Abstract
Objective:
During adolescence, neurobiological maturation occurs concurrently with social and interpersonal changes, including the initiation of alcohol and other substance use. The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA) is designed to disentangle the complex relationships between onset, escalation, and desistance of alcohol use and changes in neurocognitive functioning and neuromaturation.
Method:
A sample of 831 youth, ages 12–21 years, was recruited at five sites across the United States, oversampling those at risk for alcohol use problems. Most (83%) had limited or no history of alcohol or other drug use, and a smaller portion (17%) exceeded drinking thresholds. A comprehensive assessment of biological development, family background, psychiatric symptomatology, and neuropsychological functioning—in addition to anatomical, diffusion, and functional brain magnetic resonance imaging—was completed at baseline.
Results:
The NCANDA sample of youth is nationally representative of sex and racial/ethnic groups. More than 50% have at least one risk characteristic for subsequent heavy drinking (e.g., family history, internalizing or externalizing symptoms). As expected, those who exceeded drinking thresholds (n = 139) differ from those who did not (n = 692) on identified factors associated with early alcohol use and problems.
Conclusions:
NCANDA successfully recruited a large sample of adolescents and comprehensively assessed psychosocial functioning across multiple domains. Based on the sample’s risk profile, NCANDA is well positioned to capture the transition into drinking and alcohol problems in a large portion of the cohort, as well as to help disentangle the associations between alcohol use, neurobiological maturation, and neurocognitive development and functioning.
THE DYNAMIC BIOLOGICAL, COGNITIVE, and psychosocial changes of adolescence result in a developmental focal point, highlighted by processes that progress or regress desynchronously over time as individuals transition into young adulthood (Dahl, 2004; Forbes & Dahl, 2010; Masten et al., 2008; Spear, 2000). Development results from the interplay of biological and psychosocial processes, including accumulating experience (e.g., acquisition of academic skills), biological maturation (e.g., onset of puberty), and changing life roles (e.g., independence from family of origin). A hallmark of adolescence is a heightened propensity for sensation seeking and risk taking that can result in negative consequences (Chein et al., 2011; Steinberg, 2005, 2007), such as heavy alcohol consumption (Casey & Jones, 2010; Jacobus & Tapert, 2013; Norman et al., 2011; Urošević et al., 2015; Witt, 2010), as rates of alcohol use rise dramatically from ages 14 to 18 years (Johnston et al., 2014).
The confluence of increasing alcohol consumption and brain maturation in adolescence may lead to risk for adverse effects (Masten et al., 2008). Gaining a better understanding of alcohol use effects during this crucial developmental stage has been positioned in national scientific consciousness and federal funding initiatives over the past two decades (Brown et al., 2008; Windle et al., 2008). Cross-sectional studies have identified brain structural and functional abnormalities (Cservenka et al., 2014; De Bellis et al., 2005, 2008; McQueeny et al., 2009; Nagel et al., 2005; Petit et al., 2014; Schweinsburg et al., 2010; Tapert et al., 2004), neurocognitive deficits (Brown et al., 2000; Silveri, 2014; Whelan et al., 2014; Xiao et al., 2013), and poorer psychosocial functioning (Anderson et al., 2010; Brown et al., 2008; Fergusson & Lynskey, 1996; Zucker et al., 2008) in heavydrinking adolescents relative to non-/low (NON) drinking counterparts. Deleterious effects of alcohol consumption have been associated with heavy drinking episodes (Chassin et al., 2002; McQueeny et al., 2009), defined as consuming four or more drinks on a single occasion for females and five or more drinks for males (National Institute on Alcohol Abuse and Alcoholism [NIAAA], 2004; Wechsler et al., 1995). Whether the reported abnormalities predate or result from alcohol consumption, however, is still unclear. Given the importance of disentangling risk for heavy drinking from its sequelae, the NIAAA, National Institute on Drug Abuse, National Institute of Mental Health, and National Institute of Child Health and Human Development sponsored the National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA) to examine factors associated with alcohol consumption in a large, multisite, longitudinal study that follows adolescents from before exposure through this crucial period when many typically initiate use.
NCANDA addresses the following aims: (a) determine the effects of alcohol exposure on the developmental trajectory of the adolescent human brain; (b) establish the effects of dose, duration, and age at alcohol exposure on adolescent brain development; (c) determine the extent to which alcohol’s effects on neuroanatomical and neurocognitive functioning resolve or persist with abstinence; (d) explore the role of developmental factors (e.g., pubertal stage, sleep patterns) and relevant covariates (e.g., sex, psychopathology, family history of alcoholism) in modulating alcohol’s effects on the developing adolescent brain; and (e) identify brain structural, functional, cognitive, and affective markers that may predict alcohol use disorder and other psychopathology in adolescence and young adulthood. This report provides an overview of NCANDA’s methodology and characterizes the sample at baseline. Other reports provide analysis of this sample on cognitive and motor performance, comprehensive clinical measures, sleep hygiene, regional brain macrostructure based on magnetic resonance imaging (MRI)–derived metrics (Pfefferbaum et al., 2015), regional brain white matter microstructural metrics derived from MR diffusion tensor imaging (DTI), and resting state MRI (rsMRI).
Method
To accomplish its aims, NCANDA set out to recruit 830 participants, ranging in age from 12 to 21 years, across five data collection sites: Duke University, University of Pittsburgh Medical Center (UPMC), Oregon Health & Science University (OHSU), University of California, San Diego (UCSD), and SRI International (SRI). The administrative component (UCSD) and the data analysis and informatics component (SRI) facilitated the training, quality assurance, and data integration across sites. The institutional review board at each site approved the study. Adult participants consented to participating, and minors provided written assent along with consent from a parent/legal guardian. Each data collection site administers the same core protocol, and sites work in pairs to conduct studies testing additional hypotheses (e.g., overnight sleep evaluation, recovery during monitored abstinence, behavioral inhibition, and Stroop functional MRI tasks). This paired-site design allows more hypotheses to be evaluated across the consortium with sufficient power to detect effects and provide replicability.
Youth complete a core data-acquisition protocol at baseline and three annual follow-ups, and each visit includes a neuropsychological battery; neuroimaging session (MRI, DTI, and rsMRI); and comprehensive assessment of substance use, psychiatric symptoms and diagnoses, and functioning in major life domains. One parent of each youth completes an annual interview on the youth and family environment. Parents of minors consented that youth self-reported data would not be revealed to them with the exception of disclosures of harm (e.g., suicidal/homicidal ideation or abuse), and confidentiality was reiterated to youth to confirm that information would not be shared with parents unless required by ethical standards. These efforts were taken to help facilitate accurate and open self-report. Urine samples collected for toxicology screens use a 12-panel test covering major intoxicants. At baseline, positive drug screens other than marijuana were confirmed via gas chromatography– mass spectrometry (GC/MS), and any positive drug screen that conflicts with self-reported substance use is sent for GC/MS confirmation at all other time points. Biosamples for genetic analysis are collected annually for future examination of epigenetic changes (e.g., histone modification, DNA methylation; Nestler, 2014).
Design
NCANDA uses an accelerated longitudinal design (ALD; Duncan et al., 1996, 2006; Miyazaki & Raudenbush, 2000) to sample subjects from a broad span of baseline ages, allowing for characterizing development across larger age ranges. Unlike traditional cohort designs, ALDs are able to address a much broader developmental window. As a result, ALDs may have less power to detect very small effects that occur within highly time-limited developmental windows (e.g., transition into high school) compared with fully longitudinal designs that would track an age-restricted cohort across a longer period. To maximize power to address our aims (e.g., onset of alcohol use) given the study timeframe and resources, NCANDA recruited participants ages 12–21 years, oversampling ages 12–15 years so that a large portion of the sample was tracked through critical periods of adolescent development and alcohol use initiation risk (Duncan et al., 2006). One methodological challenge in characterizing brain development and behavioral trajectories involves the ability to capture, characterize, and explain within- and between-subject variations in trajectories. ALDs address within- and between-subject variations by accounting for age cohort effects (Duncan et al., 1996; Miyazaki & Raudenbush, 2000), here specifically for differences in onset, dose, and duration of alcohol exposure across subjects. Shorter-term trajectories derived from different individuals can be “spliced” together to form longitudinal trajectories spanning the entire age range of the sample.
The majority of recruited youth had no history of heavy drinking; however, strategically allowing a small proportion of subjects from older age cohorts to exceed the alcohol use thresholds enables estimation of long-term trajectories representing a continuum from nondrinking to heavy drinking on an accelerated time scale. Sampling heavier drinkers does not reduce power for estimating the effects of transitions from pre- to post-alcohol exposure on outcomes (estimated power of .99 with a moderate effect size). Therefore, our ALD maximizes power to detect onset of drinking, by oversampling individuals with high probability of onset based on age, so that we can model the effects of drinking onset and acceleration over a much larger age span than the 4 years of the study. Trajectory analyses examining the effects of substance use on neurocognition and neurodevelopment will be assessed primarily in general additive models (Hastie & Tibshirani, 1986; Wood, 2006, 2011).
Each site endeavored to collect a community sample reflective of local racial/ethnic distributions of their county with equal sex proportions in each age group. To ensure sufficient inclusion of participants who would likely begin drinking heavily during the study, youth at greater risk for heavy drinking were preferentially recruited to comprise roughly 50% of the sample based on screening evidence of (a) early experimentation with alcohol (i.e., first full drink before age 15 years; Grant & Dawson, 1997), (b) family history of alcohol or other drug problems (Edenberg et al., 1998; Schuckit & Smith, 1996), (c) endorsement of one or more externalizing (e.g., conduct disorder) symptoms (Brown et al., 1996; Myers et al., 1995; Slutske et al., 1998), and/or (d) endorsement of two or more internalizing symptoms (Chassin et al., 2002; Hussong et al., 2011).
