Abstract
Objective:
The purpose of this study was to clarify inconsistent findings regarding the acute cognitive effects of subintoxicating alcohol doses (i.e., <80 mg/dl) by controlling for and evaluating variables that might modulate dose-related outcomes.
Method:
The current study examined the effects of sex/gender and alcohol concentration on select cognitive functions in 94 individuals (49 men) between 25 and 35 years of age. Participants were randomly assigned to one of three dose conditions: target peak breath alcohol concentration of 0 mg/dl (placebo), 40 mg/dl (low), or 65 mg/dl (moderate). After beverage consumption, they completed tasks assessing psychomotor, set-shifting, and working memory ability.
Results:
Analyses revealed no significant effect of dose for any cognitive domain. A trend-level effect of dose on psychomotor performance was observed, with the low-dose group performing somewhat better than the moderate-dose and placebo groups. No sex main effects or interactions were revealed.
Conclusions:
Consistent with our previous studies, these data suggest that low and moderate doses of alcohol may not compromise cognitive ability in non–problem drinkers under certain task conditions. Given the outcomes, sex differences cannot be meaningfully addressed. Future consideration of potentially influential variables and assessment of similarly well-defined cohorts might yield a clearer interpretation of alcohol’s behavioral consequences.
The current understanding of alcohol’s acute effects is largely based on breath alcohol concentrations (BrACs) associated with legal intoxication in the United States (i.e., ≥80 mg/dl). The behavioral effects of lower concentrations are less frequently studied. This gap in the literature is important to address, because lower levels are commonly achieved by non–problem drinkers in social-drinking settings.
Among acute alcohol investigations of subintoxicating doses (i.e., low and moderate), findings are largely inconsistent. Using a variety of cognitive tasks, low- and moderate-dose alcohol, relative to placebo, has produced deficient (Bisby et al., 2009; Field et al., 2010; Friedman et al., 2011), facilitated (Gilbertson et al., 2009; Sklar et al., 2012), and similar (Dry et al., 2012; Guillot et al., 2010; Milani & Curran, 2000) performance. Contradictory findings are also reported for identical tasks and similar alcohol concentrations (e.g., Dry et al., 2012, vs. Gilbertson et al., 2009).
Inconsistencies could be attributable to a number of factors, including age (Sklar et al., 2012), drinking patterns (Weissenborn & Duka, 2003), and targeted BrACs (Dry et al., 2012). Another potential but understudied influence is sex/gender. Although evidence suggests that BrACs of 80 mg/dl or more might differentially affect men and women (Fillmore & Weafer, 2004; Miller et al., 2009), few investigations have considered sex when evaluating lower doses (for a review, see Nixon et al., 2014).
The current study focused on two of these factors, sex and dose, in a well-defined sample of moderate drinkers (meeting U.S. Department of Agriculture/U.S. Department of Health and Human Services, 2010, guidelines) within a restricted age range. Target BrACs were 0 mg/dl (placebo), 40 mg/dl (low dose), and 65 mg/dl (moderate dose), which have produced differential effects in other cohorts (Gilbertson et al., 2009; Hoffman et al., 2015). Psychomotor, set-shifting, and working memory functions were investigated. These cognitive domains have previously demonstrated sensitivity to BrACs as low as 20 mg/dl (for a review, see Moskowitz & Fiorentino, 2000).
Method
Participants
Participants (N = 94; 49 men), ages 25–35 years (M = 27.65, SD = 2.66), were recruited from communities in North Central Florida. As part of a larger investigation examining age and alcohol interactions, a portion of the participants were included in previous reports (Boissoneault et al., 2014, n = 51; Sklar et al., 2014, n = 36). Participants were primarily White (72.3%) with 13–18 years of education (M = 16.70, SD = 1.18). They provided written informed consent before participation and were compensated. All procedures were approved by the University of Florida Medical Institutional Review Board.
