Abstract
Fast scan cyclic voltammetry is commonly used for measuring the kinetics of dopamine release and uptake. For experiments using an anesthetized preparation, urethane is preferentially used because it does not alter dopamine uptake kinetics compared to freely moving animals. Unfortunately, urethane is highly toxic, can induce premature death during experiments, and cannot be used for recovery surgeries. Isoflurane is an alternative anesthetic that is less toxic than urethane, produces a stable level of anesthesia over extended periods, and is often used for recovery surgeries. Despite these benefits, the effects of isoflurane on dopamine release and uptake have not been directly characterized. In the present studies, we assessed the utility of isoflurane for voltammetry experiments by testing dopamine signaling parameters under baseline conditions, after treatment with the dopamine uptake inhibitor cocaine, and after exposure to increasing concentrations of isoflurane. Our results indicate that surgical levels of isoflurane do not significantly alter terminal mechanisms of dopamine release and uptake over prolonged periods of time. Consequently, we propose that isoflurane is an acceptable anesthetic for voltammetry experiments, which in turn permits the design of studies in which dopamine signaling is examined under anesthesia prior to recovery and subsequent experimentation in the same animals.
Keywords: Fast scan cyclic voltammetry, cocaine, urethane, dopamine transporter, nucleus accumbens
Introduction
Fast scan cyclic voltammetry (FSCV) is a powerful technique for measuring catecholamine release and uptake kinetics, and is commonly used to study neurochemical, pharmacological, and genetic manipulations in anesthetized animals [3, 6, 17, 19]. FSCV experiments performed under anesthesia have preferentially used urethane, an anesthestic thought to produce its effects through interaction with ligand gated ion channels in a manner similar to many others [9, 12, 25]. Importantly, however, urethane is the only anesthetic which has been shown to produce anesthesia without significantly changing basal dopamine (DA) uptake kinetics when compared to awake animals [7, 20], thus it is the preferred anesthesia for use with in vivo voltammetry [8]. Despite this benefit, there are also significant disadvantages to the use of urethane. In particular urethane is a carcinogen, produces cardiomyopathy, and respiratory deficits, and is toxic to the liver, kidney, gastrointestinal and nervous systems [10, 13, 27]; because of these effects urethane can lead to death at any point during an experiment, and is limited to use in terminal studies.
Isoflurane is an inhalation anesthetic that offers several advantages over urethane. Isoflurane is far less toxic than urethane, can be rapidly adjusted to maintain appropriate levels of anesthesia for extended periods, and can be used for recovery experiments. Recent evidence suggests that isoflurane may produce anesthetic hypnosis by interacting with ligand-gated ion channels [12] to promote the activation of endogenous sleep circuitry [15]. A limited number of studies have examined the effects of isoflurane on DA signaling. Unfortunately, many of these studies either administered isoflurane with N2O, an agent that produces changes in DA signaling on its own [11, 21], or used concentrations of isoflurane that are higher than necessary to maintain surgical level anesthesia (2–6%). For example, studies in which isoflurane was administered in combination with N2O indicate that extracellular DA concentrations are elevated through interactions with the DA transporter (DAT) [16, 22]. Likewise, 2.5% isoflurane elevates extracellular DA levels in vivo [1, 23]) and 4–6% isoflurane has been shown to induce DAT internalization in vitro [23]. By comparison, lower concentrations of isoflurane did not significantly alter extracellular DA levels [1], and were reported as insufficient to reliably produce DAT internalization [23] in these same studies. Together, these observations indicate that low concentrations of isoflurane administered with air may not significantly alter DA signaling, and thus may serve as an acceptable anesthetic for use in FSCV experiments.
We tested the utility of isoflurane as an anesthetic for use in FSCV experiments by characterizing DA release and uptake parameters in the nucleus accumbens core (NAc). We compared baseline DA uptake and release kinetics and the effects of the DAT inhibitor cocaine, ex vivo, in tissue prepared from unanesthetized animals or animals that received urethane or isoflurane anesthesia. We then compared baseline DA uptake kinetics and release as well as the effects of cocaine in vivo in animals anesthetized with isoflurane or urethane. Last, we characterized the effects of isoflurane exposure duration and isoflurane concentration on DA release and uptake parameters in vivo.