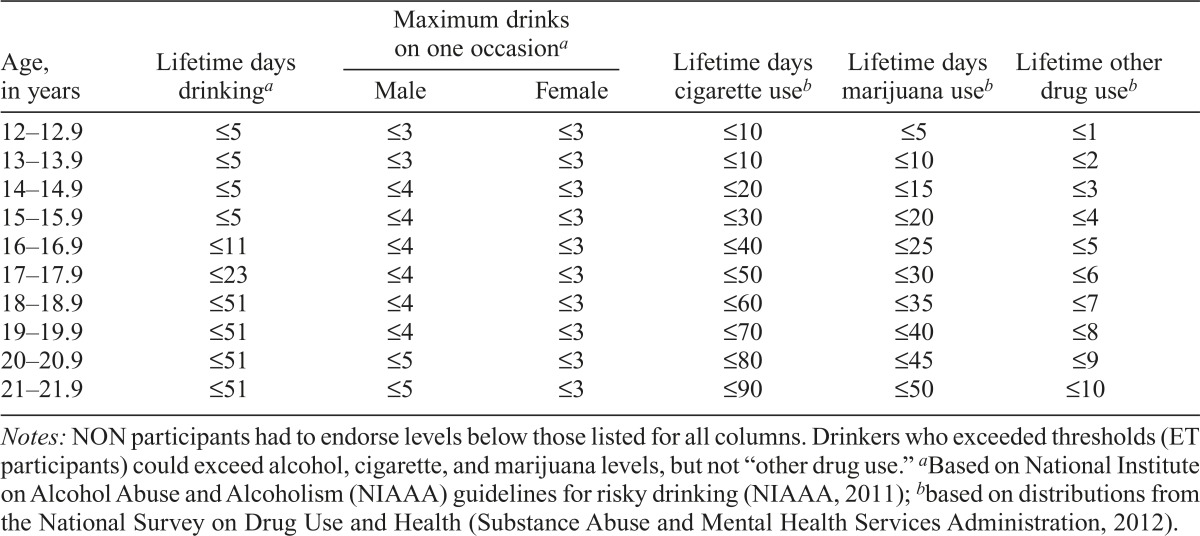
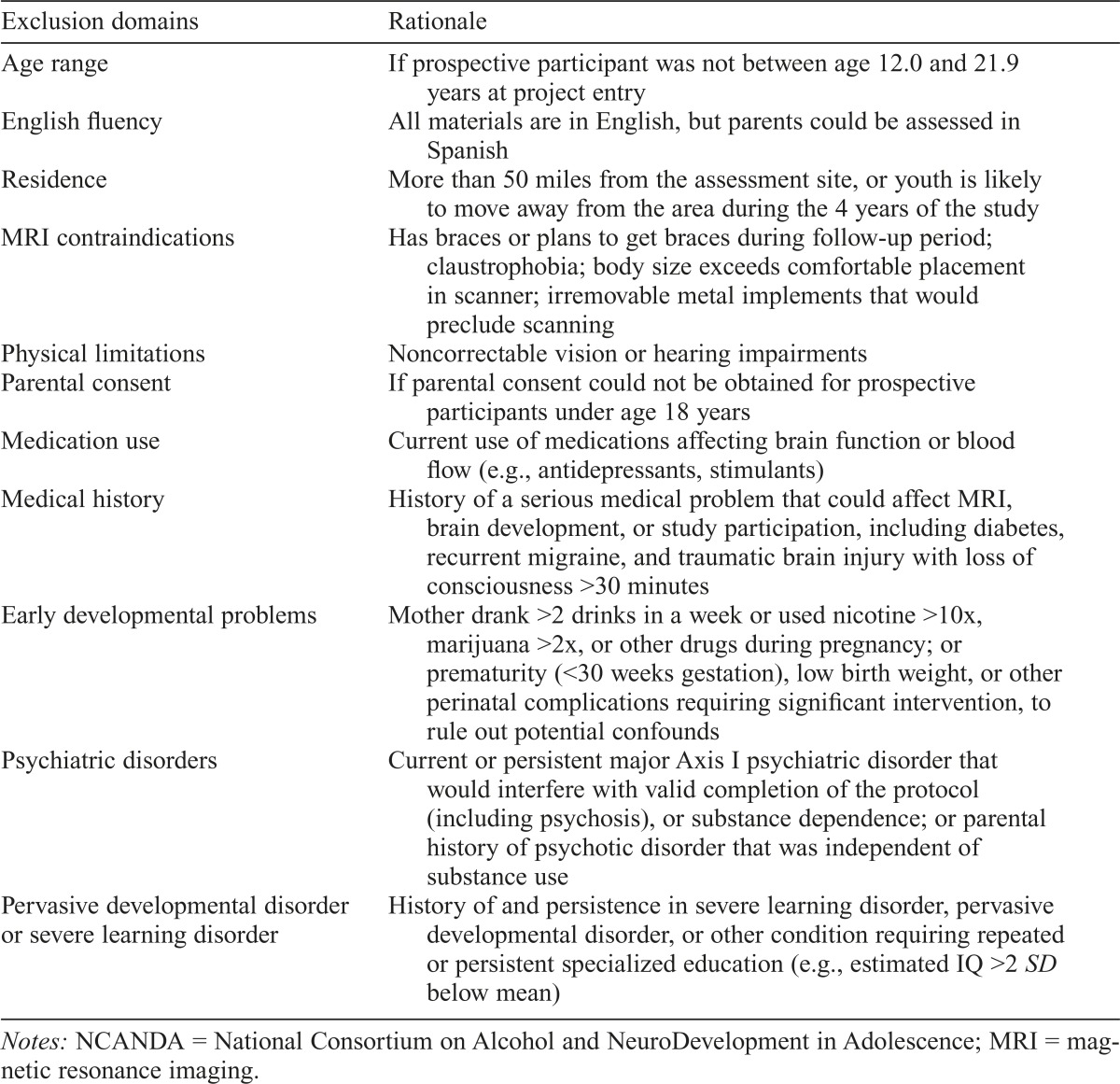
The majority of participants were required to have limited exposure to alcohol or other drugs (Table 1), because a primary aim was to determine neural changes following the onset of heavy alcohol use. In addition, participants were excluded based on factors that may confound detecting the main effect of interest, compromise valid completion of the protocol, or impede the ability to follow participants over 5 years (Table 2). Although exclusion criteria were minimized to increase our ability to recruit a representative sample, some criteria likely led to the exclusion of elements of the population (e.g., no psychotropic medications, required parental involvement, English proficiency). A limited number (17%) of youth who exceeded drinking thresholds (ET) were enrolled. ET drinkers were allowed to exceed marijuana and nicotine exposure criteria but required to meet all other eligibility criteria. To maximize recruitment of NON youth who met the inclusion criteria, recruitment of ET drinkers was titrated so that the majority entered the study during the second half of the recruitment period.
Table 1.
Substance use inclusion criteria to be classified as non-/low drinker (NON) at baseline
| Age, in years | Lifetime days drinkinga | Maximum drinks on one occasiona |
Lifetime days cigarette useb | Lifetime days marijuana useb | Lifetime other drug useb | |
| Male | Female | |||||
| 12–12.9 | ≤5 | ≤3 | ≤3 | ≤10 | ≤5 | ≤1 |
| 13–13.9 | ≤5 | ≤3 | ≤3 | ≤10 | ≤10 | ≤2 |
| 14–14.9 | ≤5 | ≤4 | ≤3 | ≤20 | ≤15 | ≤3 |
| 15–15.9 | ≤5 | ≤4 | ≤3 | ≤30 | ≤20 | ≤4 |
| 16–16.9 | ≤11 | ≤4 | ≤3 | ≤40 | ≤25 | ≤5 |
| 17–17.9 | ≤23 | ≤4 | ≤3 | ≤50 | ≤30 | ≤6 |
| 18–18.9 | <51 | ≤4 | ≤3 | ≤60 | ≤35 | ≤7 |
| 19–19.9 | ≤51 | ≤4 | ≤3 | ≤70 | ≤40 | ≤8 |
| 20–20.9 | ≤51 | ≤5 | ≤3 | ≤80 | ≤45 | ≤9 |
| 21–21.9 | ≤51 | ≤5 | ≤3 | ≤90 | ≤50 | ≤10 |
Notes: NON participants had to endorse levels below those listed for all columns. Drinkers who exceeded thresholds (ET participants) could exceed alcohol, cigarette, and marijuana levels, but not “other drug use.”
Based on National Institute on Alcohol Abuse and Alcoholism (NIAAA) guidelines for risky drinking (NIAAA, 2011);
based on distributions from the National Survey on Drug Use and Health (Substance Abuse and Mental Health Services Administration, 2012).
Table 2.
Exclusionary criteria for NCANDA study participation at baseline
| Exclusion domains | Rationale |
| Age range | If prospective participant was not between age 12.0 and 21.9 years at project entry |
| English fluency | All materials are in English, but parents could be assessed in Spanish |
| Residence | More than 50 miles from the assessment site, or youth is likely to move away from the area during the 4 years of the study |
| MRI contraindications | Has braces or plans to get braces during follow-up period; claustrophobia; body size exceeds comfortable placement in scanner; irremovable metal implements that would preclude scanning |
| Physical limitations | Noncorrectable vision or hearing impairments |
| Parental consent | If parental consent could not be obtained for prospective participants under age 18 years |
| Medication use | Current use of medications affecting brain function or blood flow (e.g., antidepressants, stimulants) |
| Medical history | History of a serious medical problem that could affect MRI, brain development, or study participation, including diabetes, recurrent migraine, and traumatic brain injury with loss of consciousness >30 minutes |
| Early developmental problems | Mother drank >2 drinks in a week or used nicotine >10x, marijuana >2x, or other drugs during pregnancy; or prematurity (<30 weeks gestation), low birth weight, or other perinatal complications requiring significant intervention, to rule out potential confounds |
| Psychiatric disorders | Current or persistent major Axis I psychiatric disorder that would interfere with valid completion of the protocol (including psychosis), or substance dependence; or parental history of psychotic disorder that was independent of substance use |
| Pervasive developmental disorder or severe learning disorder | History of and persistence in severe learning disorder, pervasive developmental disorder, or other condition requiring repeated or persistent specialized education (e.g., estimated IQ >2 SD below mean) |
Notes: NCANDA = National Consortium on Alcohol and NeuroDevelopment in Adolescence; MRI = magnetic resonance imaging.
Participants were recruited through announcements distributed to student populations at local schools and colleges, public notices, and targeted catchment-area calling. Interested participants and one biological parent completed a phone screen that assessed eligibility and preliminary characterization of risk factors. Screening was conducted in a stepwise, prioritized order in which MRI contraindications, physical limitations, and parental availability/consent were assessed first; substance use history was assessed second; and medical conditions/medication use, prenatal alcohol/ drug exposure, and learning disabilities were assessed last. If a participant was ineligible based on one screening element, screening ceased and additional factors were not assessed. Participants who appeared eligible based on the telephone interview were invited for an in-person interview. Preliminary risk factor and eligibility classifications were confirmed through extensive in-person interviews. Recruitment data were reviewed monthly to ensure adherence to recruitment targets (e.g., 50% female within each age group; 50% endorsing at least one high-risk criterion) and site-specific targets (e.g., local racial/ethnic distributions). Participants were compensated for completing all baseline sessions, and parents were compensated for completing the baseline interview, with total compensation ranging from $200 to $225 per family across sites. Participants discovered to be ineligible (n = 50) received partial compensation based on the measures completed before exclusion.
