Abstract
HLA-matched related donor (MRD) hematopoietic stem cell transplantation (HSCT) is a well-established therapy for patients with sickle cell disease (SCD); however, experience using alternative donors, including haploidentical donors, in HSCT for SCD is limited. We report the long-term outcomes of 22 pediatric patients who underwent related donor HSCT for SCD at St. Jude Children’s Research Hospital, either a myeloablative sibling MRD HSCT (n = 14) or reduced-intensity parental haploidentical donor HSCT (n = 8). The median patient age was 11.0 ± 3.9 years in the MRD graft recipients and 9.0 ± 5.0 years in the haploidentical donor graft recipients. The median follow-up was 9.0 ± 2.3 years, with an overall survival (OS) of 93% and a recurrence/graft failure rate of 0%, for the MRD cohort and 7.4 ± 2.4 years, with an OS of 75%, disease-free survival of 38%, and disease recurrence of 38%, for the haploidentical donor cohort. We report the long-term hematologic response and organ function in patients undergoing MRD or haploidentical donor HSCT for severe SCD. Our data demonstrate long-term hematologic improvements after HSCT with sustained engraftment, and confirm that HSCT offers long-term protection from common complications of SCD, including stroke, pulmonary hypertension, acute chest, and nephropathy, regardless of donor source.
Keywords: Sickle cell disease, Reduced-intensity regimen, Haploidentical donor, Matched sibling donor, Long-term follow-up
INTRODUCTION
Currently allogeneic hematopoietic stem cell transplantation (HSCT) is the sole curative option for sickle cell disease (SCD). The majority of HSCTs performed for SCD involve transplantation of HLA-matched related donor (MRD) grafts after a myeloablative preparative regimen. Several previous studies have reported excellent outcomes in recipients of MRD HSCT for SCD, with >80% disease-free survival (DFS) and >90% overall survival (OS) [1–4]. Moreover, recipients who achieve durable engraftment of donor cells do not experience the continual vaso-occlusive damage related to SCD [3–9].
Although these results are encouraging, 85% of patients with SCD who meet the criteria for an HSCT do not have an appropriate MRD [10]. This lack of MRD availability has been a major barrier to HSCT for patients with severe SCD. Recently, alternative donor sources for HSCT, including cord blood and HLA-haploidentical donors, have been explored for patients with SCD [11,12].
Bolaños-Meade et al. [11] recently reported the outcomes of 14 patients with SCD who underwent haploidentical donor HSCT with a nonmyeloablative conditioning regimen. Eight of the 14 patients engrafted and were asymptomatic at a median follow-up of 2.1 years. Graft failure (43%) remained a major obstacle, but no serious toxicities were reported. These early reports suggest the potential for using a reduced-intensity haploidentical HSCT platform to treat SCD. Further studies on approaches to decreasing graft failure and continual long-term follow-up are needed to establish haploidentical donor HSCT as a safe, effective alternative treatment for SCD.
The effects of MRD HSCT on other organs have been reported through retrospective reviews of available data from large multicenter studies [4–8,13–17]. Although comprehensive neurocognitive studies performed after HSCT have reported stable findings, no prospective evaluations performed both before and after HSCT have been reported to date [7]. Moreover, long-term prospective imaging, neurocognitive, and pulmonary function studies are needed in patients with SCD undergoing reduced-intensity conditioning haploidentical HSCT. Finally, pulmonary hypertension is associated with a high risk of mortality [18,19] and should be followed in patients undergoing MRD HSCT or haploidentical donor HSCT. A comprehensive evaluation of central nervous system, pulmonary, cardiac, renal, endocrine, growth, and gonadal toxicities in patients undergoing haploidentical HSCT for SCD is currently just emerging. Here we report the long-term outcomes of pediatric patients who underwent reduced-intensity haploidentical donor HSCT or myeloablative MRD HSCT, with similar hematologic responses and organ function in patients with durable engraftment.
MATERIALS AND METHODS
Patients
All 22 patients were enrolled in a St Jude Children’s Research Hospital Institutional Review Board–approved study (SCALLO or FAMSCT/SCDHAP). Consent was granted in accordance with the Declaration of Helsinki, and each patient or a parent or guardian signed an Institutional Review Board–approved informed consent form before receiving treatment on a Phase II clinical trial registered at ClinicalTrials.gov (NCT00186810 and NCT00152113). For MRD HSCT, patients age ≤21 years with symptomatic SCD genotype SS, SC, or Sβ0 with an MRD or related umbilical cord blood (UCB) donor with hemoglobin genotype AA or AS were considered for HSCT. HLA typing at the molecular level was performed, and all donors were fully matched as described previously [20]. All patients were required to meet the eligibility criteria as reported previously, and any patient with extensive end-organ damage was excluded [21]. An additional eligibility criterion for patients undergoing haploidentical donor HSCT was a previous cerebrovascular accident (CVA).
Donors and Grafts
MRD grafts consisted of fresh harvested bone marrow (BM) with a collection target of 4 × 108 total nucleated cells/kg. Cryopreserved MRD cords blood units could be used as well. For haploidentical grafts, preference was given to donors who were natural killer cell Ig-like receptor–mismatched with recipients. The haploidentical donor grafts consisted of a granulocyte colony-stimulating factor–mobilized apheresis product that was CD34+-selected, cryopreserved, and administered on day 0. The donor also underwent a second cycle of granulocyte colony-stimulating factor–primed apheresis to provide a fresh CD3+-depleted product to reach the CD34+ cell target dose of 5 × 106 cells/kg. Thus, the product consisted of a CD34+-selected product infused on day 0 and a CD3+-depleted product infused on day +1 using the CliniMACS device, as described previously [22,23]. A fixed CD3+ T cell dose of approximately 1.0 × 105 cells/kg was targeted for infusion.
HSCT Conditioning and GVHD Prophylaxis Regimens
For MRD HSCT, patients were conditioned with targeted busulfan, cyclophosphamide (200 mg/kg), and horse antithymocyte globulin (20 mg/kg). Busulfan was given i.v. or orally 4 times daily at a starting dose of 37.5 mg/m2 for 4 consecutive days. Busulfan concentrations was measured after the first dose, and subsequent doses were adjusted to achieve a targeted systemic exposure with an area under the receiver-operating characteristic curve of 6000 ng/mL/hr, equivalent to approximately 1500 uM. Busulfan concentration was monitored daily throughout the course of therapy.
GVHD prophylaxis included cyclosporine beginning on day −2 and methotrexate at 15 mg/m2 on days +1, +3, and +6 and 10 mg/m2 on day +11. Patients receiving a UCB graft were given cyclosporine without methotrexate. In patients who developed GVHD, methylprednisolone was added at an initial dose of 1 mg/kg and increased in the event of persistent or progressive GVHD.
The first 3 haploidentical HSCT recipients received fludarabine (150–200 mg/m2), thiotepa (10 mg/kg), targeted busulfan (900 ng/mL for 4 days), rabbit antithymocyte globulin (10 mg/kg for 3 days), and muromonab-CD3 (Orthoclone OKT3; 0.1 mg/kg maximum over days +1 to +20). The next 5 patients received hydroxyurea and azathioprine approximately 3 months before HSCT, followed by busulfan (900 ng/mL for 4 days), thiotepa (10 mg/kg), cyclophosphamide (200 mg/kg), and OKT3 (0.1 mg/kg maximum on days −10 to +17), as well as mycophenolate mofetil for GVHD prophylaxis.