Methods
Animals
Male Sprague-Dawley rats (350–420g, Harlan, Frederick, MD) were given ad libitum access to food and water and kept on a 12:12hr light:dark cycle (lights on at 7:00h). All protocols and animal care procedures were maintained in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals: Eighth Edition (The National Academies Press, Washington, DC, 2011) and approved by the Institutional Animal Care and Use Committee at Drexel University College of Medicine.
Ex vivo voltammetry
Striatal slices were prepared from animals that were sacrificed by decapitation alone, or by decapitation after being fully anesthetized with urethane or isoflurane (n = 6 for all groups). Animals treated with urethane received an intraperitoneal (i.p.) injection of 1.5 – 1.7 g/kg urethane (dissolved in water; Sigma-Aldrich, St. Louis, MO), while animals treated with isoflurane were placed in a gas chamber and exposed to 2.5% isoflurane (99.9%, Piramal Critical Care, Bethlehem, PA) flushed with air (~21% O2) for 3 min to induce anesthesia, and then maintained at 1.5% isoflurane for an additional 30 min.
Brains were removed following decapitation and then transferred into oxygenated ice-cold artificial cerebral spinal fluid (aCSF) containing (in mM) NaCl (126), KCl (2.5), NaH2PO4 (1.2), CaCl2 (2.4), MgCl2 (1.2), NaHCO3 (25), glucose (11), l-ascorbic acid (0.4), pH adjusted to 7.4. A vibrating microtome was used to produce 300 μm thick coronal sections containing the NAc, which were then transferred into a testing chamber and flushed with aCSF (32°C) flowed at 1 ml/min. Following 30 min of equilibration, a carbon fiber microelectrode (150–200 μm length × 7 μm diameter) and a bipolar stimulating electrode (Plastics One, Roanoke, VA) were placed in the NAc core. DA release was elicited every 5 min using a single electrical pulse (400 μA, 4 msec, monophasic), and was recorded as previously described [3]. After recording three stable responses (less than 10% variation) cocaine (obtained from the National Institute on Drug Abuse) was cumulatively applied (0.3–30 μM) to the tissue.
In vivo voltammetry
For one group of animals, anesthesia was induced with an i.p. injection of 1.5–1.7 g/kg urethane which produced stable anesthesia throughout the duration of experiment. For a second group of animals, anesthesia was induced by placing animals in a gas chamber perfused with 2.5% isoflurane flushed with air for 3 min and then maintained at a constant 1.5% isoflurane which is the lowest concentration that consistently maintained a surgical level of anesthesia (indicated by loss of paw withdrawal reflex).
Rats used to investigate the effects of cocaine were implanted with an intravenous (i.v.) catheter into the jugular vein, while rats used to investigate the effects of various isoflurane concentrations were not. Rats were placed into a stereotaxic apparatus and implanted with a bipolar stimulating electrode aimed at the ventral tegmental area (VTA; 5.3 P, +1.0 L), a carbon fiber microelectrode aimed above the NAc core (+1.3 A, +1.3 L) and a reference electrode in contralateral cortex as described previously [4, 18].
Following surgery, the carbon fiber microelecrode was initially positioned in the caudate nucleus (−4.5 V), and the stimulating electrode was driven into the VTA (−7.2 to −7.6 V) until a 1 sec, 60 Hz, 4 ms monophasic (~600 μA) stimulation train elicited a robust DA signal. Once stimulator and carbon fiber electrodes were positioned to achieve adequate dopamine release the carbon fiber recording electrode was lowered into the NAc (−6.5 V) which yields lower and slower dopamine release and uptake [2–4]. Release was then elicited every 5 min. Three baseline responses were recorded (less than 10% variation), and treatment began immediately after baseline collections were completed.
The first set of in vivo voltammetry experiments was designed to compare the effects of cocaine in animals anesthetized with urethane or isoflurane. Cocaine injections (1.5 mg/kg) were experimenter-delivered (5 sec, ~200 μl i.v. bolus) after the final baseline recording in the NAc. Electrically-stimulated DA responses were acquired at 30 and 60 sec post-cocaine injection, and every 5 min thereafter [4].