Clinical measures
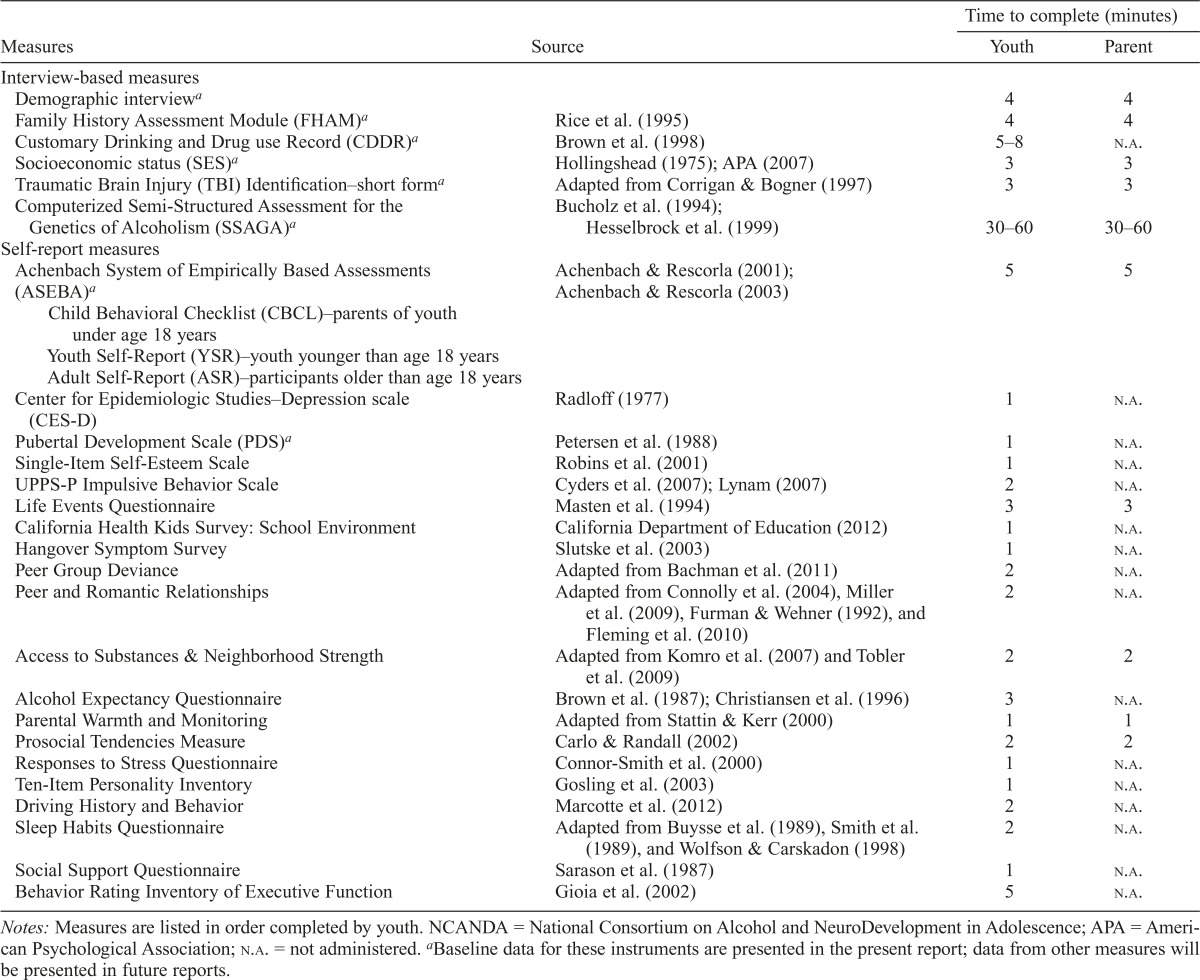
Measures were selected to assess substance use, psychiatric symptoms and diagnoses, familial and social environment, education, personality factors, biological and developmental factors, and basic demographics (see Table 3 for complete list of measures). The evaluation was designed to assess potential antecedent conditions related to substance use and gather data to ensure homogeneity between risk groups, or to control for differences statistically if needed. Additional measures addressing hypotheses—such as the effects of sleep, childhood trauma, parenting, peer relationships, and neighborhood characteristics on alcohol use—will be presented in subsequent reports. Measures were collected over 1–2 days with regular breaks and provisions (e.g., snacks/drinks) to minimize fatigue during data collection.
Table 3.
Comprehensive measures administered in NCANDA protocol
| Measures | Source | Time to complete (minutes) Youth Parent |
|
| Interview-based measures | |||
| Demographic interviewa | 4 | 4 | |
| Family History Assessment Module (FHAM)a | Rice et al. (1995) | 4 | 4 |
| Customary Drinking and Drug use Record (CDDR)a | Brown et al. (1998) | 5–8 | N.A. |
| Socioeconomic status (SES)a | Hollingshead (1975); APA (2007) | 3 | 3 |
| Traumatic Brain Injury (TBI) Identification–short forma | Adapted from Corrigan & Bogner (1997) | 3 | 3 |
| Computerized Semi–Structured Assessment for the | Bucholz et al. (1994); | ||
| Genetics of Alcoholism (SSAGA)a | Hesselbrock et al. (1999) | 30–60 | 30–60 |
| Self-report measures | |||
| Achenbach System of Empirically Based Assessments (ASEBA)a | Achenbach & Rescorla (2001); Achenbach & Rescorla (2003) | 5 | 5 |
| Child Behavioral Checklist (CBCL)–parents of youth under age 18 years | |||
| Youth Self-Report (YSR)–youth younger than age 18 years | |||
| Adult Self-Report (ASR)–participants older than age 18 years | |||
| Center for Epidemiologic Studies–Depression scale (CES-D) | Radloff (1977) | 1 | n.a. |
| Pubertal Development Scale (PDS)a | Petersen et al. (1988) | 1 | n.a. |
| Single-Item Self-Esteem Scale | Robins et al. (2001) | 1 | n.a. |
| UPPS-P Impulsive Behavior Scale | Cyders et al. (2007); Lynam (2007) | 2 | n.a. |
| Life Events Questionnaire | Masten et al. (1994) | 3 | 3 |
| California Health Kids Survey: School Environment | California Department of Education (2012) | 1 | n.a. |
| Hangover Symptom Survey | Slutske et al. (2003) | 1 | n.a. |
| Peer Group Deviance | Adapted from Bachman et al. (2011) | 2 | n.a. |
| Peer and Romantic Relationships | Adapted from Connolly et al. (2004), Miller et al. (2009), Furman & Wehner (1992), and Fleming et al. (2010) | 2 | n.a. |
| Access to Substances & Neighborhood Strength | Adapted from Komro et al. (2007) and Tobler et al. (2009) | 2 | 2 |
| Alcohol Expectancy Questionnaire | Brown et al. (1987); Christiansen et al. (1996) | 3 | n.a. |
| Parental Warmth and Monitoring | Adapted from Stattin & Kerr (2000) | 1 | 1 |
| Prosocial Tendencies Measure | Carlo & Randall (2002) | 2 | 2 |
| Responses to Stress Questionnaire | Connor-Smith et al. (2000) | 1 | n.a. |
| Ten-Item Personality Inventory | Gosling et al. (2003) | 1 | n.a. |
| Driving History and Behavior | Marcotte et al. (2012) | 2 | n.a. |
| Sleep Habits Questionnaire | Adapted from Buysse et al. (1989), Smith et al. (1989), and Wolfson & Carskadon (1998) | 2 | n.a. |
| Social Support Questionnaire | Sarason et al. (1987) | 1 | n.a. |
| Behavior Rating Inventory of Executive Function | Gioia et al. (2002) | 5 | n.a. |
Notes: Measures are listed in order completed by youth. NCANDA = National Consortium on Alcohol and NeuroDevelopment in Adolescence; APA = American Psychological Association; n.a. = not administered.
Baseline data for these instruments are presented in the present report; data from other measures will be presented in future reports.
Quality assurance of clinical measures
The NCANDA Administrative Component coordinated in-person and teleconference training to ensure reliability across sites and conducted training and reliability checks for the entire core battery. Training manuals were created for the clinical protocol, including an overview and “frequently asked questions” for each measure, and an expert on the clinical interview (Semi-Structured Assessment of the Genetics of Alcoholism [SSAGA]) provided video-recorded training. Each site established a senior-level staff member (i.e., Ph.D./M.D.) who oversaw implementation of training disseminated by the administrative component. For clinical interview and neuropsychological assessments, training included repeated mock sessions observed by senior staff, who then provided feedback. Interview administrators had to be approved by properly completing an interview with a mock participant observed by a senior-level staff member. Crucially, senior trainers complete annual visits to each site to check for interviewer drift and confirm proper training of new staff members. During these annual visits, trainers spend 2–3 days at each site observing staff and providing oral and written feedback. Any practices identified as needing remediation are addressed immediately, discussed with the administrative component principal investigators (PIs), and documented for site PIs and staff.
Data processing pipeline
The infrastructure for data collection includes the Research Electronic Data Capture (REDCap) system (http://project-redcap.org), extensible Neuroimaging Archive Toolkit (XNAT; http://www.xnat.org), LimeSurvey (http://www.limesurvey.org/), Blaise (http://www.blaise.com), ePrime (http://www.pstnet.com/eprime.cfm), and University of Pennsylvania Web-based Computerized Neurocognitive Battery (https://webcnp.med.upenn.edu/). All data collected via laptop computers were automatically merged onto a REDCap server hosted at the data analysis component. Test scores not collected through REDCap direct data entry were automatically uploaded from collection sites to REDCap via Subversion (https://subversion.apache.org/), a secure and encrypted data uploading system (see Rohlfing et al., 2014). Imaging data were uploaded from site-specific Picture Archiving and Communication Systems to an XNAT server hosted at SRI. All data underwent quality control checks, including automatically generated scores and a radiologist report. Quality control–processed imaging and corresponding nonimaging data for each session were combined and uploaded onto REDCap to generate biweekly data integrity reports with site consultation to resolve scoring irregularities. Data in this manuscript are based on a quality-reviewed, locked data release provided by the data analysis component (version: NCANDA_DATA_00010).
Data analysis
The current results characterize the NCANDA sample at baseline. Demographic data were examined using correlations, t tests, analyses of covariance, and chi-square analyses to compare the categories of interest and to compare to epidemiological data (e.g., U.S. Census). Descriptions of the sample are split into NON and ET groups to differentiate the portion of the sample with low exposure to alcohol who may transition to heavy use during the course of the study and those who were included to increase the power of the ALD for predicting use trajectories.
Results
Demographics of the sample
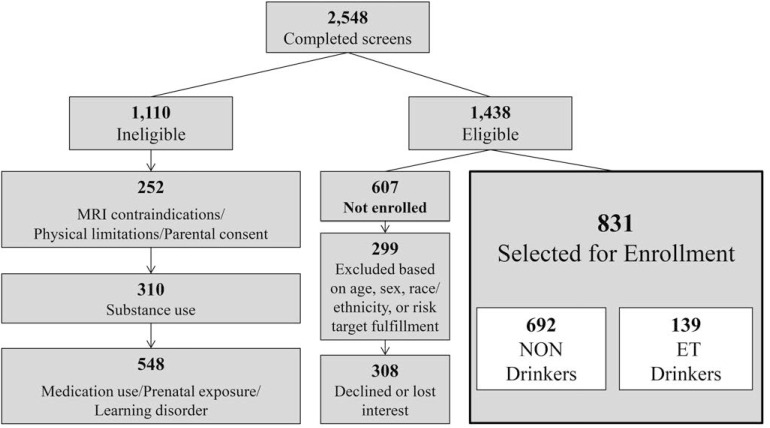
More than 7,500 individuals contacted NCANDA sites for screening in response to school-, mail-, and telephone-based recruitment procedures. A total of 2,548 target participants (as well as one biological parent) completed a screening interview, ultimately yielding a sample of 831 participants (Figure 1). The sample includes 692 (83%) individuals who met the inclusion criteria as NON drinkers and 139 (17%) ET drinkers were included to represent a range of drinking for future trajectory analyses. Because of the finite budget and sample size for each site, ample screening was conducted to facilitate oversampling for risk, matching sex within age groups, and meeting enrollment targets for age and racial/ethnic groups. An additional 607 participants met the eligibility criteria after screening but were not enrolled in the study because they declined or lost interest (n = 308) or because enrollment targets for age, sex, or racial/ethnic categories had already been fulfilled (n = 299; Figure 1).
Figure 1.
Recruitment overview across five data collection sites within the National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA) consortium. More than 7,500 individuals made initial contact or began screening, and sufficient screening was completed to make inclusion/exclusion decisions for 2,548 (i.e., “completed screens”). Exclusionary factors were assessed in a stepwise, prioritized order depicted in the figure from top to bottom such that individuals who were excluded because of higher order factors were not assessed on subsequent criteria. The enrolled sample (N = 831) includes (n = 692) non-/low (NON) drinkers and (n = 139) drinkers who exceeded thresholds (ET). MRI = magnetic resonance imaging.