Supportive Therapy
Patients who were not receiving long-term transfusion therapy underwent a partial exchange transfusion to achieve a hemoglobin S fraction ≤30% before HSCT. Strategies to prevent neurologic complications included anticonvulsant prophylaxis with phenytoin during busulfan administration and continuing for 6 months after HSCT, along with strict control of hypertension and magnesium levels. Hemoglobin concentration was maintained at 9–11 g/dL, and platelet count was maintained at ≥50,000/μL. Before HSCT, patients were started and maintained on oral penicillin V-K. Oral trimethoprim and sulfamethoxazole was provided for Pneumocystis jiroveci prophylaxis. Cytomegalovirus (CMV)-seropositive patients or patients with a CMV-seropositive donor received acyclovir prophylaxis on day +1 to day +120. Viral reactivation of CMV and Epstein-Barr virus were monitored weekly for detection of DNA copy number by PCR. CMV reactivation was treated with gancyclovir or foscarnet. Epstein-Barr virus reactivation and lymphoproliferative disorder were treated with monoclonal anti-CD20 antibody.
Engraftment and Donor Chimerism
Engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count (ANC) ≥500/μL. Hematopoietic cell chimerism was identified by studies of restriction fragment-length polymorphisms or variable number tandem repeats (VNTRs) in DNA. BM aspirates were obtained at the time of engraftment or around day +21. Peripheral blood samples for VNTR analysis were obtained starting at the appearance of signs of ANC recovery or by day +21, whichever occurred first. Thereafter, VNTR analysis was performed weekly up to day +120, then monthly up to 1 year, and then annually. After engraftment, patients with stable donor chimerism who subsequently developed aplasia, cytopenia, or an ANC <500/mm3 were eligible to receive an additional CD34+ stem cell “boost” of 5 × 106 CD34+ cells/kg. In addition, patients with declining donor chimerism without aplasia or cytopenia were eligible to receive up to 3 donor lymphocyte infusions (DLIs) at 2.5 × 104 CD3+ cells/kg/dose in accordance with protocol.
Assessment of Disease Response and Organ Function
Before HSCT, patients underwent a complete blood count (CBC) with WBC differential, platelet count, reticulocyte count, hemoglobin electrophoresis, and quantification of hemoglobin F, A1, and S, ferritin, and iron. CBC with WBC differential was performed a minimum of 3 times per week until the first hospital discharge after HSCT. Reticulocyte count, hemoglobin electrophoresis, and quantification of hemoglobin F, A1, and S were obtained yearly thereafter. Ferritin and iron levels were measured every 3 months and then annually after the patient had been transfusion-independent for 1 year. Patients with evidence of iron overload and persistent elevated ferritin levels after successful HSCT were considered for phlebotomy and/or chelation therapy.
Transcranial Doppler (TCD) was performed before and at 6, 12, and 24 months after HSCT. Prospective magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), neuropsychological testing, and neurologic examinations were performed on all patients before and at day +180 after HSCT, and yearly thereafter. Patients were imaged using sagittal T1-, axial inversion recovery T2-, and FLAIR-weighted sequences on a 1.5-T imager. Comprehensive neuropsychological evaluations were obtained before and at 1, 3, and 5 years after HSCT. Neuropsychiatric evaluation included age-appropriate assessment of intelligence or developmental quotient for all patients. For patients of school age and older, comprehensive assessment of academic achievement, attention, memory, visual-motor, and visual-perceptual skills was performed.
All patients underwent organ function studies before HSCT. Pulmonary function tests (PFTs), including total lung capacity, forced vital capacity (FVC), and diffusion capacity of the lung for carbon monoxide (DLCO), were performed yearly. Cardiac function was evaluated by echocardiography and electrocardiography (ECG) annually. Renal function was assessed by 24-hour urine protein and creatinine clearance (CrCl) and/or technetium 99m-labeled diethylenetriamine penta-acetic acid (Tc99m DPTA) plasma clearance at approximately day +120 and annually thereafter. Liver biopsy analysis, including measurement of liver iron content, was performed if the ferritin level was >1000 mg/mL. Liver-spleen scans were performed at approximately 3, 12, and 24 months after HSCT. Endocrine function tests, including thyroid function tests and measurement of luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol, and testosterone, were performed annually after HSCT. Bone age films were obtained annually until maturation. All patients underwent annual imaging studies by MRI and/or plain film of the hips to assess the risk of osteonecrosis. Bone mineral density (BMD) and BMD z-score of the whole body and lumbar spine were monitored yearly with computed tomography bone density and dual-energy X-ray absorptiometry scans.
Statistical Analysis
Statistical analyses were performed to summarize results for the MRD cohort. Descriptive results are provided for patients who underwent haploidentical HSCT. The Kaplan-Meier method was used to estimate OS and DFS; events studied included death, graft rejection, and recurrence of SCD. The Student t-test was applied to each of variables to evaluate for any significant changes after HSCT. Descriptive statistics were computed for each function variable.
RESULTS
Patients
Twenty-two patients with SCD underwent related donor HSCT at St. Jude Children’s Research Hospital, including 14 recipients of a sibling MRD graft and 8 recipients of a parental haploidentical donor graft (Table 1). Eighteen patients had SCD-SS, 2 had SCD-SC, and 2 had SCD-SB. The MRD cohort had a median age of 11.0 ± 3.9 years (range, 5.4–17.4 years) and included 3 females and 11 males. The indications for HSCT included a history of stroke (9 patients), recurrent acute chest syndrome (8 patients), and recurrent painful episodes (6 patients), with most patients having multiple indications. The haploidentical cohort had a median age of 7 ± 5 years (range, 4 to 17 years) and included 3 females and 5 males. All the patients had a history of symptomatic SCD and documented CVA. The infused donor products had a mean total nucleated cell count of 450 ± 680 × 106 cells/kg (range, 10–1890 × 106/kg), a CD34+ cell count of 25.4 ± 16.3 × 106/kg (range, 6–57 × 106/kg), and a CD3+ cell count of 0.07 ± 0.07 × 106/kg (range, 0.006–0.168 × 106/kg) (Table 1).
Table 1.