A second set of in vivo voltammetry experiments was designed to test DA uptake kinetics and release under increasing concentrations of isoflurane. After recording stable baselines of electrically stimulated DA release at 1.5% isoflurane, the concentration of isoflurane was increased by 0.5% and signals were monitored until 30 min of stable responses were observed (less than 10% variation). This was repeated such that 30 min of stable DA responses were recorded for 1.5, 2.0, 2.5 and 3.0% isoflurane in each animal. Average values recorded from the final three stimulations were used for statistical analysis.
Data Analysis
The total concentration of electrically-stimulated DA release and transporter-mediated uptake kinetics, including maximal uptake rate (Vmax) and apparent affinity of endogenous DA (Km) for the DA transporter were monitored. DA overflow curves were fitted to a Michaelis–Menten-based kinetic model [24] using Demon Voltammetry and Analysis Software [26]. Changes in uptake and release were obtained by setting baseline Km values (prior to any treatment) to 0.17 – 0.19 μM and establishing a baseline Vmax individually for each subject. For cocaine experiments, Vmax was held constant for the remainder of the experiment and alterations in uptake were attributed to changes in apparent Km. For studies on the concentration effects of isoflurane, Km values were held constant throughout the experiment and thus alterations in uptake were attributed to changes in Vmax. The concentration of stimulated DA release was assessed by comparing the current at the peak oxidation potential for DA in voltammograms with electrode calibrations of 3 μM of DA.
Statistics were carried out using SPSS version 22 (IBM, Armonk, NY). Ex vivo baseline measurements for all groups were compared using one-way ANOVA, and in vivo baseline measurements were compared with independent samples t-tests. The effects of cocaine for both ex vivo and in vivo experiments were tested with two-way ANOVA with repeated measures. Correlation for duration of exposure to isoflurane and Vmax was tested with Pearson’s correlation test, and the concentration effects of isoflurane were tested with one-way ANOVA with repeated measures. All results were verified with retrospective power analysis using G-Power [5]. Alpha was set to 0.05. Effect size was estimated based on the previous reports of high concentration isoflurane effects described above and the standard deviation observed in the current studies. All null results hold greater than 80% power.
Results
Urethane and isoflurane do not alter baseline dopamine signaling or the effects of cocaine ex vivo
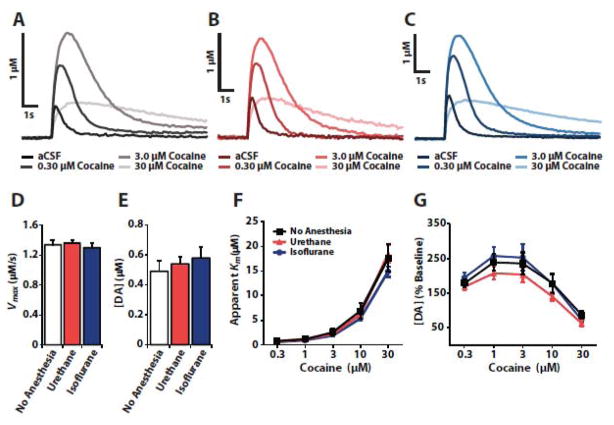
We first sought to determine if isoflurane or urethane alters terminal mechanisms of DA release and uptake. To this end, we compared baseline DA uptake kinetics and release and cocaine-induced DA uptake inhibition in the NAc of tissue sections prepared from unanesthetized rats or from rats that were anesthetized with either urethane or isoflurane (Fig. 1). These experiments showed no significant difference in baseline uptake rates (n = 6 per group, F(2, 15) = 0.354, p=0.707) or DA release (n=6 per group, F(2,15) = 0.455, p=0.643) across conditions. Further, we observed no differences in cocaine-induced DA uptake inhibition (n = 6 per group, F(2,15) = 0.912, p = 0.423), or changes in DA release (n = 6 per group, F(2,15) = 0.892, p = 0.431) between groups. Additionally, no interactions were observed between anesthetic group and cocaine effects on DA uptake inhibition (n=6 per group, F(8,60) = 0.703, p = 0.688) or DA release (n = 6 per group, F(8,60) = 0.906, p = 0.517).
Figure 1.