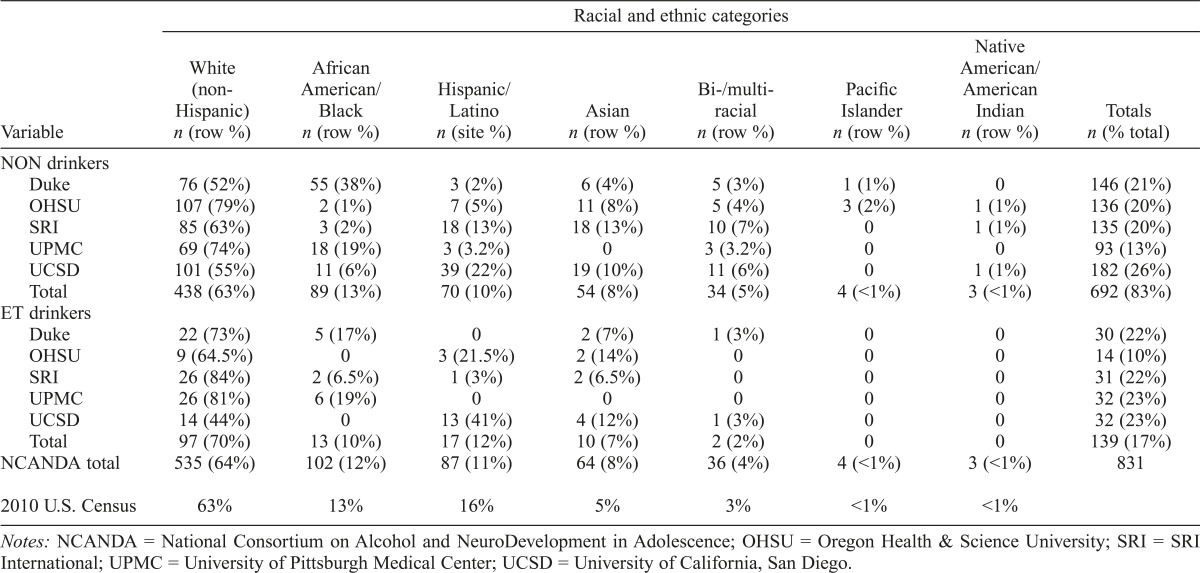
Each site contributed 15%–26% of the sample. The sample was distributed across age groups and matched for sex, with the largest proportion (44%) from the 12- to 14-year-old age group. There were no significant age group or sex differences across sites. The sample is roughly equivalent to reported census numbers (Humes et al., 2011; see the last row in Table 4 for 2010 census comparisons). By design, compared with NON drinkers, the ET sample was biased toward the oldest age group, with more than 60% ages 18–21 years, χ2(2) = 130.2, p < .001. The trajectories of ET drinkers in this oldest age group will approximate the end trajectories for individuals in the younger age groups, thus facilitating longitudinal trajectory analyses.
Table 4.
Racial and ethnic description of NCANDA sample for non-/low (NON) drinkers and drinkers who exceeded thresholds (ET) at baseline (N = 831) with comparison to 2010 U.S. Census data
| Racial and ethnic categories |
||||||||
| Variable | White (non-Hispanic) n (row %) | African American/ Black n (row %) | Hispanic/ Latino n (site %) | Asian n (row %) | Bi-/multi-racial n (row %) | Pacific Islander n (row %) | Native American/ American Indian n (row %) | Totals n (% total) |
| NON drinkers | ||||||||
| Duke | 76 (52%) | 55 (38%) | 3 (2%) | 6 (4%) | 5 (3%) | 1 (1%) | 0 | 146 (21%) |
| OHSU | 107 (79%) | 2 (1%) | 7 (5%) | 11 (8%) | 5 (4%) | 3 (2%) | 1 (1%) | 136 (20%) |
| SRI | 85 (63%) | 3 (2%) | 18 (13%) | 18 (13%) | 10 (7%) | 0 | 1 (1%) | 135 (20%) |
| UPMC | 69 (74%) | 18 (19%) | 3 (3.2%) | 0 | 3 (3.2%) | 0 | 0 | 93 (13%) |
| UCSD | 101 (55%) | 11 (6%) | 39 (22%) | 19 (10%) | 11 (6%) | 0 | 1 (1%) | 182 (26%) |
| Total | 438 (63%) | 89 (13%) | 70 (10%) | 54 (8%) | 34 (5%) | 4 (<1%) | 3 (<1%) | 692 (83%) |
| ET drinkers | ||||||||
| Duke | 22 (73%) | 5 (17%) | 0 | 2 (7%) | 1 (3%) | 0 | 0 | 30 (22%) |
| OHSU | 9 (64.5%) | 0 | 3 (21.5%) | 2 (14%) | 0 | 0 | 0 | 14 (10%) |
| SRI | 26 (84%) | 2 (6.5%) | 1 (3%) | 2 (6.5%) | 0 | 0 | 0 | 31 (22%) |
| UPMC | 26 (81%) | 6 (19%) | 0 | 0 | 0 | 0 | 0 | 32 (23%) |
| UCSD | 14 (44%) | 0 | 13 (41%) | 4 (12%) | 1 (3%) | 0 | 0 | 32 (23%) |
| Total | 97 (70%) | 13 (10%) | 17 (12%) | 10 (7%) | 2 (2%) | 0 | 0 | 139 (17%) |
| NCANDA total | 535 (64%) | 102 (12%) | 87 (11%) | 64 (8%) | 36 (4%) | 4 (<1%) | 3 (<1%) | 831 |
| 2010 U.S. Census | 63% | 13% | 16% | 5% | 3% | <1% | <1% | |
Notes: NCANDA = National Consortium on Alcohol and NeuroDevelopment in Adolescence; OHSU = Oregon Health & Science University; SRI = SRI International; UPMC = University of Pittsburgh Medical Center; UCSD = University of California, San Diego.
Family background.
The majority of the sample resides with one or more of their parents (95% of age 18 years or younger, and 88% overall). A greater proportion of ET drinkers reported living away from parents (e.g., alone, with roommates, or in a dormitory), even if older than age 18 years (30% of NON vs. 60% of ET drinkers), χ2(1) = 11.8, p < .001. Most participants were from families of married parents (73%), whereas 21% reported that their parents were divorced or separated, and 6% reported having a single parent. NON and ET drinkers did not differ in proportion of unmarried, married, or divorced parents.
Socioeconomic status.
Socioeconomic status (SES) is determined by parental education level and income. The sample spans a wide range, with 20% reporting parents with education below a college degree, 27% with at least one parent attaining a college degree, and 53% with at least one parent with education greater than a college degree. Annual family income ranged from below $12,000 to greater than $200,000, and 18% reported income below $50,000 per year. Of note, 11% of the sample did not know or declined to provide family income data. The median income in the United States at the time of study entry (2013) was $52,250, and the median incomes for the metropolitan areas surrounding data collection sites ranged from $50,988 (Pittsburgh) to $90,786 (Silicon Valley). As expected, the sample collected near Silicon Valley (SRI) had the highest annual family income, F(4, 733) = 14.4, p < .001, and parental education, F(4, 759) = 8.9, p < .001, but neither SES indicator differed among the other sites. ET drinkers reported higher annual family income, t(736) = -2.4, p = .02, but drinking groups did not differ on parental education.
Current health and psychosocial functioning
Health and academic data were gathered in the demographic survey from both youth and one parent, including aspects of functioning associated with initiation of drinking in this age range (e.g., Anderson & Brown, 2011; Brown et al., 2008; Zucker et al., 2008). Most (85%) participants had body mass indices in the normal range (2 SD of means for age and sex; Centers for Disease Control and Prevention, 2001); 2% were under and 13% were over normal body mass index ranges, with no differences between NON and ET drinkers. Participants were generally in good health, and only 5% of the sample reported visiting a doctor or hospital in the last year for a serious medical problem, with no differences between NON and ET drinkers. Pubertal development positively correlated with age (r = .64, p < .001) and differed by sex, as expected (female > male; t(817) = 11.8, p < .001). Although ET drinkers had a greater ratio of late-pubertal and post-pubertal compared with NON drinkers, χ2(4) = 59.9, p < .001, drinking group was not related to pubertal stage after we controlled for age and sex, F(4, 814) = 0.5, n.s.
Educational levels of participants ranged from fourth grade through college graduates and did not differ between drinking groups after we controlled for age. Participants reported earning mainly above-average grades, with a mean grade point average of 3.5 on a 4.0 scale that did not differ by drinking group or age, thus indicating that the sample is relatively high achieving.
Substance use.
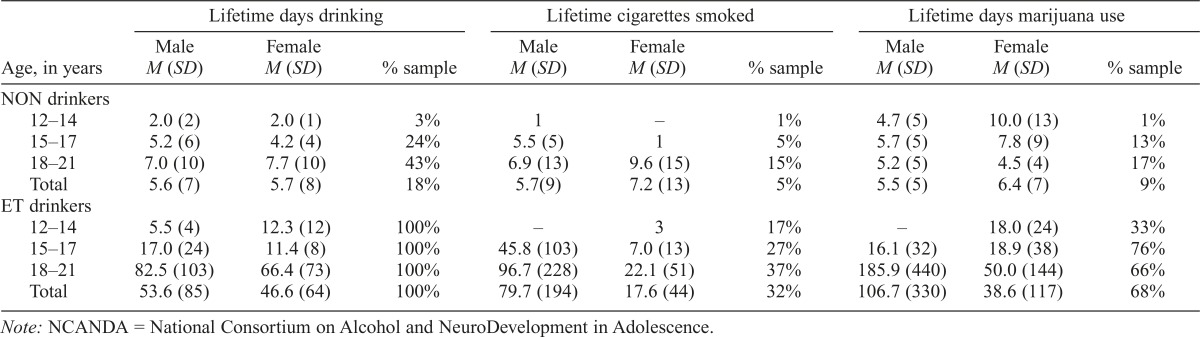
Participants completed the Customary Drinking and Drug-use Record (Brown et al., 1998) to characterize their past and current alcohol and substance use. In line with inclusion criteria, NON drinkers reported limited alcohol, tobacco, and marijuana experience (Table 5). A small number of NON drinkers (1.4%; n =10) reported some experience with other drugs—including synthetic marijuana, amphetamines, Ecstasy, and opiates—at levels below those listed in Table 1 and commensurate with the norms from recent epidemiological surveys (Substance Abuse and Mental Health Services Administration [SAMHSA], 2014). ET drinkers reported significantly more alcohol, cigarette, and marijuana use than NON drinkers (Table 5), and 13.7% (n = 19) reported experience with other drugs—including spice, amphetamines, Ecstasy, hallucinogens, and inhalants —which fell within eligibility criteria (Table 1). By design, 92% of ET drinkers reported heavy drinking experience (i.e., ≥4 drinks for female and ≥5 drinks for male youth), with 85% reporting a heavy episode in the last year and 33% in the past month. The rate of ET past-month heavy drinking is higher than epidemiological survey data, as expected: 38% versus 29% in 18- to 20-year-olds; 24% versus 13% in 15- to 17-year-olds (SAMHSA, 2015).
Table 5.