Patient and Graft Characteristics, Engraftment, GVHD, and Follow-Up
| Patient | Age, yr | Sex | Diagnosis | Donor | Indication | HLA Match |
TNC, × 108/kg |
CD3, × 106/kg |
ANC, Days |
Graft | aGVHD | cGVHD | Status | Follow- Up, yr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 14.6 | M | SCD-SS | MRD BM | CVA/AC | 6/6 | 4.64 | 12 | Stable | Grade III | Severe lung | Expired | (1.2) | |
| 2 | 14.5 | M | SCD-SB | MRD BM | CVA/AC | 6/6 | 3.20 | 16 | Stable | Grade II | Moderate GI | Alive | 7.3 | |
| 3 | 9.2 | F | SCD-SS | MRD BM | CVA/AC | 6/6 | 3.23 | 17 | Stable | None | None | Alive | 12.3 | |
| 4 | 6 | M | SCD-SS | MRD BM | AC | 6/6 | 5.75 | 19 | Stable | Grade I | None | Alive | 6.1 | |
| 5 | 10.5 | M | SCD-SS | MRD BM | TCD | 6/6 | 2.14 | 15 | Stable | None | None | Alive | 5.2 | |
| 6 | 14.3 | M | SCD-SS | MRD BM | AC | 6/6 | 2.85 | 24 | Stable | None | None | Alive | 10.9 | |
| 7 | 14.6 | F | SCD-SS | MRD BM | AC/TCD | 6/6 | 3.29 | 15 | Stable | None | None | Alive | 9.3 | |
| 8 | 5.8 | M | SCD-SC | MRD BM | TCD | 6/6 | 3.31 | 14 | Stable | None | None | Alive | 5.9 | |
| 9 | 5.4 | M | SCD-SS | MRD BM | CVA | 6/6 | 3.94 | 28 | Stable | None | None | Alive | 11.0 | |
| 10 | 8.8 | M | SCD-SS | MRD BM | CVA | 6/6 | 3.00 | 16 | Stable | None | None | Alive | 12.2 | |
| 11 | 13.7 | M | SCD-SS | MRD BM | CVA | 6/6 | 3.21 | 23 | Stable | None | None | Alive | 7.9 | |
| 12 | 17.4 | M | SCD-SS | MRD BM | CVA/AC | 6/6 | 3.29 | 14 | Stable | Grade II | Moderate GI | Alive | 8.8 | |
| 13 | 8.5 | F | SCD-SB | MRD cord blood | CVA/AC | 6/6 | 0.45 | 23 | Stable | Grade I | None | Alive | 6.0 | |
| 14 | 11.5 | F | SCD-SS | MRD BM | TCD/AC | 6/6 | 4.71 | 15 | Stable | Grade III | None | Alive | 10.0 | |
| 15 | 8.5 | F | SCD-SS | Haplo; father | CVA | 3/6 | 0.32 | 0.009 | 12 | Stable; DLI on day +107 | None | None | Alive | 8.9 |
| 16 | 6.9 | M | SCD-SS | Haplo; father | CVA | 3/6 | 0.18 | 0.010 | 12 | Rejection; CD34 boost | None | — | Alive with SCD | 7.8 |
| 17 | 13.4 | M | SCD-SB | Haplo; father | CVA | 3/6 | 0.18 | 0.013 | 13 | Stable after rejection; CD34 boost | Grade I | None | Alive | 9.0 |
| 18 | 17.1 | F | SCD-SS | Haplo; father | CVA | 3/6 | 4.52 | 0.168 | 11 | Stable | Grade I | Extensive; severe lung | Expired | (0.9) |
| 19 | 4.2 | M | SCD-SS | Haplo; mother | CVA | 3/6 | 10.16 | 0.149 | 13 | Stable | Grade II | Limited; mild skin | Alive | 6.9 |
| 20 | 16.9 | M | SCD-SB | Haplo; mother | CVA | 3/6 | 1.541 | 0.100 | 14 | Rejection; DLI on day +51; CD34 boost | None | — | Alive with SCD | 3.7 |
| 21 | 5.8 | M | SCD-SS | Haplo; father | CVA | 4/6 | 18.90 | 0.100 | 16 | Rejection; auto-HSCT | None | — | Alive with SCD | 2.8 |
| 22 | 9.5 | F | SCD-SS | Haplo; mother | CVA | 4/6 | 0.14 | 0.006 | 11 | Stable; DLI on day +70 | Grade II | Extensive; severe lung | Expired | (2.3) |
AC indicates acute chest; Haplo, haploidentical; TNC, total nucleated cells; CD34+ boost, 5 × 106–108 cells/kg; DLI dose, 0.025 × 106 CD3+ cells/kg.
Outcome
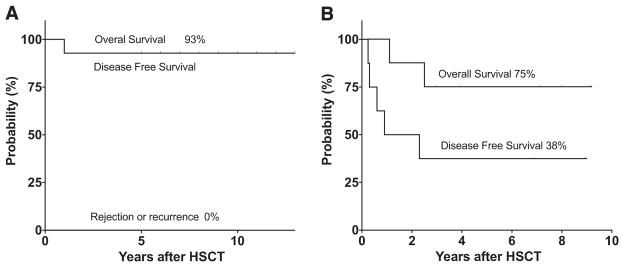
After a median of 9.0 ± 2.3 years (range, 5.9 to 12.3 years), 13 of the 14 patients in the MRD cohort were alive, at a median age of 21 ± 6.2 years (range, 12 to 33 years). One patient died at 15 months post-HSCT from complications related to chronic GVHD (cGVHD). Kaplan-Meier estimates of OS and DFS were both 93%, and there were no cases of graft failure/disease recurrence (Figure 1A).
Figure 1.
Kaplan-Meier estimates of OS and DFS after MRD HSCT and haploidentical HSCT. OS and DFS were determined with events defined as death, graft rejection, or recurrence of SCD after MRD HSCT (A) or haploidentical HSCT (B). Tick marks represent surviving patients and indicate the duration of follow-up after transplantation. The percentages above each curve indicate the estimates of OS, DFS, and cumulative incidence of graft rejection or recurrence of disease for the entire cohort.
After a median of 7.4 ± 2.7 years (range, 2.8 to 9 years), 6 of the 8 patients (75%) were alive, at a median age of 21 ± 6.2 years (range, 12 to 33 years). Three of the 8 patients (38%) experienced sustained engraftment and remained disease-free. Two patients (25%) died from complications related to chronic GVHD, at 0.9 and 2.3 years after HSCT. Graft failure and SCD recurrence occured in 3 patients (38%). Kaplan-Meier estimates of OS and DFS were 75% and 38%, respectively (Figure 1B).
Engraftment and Chimerism
All MRD HSCT recipients engrafted; the median time to engraftment with an ANC ≥500/μL was 16.0 ± 4.7 days (range, 15 to 32 days), and the median time to a platelet count ≥20,000/μL was 28.0 ± 17.5 days (range, 11 to 40 days). At 5 years post-HSCT, all surviving patients demonstrated sustained engraftment with ≥85% donor-derived blood cells on chimerism tests.
All 8 haploidentical HSCT recipients achieved donor engraftment with 100% donor chimerism, with a median time to engraftment of 12.5 ± 1.7 days (range, 10 to 14 days) and a median time to a platelet count ≥20,000/μL of 19.0 ± 15.4 days (range, 17 to 49 days). Thereafter, 4 patients (50%) developed evidence of graft rejection at a median of 30 days (range, 22 to 44 days) and required additional stem cell infusion. After this infusion, 1 of the 4 patients recovered with sustained 100% donor chimerism, whereas the other 3 patients progressed to graft failure with recurrence of disease. Donor engraftment was sustained in 5 patients (62%), and graft failure occurred in 3 patients (38%).
GVHD
Four MRD HSCT recipients (28%) developed grade II–IV acute GVHD (aGVHD), and 3 patients (21%) developed cGVHD. One patient with extensive or severe cGVHD of the lung died of this complication; the other patients with GVHD recovered and discontinued immunosuppressive therapy.
Four of the 5 engrafted haploidentical HSCT recipients developed aGVHD. Two patients were limited to grade I aGVHD, and the other 2 had grade II aGVHD. Three of the 4 patients developed cGVHD: 1 with limited skin involvement and the other 2 with extensive severe cGVHD of the lung. The latter 2 patients died from complications of cGVHD (Table 1). One of these 2 patients had received the highest CD3+ dose in the graft, and the other patient developed severe chronic lung GVHD after receiving a DLI.
Hematologic Disease Response
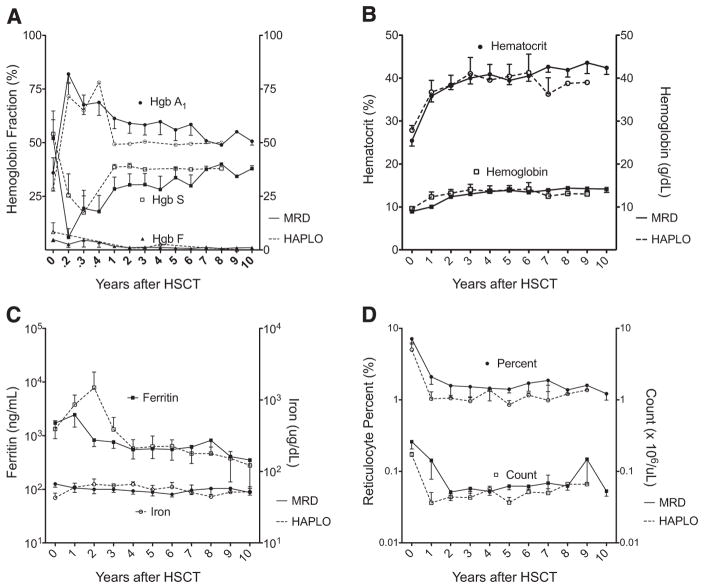
All patients with sustained engraftment became transfusion-independent with evidence of normal erythropoiesis, decreased hemolysis, and iron burden. Hematologic response values after MRD and haploidentical HSCT are presented in Table 1. After HSCT, recipients demonstrated a significant increase in median hemoglobin A1 levels and decreases in median hemoglobin S and hemoglobin F levels (Figure 2A), along with significant increases in median total hemoglobin and hematocrit levels (Figure 2B). Furthermore, there was a decline in the indices of hemolysis after HSCT. Compared with initial studies, there were significant decreases in median bilirubin, reticulocyte, and lactate dehydrogenase levels (Figure 2C), along with a significant decrease in iron load and a nonsignificant decrease in mean ferritin level (Figure 2D).