Urethane and isoflurane do not alter baseline dopamine uptake kinetics and release or the effects of cocaine ex vivo. Representative traces recorded from tissue prepared from (A) unanesthetized rats and rats anesthetized with (B) urethane or (C) isoflurane. Shown are mean ± SEM of (D) baseline Vmax, and (E) baseline DA release, as well as cocaine effects on (F) apparent Km and (G) DA release.
Dopamine signaling is similar in urethane- and isoflurane-anesthetized rats in vivo
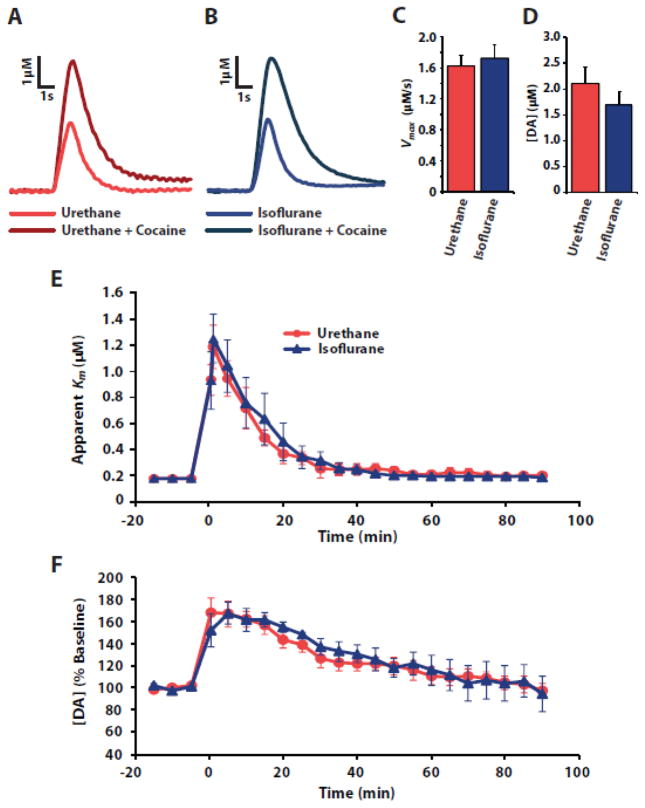
Given that exposure to isoflurane does not alter DA signaling ex vivo, we next compared baseline DA uptake kinetics and release as well as changes in DA uptake in response to cocaine in animals anesthetized with urethane or 1.5% isoflurane (Fig. 2). Vmax and DA release in urethane-anesthetized animals were similar to previously reported values [4, 18]. Similar to the ex vivo studies, no significant differences were observed across anesthetic conditions for Vmax (urethane n = 6, isoflurane n = 13, t(17) = −0.341, p = 0.0.737) or DA release (urethane n = 6, isoflurane n=13, t(17) = −0.971, p = 0.344). Likewise, no differences were observed between urethane- and isoflurane-treated animals for cocaine-induced uptake inhibition (n = 6 per group, F(1,22) = 0.1774, p = 1.0) or changes in DA release (n = 6 per group, F(1,22) = 0.2258, p = 0.99).
Figure 2.
In vivo baseline dopamine uptake and release as well as the effects of cocaine are similar between rats anesthetized with urethane or isoflurane. Representative traces recorded before and after cocaine in animals anesthetized with (A) urethane or (B) isoflurane. Shown are mean ± SEM of (C) baseline Vmax, (D) baseline DA release, and cocaine effects on (E) apparent Km and (F) DA release across time.