Substance use in NCANDA sample by sex, age group, and for non-/low (NON) drinkers (n = 692) and drinkers who exceeded thresholds (ET; n = 139) at baseline
| Lifetime days drinking |
Lifetime cigarettes smoked |
Lifetime days marijuana use |
|||||||
| Age, in years | Male M (SD) | Female | % sample | Male M (SD) | Female M (SD) | % sample | Male M (SD) | Female M (SD) | % sample |
| NON drinkers | |||||||||
| 12–14 | 2.0 (2) | 2.0 (1) | 3% | 1 | – | 1% | 4.7 (5) | 10.0 (13) | 1% |
| 15–17 | 5.2 (6) | 4.2 (4) | 24% | 5.5 (5) | 1 | 5% | 5.7(5) | 7.8 (9) | 13% |
| 18–21 | 7.0 (10) | 7.7 (10) | 43% | 6.9 (13) | 9.6 (15) | 15% | 5.2(5) | 4.5 (4) | 17% |
| Total | 5.6 (7) | 5.7 (8) | 18% | 5.7(9) | 7.2 (13) | 5% | 5.5 (5) | 6.4 (7) | 9% |
| ET drinkers | |||||||||
| 12–14 | 5.5 (4) | 12.3 (12) | 100% | – | 3 | 17% | – | 18.0 (24) | 33% |
| 15–17 | 17.0 (24) | 11.4 (8) | 100% | 45.8 (103) | 7.0 (13) | 27% | 16.1 (32) | 18.9 (38) | 76% |
| 18–21 | 82.5 (103) | 66.4 (73) | 100% | 96.7 (228) | 22.1 (51) | 37% | 185.9 (440) | 50.0 (144) | 66% |
| Total | 53.6 (85) | 46.6 (64) | 100% | 79.7 (194) | 17.6 (44) | 32% | 106.7 (330) | 38.6 (117) | 68% |
Note: NCANDA = National Consortium on Alcohol and NeuroDevelopment in Adolescence.
Risk characteristics for future substance use and disorders
Four a priori risk domains for NCANDA were early alcohol experience, family history of alcohol/other drug problems, externalizing symptoms, and internalizing symptoms. As noted above, each of these risk factors has been associated with increased alcohol use and problems, and it was crucial for NCANDA to oversample adolescents endorsing these factors to increase the likelihood of capturing transitions to heavy drinking during the study. In the NON group, 47% carry at least one risk factor for heavy alcohol use. Although these risk domains are associated with different mechanisms of risk for alcohol use and problems, endorsing more than one factor increases the risk. Among NON drinkers, 17% endorsed two or more risk factors. The ET group contained 70% risk-positive individuals and 34% with more than one risk factor.
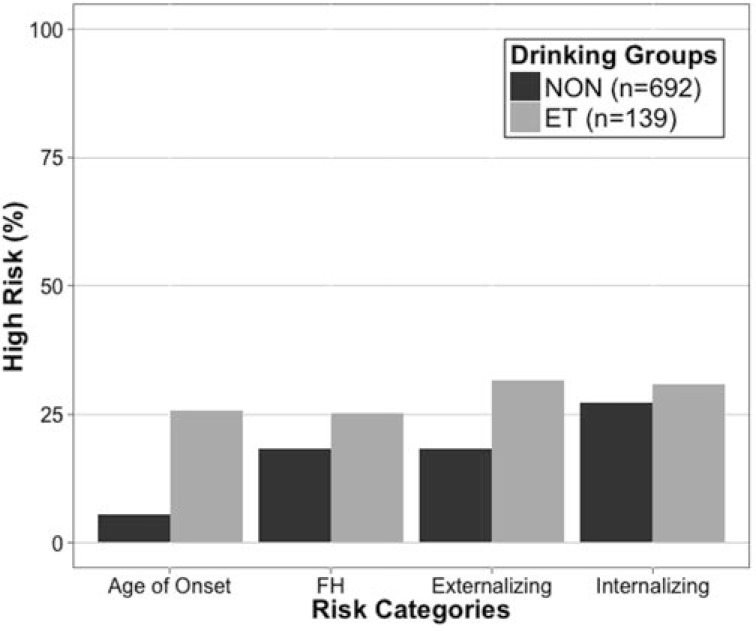
Early onset of alcohol use. Consuming one’s first full drink before age 15 years is associated with more severe negative consequences and a higher prevalence of alcohol problems later in life (Grant & Dawson, 1997). A greater proportion of ET drinkers compared with the NON group reported drinking onset before age 15 years (25% vs. 6%; Figure 2), χ2(1) = 57.9, p < .001, with no differences between the sexes.
Figure 2.
Prevalence of risk factors for alcohol use and problems, including alcohol use onset before age 15 (age of onset), family history of alcohol or other drug problems (FH), endorsement of externalizing symptoms (externalizing), and endorsement of internalizing symptoms (internalizing) for non-/low (NON) drinkers (n = 692) and drinkers who exceeded thresholds (ET; n = 139). Y-axis values represent the percentage of each group who endorsed risk factors.
Family history of substance use disorders was assessed with the Family History Assessment Module (Rice et al., 1995). Family history–positive participants had (a) at least one biological parent with problems indicative of an alcohol/other drug use disorder (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; American Psychiatric Association, 1994); (b) two or more biological grandparents with significant problems indicative of an alcohol/other drug disorder; or (c) one or more biological grandparents and two or more other biological second-degree relatives (e.g., aunt, uncle) with significant problems indicative of an alcohol/other drug disorder (Bierut et al., 2002; Rice et al., 1995). Using these criteria, 17% of the sample was positive for familial alcohol use problems, and 8% was positive for familial drug use problems. The proportion of family history–positive participants did not differ between drinking groups (NON: 18% positive for family history of alcohol/other drugs problems; ET: 25% positive for family history of alcohol/other drugs problems; Figure 2).
Externalizing symptoms.
Participants and one parent (for youth younger than age 18 years) completed a computerized SSAGA (Bucholz et al., 1994; Hesselbrock et al., 1999), which was shortened from the full-length version, assessing Axis I diagnoses (e.g., mood, anxiety, and conduct disorders; American Psychiatric Association, 1994, 2013), many of which are risk factors associated with onset or acceleration of substance use. As such, counts of symptom endorsements, rather than full diagnosis, were used to characterize potential future risk. Specifically, endorsing one or more symptoms of conduct disorder/antisocial personality disorder indexed risk for alcohol misuse (Brown et al., 1996; Slutske et al., 1998). Participants and one parent (for participants under age 18) completed the self-report instruments from the Achenbach System of Empirically Based Assessments (ASEBA; Achenbach & Rescorla, 2001, 2003), and symptom endorsement is summarized in externalizing scale t scores for age and sex. Participants who scored in the borderline or clinical range (i.e., t score ≥ 60) were considered high risk for alcohol use and problems.
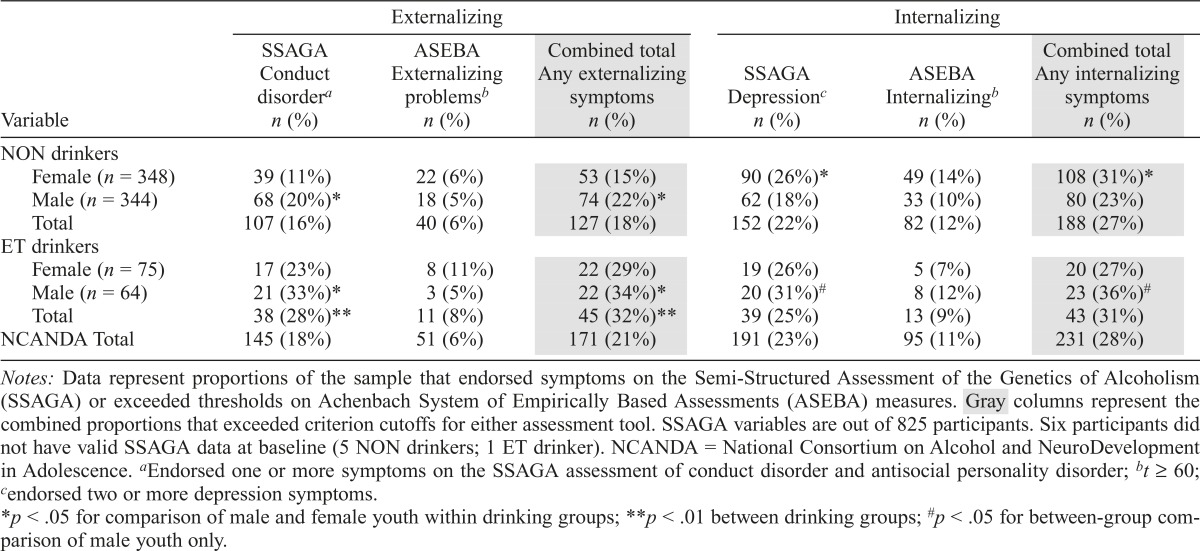
For externalizing symptoms, the ASEBA instruments sample common behaviors (e.g., bragging, arguing, stubbornness) in addition to more severe clinical behaviors (e.g., sets fires), whereas the SSAGA samples more severe clinical behaviors (e.g., hurting animals, using a weapon in a fight). Despite these differences, the reports compared within respondent were correlated (Youth/Teens [youth self-report/adult self-report with SSAGA]: r = .36, p < .001; Parents [Child Behavioral Checklist with SSAGA]: r = .38, p < .001). In total, 21% of the sample endorsed externalizing symptoms on one of the two scales (Table 6; Figure 2), with a higher proportion of male than female, χ2(1) = 4.3, p < .05, and ET than NON, χ2(1) = 12.5, p < .01, youth endorsing these symptoms. A higher proportion of male, χ2(1) = 10.5, p < .01, and ET, χ2(1) = 11.6, p < .01, youth endorsed externalizing symptoms on the SSAGA (Table 6), but the less severe ASEBA externalizing problems did not differ by sex or drinking group.
Table 6.
Internalizing and externalizing disorder symptom endorsement on NCANDA sample for non-/low (NON; n = 692) drinkers and drinkers who exceeded thresholds (ET; n = 139) at baseline
| Variable | Externalizing |
Internalizing |
||||
| SSAGA Conduct disordera n (%) | ASEBA Externalizing problemsb n (%) | Combined total Any externalizing symptoms n (%) | SSAGA Depressionc n (%) | ASEBA Internalizingb n (%) | Combined total Any internalizing symptoms n (%) | |
| NON drinkers | ||||||
| Female (n = 348) | 39 (11%) | 22 (6%) | 53 (15%) | 90 (26%)* | 49 (14%) | 108(31%)* |
| Male (n = 344) | 68 (20%)* | 18(5%) | 74 (22%)* | 62(18%) | 33 (10%) | 80 (23%) |
| Total | 107 (16%) | 40 (6%) | 127 (18%) | 152(22%) | 82 (12%) | 188(27%) |
| ET drinkers | ||||||
| Female (n = 75) | 17 (23%) | 8(11%) | 22 (29%) | 19 (26%) | 5 (7%) | 20 (27%) |
| Male (n = 64) | 21 (33%)* | 3 (5%) | 22 (34%)* | 20(31%)# | 8 (12%) | 23 (36%)# |
| Total | 38 (28%)** | 11 (8%) | 45 (32%)** | 39 (25%) | 13 (9%) | 43 (31%) |
| NCANDA Total | 145 (18%) | 51 (6%) | 171 (21%) | 191 (23%) | 95 (11%) | 231 (28%) |
Notes: Data represent proportions of the sample that endorsed symptoms on the Semi-Structured Assessment of the Genetics of Alcoholism (SSAGA) or exceeded thresholds on Achenbach System of Empirically Based Assessments (ASEBA) measures. Gray columns represent the combined proportions that exceeded criterion cutoffs for either assessment tool. SSAGA variables are out of 825 participants. Six participants did not have valid SSAGA data at baseline (5 NON drinkers; 1 ET drinker). NCANDA = National Consortium on Alcohol and NeuroDevelopment in Adolescence.