Figure 2.
Disease response after MRD HSCT (solid lines) and haploidentical HSCT (dashed lines) for SCD. Mean ± SEM values are plotted over time after MRD HSCT and haploidentical HSCT for hemoglobin and hematocrit (A), hemoglobin fractions (B), reticulocyte index and percent (C), and iron and ferritin levels (D).
Central Nervous System
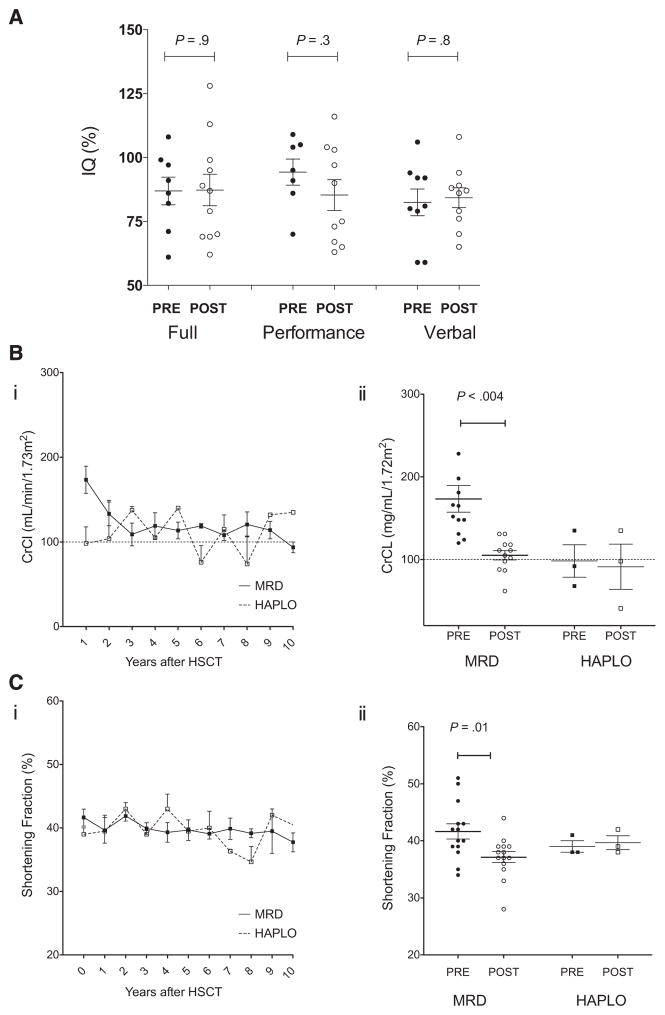
Prospective MRI/MRA, TCD, neuropsychological testing, and neurologic examinations were performed before and then annually after HSCT to evaluate the effect of HSCT on neurologic status in children with SCD (Table 2). Neuroimaging before HSCT revealed evidence of cerebral infarction and vasculopathy in the patients with documented CVA. Four patients experienced seizure activity during the first year after HSCT, including 1 patient with a history of seizure. One patient sustained a subarachnoid hemorrhage on day +16 after HSCT that resolved without complications. MRI/MRA results confirmed a previous finding that parenchymal changes can continue despite normal erythropoiesis during the first 3 years after HSCT. By 5 years after HSCT, no patient with sustained engraftment exhibited any clinical evidence of CVA or progression on imaging studies. MRI showed improvement in white matter changes, and MRA revealed stable or even improved vessel abnormalities (Table 3). TCD studies performed before and after MRD HSCT showed a significant decrease in maximal velocity, from 170 ± 16 cm/s to 81 ± 18 cm/s (P = .001) (Table 1). All TCD studies were normal at the last evaluation. Comprehensive neuropsychiatric evaluations were performed in 11 of the 13 patients before and at 1, 3, and 5 years after HSCT. (Testing was limited in 2 patients in whom English was not their first language.) The results confirm stable cognitive function after HSCT, with no significant decreases in full, performance, or verbal IQ scores (Table 1 and Figure 3A).
Table 2.
Characteristics of Patients before and after HSCT
| Pre-HCST
|
Post-HSCT
|
Difference
|
|||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | P Value | ||
| Age, yr | MRD | 11.1 ± 4.7 | 5.4–17.4 | 21.5 ± 8.1 | 12–32 | ||
| Haploidentical | 10.3 ± 5 | 4.2–17.1 | |||||
| Hemoglobin, g/dL | MRD | 7.8 ± 1.0 | 6–10 | 14.2 ± 1.5 | 11.5–17 | 6.3 ± 1.0 | <.0001 |
| Haploidentical | 9.6 ± 0.6 | 9–10 | 13.6 ± 2.9 | 11–16 | |||
| Hematocrit, % | MRD | 26 ± 5 | 19–32 | 42 ± 5 | 33–48 | 16 ± 6 | <.0001 |
| Haploidentical | 28 ± 1.9 | 26–30 | 40 ± 8.3 | 32–49 | |||
| Hemoglobin A, % | MRD | 33 ± 28 | 0–64 | 59 ± 15 | 47–90 | 26 ± 33 | .0055 |
| Haploidentical | 38 ± 38 | 0–77 | 50.2 ± 1 | 50–51 | |||
| Hemoglobin S, % | MRD | 53 ± 27 | 21–97 | 29 ± 16 | 0–40 | 24 ± 32 | .0087 |
| Haploidentical | 45.4 ± 30.8 | 13–75 | 37 ± 1 | 37–38 | |||
| Hemoglobin F, % | MRD | 6.6 ± 5.6 | 1.6–22 | 0.4 ± 0.6 | 0–1.4 | 6.2 ± 5.5 | .0004 |
| Haploidentical | 9.7 ± 5.8 | 2.1–16 | 1.2 ± 1.4 | 0–2.7 | |||
| Reticulocytes, × 103/mL | MRD | 293 ± 211 | 104–706 | 90 ± 114 | 29–471 | 168 ± 245 | .0049 |
| Haploidentical | 170 ± 240 | 156–198 | 45 ± 30 | 11–66 | |||
| Bilirubin, mg/dL | MRD | 4.5 ± 4.6 | 1.1–19.3 | 0.4 ± 0.3 | 0.2–1.2 | 4.1 ± 4.6 | .0025 |
| Haploidentical | 3.2 ± 1.3 | 1.7–4.1 | 0.3 ± 0.3 | 0.1–0.6 | |||
| Lactate dehydrogenase, IU/L | MRD | 1249 ± 538 | 455–2138 | 181 ± 46 | 129–290 | 1068 ± 565 | <.0001 |
| Haploidentical | 1630 ± 708 | 1065–2424 | 220 ± 48 | 188–276 | |||
| Ferritin, ng/mL | MRD | 1722 ± 3068 | 46–12,239 | 462 ± 602 | 56–1720 | 1259 ± 3121 | .15 |
| Haploidentical | 1334 ± 1001 | 644–2480 | 331 ± 152 | 230–507 | |||
| Iron, ng/mL | MRD | 132 ± 63 | 44–214 | 91 ± 41 | 34–171 | 35 ± 65 | .03 |
| Haploidentical | 70 ± 27 | 42–96 | 90 ± 38 | 51–128 | |||
| CrCl, mL/min/1.73 m2 | MRD | 173 ± 56 | 120–320 | 101 ± 24 | 57–131 | 60 ± 54 | .004 |
| Haploidentical | 98 ± 33 | 68–135 | 91 ± 47 | 41–135 | |||
| TCD, cm/s | MRD | 170 ± 16 | 125–222 | 89 ± 9 | 65–118 | 81 ± 18 | .001 |
| Haploidentical | 127 ± 17 | 115–139 | 88 | — | |||
| Shortening fraction, % | MRD | 42 ± 5 | 34–51 | 37 ± 4 | 28–44 | 37 ± 4 | .01 |
| Haploidentical | 39 ± 2 | 38–41 | 40 ± 2 | 38–42 | |||
| FVC, % | MRD | 80 ± 18 | 45–111 | 80 ± 12 | 52–101 | 3 ± 12 | .80 |
| Haploidentical | 72 ± 40 | 34–114 | 71 ± 12 | 57–80 | |||
| DLCO | MRD | 99 ± 6 | 73–126 | 77 ± 6 | 45–124 | 22 ± 9 | .02 |
| Haploidentical | 72 ± 23 | 34–114 | 96 ± 25 | 71–121 | |||
| FEV1 | MRD | 73 ± 5 | 36–95 | 78 ± 6 | 41–102 | 5 ± 7 | .50 |
| Haploidentical | 65 ± 14 | 37–82 | 78 ± 2 | 74–80 | |||
| FEV1/FVC | MRD | 0.93 ± 0.18 | 0.66–1.16 | 0.95 ± 0.21 | 0.66–1.34 | 0.02 ± 0.09 | .80 |