Participants were excluded if reporting medical conditions/use of medications that contraindicated alcohol consumption and/or if they met criteria for significant psychiatric disorders according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (American Psychiatric Association, 1994), including substance abuse and dependence (Robins et al., 2000). Participants completed paper-and-pencil assessments regarding demographics, affective state, substance use, and alcohol consumption (i.e., quantity–frequency index [QFI]: average oz. of absolute ethanol consumed daily over the past 6 months; modified from Cahalan et al., 1969).
Laboratory protocol
Participants were informed of the study objectives and the chance of receiving an alcoholic (2/3) or placebo (1/3) beverage. Participants abstained from alcohol and sleep aids for 24 hours, avoided the use of sedating allergy medications on testing day, and fasted for 4 hours before their session. A negative breath alcohol test and urine toxicology screen was required for participation. One hour before beverage administration, participants consumed a 220 kcal snack.
Alcohol administration
Within sex, participants were randomly assigned to one of three dose conditions: target peak BrAC of 0 mg/dl (placebo), 40 mg/dl (low), or 65 mg/dl (moderate). Beverages were mixed according to previously published procedures (Fillmore et al., 2008; Sklar & Nixon, 2014), administered in a double-blind fashion, and consumed within 5 minutes. For active-dose groups, a volume of medical-grade ethanol was added to a vehicle solution using a modified Widmark calculation (Watson et al., 1981). The placebo group received vehicle-only beverages of equal volume.
BrACs were obtained 10, 25, and 60 minutes after beverage consumption (Intoxylizer 400PA; CMI, Inc., Owensboro, KY). To sustain target peak BrACs, participants with 25-minute BrACs below 50% of their target (six participants: 5/6 low-dose group) received an active “booster” beverage (i.e., vehicle + half of participants’ original alcohol dose). To maintain double-blind procedures, all other participants received a vehicle-only booster. This booster method demonstrates maintenance of group-appropriate BrACs over time (Gilbertson et al., 2009; Sklar et al., 2012). Task performance did not significantly differ between dose/sex-matched active and placebo booster recipients (ps > .05; low-dose group: Cohen’s d for all tasks < .10).
All beverages were misted with ethanol to promote alcohol expectancy. To assess placebo effectiveness, participants indicated their perceived dose assignment (active or placebo) after testing.
Cognitive testing
The Trail Making Test Part A (TMT-A) and Part B (TMT-B; Reitan & Wolfson, 1993) were used to investigate psychomotor and set-shifting abilities, respectively. Working memory was assessed via a visual remember/ignore task (working memory task [WMT]; Gazzaley et al., 2005b). Our laboratory has shown that these tasks are sensitive to low- and moderate-dose alcohol’s effects in other samples (e.g., Gilbertson et al., 2009; Hoffman et al., 2015).
The TMT-A and TMT-B were administered 15 minutes after initial beverage consumption. The TMT-A required the connection of numerically ordered dots; TMT-B required alternation between numbers and letters. If errors occurred, participants were corrected and proceeded from their last correct move. Accuracy and speed were equally emphasized, and the dependent variable was time to completion.
Participants performed the WMT 35 minutes after beverage consumption. The WMT consisted of two remember/ignore instructional blocks (“face” and “scene” condition) and 20 trials per block. In each trial, a set of two faces and two scenes was presented sequentially. Participants were instructed to remember faces/ignore scenes for the face condition and to remember scenes/ignore faces for the scene condition. After each set, a fixation cross was presented (9 seconds), followed by a condition-relevant probe (face or scene). Thereafter, participants indicated if the probe was absent or present in the preceding set (see Hoffman et al., 2015, for detailed WMT parameters). Participants were informed that accuracy and speed were equally important.