Endorsed one or more symptoms on the SSAGA assessment of conduct disorder and antisocial personality disorder;
t ≥ 60;
Endorsed two or more depression symptoms.
p < .05 for comparison of male and female youth within drinking groups;
p < .01 between drinking groups;
p < .05 for between-group comparison of male youth only.
Internalizing symptoms were assessed with the SSAGA (i.e., two or more depression symptoms) and ASEBA instruments (i.e., Internalizing Scale t score ≥ 60), and participants who met the criteria (28% of the total sample; Table 6 and Figure 2) were considered at risk for alcohol use and problems (Hussong et al., 2011). Internalizing disorder symptoms did not differ by sex overall; however, a higher proportion of NON female compared with male youth endorsed symptoms, χ2(1) = 5.3, p < .05 (Table 6). Male ET youth endorsed internalizing symptoms at a higher rate than NON male youth, χ2(1) = 4.6, p <.01 (Table 6).
Depression symptoms were endorsed on the SSAGA by 23% of the sample, with a higher proportion of female than male participants within the NON group, χ2(1) = 6.3, p < .05 (Table 6), and no differences between the sexes in the ET group. As with overall internalizing symptoms, male ET participants endorsed depression symptoms on the SSAGA at a higher rate than their male NON counterparts, χ2(1) = 5.8, p < .05 (Table 6). On the ASEBA Internalizing Scale, 11% of the sample scored in the borderline or clinical range, indicating endorsement of mood or anxiety symptoms above the normal range, which did not differ by sex or drinking group (Table 6).
Discussion
Description of the baseline NCANDA sample serves as the foundation from which the effects of exposure to heavy drinking on adolescent development and functioning can subsequently be measured. The sample includes a large proportion of individuals who endorsed familial factors as well as psychiatric, personality, and behavioral phenotypes associated with increased risk for substance use initiation. The broad range of risk and resilience factors assessed by the full clinical battery will allow for the effects of multiple domains to be examined in concert when evaluating the influence of alcohol on brain development during the adolescent years. For example, the relationship between externalizing/conduct disorder symptoms and risk for alcohol use has been described for many years (Anderson et al., 2010; Slutske et al., 1998), but the emergence of symptoms over the course of adolescence and the interaction of externalizing symptoms with emerging symptoms from other domains have not been fully examined in such a large sample and with the breadth of assessments in the current study (Hussong et al., 2011; Oshri et al., 2011). Based on previous reports associating these risk domains with drinking during adolescence, the NCANDA sample is well positioned to capture transitions to heavy alcohol consumption during the 4 years of data collection and examine brain changes in relation to use characteristics. Furthermore, NCANDA used extensive training and quality control procedures to ensure reliable data collection across the consortium and serves as a model for future large-scale, multisite studies examining adolescent development and substance use.
An advantage of the NCANDA study design is the ability to assess coherence and deviations in trajectories of brain, biology, neurocognitive performance, and behavior. The cross-sectional characterization of the baseline sample in the present report reflects the initiation points for the trajectories that will emerge over the course of the study. Tracking behavioral and neuroanatomical trajectories simultaneously over time will help clarify independent and related causal pathways associated with adolescent development and the initiation and exacerbation of heavy drinking (Hanson et al., 2011). For example, comparisons of pubertal development and brain maturation concurrently with social and neurocognitive changes will allow for assessment of differential influences of each factor on the emergence of heavy episodic drinking, extending recent work on normal adolescent development (Forbes & Dahl, 2010; Mills et al., 2014). Characterizing these trajectories will provide insight into the chronological development of comorbidity and continuity and discontinuity in the escalation of alcohol use during adolescence (cf. Beauchaine & McNulty, 2013), as well as the impact of cumulative risks on development and functioning. The baseline description provided here highlights the need to evaluate variations in samples across sites (e.g., racial/ethnic distributions, SES) as possible covariates for future analyses.
Several limitations should be considered. First, the level of commitment from both participants and their parents may have dissuaded some families from enrolling in the study. This may have contributed to the relatively high achieving and middle- to upper-middle-class sample. Second, the ALD approach used in NCANDA was determined to be optimal given the time and funding constraints, and to adequately address the aims of the project; however, a fully longitudinal design would have offered advantages in modeling within- subject variations over time. Third, the current report compares ET and NON drinkers in a cross-sectional approach, which is necessary for presenting the baseline characteristics, but which is not an ideal comparison because we do not have pre-drinking baseline data on the ET group.
NCANDA intends to address the effects of alcohol use on developmental trajectories of brain structure and function. The broad domains of assessment included in the consortium will allow scientists to identify antecedent conditions influencing the development of heavy drinking and to quantify the relative importance of risk factors within specific subsamples. For example, we hypothesize that female adolescents will exhibit greater neurocognitive deviations resulting from accelerating alcohol use (Squeglia et al., 2011, 2012), whereas male adolescents will exhibit a greater association of externalizing disorder symptoms and a propensity for risk taking with increased heavy drinking (Dayan et al., 2010; Galvan et al., 2007; Noël, 2014; Schneider et al., 2012; Steinberg, 2007). Quantifying relative influences of alcohol use antecedents for subpopulations of the NCANDA sample may also contribute to the development of targeted intervention and prevention strategies (e.g., Conrod et al., 2013). Furthermore, NCANDA will evaluate protective factors that decrease the probability of progression to problematic alcohol involvement and factors associated with resilience to deleterious effects of heavy drinking (Bekman et al., 2013; Brumback et al., 2015; Winward et al., 2014). Adolescents exhibit malleability of behavior and neurocognition and brain plasticity that make this period of development one rife with opportunities and challenges (Crone & Dahl, 2012; Dahl, 2004). Understanding the neural and behavioral risk factors for, and consequences of, adolescent substance use will help improve public health information for youth, parents, and policy makers and provide a basis on which targeted prevention and intervention programs can be developed.
Footnotes
This work was supported by the U.S. National Institute on Alcohol Abuse and Alcoholism with co-funding from the National Institute on Drug Abuse, the National Institute of Mental Health, and the National Institute of Child Health and Human Development [NCANDA grant numbers: AA021695 (to Sandra A. Brown and Susan F. Tapert), AA021697 (to Adolf Pfefferbaum and Kilian M. Pohl), AA021692 (to Susan F. Tapert), AA021681 (to Michael D. De Bellis), AA021690 (to Duncan B. Clark), AA021691 (to Bonnie J. Nagel), AA021696 (to Ian M. Colrain and Fiona C. Baker)], K24 DA028773 (to Michael D. De Bellis), and T32 AA013525 (to Ty Brumback). The authors thank the NCANDA Scientific Advisory Board—specifically Kenneth Sher, Andrea Hussong, Robert Zucker, and Raquel Gur—for their input on the design and methods of the study, all NCANDA study staff, and the youth and parents who participate in this study.
References
- Achenbach T. M., Rescorla L. A. Manual for the ASEBA School– Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- Achenbach T. M., Rescorla L. A. Manual for the ASEBA Adult Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2003. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5 th ed. Arlington, VA: Author; 2013. doi:10.1176/appi.books.9780890425596. [Google Scholar]
- American Psychological Association, Task Force on Socioeconomic Status. Report of theAPA Task Force on Socioeconomic Status. Washington, DC: Author; 2007. [Google Scholar]
- Anderson K. G., Brown S. A. Middle school drinking: Who, where, and when. Journal of Child & Adolescent Substance Abuse. 2011;20:48–62. doi: 10.1080/1067828X.2011.534362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. G., Ramo D. E., Cummins K. M., Brown S. A. Alcohol and drug involvement after adolescent treatment and functioning during emerging adulthood. Drug and Alcohol Dependence. 2010;107:171–181. doi: 10.1016/j.drugalcdep.2009.10.005. doi:10.1016/j.drugalcdep.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachman J. G., Johnston L. D., O’Malley P. M. Monitoring the Future: Questionnaire responses from the nation’s high school seniors, 2010. Ann Arbor, MI: Institute for Social Research; 2011. [Google Scholar]
- Beauchaine T. P., McNulty T. Comorbidities and continuities as ontogenic processes: Toward a developmental spectrum model of externalizing psychopathology. Development and Psychopathology, 25, 25th Anniversary Special Issue 4, pt2. 2013:1505–1528. doi: 10.1017/S0954579413000746. doi:10.1017/S0954579413000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekman N. M., Winward J. L., Lau L. L., Wagner C. C., Brown S. A. The impact of adolescent binge drinking and sustained abstinence on affective state. Alcoholism: Clinical and Experimental Research. 2013;37:1432–1439. doi: 10.1111/acer.12096. doi:10.1111/acer.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut L. J., Saccone N. L., Rice J. P., Goate A., Foroud T., Edenberg H., Reich T. Defining alcohol-related phenotypes in humans. The Collaborative Study on the Genetics of Alcoholism. Alcohol Research & Health. 2002;26:208–213. [PMC free article] [PubMed] [Google Scholar]
- Brown S. A., Christiansen B. A., Goldman M. S. The Alcohol Expectancy Questionnaire: An instrument for the assessment of adolescent and adult alcohol expectancies. Journal of Studies on Alcohol. 1987;48:483–491. doi: 10.15288/jsa.1987.48.483. doi:10.15288/jsa.1987.48.483. [DOI] [PubMed] [Google Scholar]
- Brown S. A., Gleghorn A., Schuckit M. A., Myers M. G., Mott M. A. Conduct disorder among adolescent alcohol and drug abusers. Journal of Studies on Alcohol. 1996;57:314–324. doi: 10.15288/jsa.1996.57.314. doi:10.15288/jsa.1996.57.314. [DOI] [PubMed] [Google Scholar]
- Brown S. A., McGue M., Maggs J., Schulenberg J., Hingson R., Swartzwelder S., Murphy S. A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics. 2008;121(Supplement 4):S290–S310. doi: 10.1542/peds.2007-2243D. doi:10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. A., Myers M. G., Lippke L., Tapert S. F., Stewart D. G., Vik P. W. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. doi:10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown S. A., Tapert S. F., Granholm E., Delis D. C. Neurocognitive functioning of adolescents: Effects of protracted alcohol use. Alcoholism: Clinical and Experimental Research. 2000;24:164–171. doi:10.1111/j.1530-0277.2000.tb04586.x. [PubMed] [Google Scholar]
- Brumback T., Squeglia L. M., Jacobus J., Pulido C., Tapert S. F., Brown S. A. Adolescent heavy drinkers’ amplified brain responses to alcohol cues decrease over one month of abstinence. Addictive Behaviors. 2015;46:45–52. doi: 10.1016/j.addbeh.2015.03.001. doi:10.1016/j.addbeh.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz K. K., Cadoret R., Cloninger C. R., Dinwiddie S. H., Hesselbrock V. M., Nurnberger J. I., Jr., Schuckit M. A. A new, semi-structured psychiatric interview for use in genetic linkage studies: A report on the reliability of the SSAGA. Journal ofStudies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. doi:10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Buysse D., Reynolds C., Monk T., Berman S., Kupfer D. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- California Department of Education. California Healthy Kids Survey. San Francisco, CA: WestEd (Health and Human Development Department: Safe and Health Kids Program Office); 2012. Retrieved from http://chks.wested.org. [Google Scholar]
- Carlo G., Randall B. A. The development of a measure of prosocial behaviors for late adolescents. Journal of Youth and Adolescence. 2002;31:31–44. doi:10.1023/A:1014033032440. [Google Scholar]
- Casey B. J., Jones R. M. Neurobiology of the adolescent brain and behavior: Implications for substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:1189–1201. doi: 10.1016/j.jaac.2010.08.017. , quiz 1285. doi:10.1016/j.jaac.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Data table of BMI-for-age charts. 2001 Retrieved from http://www.cdc.gov/growthcharts/html_charts/bmiagerev.htm.