| Haploidentical | 0.97 ± 1.04 | 0.66–1.34 | 1.02 ± 0.4 | 0.99–1.07 | |||
| Full IQ | MRD | 87 ± 5 | 61–108 | 87 ± 6 | 62–128 | 0.4 ± 8 | .90 |
| Performance IQ | MRD | 94 ± 5 | 70–109 | 85 ± 6 | 63–116 | 9 ± 8 | .30 |
| Verbal IQ | MRD | 85 ± 6 | 59–106 | 84 ± 4 | 65–108 | 2 ± 6 | .70 |
Table 3.
CNS Evaluation before and after HSCT
| Clinical
|
MRI/MRA
|
||
|---|---|---|---|
| Pre-HSCT | Post-HSCT | Pre-HSCT | Post-HSCT |
| MRD | |||
| CVA | Normal | Encephalomalacia; significant stenosis | Stable; normal MRA |
| CVA | Normal | Multiple lacunar infarcts, circle of Willis occlusion | Stable |
| CVA | Normal | Lacunar infarcts; moyamoya disease; severe arterio-occlusive disease | Stable |
| CVA | Normal | Small lacune; intracranial tortuosity | Stable |
| CVA | Normal | Perivascular demyelination; mild tortuosity | Stable |
| CVA | Seizure | Lacunar infarcts; significant stenosis | Stable |
| CVA | Seizure | Multiple lacunar infarctions; abnormal MRA | Stable |
| Normal | Seizure | Leukoenchephalopathy; normal MRA | Stable |
| Normal | Subarachnoid hemorrhage | Normal brain; intracranial vessel tortuosity | Stable |
| Normal | Normal | Normal brain; intracranial vessel tortuosity | Stable |
| Normal | Normal | Mild leukoencephalopathy; normal MRA | Normal brain; normal MRA |
| Normal | Normal | Punctate lacune; mild stenosis | Improved leukomalacia; normal MRA |
| Haploidentical | |||
| CVA | Normal | Encephalomalacia; significant stenosis | Stable |
| CVA | Normal | Multiple lacunar infarctions; abnormal MRA | Stable |
| CVA | Normal | Encephalomalacia; significant stenosis | Stable |
Figure 3.
IQ, renal function, and cardiac function before and after HSCT. IQ (A), renal function (B), and cardiac function (C) were monitored before (solid symbols) and yearly after (open symbols) MRD (●, ○) and haploidentical (HAPLO) (■, □) HSCT. Mean ± SEM values are plotted over time after HSCT for CrCl (Bi) and shortening fraction (Ci), and median and SEM values for CrCl (Bii) and shortening fraction (Cii) before HSCT are compared with the values measured at the most recent evaluation.
Renal and Cardiac Function
The mean CrCl value was 158 ± 55.7 mL/min/1.73 m2 (range, 120 to 320 mL/min/1.73 m2) in the MRD cohort and 98 ± 33 mL/min/1.73 m2 in the haploidentical HSCT. At the most recent evaluation, the mean CrCl in the MRD cohort was 103.5 ± 24 mL/min/1.73 m2 (range, 57 to 131 mL/min/1.73 m2), for a significant difference of 60 ± 55 mL/min/1.73 m2 (P = .004) (Table 1). In 2 MRD HSCT recipients, renal function values decreased to below-normal levels (Figure 3B). At the last evaluation, all patients had normal urinanalysis results, with no proteinuria or hematuria. Before undergoing HSCT, all patients had normal cardiac function, with a median shortening fraction of 41% ± 5% (range, 34% to 51%), and at the most recent evaluations the median shortening fraction was 37.5% ± 4% (range, 28% to 44%), demonstrating a significant difference between the 2 time points (P = .001). No haploidentical HSCT recipient exhibited a change in shortening fraction (Figure 3C). Three patients had mild tricuspid insufficiency, and 1 patient had trace pulmonary insufficiency, but no patient had evidence of pulmonary hypertension.
Pulmonary Function
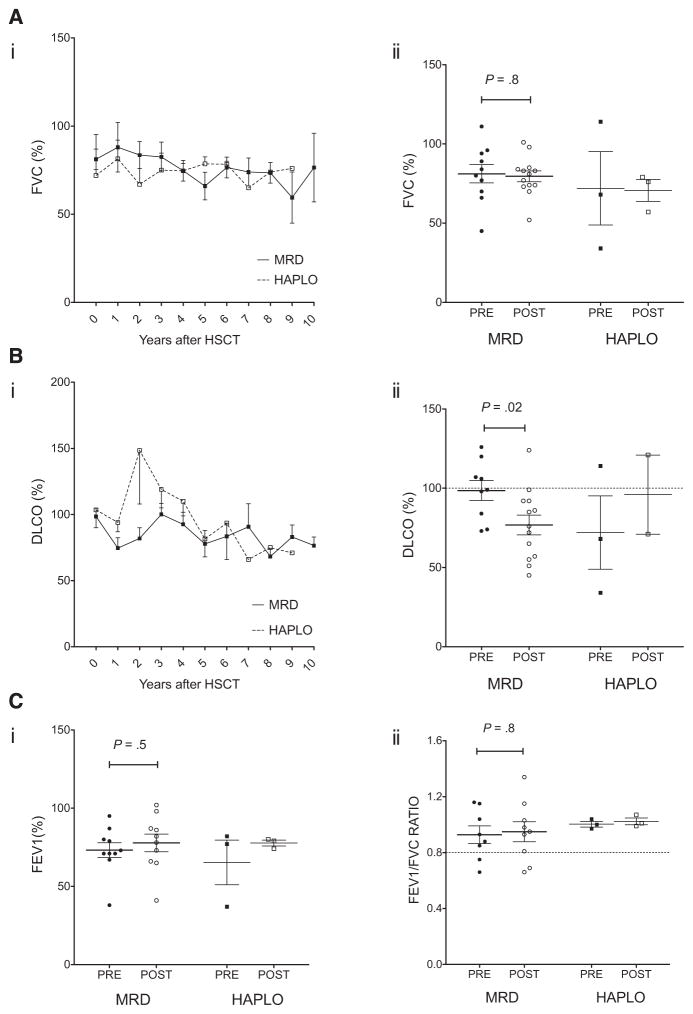
Among the 16 patients treated successfully for SCD, 12 were age >6 years at the time of HSCT and had baseline PFT data available. The other 4 patients underwent PFTs when able to perform the studies. Three patients underwent PFTs at 1 year after HSCT and 1 patient did so at 3 years after HSCT. There was no significance difference in the mean predicted FVC, FEV1, and FEV1/FVC ratio between baseline studies and the most recent evaluation (Table 2); however, in the MRD cohort, the mean DLCO declined from 99% ± 6% to 77% ± 7% (P = .02) after HSCT (Table 2 and Figure 4). Before HSCT, 6 patients had normal PFT values, 5 had a restrictive pattern, and 1 had a combined restrictive/obstructive pattern. Three of the 4 patients who underwent PFTs performed after HSCT exhibited a restrictive pattern, whereas the other patient had normal values. The 7 patients with normal initial PFT values maintained normal lung function after HSCT. Among the 9 patients who had restrictive disease at the initial examination, 1 patient had improved function, 4 patients had persistent restrictive changes, and 4 patients developed a combined restrictive/obstructive pattern, as documented by decreased FEV1, FVC, and FEV1/FVC ratio values.