In vivo DA signaling is stable after prolonged exposure to isoflurane but changes across isoflurane concentration
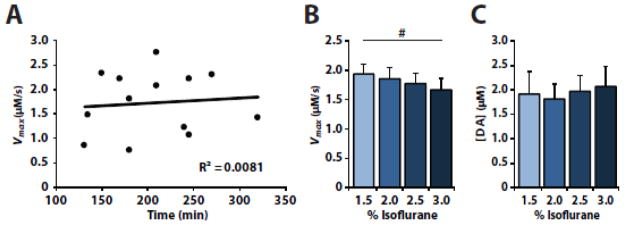
Previous studies have shown that changes in DA signaling occur when rats are administered concentrations of isoflurane that are higher than those used herein [1, 23]. To determine if the degree of isoflurane exposure alters DA release and uptake in vivo, we examined the effects of isoflurane exposure duration and isoflurane concentration on DA signaling (Fig. 3). We tested if the duration of exposure to 1.5% isoflurane produced changes in maximal DA uptake rate by comparing Vmax after various exposure times across separate animals, and observed no correlation between exposure time and Vmax (n = 13, R2 = 0.008, p = 0.770). We also examined the effect of varying isoflurane concentrations on DA uptake and release. We found that increasing concentrations of isoflurane significantly decreased Vmax (n = 6, F(3, 15) = 3.674, p = 0.036), although no significance was seen at any individual concentration (Dunnet’s post hoc with 1.5% isoflurane as a control). Moreover, increasing isoflurane concentration did not significantly alter DA release (n = 6, F(3, 15) = 0.621, p = 0.612).
Figure 3.
In vivo dopamine uptake kinetics are stable under isoflurane after prolonged exposure but not across increasing concentration. (A) A scatterplot with linear regression of baseline Vmax recorded from different animals at after various isoflurane exposure times. Shown are (B) Vmax and (C) DA release after increasing concentrations of isoflurane. # indicates a significant main effect (p < 0.05).
Discussion
Our observations indicate that maintenance concentrations of isoflurane are an acceptable anesthetic for use in ex vivo and in vivo voltammetry experiments. First, we found that DA release and uptake kinetics, ex vivo, are similar between untreated rats and rats anesthetized with urethane or 1.5% isoflurane under baseline conditions and in response to cocaine. We then verified that baseline DA uptake kinetics and release as well as changes in DA uptake in response to cocaine are comparable between animals anesthetized with 1.5% isoflurane or with urethane. Last, we determined that DA uptake remains stable over extended exposure to 1.5% isoflurane, but is altered by increasing concentration of isoflurane.
Anesthetic effects on baseline dopamine signaling
Our ex vivo results indicate that neither 1.5% isoflurane nor urethane significantly alters terminal mechanisms of DA release and uptake, however in vivo studies have indicated that although urethane does not alter DA uptake kinetics it does reduce stimulated DA release [7, 20]. This in vivo effect may be mediated by altered inhibitory balance at DA cell bodies, and urethane and isoflurane may produce this effect through similar actions at ligand gated ion channels in the central nervous system [9, 12]. Accordingly, we found no differences in DA uptake rates or release between urethane- and isoflurane-anesthetized animals. Together, these observations suggest the possibility that urethane and maintenance concentrations of isoflurane may alter DA signaling through similar actions at DA cell bodies. It should be noted, however, that our observations are limited to a comparison between acute isoflurane and urethane exposure, and thus our findings do not provide any evidence regarding DA signaling following recovery from anesthesia.
Anesthetic interaction with cocaine
FSCV experiments using urethane anesthesia have often been used to investigate DA changes in response to cocaine [2, 3, 14, 18], but the extent to which urethane itself alters cocaine’s effect on DAT remains unknown [8]. Here we demonstrate that neither urethane nor isoflurane alters cocaine’s effects ex vivo. This may appear to be in contrast to previous investigations that suggested isoflurane enhances the effect of DAT inhibitors, including cocaine [16, 22]. Those studies, however, used isoflurane mixed with 70% N2O, an agent that has been shown to alter DA signaling on its own [11, 21], and thus the extent to which these effects are attributable to N2O versus isoflurane remains unclear. Whether urethane or isoflurane alter the effects of cocaine in vivo remains an open question, however, our ex vivo results indicate that exposure to these anesthetics does not alter the action of cocaine on DA terminals and our in vivo results suggest that any changes in cocaine’s action are similar between urethane and isoflurane.
Concentration effects of isoflurane on dopamine signaling
It is important to note that the utility of isoflurane for FSCV is likely restricted to low maintenance concentrations. High concentrations of isoflurane (4 – 6%) have been shown to directly induce the internalization of DAT in vitro [23], which should correspond with decreases in Vmax [6]. Indeed, we found modest decreases in Vmax with increasing concentration of isoflurane. The highest concentrations tested herein (2.5 and 3.0%), however, are typically lethal over prolonged exposure, and thus are inappropriate and unnecessarily high for use with FSCV. Therefore, these results indicate that FSCV experiments that use isoflurane anesthesia should be performed with the lowest concentration of isoflurane that produces a surgical level of anesthesia.