The WMT was applied to investigate age and alcohol interactions in our larger study. It is a laboratory assessment that demonstrates sensitivity for measuring working memory processes (Gazzaley et al., 2005a). Our previous work revealed dose and sex effects with this task for a different sample of moderate drinkers (Hoffman et al., 2015). The dependent variable was efficiency (calculated as % accurate / mean reaction time for accurate trials). This process-oriented measure is demonstrated to be relatively stable for a given participant and task (Thorne, 2006) and has been used effectively in our previous investigations (e.g., Hoffman et al., 2015; Sklar etal., 2012).
Statistical analysis
Descriptive and dependent variables were subject to Pearson correlations to identify potentially confounding relationships. Demographic, affective, and substance use data were investigated with two (sex) × three (dose) analyses of variance (ANOVAs) to ensure adequate randomization.
Mixed-effects ANOVAs were used to test for BrAC differences between sexes and active-dose groups across time and to assess sex and dose effects on WMT efficiency across task condition. TMT-A and TMT-B outcomes were analyzed with separate two (sex) × three (dose) ANOVAs. Bonferroni correction was applied for post hoc comparisons. To eliminate the influence of outliers, participants who performed 2.5 deviations from the mean on the TMT-A (four participants; one placebo, one low-dose, and two moderate-dose groups) and TMT-B (one participant; moderate-dose group) were excluded from individual task analyses. In addition, one participant (low-dose group) exhibited difficulty understanding WMT instructions and was excluded from WMT analyses. Remaining participants performed at levels above chance (>50% accuracy), indicating an understanding of task demands. One participant (accounted for, above) was excluded from more than one analysis (TMT-A and TMT-B). This participant completed the TMTs exceptionally quickly and admitted to having prior experience with them. No other overlap was observed for participant exclusion. Effect sizes were calculated for cognitive performance measures to better characterize results. SAS Version 9.3 (SAS Institute Inc., Cary, NC) was used for all statistical operations.
Results
Descriptive variables