Figure 4.
Pulmonary function before and after HSCT. PFTs, including FVC (A), DLCO (B), FEV1 (C), and FEV1/FVC ratio (D), were monitored before (solid symbols) and yearly after (open symbols) HSCT. Mean ± SEM values are plotted over time after MRD (●, ○) and haploidentical (HAPLO) (■, □) HSCT for FVC (Ai) and DLCO (Bi). The median ± SEM values before HSCT or first available values (closed symbols) are compared with the most recent value (open symbol) for FVC (Aii), DLCO (Bii), FEV1 (Ci), and FEV1/FVC ratio (Cii).
Liver and Spleen Function
Liver and spleen scans were performed in 12 patients. The 3 patients with documented splenectomy did not undergo these studies. Three patients who had minimal splenic uptake before HSCT demonstrated improved splenic uptake/regeneration after HSCT. One patient with no splenic uptake before HSCT exhibited increased splenic function, with evidence of splenic regeneration at day +160 and 1 year after HSCT. The other patients with no splenic uptake did not recover any function. Nine patients who had a normal liver scan before HSCT also had a normal scan at the last evaluation. The 3 patients with documented mild to moderate hepatomegaly before HSCT also had a normal liver scan after HSCT (Table 4).
Table 4.
Results of Liver Spleen Scans before and after HSCT
| Age Pre-HSCT, y | Spleen
|
Liver
|
|||
|---|---|---|---|---|---|
| Pre-HSCT | Post-HSCT | Pre-HSCT | Post-HSCT | ||
| MRD | 14.6 | Normal | Normal | Normal | Normal |
| MRD | 5.8 | Normal | Normal | Normal | Normal |
| MRD | 9.2 | Minimal uptake | Recovered | Normal | Normal |
| MRD | 5.4 | Minimal uptake | Mild regeneration | Normal | Normal |
| MRD | 6.0 | None | Mild regeneration | Normal | Normal |
| MRD | 14.3 | None | None | Normal | Normal |
| MRD | 8.8 | None | None | Hepatomegaly | Normal |
| MRD | 10.5 | None | None | Hepatomegaly | Normal |
| MRD | 14.5 | None | None | Hepatomegaly | Normal |
| Haplo | 8.5 | None | None | Normal | Normal |
| Haplo | 13.4 | None | None | Normal | Normal |
| Haplo | 4.2 | Minimal uptake | Mild regeneration | Normal | Normal |
Haplo indicates haploidentical.
Hypothalamic-Pituitary (Adrenal, Thyroid, and Gonadal) Function
Endocrine function was monitored in all survivors after HSCT. All patients were age ≥12 years at the last evaluation. The median age was 20.3 ± 7.1 years (range, 12 to 33 years) for the 9 males (69%) and 18.3 ± 3.3 years (range, 15 to 22 years) for the 4 females (31%). Five of the 9 males exhibited normal gonadal function, with normal LH, FSH, and testosterone levels. Three males had evidence of hypogonadism, with elevated gonadotropin, LH, and FSH levels but normal testosterone levels. One patient developed primary hypogonadism and was started on testosterone therapy. Two of the 4 females developed ovarian failure with elevated gonadotropin and low estradiol levels requiring replacement therapy. The other 2 females had normal laboratory results and menstrual cycles, and 1 of the women was 5 months pregnant at her last evaluation.
Growth and Bone
Growth was monitored based on skeletal bone age, growth velocity, and somatomedin C or insulin-like growth factor 1 levels. All 13 of the surviving patients underwent annual skeletal bone age studies until skeletal maturation was complete. Seven of the 13 patients (54%) had no evidence of abnormal skeletal development detected on initial or follow-up studies. Five of the 13 patients with delayed bone age on the initial study exhibited normal bone age by 2 years after HSCT. One patient with cGVHD who had normal bone age before HSCT exhibited delayed bone age and low/normal insulin-like growth factor 1 levels and developed severe osteoporosis after HSCT. No survivor demonstrated hypothyroidism or adrenal insufficiency at the last evaluation. All patients had normal thyroid-stimulating hormone, thyroxine, and free thyroxine before and after HSCT. At the last evaluation, cortisol levels were normal in all patients screened for adrenal insufficiency (n = 10). Adrenocorticotropic hormone and metyrpone stimulation studies were normal in patients considered at increased risk for insufficiency (n = 7).
All patients underwent imaging studies (MRI or radiography) to evaluate the risk of osteonecrosis. Before HSCT, 3 patients had evidence of avascular necrosis (AVN) at common sites, including the femoral head, knee, and/or sacrum; these patients had stable findings by 3 years after HSCT, (Table 5). Two patients developed AVN (1 knee and 1 hip) at 2 years after HSCT. By 5 years after HSCT, all patients with AVN had stable lesions, and no additional lesions were detected.
Table 5.