- Chassin L., Pitts S. C., Prost J. Binge drinking trajectories from adolescence to emerging adulthood in a high-risk sample: Predictors and substance abuse outcomes. Journal of Consulting and Clinical Psychology. 2002;70:67–78. doi:10.1037/0022-006X.70.1.67. [PubMed] [Google Scholar]
- Chein J., Albert D., O’Brien L., Uckert K., Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science. 2011;14:F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. doi:10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen B. A., Goldman M. S., Brown S. A. Alcohol Expectancy Questionnaire Adolescent Form. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 1996. [Google Scholar]
- Connolly J., Craig W., Goldberg A., Pepler D. Mixed gender groups, dating, and romantic relationships in early adolescence. Journal of Research on Adolescence. 2004;14:185–207. doi:10.1111/j.1532-7795.2004.01402003.x. [Google Scholar]
- Connor-Smith J. K., Compas B. E., Wadsworth M. E., Thomsen A. H., Saltzman H. Responses to stress in adolescence: Measurement of coping and involuntary stress responses. Journal of Consulting and Clinical Psychology. 2000;68:976–992. doi:10.1037/0022-006X.68.6.976. [PubMed] [Google Scholar]
- Conrod P. J., O’Leary-Barrett M., Newton N., Topper L., Castellanos–Ryan N., Mackie C., Girard A. Effectiveness of a selective, personality-targeted prevention program for adolescent alcohol use and misuse: A cluster randomized controlled trial. JAMA Psychiatry. 2013;70:334–342. doi: 10.1001/jamapsychiatry.2013.651. doi:10.1001/jamapsychiatry.2013.651. [DOI] [PubMed] [Google Scholar]
- Corrigan J. D., Bogner J. Initial reliability and validity of the Ohio State University TBI Identification Method. Journal of Head Trauma Rehabilitation. 2007;22:318–329. doi: 10.1097/01.HTR.0000300227.67748.77. doi:10.1097/01.HTR.0000300227.67748.77. [DOI] [PubMed] [Google Scholar]
- Crone E. A., Dahl R. E. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13:636–650. doi: 10.1038/nrn3313. doi:10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Cservenka A., Fair D. A., Nagel B. J. Emotional processing and brain activity in youth at high risk for alcoholism. Alcoholism: Clinical and Experimental Research. 2014;38:1912–1923. doi: 10.1111/acer.12435. doi:10.1111/acer.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders M. A., Smith G. T., Spillane N. S., Fischer S., Annus A. M., Peterson C. Integration of impulsivity and positive mood to predict risky behavior: Development and validation of a measure of positive urgency. Psychological Assessment. 2007;19:107–118. doi: 10.1037/1040-3590.19.1.107. doi:10.1037/1040-3590.19.1.107. [DOI] [PubMed] [Google Scholar]
- Dahl R. E. Adolescent brain development: A period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. doi:10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Dayan J., Bernard A., Olliac B., Mailhes A.-S., Kermarrec S. Journal of Physiology. Vol. 104. Paris: 2010. Adolescent brain development, risk-taking and vulnerability to addiction; pp. 279–286. doi:10.1016/j.jphysparis.2010.08.007. [DOI] [PubMed] [Google Scholar]
- De Bellis M. D., Narasimhan A., Thatcher D. L., Keshavan M. S., Soloff P., Clark D. B. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcoholism: Clinical and Experimental Research. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. doi:10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- De Bellis M. D., Van Voorhees E., Hooper S. R., Gibler N., Nelson L., Hege S. G., MacFall J. Diffusion tensor measures of the corpus callosum in adolescents with adolescent onset alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2008;32:395–404. doi: 10.1111/j.1530-0277.2007.00603.x. doi:10.1111/j.1530-0277.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S. C., Duncan T. E., Hops H. Analysis of longitudinal data within accelerated longitudinal designs. Psychological Methods. 1996;1:236–248. doi:10.1037/1082-989X.1.3.236. [Google Scholar]
- Duncan S. C., Duncan T. E., Strycker L. A. Alcohol use from ages 9 to 16: A cohort-sequential latent growth model. Drug and Alcohol Dependence. 2006;81:71–81. doi: 10.1016/j.drugalcdep.2005.06.001. doi:10.1016/j.drugalcdep.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg H. J., Foroud T., Koller D. L., Goate A., Rice J, Van Eerdewegh P., Begleiter H. A family-based analysis of the association of the dopamine D2 receptor (DRD2) with alcoholism. Alcoholism: Clinical and Experimental Research. 1998;22:505–512. doi:10.1111/j.1530-0277.1998.tb03680.x. [PubMed] [Google Scholar]
- Fergusson D. M., Lynskey M. T. Alcohol misuse and adolescent sexual behaviors and risk taking. Pediatrics. 1996;98:91–96. [PubMed] [Google Scholar]
- Fleming C. B., White H. R., Catalano R. F. Romantic relationships and substance use in early adulthood: An examination of the influences of relationship type, partner substance use, and relationship quality. Journal of Health and Social Behavior. 2010;51:153–167. doi: 10.1177/0022146510368930. doi:10.1177/0022146510368930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E. E., Dahl R. E. Pubertal development and behavior: Hormonal activation of social and motivational tendencies. Brain and Cognition. 2010;72:66–72. doi: 10.1016/j.bandc.2009.10.007. doi:10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman W., Wehner E. A. Dating History Questionnaire. Unpublished measure. University of Denver; Denver, CO: 1992. [Google Scholar]
- Galvan A., Hare T., Voss H., Glover G., Casey B. J. Risk-taking and the adolescent brain: Who is at risk? Developmental Science. 2007;10:F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. doi:10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Gioia G. A., Isquith P. K., Retzlaff R. D., Espy K. A. Confirmatory factor analysis of the Behavior Rating Inventory of Executive Function (BRIEF) in a clinical sample. Child Neuropsychology. 2002;8:249–257. doi: 10.1076/chin.8.4.249.13513. doi:10.1076/chin.8.4.249.13513. [DOI] [PubMed] [Google Scholar]
- Gosling S. D., Rentfrow P. J., Swann W. B., Jr. A very brief measure of the Big-Five personality domains. Journal of Research in Personality. 2003;37:504–528. doi:10.1016/S0092-6566(03)00046-1. [Google Scholar]
- Grant B. F., Dawson D. A. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of Substance Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. doi:10.1016/S0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Hanson K. L., Cummins K., Tapert S. F., Brown S. A. Changes in neuropsychological functioning over 10 years following adolescent substance abuse treatment. Psychology of Addictive Behaviors. 2011;25:127–142. doi: 10.1037/a0022350. doi:10.1037/a0022350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie T., Tibshirani R. Generalized additive models. Statistical Science. 1986;1:297–310. doi: 10.1177/096228029500400302. doi:10.1214/ss/1177013604. [DOI] [PubMed] [Google Scholar]
- Hesselbrock M., Easton C., Bucholz K. K., Schuckit M., Hesselbrock V. A validity study of the SSAGA—A comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. doi:10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. B. Unpublished manuscript. Yale University; New Haven, CT: 1975. Four factor index of social position. [Google Scholar]
- Humes K. R., Jones N. A., Ramirez R. R. Overview of race and Hispanic origin: 2010. (Report Number: C2010BR-02.) Washington, D.C: U.S. Census Bureau; 2011. Retrieved from: http://www.census.gov/library/publications/2011/dec/c2010br-02.html. [Google Scholar]
- Hussong A. M., Jones D. J., Stein G. L., Baucom D. H., Boeding S. An internalizing pathway to alcohol use and disorder. Psychology of Addictive Behaviors. 2011;25:390–404. doi: 10.1037/a0024519. doi:10.1037/a0024519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J., Tapert S. F. Neurotoxic effects of alcohol in adolescence. Annual Review of Clinical Psychology. 2013;9:703–721. doi: 10.1146/annurev-clinpsy-050212-185610. doi:10.1146/annurev-clinpsy-050212-185610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L. D., O’Malley P. M., Miech R. A., Bachman J. G., Schulenberg J. E. Monitoring the Future national survey results on drug use: 2013 Overview, Key Findings on Adolescent Drug Use. Ann Arbor, MI: Institute for Social Research, The University of Michigan; 2014. [Google Scholar]
- Komro K. A., Maldonado-Molina M. M., Tobler A. L., Bonds J. R., Muller K. E. Effects of home access and availability of alcohol on young adolescents’ alcohol use. Addiction. 2007;102:1597–1608. doi: 10.1111/j.1360-0443.2007.01941.x. doi:10.1111/j.1360-0443.2007.01941.x. [DOI] [PubMed] [Google Scholar]
- Lynam D. R., Smith G. T., Cyders M. A., Fischer S., Whiteside S. P. The UPPS-P: Assessing five personality pathways to impulsive behavior. 2007 Unpublished Technical Report. [Google Scholar]
- Marcotte T. D., Bekman N. M., Meyer R. A., Brown S. A. High-risk driving behaviors among adolescent binge drinkers. American Journal of Drug and Alcohol Abuse. 2012;38:322–327. doi: 10.3109/00952990.2011.643981. doi:10.3109/00952990.2011.643981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten A. S., Faden V. B., Zucker R. A., Spear L. R. Underage drinking: A developmental framework. Pediatrics. 2008;121(Supplement 4):S235–S251. doi: 10.1542/peds.2007-2243A. doi:10.1542/peds.2007-2243A. [DOI] [PubMed] [Google Scholar]
- Masten A. S., Neemann J., Andenas S. Life events and adjustment in adolescents: The significance of event independence, desirability, and chronicity. Journal of Research on Adolescence. 1994;4:71–97. doi:10.1207/s15327795jra0401_5. [Google Scholar]
- McQueeny T., Schweinsburg B. C., Schweinsburg A. D., Jacobus J., Bava S., Frank L. R., Tapert S. F. Altered white matter integrity in adolescent binge drinkers. Alcoholism: Clinical and Experimental Research. 2009;33:1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. doi:10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S., Lansford J. E., Costanzo P., Malone P. S., Golonka M., Killeya-Jones L. A. Early adolescent romantic partner status, peer standing, and problem behaviors. Journal of Early Adolescence. 2009;29:839–861. doi: 10.1177/0272431609332665. doi:10.1177/0272431609332665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K. L., Lalonde F., Clasen L. S., Giedd J. N., Blakemore S. J. Developmental changes in the structure of the social brain in late childhood and adolescence. Social Cognitive and Affective Neuroscience. 2014;9:123–131. doi: 10.1093/scan/nss113. doi:10.1093/scan/nss113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y., Raudenbush S. W. Tests for linkage of multiple cohorts in an accelerated longitudinal design. Psychological Methods. 2000;5:44–63. doi: 10.1037/1082-989x.5.1.44. doi:10.1037/1082-989X.5.1.44. [DOI] [PubMed] [Google Scholar]
- Myers M. G., Brown S. A., Mott M. A. Preadolescent conduct disorder behaviors predict relapse and progression of addiction for adolescent alcohol and drug abusers. Alcoholism: Clinical and Experimental Research. 1995;19:1528–1536. doi: 10.1111/j.1530-0277.1995.tb01019.x. doi:10.1111/j.1530-0277.1995.tb01019.x. [DOI] [PubMed] [Google Scholar]
