiger P, et al. 2006. Fetal microchimerism in Hashimoto's thyroiditis: a quantitative approach. Eur J Endocrinol 154: 237–41. [DOI] [PubMed] [Google Scholar]
- 69. Lepez T, Vandewoestyne M, Hussain S, Van Nieuwerburgh F, et al. 2011. Fetal microchimeric cells in blood of women with an autoimmune thyroid disease. PLoS One 6: e29646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Renné C, Ramos Lopez E, Steimle‐Grauer SA, Ziolkowski P, et al. 2004. Thyroid fetal male microchimerisms in mothers with thyroid disorders: presence of Y‐chromosomal immunofluorescence in thyroid‐infiltrating lymphocytes is more prevalent in Hashimoto's thyroiditis and Graves' disease than in follicular adenomas. J Clin Endocrinol Metab 89: 5810–4. [DOI] [PubMed] [Google Scholar]
- 71. Ando T, Imaizumi M, Graves PN, Unger P, et al. 2002. Intrathyroidal fetal microchimerism in Graves' disease. J Clin Endocrinol Metab 87: 3315–20. [DOI] [PubMed] [Google Scholar]
- 72. Benoit D. 2004. Infant‐parent attachment: definition, types, antecedents, measurement and outcome. Paediatr Child Health 9: 541–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Carter CS. 2014. Oxytocin pathways and the evolution of human behavior. Annu Rev Psychol 65: 17–39. [DOI] [PubMed] [Google Scholar]
- 74. Keverne EB. 2014. Significance of epigenetics for understanding brain development, brain evolution and behaviour. Neuroscience 264: 207–17. [DOI] [PubMed] [Google Scholar]
- 75. Chan WFN, Gurnot C, Montine TJ, Sonnen JA, et al. 2012. Male microchimerism in the human female brain. PLoS One 7: e45592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kendall‐Tackett K. 2007. A new paradigm for depression in new mothers: the central role of inflammation and how breastfeeding and anti‐inflammatory treatments protect maternal mental health. Int Breastfeed J 2: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gürel a S, Gürel H. 2000. The evaluation of determinants of early postpartum low mood: the importance of parity and inter‐pregnancy interval. Eur J Obstet Gynecol Reprod Biol 91: 21–4. [DOI] [PubMed] [Google Scholar]
- 78. Demirbek B, Yurt E. 2011. Can microchimerism find itself a place in psychiatric research? Psikiyatr Guncel Yaklasimlar Curr Approache Psychia 3: 296–308. [Google Scholar]
- 79. Artlett CM, Smith JB, Jimenez SA. 1998. Identification of fetal DNA and cells in skin lesions from women with systemic sclerosis. N Engl J Med 338: 1186–91. [DOI] [PubMed] [Google Scholar]
- 80. Lambert NC, Lo YMD, Erickson TD, Tylee TS, et al. 2002. Male microchimerism in healthy women and women with scleroderma: cells or circulating DNA? A quantitative answer. Blood 100: 2845–51. [DOI] [PubMed] [Google Scholar]
- 81. Endo Y, Negishi I, Ishikawa O. 2002. Possible contribution of microchimerism to the pathogenesis of Sjögren's syndrome. Rheumatology 41: 490–5. [DOI] [PubMed] [Google Scholar]
- 82. Abbud Filho M, Pavarino‐Bertelli EC, Alvarenga MPS, Fernandes IMM, et al. 2002. Systemic lupus erythematosus and microchimerism in autoimmunity. Transplant Proc 34: 2951–2. [DOI] [PubMed] [Google Scholar]
- 83. Kekow M, Barleben M, Drynda S, Jakubiczka S, et al. 2013. Long‐term persistence and effects of fetal microchimerisms on disease onset and status in a cohort of women with rheumatoid arthritis and systemic lupus erythematosus. BMC Musculoskelet Disord 14: 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rak JM, Maestroni L, Balandraud N, Guis S, et al. 2009. Transfer of the shared epitope through microchimerism in women with rheumatoid arthritis. Arthritis Rheum 60: 73–80. [DOI] [PubMed] [Google Scholar]