Overall, participants indicated minimal negative affect (Beck Depression Inventory-II; Beck, 1996; M = 2.55, SD = 2.85), minimal state anxiety (State Anxiety Inventory; Spielberger, 1983; M = 40.68, SD = 5.33), and moderate alcohol consumption levels (QFI [0.6 = 1 standard drink/day]; M = 0.35, SD = 0.25). Demographic, affective, and alcohol use variables did not significantly differ between dose groups (ps > .20). Although sex differences were observed for QFI, t(92) = 3.03, p = .003, and education, t(92) = 2.78, p = .007, they had no statistically significant relationship with the dependent measures of interest (ps > .17). Daily alcohol consumption was higher for men (M = 0.42, SD = 0.28) than for women (M = 0.27, SD = 0.19). Results reflect a difference of less than half a drink per day. Furthermore, women had approximately 1 more year of education than men (M = 17.04, SD = 0.98, vs. M = 16.39, SD = 1.27, respectively). Given their lack of influence on experimental outcomes, these differences were not pursued further.
Breath alcohol concentration and placebo effectiveness
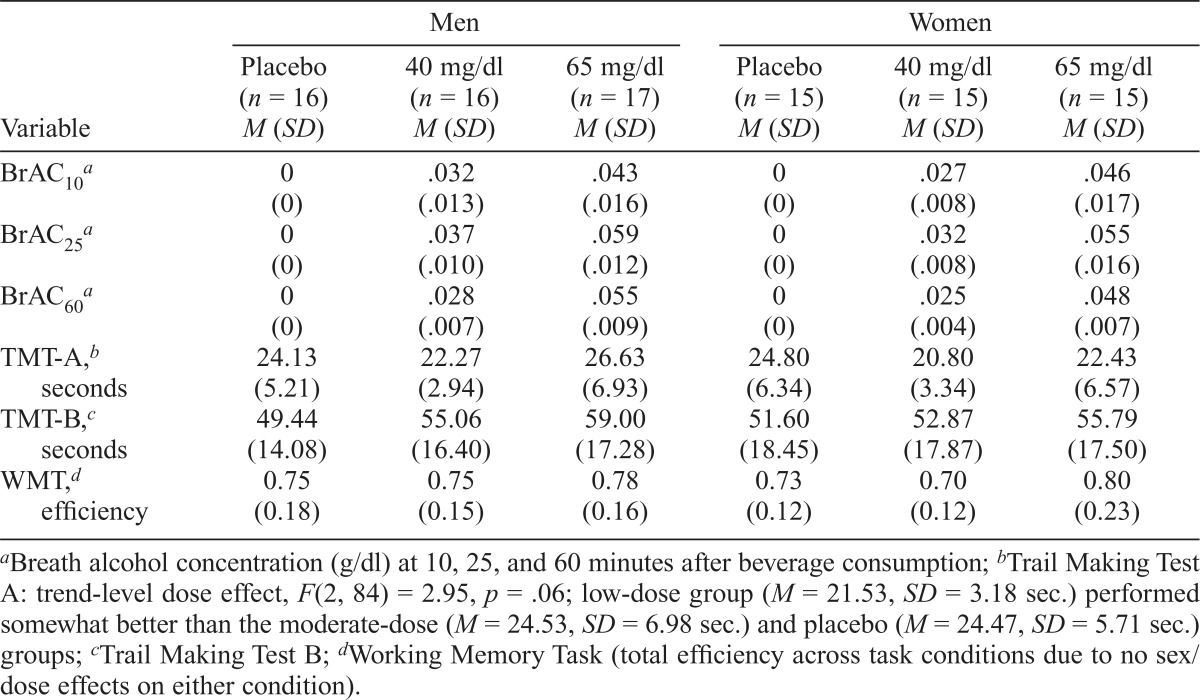
As anticipated, BrACs significantly differed between the low- and moderate-dose groups, F(1, 59) = 96.89, p < .0001. No main effect of sex (p > .08), Sex × Dose interaction (p > .66), or Sex × Dose × Time interaction (p > .19) was observed. Average BrACs are presented in Table 1 by sex, dose, and time.
Table 1.
Breath alcohol concentrations (BrAC) and task performance
| Men |
Women |
|||||
| Variable | Placebo (n = 16) M (SD) | 40 mg/dl (n = 16) M (SD) | 65 mg/dl (n = 17) M (SD) | Placebo (n = 15) M (SD) | 40 mg/dl (n = 15) M (SD) | 65 mg/dl (n = 15) M (SD) |
| BrAC10a | 0 (0) | .032 (.013) | .043 (.016) | 0 (0) | .027 (.008) | .046 (.017) |
| BrAC25a | 0 (0) | .037 (.010) | .059 (.012) | 0 (0) | .032 (.008) | .055 (.016) |
| BrAC60a | 0 (0) | .028 (.007) | .055 (.009) | 0 (0) | .025 (.004) | .048 (.007) |
| tmt-a,b seconds | 24.13 (5.21) | 22.27 (2.94) | 26.63 (6.93) | 24.80 (6.34) | 20.80 (3.34) | 22.43 (6.57) |
| tmt-b,c seconds | 49.44 (14.08) | 55.06 (16.40) | 59.00 (17.28) | 51.60 (18.45) | 52.87 (17.87) | 55.79 (17.50) |
| WMT,d efficiency | 0.75 (0.18) | 0.75 (0.15) | 0.78 (0.16) | 0.73 (0.12) | 0.70 (0.12) | 0.80 (0.23) |
Breath alcohol concentration (g/dl) at 10, 25, and 60 minutes after beverage consumption;
Trail Making Test A: trend-level dose effect, F(2, 84) = 2.95, p = .06; low-dose group (M = 21.53, SD = 3.18 sec.) performed somewhat better than the moderate-dose (M = 24.53, SD = 6.98 sec.) and placebo (M = 24.47, SD = 5.71 sec.) groups;
Trail Making Test B;
Working Memory Task (total efficiency across task conditions due to no sex/dose effects on either condition).