- Nagel B. J., Schweinsburg A. D., Phan V, Tapert S. F. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Research: Neuroimaging. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. doi:10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. NIAAA Council approves definition of binge drinking. NIAAA Newsletter (Vol. 3) Bethesda, MD: Author; 2004. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Alcohol screening and brief intervention for youth: A practitioner’s guide (NIH Publication No. 11-7805.) Bethesda, MD: Author; 2011. Retrieved from http://www.niaaa.nih.gov/YouthGuide. [Google Scholar]
- Nestler E. J. Epigenetic mechanisms of drug addiction. Neuropharmacology. 2014;76 Part B:259–268. doi: 10.1016/j.neuropharm.2013.04.004. doi:10.1016/j.neuropharm.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noël X. Why adolescents are at risk of misusing alcohol and gambling. Alcohol and Alcoholism. 2014;49:165–172. doi: 10.1093/alcalc/agt161. doi:10.1093/alcalc/agt161. [DOI] [PubMed] [Google Scholar]
- Norman A. L., Pulido C., Squeglia L. M., Spadoni A. D., Paulus M. P, Tapert S. F. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug and Alcohol Dependence. 2011;119:216–223. doi: 10.1016/j.drugalcdep.2011.06.019. doi:10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshri A., Rogosch F. A., Burnette M. L., Cicchetti D. Developmental pathways to adolescent cannabis abuse and dependence: Child maltreatment, emerging personality, and internalizing versus externalizing psychopathology. Psychology of Addictive Behaviors. 2011;25:634–644. doi: 10.1037/a0023151. doi:10.1037/a0023151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A. C., Crockett L., Richards M., Boxer A. A selfreport measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. doi:10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Petit G., Maurage P, Kornreich C., Verbanck P, Campanella S. Binge drinking in adolescents: A review of neurophysiological and neuroimaging research. Alcohol and Alcoholism. 2014;49:198–206. doi: 10.1093/alcalc/agt172. doi:10.1093/alcalc/agt172. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A., Rohlfing T., Pohl K. M., Lane B., Chu W., Kwon D., Sullivan E. V. Adolescent development of cortical and white matter structure in the NCANDA sample: Role of sex, ethnicity, puberty, and alcohol drinking. Cerebral Cortex (advance access) 2015 doi: 10.1093/cercor/bhv205. doi:10.1093/cercor/bhv205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L. S. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi:10.1177/014662167700100306. [Google Scholar]
- Rice J. P., Reich T., Bucholz K. K., Neuman R. J., Fishman R., Rochberg N., Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Vol. 19. Alcoholism: Clinical and Experimental Research; 1995. pp. 1018–1023. doi:10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Robins R. W., Hendin H. M., Trzesniewski K. H. Measuring global self-esteem: Construct validation of a single-item measure and the Rosenberg Self-Esteem Scale. Personality and Social Psychology Bulletin. 2001;27:151–161. doi:10.1177/0146167201272002. [Google Scholar]
- Rohlfing T., Cummins K., Henthorn T., Chu W., Nichols B. N. N-CANDA data integration: Anatomy of an asynchronous infrastructure for multi-site, multi-instrument longitudinal data capture. Journal of the American Medical Informatics Association. 2014;21:758–762. doi: 10.1136/amiajnl-2013-002367. doi:10.1136/amiajnl-2013-002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarason I. G., Sarason B. R., Shearin E. N., Pierce G. R. A brief measure of social support: Practical and theoretical implications. Journal of Social and Personal Relationships. 1987;4:497–510. doi:10.1177/0265407587044007. [Google Scholar]
- Schneider S., Peters J., Bromberg U., Brassen S., Miedl S. F, Banaschewski T., Büchel C. the IMAGEN Consortium. Risk taking and the adolescent reward system: A potential common link to substance abuse. American Journal of Psychiatry. 2012;169:39–46. doi: 10.1176/appi.ajp.2011.11030489. doi:10.1176/appi.ajp.2011.11030489. [DOI] [PubMed] [Google Scholar]
- Schuckit M. A., Smith T. L. An 8-year follow-up of 450 sons of alcoholic and control subjects. Archives of General Psychiatry. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. doi:10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Schweinsburg A. D., McQueeny T., Nagel B. J., Eyler L. T., Tapert S. F. A preliminary study of functional magnetic resonance imaging response during verbal encoding among adolescent binge drinkers. Alcohol. 2010;44:111–117. doi: 10.1016/j.alcohol.2009.09.032. doi:10.1016/j.alcohol.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri M. Platform presentations: NBTS 03: Neurobiological and neuropsychological consequences of substance abuse in adolescents. Neurotoxicology and Teratology. 2014;43:76. doi:10.1016/j.ntt.2014.04.006. [Google Scholar]
- Slutske W. S., Heath A. C., Dinwiddie S. H., Madden P, A. F, Bucholz K. K., Dunne M. P. Martin N. G. Common genetic risk factors for conduct disorder and alcohol dependence. Journal of Abnormal Psychology. 1998;107:363–374. doi: 10.1037//0021-843x.107.3.363. doi:10.1037/0021-843X.107.3.363. [DOI] [PubMed] [Google Scholar]
- Slutske W. S., Piasecki T. M., Hunt-Carter E. E. Development and initial validation of the Hangover Symptoms Scale: Prevalence and correlates of hangover symptoms in college students. Alcoholism: Clinical and Experimental Research. 2003;27:1442–1450. doi: 10.1097/01.ALC.0000085585.81711.AE. doi:10.1097/01.ALC.0000085585.81711.AE. [DOI] [PubMed] [Google Scholar]
- Smith C. S., Reilly C., Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. Journal of Applied Psychology. 1989;74:728–738. doi: 10.1037/0021-9010.74.5.728. doi:10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- Spear L. P. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. doi:10.1016/S0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Squeglia L. M., Pulido C., Wetherill R. R., Jacobus J., Brown G. G., Tapert S. F. Brain response to working memory over three years of adolescence: Influence of initiating heavy drinking. Journal of Studies on Alcohol and Drugs. 2012;73:749–760. doi: 10.15288/jsad.2012.73.749. doi:10.15288/jsad.2012.73.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia L. M., Schweinsburg A. D., Pulido C., Tapert S. F. Adolescent binge drinking linked to abnormal spatial working memory brain activation: Differential gender effects. Alcoholism: Clinical and Experimental Research. 2011;35:1831–1841. doi: 10.1111/j.1530-0277.2011.01527.x. doi:10.1111/j.1530-0277.2011.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stattin H., Kerr M. Parental monitoring: A reinterpretation. Child Development. 2000;71:1072–1085. doi: 10.1111/1467-8624.00210. doi:10.1111/1467-8624.00210. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Sciences. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. doi:10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: New perspectives from brain and behavioral science. Current Directions in Psychological Science. 2007;16:55–59. doi:10.1111/j.1467-8721.2007.00475.x. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings (Vol. NSDUH Series H-44) Rockville, MD: Author; 2012. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings (Vol. NSDUH Series H-48) Rockville, MD: Author; 2014. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Behavioral Health Barometer, United States, 2014. Rockville, MD: Author; 2015. [PubMed] [Google Scholar]
- Tapert S. F., Pulido C., Paulus M. P., Schuckit M. A., Burke C. Level of response to alcohol and brain response during visual working memory. Journal of Studies on Alcohol. 2004;65:692–700. doi: 10.15288/jsa.2004.65.692. doi:10.15288/jsa.2004.65.692. [DOI] [PubMed] [Google Scholar]
- Tobler A. L., Komro K. A., Maldonado-Molina M. M. Relationship between neighborhood context, family management practices and alcohol use among urban, multi-ethnic, young adolescents. Prevention Science. 2009;10:313–324. doi: 10.1007/s11121-009-0133-1. doi:10.1007/s11121-009-0133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urošević S., Collins P, Muetzel R., Schissel A., Lim K. O., Luciana M. Effects of reward sensitivity and regional brain volumes on substance use initiation in adolescence. Social Cognitive and Affective Neuroscience. 2015;10:106–113. doi: 10.1093/scan/nsu022. doi:10.1093/scan/nsu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler H., Dowdall G. W., Davenport A., Rimm E. B. A gender-specific measure of binge drinking among college students. American Journal of Public Health. 1995;85:982–985. doi: 10.2105/ajph.85.7.982. doi:10.2105/AJPH.85.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R., Watts R., Orr C. A., Althoff R. R., Artiges E., Banaschewski T., Garavan H. the IMAGEN Consortium. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512:185–189. doi: 10.1038/nature13402. doi:10.1038/nature13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle M., Spear L. P, Fuligni A. J., Angold A., Brown J. D., Pine D., Dahl R. E. Transitions into underage and problem drinking: Developmental processes and mechanisms between 10 and 15years of age. Pediatrics, 121, Supplement 4. 2008:S273–S289. doi: 10.1542/peds.2007-2243C. doi:10.1542/peds.2007-2243C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winward J. L., Bekman N. M., Hanson K. L., Lejuez C. W, Brown S. A. Changes in emotional reactivity and distress tolerance among heavy drinking adolescents during sustained abstinence. Alcoholism: Clinical and Experimental Research. 2014;38:1761–1769. doi: 10.1111/acer.12415. doi:10.1111/acer.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt E. D. Research on alcohol and adolescent brain development: Opportunities and future directions. Alcohol. 2010;44:119–124. doi: 10.1016/j.alcohol.2009.08.011. doi:10.1016/j.alcohol.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Wolfson A. R., Carskadon M. A. Sleep schedules and daytime functioning in adolescents. Child Development. 1998;69:875–887. [PubMed] [Google Scholar]
- Wood S. N. Low-rank scale-invariant tensor product smooths for generalized additive mixed models. Biometrics. 2006;62:1025–1036. doi: 10.1111/j.1541-0420.2006.00574.x. doi:10.1111/j.1541-0420.2006.00574.x. [DOI] [PubMed] [Google Scholar]
- Wood S. N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society: Series B. Statistical Methodology. 2011;73:3–36. doi:10.1111/j.1467-9868.2010.00749.x. [Google Scholar]
- Xiao L., Bechara A., Gong Q., Huang X., Li X., Xue G., Johnson C. A. Abnormal affective decision making revealed in adolescent binge drinkers using a functional magnetic resonance imaging study. Psychology of Addictive Behaviors. 2013;27:443–454. doi: 10.1037/a0027892. doi:10.1037/a0027892. [DOI] [PubMed] [Google Scholar]
- Zucker R. A., Donovan J. E., Masten A. S., Mattson M. E., Moss H. Early developmental processes and the continuity of risk for underage drinking and problem drinking. Pediatrics, 121, Supplement 4. 2008:S252–S272. doi: 10.1542/peds.2007-2243B. doi:10.1542/peds.2007-2243B. [DOI] [PMC free article] [PubMed] [Google Scholar]