Osteonecrosis and BMD before and after HSCT
| MRI AVN
|
BMD
|
Clinical | ||
|---|---|---|---|---|
| Pre-HSCT | Post-HSCT | Pre-HSCT | Post-HSCT | |
| Normal | Normal | Normal | Normal | |
| Normal | Normal | Mild | Normal | |
| Normal | Normal | Mild | Normal | |
| Normal | Normal | Mild | Mild | |
| Normal | Normal | Mild | Mild | |
| Normal | Normal | Mild | Moderate | |
| Normal | Normal | Moderate | Normal | |
| Normal | Normal | Moderate | Mild | Fracture |
| Normal | Normal | Severe BMD | Moderate | Osteoporosis |
| Knee | Stable | Moderate | Moderate | |
| Femoral head | Normal | Moderate | Moderate improved | Osteoporosis |
| Femoral head | Normal | Moderate | Moderate improved | |
| Sacrum and knee | Knee | Severe | Severe | Osteoporosis and fracture |
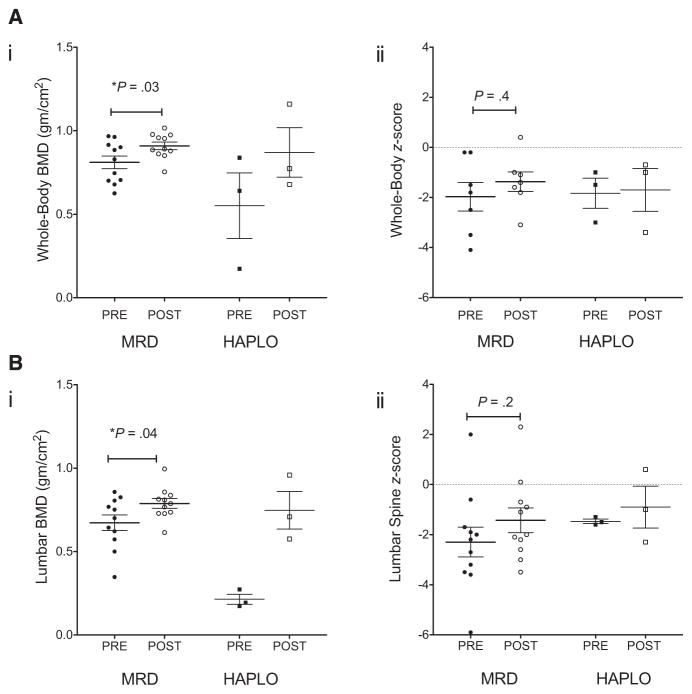
Osteoporosis was monitored yearly with computed tomography bone density and dual-energy X-ray absorptiometry scans (Figure 5). The median whole-body BMD values were 151 ± 41 (range, 72 to 210) and 151 ± 22 (range, 128 to 200), and median lumbar spine BMD values were 0.70 ± 0.16 (range, 0.35–0.86) and 0.79 ± 0.96 (range, 0.61 to 1.0). Median BMD z-scores were −1.5 ± 1.0 (range, −2.21 to 1.1) and −1.3 ± 1.5 (range, −4.6 to 1.6) for whole body and −2.2 ± 2.0 (range, −5.9 to 2.0) and −2.0 ± 1.6 (range, −3.5 to 2.3) for lumbar spine.
Figure 5.
Bone density before and after HSCT. Whole-body (A) and vertebral body (B) BMD (i) and BMD z-scores (ii) were monitored before (solid symbols) and after (open symbols) HSCT. Median ± SEM values for the MRD (●, ○) and haploidentical (HAPLO) (■, □) recipients are compared with those measured at the most recent evaluation.
DISCUSSION
This study is the first comprehensive evaluation of quality of survival after successful related-donor (haploidentical or MSD) HSCT for SCD. Hansbury et al. [24] previously described our institutional experience of finding an unaffected MRD (7%) for patients with severe SCD, and the use of haploidentical donors expands the pool of patients eligible for HSCT. Here we report the long-term follow-up results of routine assessments of organ function, particularly of the brain, lung, heart, liver, spleen, kidney, bone, and endocrine system.
Recipients of haploidentical HSCT had a much higher and significant risk for graft rejection compared with MRD HSCT recipients. However, their risk of rejection was similar to that of patients who underwent reduced-intensity conditioning haploidentical HSCT reported by Bolaños-Meade et al. [11]. Unfortunately, we found a greater risk for GVHD, particularly extensive cGVHD, which led to increased mortality in our study. Our haploidentical grafts were based on CD3+ depletion and CD34+ selection. Patients who received high doses of CD3+ T cells or DLI were at significant risk for increased mortality. Although the small number of patients in both studies limit conclusive results, the findings suggest that a preparative regimen with post-transplantation cyclophosphamide may be more effective than T cell depletion in preventing GVHD.
In this study, patients who successfully engrafted after haploidentical HSCT had beneficial outcomes similar to those seen in our MRD HSCT recipients. Our findings confirm that children with CVA are protected from further CVA after successful haploidentical HSCT. A previous report suggested that early progressive imaging changes noted after HSCT may be due to the natural history of neurovascular injury, including reactive gliosis related to the initial event [17]. It was hypothesized that these early changes were not new findings, but rather reflected the evolution of cerebrovascular disease that existed before HSCT. Our study supports these findings, with long-term follow-up demonstrating improvement and stabilization of cerebral lacunae or leukoencephaly by 5 years after HSCT in both the MRD and haploidentical donor cohorts. Furthermore, we confirmed that persistent brain MRI changes were not associated with progressive neurocognitive deficits. The routine neuropsychologic testing showed stable neurocognitive function with no appreciable change in IQ after HSCT. In the series reported here, individuals with silent cerebral infarction were protected from progressive changes on brain MRI, and some patients experienced resolution of abnormalities documented before HSCT. Improvements in vessel abnormalities documented on MRI/MRA were noted after HSCT, with 2 patients demonstrating diminished vessel disease; all patients with abnormal TCD velocities before HSCT had normal velocities after HSCT.
Previous studies have reported no significant decline in pulmonary function after HSCT [7,13]. Here we found a significant decline in DLCO in our MRD cohort. We assume that this decline is associated with busulfan toxicity rather than with progression of vascular damage from SCD, civen the lack of evidence of other vascular occlusive damage in the lungs. Some patients developed decreased FEV1 level and FEV1/FVC ratio, consistent with obstructive disease. Although obstructive changes observed after HSCT may be associated with the use of busulfan in the conditioning regimen [5], a growing body of evidence suggests that the progressive pulmonary toxicity of SCD may be restrictive or obstructive pulmonary disease [25,26]. The available data strongly suggest that the decline in pulmonary function due to SCD vasculopathy is halted by the establishment of normal erythropoiesis, regardless of donor source.
Our HSCT recipients demonstrated significant declines in renal function after transplantation, as demonstrated by decreased CrCl values. Although our cohort’s median CrCl value after HSCT was in the normal range, 2 patients had values clearly below the normal range. Furthermore, there was significant difference in the median CrCl values before HSCT and that measured at the last evaluation; however, no patient had evidence of proteinuria or hematuria on urinanalysis.
Although some patients had evidence of mild tricuspid regurgitation, their jet velocities were normal, with no clinical impact on cardiac function. We found no evidence of pulmonary hypertension in any of our patients. Of the 16 patients treated successfully (median age, 20 years), 85% were past late adolescence (>17 years), and 70% were age >20 years. This suggests that the abrogation of hemolysis by successful HSCT decreases the risk of developing pulmonary hypertension in patients with SCD.
Patients with SCD are at increased risk for AVN and osteoporosis [27]. Most of our patients had significant osteoporosis with very low BMD values before HSCT. Patients with documented AVN before HSCT demonstrated some improvement after HSCT, but although BMD and BMD z-score values increased after HSCT, these patients remained at high risk for osteoporosis. Furthermore, females with hypogonadism had significant osteoporosis unless treated with hormone therapy. Hip MRI performed before HSCT documented abnormal marrow intensity associated with SCD. These findings were all normalized after HSCT. In summary, we observed improvement of AVN with modest increase in BMD after both MRD and haploidentical donor HSCT.
Our patients with delayed bone growth all demonstrated normalized growth after HSCT, if not complicated by cGVHD. Endocrine function studies showed no increased risk for thyroid or adrenal insufficiency; however, a significant proportion of patients (30%) developed gonadal insufficiency after HSCT and required treatment with hormone replacement therapy. Taken together, these observations tend to confirm the gonadal toxicity associated with exposure to myeloablative doses of busulfan [6].
In summary, HSCT offers long-term protection from clinical and subclinical vaso-occlusion associated with SCD, regardless of donor source. Complications that commonly develop in patients with SCD, including stroke, pulmonary hypertension, acute chest, proteinuria, and hematuria, were not observed in our patients after successful HSCT; however, the patients who underwent myeloablative MRD HSCT demonstrated progressive declines in renal, pulmonary, and cardiac function over time that have not been reported previously. Our data suggest that the declining organ function is likely due to the conditioning regimens rather than to complications of SCD. Whether patients with SCD are at greater risk for declining organ function, and whether reduced-intensity conditioning regimens may offer better outcomes after HSCT, require additional investigation. Although our study was limited by number of patients, 100% of the patients underwent extensive pre- and post-HSCT evaluation. This might have facilitated the better detection of declining organ function compared with large retrospective multi-institutional studies. We hope that this report encourages further long-term evaluations in patients undergoing reduced-intensity haploidentical HSCT for SCD to examine whether organ function is better preserved with this modality compared with conventional MRD HSCT.