Forty-five percent of the placebo group and 97% of the active-dose groups reported that they had received alcohol. Perceived dose assignment did not significantly differ by sex for any dose group (ps > .87). Furthermore, perceived dose assignment among the placebo group did not significantly affect performance on any task (ts < 1.36, ps > .19).
Cognitive testing
TMT-A, TMT-B, and WMT results are presented by sex and dose in Table 1. TMT analyses yielded a trend level effect of dose on TMT-A: F(2, 84) = 2.95, p = .06; partial η2 = .07; Mplacebo = 24.47, SD = 5.71 seconds; Mlow = 21.53, SD = 3.18 seconds; Mmoderate = 24.53, SD = 6.98 seconds. No sex main effect (p > .15, partial η2 = .02) or Sex × Dose interaction (p > .23, partial η2 = .03) was revealed. For the TMT-B, no dose effect (p > .29, partial η2 = .03), sex effect (p > .75, partial η2 = .00), or Sex × Dose interaction (p > .80, partial η2 = .01) was observed.
Similarly, analyses of WMT efficiency did not reveal a dose effect (p > .27, partial η2 = .01), sex effect (p > .63, partial η2 = .01), or Sex × Dose interaction (p > .65, partial η2 = .01). A main effect of condition was observed, F(1, 86) = 23.70, p < .0001, partial η2 = .22. Consistent with other work (Boissoneault et al., 2014; Bollinger et al., 2011), responding was more efficient under the face condition (M = 0.82, SD = 0.21) than the scene condition (M = 0.71, SD = 0.18). No Dose × Condition (partial η2 = .02), Sex × Condition (partial η2 = .01), or Dose × Sex × Condition (partial η2 = .05) interactions were observed (ps > .12).
Discussion
This study assessed select cognitive functions in male and female moderate drinkers after the administration of a placebo or a low or moderate dose of alcohol. Our findings suggest that BrACs consistent with those of a social drinking episode for non–problem drinkers did not compromise psychomotor, set-shifting, or working memory abilities, as measured by three tasks, in this cohort. Null findings for the current study are in accord with previous acute alcohol investigations that assess a variety of cognitive functions (Dougherty et al., 2008; Leitz et al., 2009), including set-shifting (Gilbertson et al., 2009) and working memory (Tzambazis & Stough, 2000). Our results further suggest that low-dose alcohol might aid simple psychomotor ability. Although the dose effect on TMT-A failed to reach statistical significance, partial η2 suggested a moderate effect size. Mean score comparisons revealed somewhat better performance with the low dose, relative to the other doses. Consistent with this finding, low-dose alcohol-induced TMT-A facilitation has been observed by our laboratory for a different sample of similarly aged moderate drinkers (Gilbertson et al., 2009; Cohen’s d = 1.01). For the current study, an interaction between sex and dose was not observed. However, the absence of sex differences should be interpreted with caution, given that alcohol did not significantly affect performance. Sex differences might be observed with other tasks and/or larger samples. Moreover, the investigation of different BrACs, particularly those that approach intoxication, might inform future research and help determine the degree to which sex modulates alcohol’s effects at lower concentrations.
This investigation focused on only two sources of variability. Discrepancies between this study and others, demonstrating differential outcomes, could be attributable to several additional factors. The plethora of influences calls for greater attention to study parameters when making comparisons and interpretations. In reviewing findings across investigations, we must ensure that we are comparing “apples to apples.” Examining reports with similar samples and identical tasks, and evaluating outcomes with reference to BrAC at the time of task administration, rather than targeted peak, might yield a clearer picture. For example, TMT performance in populations similar to the current study’s sample seems to be largely unaffected by subintoxicating BrACs (Dry et al., 2012; Guillot et al., 2010). However, exceptions include TMT-A facilitation observed at sufficiently low BrACs (∼30 mg/dl; Gilbertson et al., 2009) and poorer TMT-B performance reported at concentrations approaching legal intoxication (∼70 mg/dl; Guillot et al., 2010). Given the current study’s BrACs at the time of TMT administration, our results lay within this distribution. Despite an emerging pattern for this particular task, discrepancies remain (e.g., Sklar et al., 2012, vs. Schulte et al., 2001), suggesting a need for systematic investigation of other modulating variables.