- 85. Aractingi S, Berkane N, Bertheau P, Le Goué C, et al. 1998. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet 352: 1898–901. [DOI] [PubMed] [Google Scholar]
- 86. Chan WFN, Atkins CJ, Naysmith D, van der Westhuizen N, et al. 2012. Microchimerism in the rheumatoid nodules of patients with rheumatoid arthritis. Arthritis Rheum 64: 380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Selva‐O'Callaghan A, Mijares‐Boeckh‐Behrens T, Prades EB, Solans‐Laqué R, et al. 2003. Lack of evidence of foetal microchimerism in female Spanish patients with systemic sclerosis. Lupus 12: 15–20. [DOI] [PubMed] [Google Scholar]
- 88. Murata H, Nakauchi H, Sumida T. 1999. Microchimerism in Japanese women patients with systemic sclerosis. Lancet 354: 220. [DOI] [PubMed] [Google Scholar]
- 89. Mosca M, Curcio M, Lapi S, Valentini G, et al. 2003. Correlations of Y chromosome microchimerism with disease activity in patients with SLE: analysis of preliminary data. Ann Rheum Dis 62: 651–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yan Z, Lambert NC, Guthrie KA, Porter AJ, et al. 2005. Male microchimerism in women without sons: quantitative assessment and correlation with pregnancy history. Am J Med 118: 899–906. [DOI] [PubMed] [Google Scholar]
- 91. Pritchard S, Hoffman AM, Johnson KL, Bianchi DW. 2011. Pregnancy‐associated progenitor cells: an under‐recognized potential source of stem cells in maternal lung. Placenta 32: S298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. O'Donoghue K, Sultan HA, Al‐Allaf FA, Anderson JR, et al. 2008. Microchimeric fetal cells cluster at sites of tissue injury in lung decades after pregnancy. Reprod Biomed Online 16: 382–90. [DOI] [PubMed] [Google Scholar]
- 93. Lambe M, Hsieh C, Trichopoulos D, Ekbom A, et al. 1994. Transient increase in the risk of breast cancer after giving birth. N Engl J Med 331: 5–9. [DOI] [PubMed] [Google Scholar]
- 94. Negro R, Mestman JH. 2011. Thyroid disease in pregnancy. Best Pract Res Clin Endocrinol Metab 25: 927–43. [DOI] [PubMed] [Google Scholar]
- 95. Weetman AP. 2010. Immunity, thyroid function and pregnancy: molecular mechanisms. Nat Rev Endocrinol 6: 311–8. [DOI] [PubMed] [Google Scholar]
- 96. Gleicher N, Barad DH. 2007. Gender as risk factor for autoimmune diseases. J Autoimmun 28: 1–6. [DOI] [PubMed] [Google Scholar]
- 97. Khashan AS, Kenny LC, Laursen TM, Mahmood U, et al. 2011. Pregnancy and the risk of autoimmune disease. PLoS One 6: e19658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sgarbi JA, Kasamatsu TS, Matsumura LK, Maciel RMB. 2010. Parity is not related to autoimmune thyroid disease in a population‐based study of Japanese‐Brazilians. Thyroid 20: 1151–6. [DOI] [PubMed] [Google Scholar]
- 99. Veenstra van Nieuwenhoven AL, Heineman MJ, Faas MM. 2003. The immunology of successful pregnancy. Hum Reprod Update 9: 347–57. [DOI] [PubMed] [Google Scholar]
- 100. Hazes JMW, Coulie PG, Geenen V, Vermeire S, et al. 2011. Rheumatoid arthritis and pregnancy: evolution of disease activity and pathophysiological considerations for drug use. Rheumatology 50: 1955–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Confavreux C, Hutchinson M, Hours MM, Cortinovis‐Tourniaire P, et al. 1998. Rate of pregnancy‐related relapse in multiple sclerosis. N Engl J Med 339: 285–91. [DOI] [PubMed] [Google Scholar]
- 102. Petri M, Howard D, Repke J. 1991. Frequency of lupus flare in pregnancy. The Hopkins Lupus Pregnancy Center experience. Arthritis Rheum 34: 1538–45. [DOI] [PubMed] [Google Scholar]
- 103. Ruiz‐Irastorza G, Lima F, Alves J, Khamashta MA, et al. 1996. Increased rate of lupus flare during pregnancy and the puerperium: a prospective study of 78 pregnancies. Br J Rheumatol 35: 133–8. [DOI] [PubMed] [Google Scholar]