Footnotes
Authorship statement: M.H. Dallas analyzed and interpreted data and wrote the manuscript. B. Triplett, D.R. Shook, C. Hartford, A. Srinivasan, J. Laver, R. Ware, and W. Leung interpreted data and wrote and edited the manuscript.
Financial disclosure: This work was supported by grants from the American Association for Cancer Research, St. Baldrick’s Foundation, Assisi Foundation of Memphis, and American Lebanese Syrian Associated Charities. There are no conflicts of interest to report.
References
- 1.Walters MC, Patience M, Leisenring W, et al. Barriers to bone marrow transplantation for sickle cell anemia. Biol Blood Marrow Transplant. 1996;2:100–104. [PubMed] [Google Scholar]
- 2.Brousseau DC, McCarver DG, Drendel AL, et al. The effect of CYP2D6 polymorphisms on the response to pain treatment for pediatric sickle cell pain crisis. J Pediatr. 2007;150:623–626. doi: 10.1016/j.jpeds.2007.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eggleston B, Patience M, Edwards S, et al. Effect of myeloablative bone marrow transplantation on growth in children with sickle cell anaemia: results of the multicenter study of haematopoietic cell transplantation for sickle cell anaemia. Br J Haematol. 2007;136:673–676. doi: 10.1111/j.1365-2141.2006.06486.x. [DOI] [PubMed] [Google Scholar]
- 4.Vermylen C, Cornu G. Bone marrow transplantation for sickle cell disease: the European experience. Am J Pediatr Hematol Oncol. 1994;16:18–21. [PubMed] [Google Scholar]
- 5.Bruno B, Souillet G, Bertrand Y, et al. Effects of allogeneic bone marrow transplantation on pulmonary function in 80 children in a single paediatric centre. Bone Marrow Transplant. 2004;34:143–147. doi: 10.1038/sj.bmt.1704549. [DOI] [PubMed] [Google Scholar]
- 6.Grigg AP, McLachlan R, Zaja J, et al. Reproductive status in long-term bone marrow transplant survivors receiving busulfancyclophosphamide (120 mg/kg) Bone Marrow Transplant. 2000;26:1089–1095. doi: 10.1038/sj.bmt.1702695. [DOI] [PubMed] [Google Scholar]
- 7.Walters MC, Hardy K, Edwards S, et al. Pulmonary, gonadal, and central nervous system status after bone marrow transplantation for sickle cell disease. Biol Blood Marrow Transplant. 2010;16:263–272. doi: 10.1016/j.bbmt.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walters MC, Patience M, Leisenring W, et al. Stable mixed hematopoietic chimerism after bone marrow transplantation for sickle cell anemia. Biol Blood Marrow Transplant. 2001;7:665–673. doi: 10.1053/bbmt.2001.v7.pm11787529. [DOI] [PubMed] [Google Scholar]
- 9.Walters MC, Sullivan KM, Bernaudin F, et al. Neurologic complications after allogeneic marrow transplantation for sickle cell anemia. Blood. 1995;85:879–884. [PubMed] [Google Scholar]
- 10.Mentzer WC, Heller S, Pearle PR, et al. Availability of related donors for bone marrow transplantation in sickle cell anemia. Am J Pediatr Hematol Oncol. 1994;16:27–29. [PubMed] [Google Scholar]
- 11.Bolaños-Meade J, Fuchs EJ, Luznik L, et al. HLA-haploidentical bone marrow transplantation with post-transplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood. 2012;120:4285–4291. doi: 10.1182/blood-2012-07-438408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruggeri A, Eapen M, Scaravadou A, et al. Umbilical cord blood transplantation for children with thalassemia and sickle cell disease. Biol Blood Marrow Transplant. 2011;17:1375–1382. doi: 10.1016/j.bbmt.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majumdar S, Robertson Z, Robinson A, et al. Outcome of hematopoietic cell transplantation in children with sickle cell disease, a single center’s experience. Bone Marrow Transplant. 2010;45:895–900. doi: 10.1038/bmt.2009.244. [DOI] [PubMed] [Google Scholar]
- 14.Panepinto JA, Walters MC, Carreras J, et al. Matched-related donor transplantation for sickle cell disease: report from the Center for International Blood and Transplant Research. Br J Haematol. 2007;137:479–485. doi: 10.1111/j.1365-2141.2007.06592.x. [DOI] [PubMed] [Google Scholar]
- 15.Parent F, Bachir D, Inamo J, et al. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med. 2011;365:44–53. doi: 10.1056/NEJMoa1005565. [DOI] [PubMed] [Google Scholar]
- 16.Walters MC, Patience M, Leisenring W, et al. Bone marrow transplantation for sickle cell disease. N Engl J Med. 1996;335:369–376. doi: 10.1056/NEJM199608083350601. [DOI] [PubMed] [Google Scholar]
- 17.Woodard P, Helton KJ, Khan RB, et al. Brain parenchymal damage after haematopoietic stem cell transplantation for severe sickle cell disease. Br J Haematol. 2005;129:550–552. doi: 10.1111/j.1365-2141.2005.05491.x. [DOI] [PubMed] [Google Scholar]
- 18.O’Callaghan DS, Savale L, Yaici A, et al. Endothelin receptor antagonists for the treatment of pulmonary arterial hypertension. Expert Opin Pharmacother. 2011;12:1585–1596. doi: 10.1517/14656566.2011.564159. [DOI] [PubMed] [Google Scholar]
- 19.Mehari A, Gladwin MT, Tian X, et al. Mortality in adults with sickle cell disease and pulmonary hypertension. JAMA. 2012;307:1254–1256. doi: 10.1001/jama.2012.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung WH, Turner V, Richardson SL, et al. Effect of HLA class I or class II incompatibility in pediatric marrow transplantation from unrelated and related donors. Hum Immunol. 2001;62:399–407. doi: 10.1016/s0198-8859(01)00220-8. [DOI] [PubMed] [Google Scholar]
- 21.Powars D, Weidman JA, Odom-Maryon T, et al. Sickle cell chronic lung disease: prior morbidity and the risk of pulmonary failure. Medicine. 1988;67:66–76. [PubMed] [Google Scholar]
- 22.Schumm M, Lang P, Taylor G, et al. Isolation of highly purified autologous and allogeneic peripheral CD34+ cells using the CliniMACS device. J Hematother. 1999;8:209–218. doi: 10.1089/106161299320488. [DOI] [PubMed] [Google Scholar]
- 23.Gordon PR, Leimig T, Mueller I, et al. A large-scale method for T cell depletion: towards graft engineering of mobilized peripheral blood stem cells. Bone Marrow Transplant. 2002;30:69–74. doi: 10.1038/sj.bmt.1703619. [DOI] [PubMed] [Google Scholar]
- 24.Hansbury EN, Schultz WH, Ware RE, et al. Bone marrow transplant options and preferences in a sickle cell anemia cohort on chronic transfusions. Pediatr Blood Cancer. 2012;58:611–615. doi: 10.1002/pbc.23304. [DOI] [PubMed] [Google Scholar]
- 25.Chien JW, Martin PJ, Gooley TA, et al. Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2003;168:208–214. doi: 10.1164/rccm.200212-1468OC. [DOI] [PubMed] [Google Scholar]
- 26.Field JJ, Glassberg J, Gilmore A, et al. Longitudinal analysis of pulmonary function in adults with sickle cell disease. Am J Hematol. 2008;83:574–576. doi: 10.1002/ajh.21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease: life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]