Conclusion
Our results demonstrate that DA release and uptake kinetics monitored under isoflurane anesthesia are similar to those observed under urethane, and thus isoflurane is a reasonable alternative anesthetic for FSCV studies. Using isoflurane for FSCV experiments increases the safety and reliability of these studies and, further, will allow investigators to design experiments in which DA signaling can be assessed in an acute anesthetized preparation prior to recovery and further experimentation.
Highlights.
Neither isoflurane nor urethane alter terminal dopamine release and uptake ex vivo
Dopamine release and uptake are similar under isoflurane or urethane in vivo
Cocaine effects on dopamine signaling are similar under isoflurane or urethane
Dopamine uptake rate remains stable after prolonged exposure to isoflurane
Isoflurane is a reasonable alternative anesthetic for use in voltammetry studies
Acknowledgments
We would like to thank Kwamie Harris and Jessica K. Shaw for assistance in manuscript preparation. These studies were supported by R01 DA031900.
Abbreviations
- FSCV
fast scan cyclic voltammetry
- DA
dopamine
- DAT
dopamine transporter
- NAc
nucleus accumbens
- aCSF
artificial cerebral spinal fluid
- VTA
ventral tegmental area
- i.p
intraperitoneal
- i.v
intravenous
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adachi YU, Yamada S, Satomoto M, Higuchi H, Watanabe K, Kazama T, Mimuro S, Sato S. Isoflurane anesthesia inhibits clozapine- and risperidone-induced dopamine release and anesthesia-induced changes in dopamine metabolism was modified by fluoxetine in the rat striatum: an in vivo microdialysis study. Neurochem Int. 2008;52:384–391. doi: 10.1016/j.neuint.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 2.España RA, Melchior JR, Roberts DC, Jones SR. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology. 2011;214:415–426. doi: 10.1007/s00213-010-2048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. The European journal of neuroscience. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.España RA, Roberts DC, Jones SR. Short-acting cocaine and long-acting GBR-12909 both elicit rapid dopamine uptake inhibition following intravenous delivery. Neuroscience. 2008;155:250–257. doi: 10.1016/j.neuroscience.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior research methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 6.Ferris MJ, Calipari ES, Yorgason JT, Jones SR. Examining the complex regulation and drug-induced plasticity of dopamine release and uptake using voltammetry in brain slices. ACS chemical neuroscience. 2013;4:693–703. doi: 10.1021/cn400026v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garris PA, Budygin EA, Phillips PE, Venton BJ, Robinson DL, Bergstrom BP, Rebec GV, Wightman RM. A role for presynaptic mechanisms in the actions of nomifensine and haloperidol. Neuroscience. 2003;118:819–829. doi: 10.1016/s0306-4522(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 8.Greco PG, Garris PA. In vivo interaction of cocaine with the dopamine transporter as measured by voltammetry. European journal of pharmacology. 2003;479:117–125. doi: 10.1016/j.ejphar.2003.08.062. [DOI] [PubMed] [Google Scholar]
- 9.Hara K, Harris RA. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesthesia and analgesia. 2002;94:313–318. doi: 10.1097/00000539-200202000-00015. table of contents. [DOI] [PubMed] [Google Scholar]
- 10.Jokinen MP, Lieuallen WG, Johnson CL, Dunnick J, Nyska A. Characterization of spontaneous and chemically induced cardiac lesions in rodent model systems: the national toxicology program experience. Cardiovascular toxicology. 2005;5:227–244. doi: 10.1385/ct:5:2:227. [DOI] [PubMed] [Google Scholar]