- 104. Pujal J‐M, Gallardo D. 2008. PCR‐based methodology for molecular microchimerism detection and quantification. Exp Biol Med Maywood NJ 233: 1161–70. [DOI] [PubMed] [Google Scholar]
- 105. Thiele K, Holzmann C, Solano ME, Zahner G, et al. 2014. Comparative sensitivity analyses of quantitative polymerase chain reaction and flow cytometry in detecting cellular microchimerism in murine tissues. J Immunol Methods 406: 74–82. [DOI] [PubMed] [Google Scholar]
- 106. Reed W, Lee TH, Vichinsky EP, Lubin BH, et al. 1998. Sample suitability for the detection of minor white cell populations (microchimerism) by polymerase chain reaction. Transfusion 38: 1041–5. [DOI] [PubMed] [Google Scholar]
- 107. Lau TK, Cheung SW, Lo PSS, Pursley AN, et al. 2014. Non‐invasive prenatal testing for fetal chromosomal abnormalities by low‐coverage whole‐genome sequencing of maternal plasma DNA: review of consecutive cases in a single center. Ultrasound Obstet Gynecol 43: 254–64. [DOI] [PubMed] [Google Scholar]
- 108. Pritchard S, Wick HC, Slonim DK, Johnson KL, et al. 2012. Comprehensive analysis of genes expressed by rare microchimeric fetal cells in the maternal mouse lung. Biol Reprod 87: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gregori S, Amodio G, Quattrone F, Panina‐Bordignon P. 2015. HLA‐G orchestrates the early interaction of human trophoblasts with the maternal niche. Front Immunol 6: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tilburgs T, Crespo ÂC, van der Zwan A, Rybalov B, et al. 2015. Human HLA‐G+ extravillous trophoblasts: immune‐activating cells that interact with decidual leukocytes. Proc Natl Acad Sci USA 112: 7219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sunami R, Komuro M, Tagaya H, Hirata S. 2010. Migration of microchimeric fetal cells into maternal circulation before placenta formation. Chimerism 1: 66–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bininda‐Emonds ORP, Cardillo M, Jones KE, MacPhee RDE, et al. 2007. The delayed rise of present‐day mammals. Nature 446: 507–12. [DOI] [PubMed] [Google Scholar]
- 113. Klintschar M, Schwaiger P, Mannweiler S, Regauer S, et al. 2001. Evidence of fetal microchimerism in Hashimoto's thyroiditis. J Clin Endocrinol Metab 86: 2494–8. [DOI] [PubMed] [Google Scholar]
- 114. Johnson KL, McAlindon TE, Mulcahy E, Bianchi DW. 2001. Microchimerism in a female patient with systemic lupus erythematosus. Arthritis Rheum 44: 2107–11. [DOI] [PubMed] [Google Scholar]
- 115. Cha D, Khosrotehrani K, Kim Y, Stroh H, et al. 2003. Cervical cancer and microchimerism. Obstet Gynecol 102: 774–81. [DOI] [PubMed] [Google Scholar]
- 116. Hromadnikova I, Kotlabova K, Pirkova P, Libalova P, et al. 2014. The occurrence of fetal microchimeric cells in endometrial tissues is a very common phenomenon in benign uterine disorders, and the lower prevalence of fetal microchimerism is associated with better uterine cancer prognoses. DNA Cell Biol 33: 40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Fassbender A, Debiec‐Rychter M, Bree RV, Vermeesch JR, et al. 2015. Lack of evidence that male fetal microchimerism is present in endometriosis. Reprod Sci 1933719115574343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Nguyen Huu S, Oster M, Avril M‐F, Boitier F, et al. 2009. Fetal microchimeric cells participate in tumour angiogenesis in melanomas occurring during pregnancy. Am J Pathol 174: 630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Hanahan D, Weinberg RA. 2000. The hallmarks of cancer. Cell 100: 57–70. [DOI] [PubMed] [Google Scholar]