- 11.Kofke WA, Stiller RL, Rose ME. Comparison of extracellular dopamine concentration in awake unstressed and postsurgical nitrous oxide sedated rats. Journal of neurosurgical anesthesiology. 1995;7:280–283. doi: 10.1097/00008506-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Krasowski MD, Harrison NL. General anaesthetic actions on ligand-gated ion channels. Cellular and molecular life sciences: CMLS. 1999;55:1278–1303. doi: 10.1007/s000180050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations. Part 3: Other systems and conclusions. Experientia. 1986;42:531–537. doi: 10.1007/BF01946692. [DOI] [PubMed] [Google Scholar]
- 14.McCutcheon JE, Cone JJ, Sinon CG, Fortin SM, Kantak PA, Witten IB, Deisseroth K, Stuber GD, Roitman MF. Optical suppression of drug-evoked phasic dopamine release. Frontiers in neural circuits. 2014;8:114. doi: 10.3389/fncir.2014.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore JT, Chen J, Han B, Meng QC, Veasey SC, Beck SG, Kelz MB. Direct activation of sleep-promoting VLPO neurons by volatile anesthetics contributes to anesthetic hypnosis. Current biology: CB. 2012;22:2008–2016. doi: 10.1016/j.cub.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Opacka-Juffry J, Ahier RG, Cremer JE. Nomifensine-induced increase in extracellular striatal dopamine is enhanced by isoflurane anaesthesia. Synapse (New York, NY) 1991;7:169–171. doi: 10.1002/syn.890070210. [DOI] [PubMed] [Google Scholar]
- 17.Park J, Takmakov P, Wightman RM. In vivo comparison of norepinephrine and dopamine release in rat brain by simultaneous measurements with fast-scan cyclic voltammetry. Journal of neurochemistry. 2011;119:932–944. doi: 10.1111/j.1471-4159.2011.07494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prince CD, Rau AR, Yorgason JT, Espana RA. Hypocretin/Orexin regulation of dopamine signaling and cocaine self-administration is mediated predominantly by hypocretin receptor 1. ACS chemical neuroscience. 2015;6:138–146. doi: 10.1021/cn500246j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson DL, Venton BJ, Heien ML, Wightman RM. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clinical chemistry. 2003;49:1763–1773. doi: 10.1373/49.10.1763. [DOI] [PubMed] [Google Scholar]
- 20.Sabeti J, Gerhardt GA, Zahniser NR. Chloral hydrate and ethanol, but not urethane, alter the clearance of exogenous dopamine recorded by chronoamperometry in striatum of unrestrained rats. Neuroscience letters. 2003;343:9–12. doi: 10.1016/s0304-3940(03)00301-x. [DOI] [PubMed] [Google Scholar]
- 21.Sakamoto S, Nakao S, Masuzawa M, Inada T, Maze M, Franks NP, Shingu K. The differential effects of nitrous oxide and xenon on extracellular dopamine levels in the rat nucleus accumbens: a microdialysis study. Anesthesia and analgesia. 2006;103:1459–1463. doi: 10.1213/01.ane.0000247792.03959.f1. [DOI] [PubMed] [Google Scholar]
- 22.Tsukada H, Nishiyama S, Kakiuchi T, Ohba H, Sato K, Harada N, Nakanishi S. Isoflurane anesthesia enhances the inhibitory effects of cocaine and GBR12909 on dopamine transporter: PET studies in combination with microdialysis in the monkey brain. Brain research. 1999;849:85–96. doi: 10.1016/s0006-8993(99)02018-1. [DOI] [PubMed] [Google Scholar]
- 23.Votaw J, Byas-Smith M, Hua J, Voll R, Martarello L, Levey AI, Bowman FD, Goodman M. Interaction of isoflurane with the dopamine transporter. Anesthesiology. 2003;98:404–411. doi: 10.1097/00000542-200302000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Wu Q, Reith ME, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J Neurosci Methods. 2001;112:119–133. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]
- 25.Yamakura T, Bertaccini E, Trudell JR, Harris RA. Anesthetics and ion channels: molecular models and sites of action. Annual review of pharmacology and toxicology. 2001;41:23–51. doi: 10.1146/annurev.pharmtox.41.1.23. [DOI] [PubMed] [Google Scholar]
- 26.Yorgason JT, Espana RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011;202:158–164. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zimmerli B, Schlatter J. Ethyl carbamate: analytical methodology, occurrence, formation, biological activity and risk assessment. Mutation research. 1991;259:325–350. doi: 10.1016/0165-1218(91)90126-7. [DOI] [PubMed] [Google Scholar]