Given the prevalence of alcohol consumption in the United States (∼57% for ages ≥21 years; Substance Abuse and Mental Health Services Administration, 2014) and the social relevance of investigating BrACs below the legal limit of intoxication, it is essential to gain a better understanding of low- and moderate-dose alcohol’s effects. Inconsistent findings suggest a need for greater control and/or evaluation of variables that modulate experimental outcomes. Identifying and acknowledging these factors might allow for a clearer interpretation of alcohol’s acute effects. The influence of sex on these effects has yet to be fully determined and merits further investigation. Ultimately, greater characterization of subintoxicating doses will advance our understanding of alcohol-related outcomes.
Study limitations
In an effort to characterize alcohol’s effects in a well-defined cohort, findings are restricted to well-educated young adult moderate drinkers. Therefore, investigation of samples with different ages, education levels, and drinking patterns is needed. In addition, each cognitive domain was assessed with a single task, and investigation was limited to two alcohol doses. Further investigation with additional doses and tasks of varying complexity will help determine the generalizability of these findings. To address the latter concern, our laboratory is currently examining the effects of subintoxicating doses on simulated driving performance.
Acknowledgments
The authors thank the participants for their willingness to partake in the study. Special thanks to Adam Gazzaley, M.D., Ph.D., who generously provided working memory task stimuli and timing parameters. The authors acknowledge Ben Lewis, Ph.D., Jeff Boissoneault, Ph.D., Alfredo Sklar, Ph.D., Layla Lincoln, and Robert Prather, who assisted in the recruiting, screening, and conducting of laboratory sessions.
Footnotes
This study was supported by National Institute on Alcohol Abuse and Alcoholism Grant R01AA019802.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Beck A. T., Steer R. A., Brown G. K. Beck Depression Inventory. 2nd ed. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- Bisby J. A., Brewin C. R., Leitz J. R., Curran H. V. Acute effects of alcohol on the development of intrusive memories. Psychopharmacology, 2009;204:655–666. doi: 10.1007/s00213-009-1496-5. doi:10.1007/s00213-009-1496-5. [DOI] [PubMed] [Google Scholar]
- Boissoneault J., Sklar A., Prather R., Nixon S. J. Acute effects of moderate alcohol on psychomotor, set shifting, and working memory function in older and younger social drinkers. Journal of Studies on Alcohol and Drugs, 2014;75:870–879. doi: 10.15288/jsad.2014.75.870. doi:10.15288/jsad.2014.75.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger J., Rubens M. T., Masangkay E., Kalkstein J., Gazzaley A. An expectation-based memory deficit in aging. Neuropsychologia, 2011;49:1466–1475. doi: 10.1016/j.neuropsychologia.2010.12.021. doi:10.1016/j.neuropsychologia.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan D., Cisin I. H., Crossley H. M. American drinking practices. New Brunswick, NJ: Rutgers Center of Alcohol Studies; 1969. [Google Scholar]
- Dougherty D. M., Marsh-Richard D. M., Hatzis E. S., Nouvion S. O., Mathias C. W. A test of alcohol dose effects on multiple behavioral measures of impulsivity. Drug and Alcohol Dependence, 2008;96:111–120. doi: 10.1016/j.drugalcdep.2008.02.002. doi:10.1016/j.drugalcdep.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dry M. J., Burns N. R., Nettelbeck T., Farquharson A. L., White J. M. Dose-related effects of alcohol on cognitive functioning. PLoS ONE, 2012;7(11):e50977. doi: 10.1371/journal.pone.0050977. doi:10.1371/journal.pone.0050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Wiers R. W., Christiansen P., Fillmore M. T., Verster J. C. Acute alcohol effects on inhibitory control and implicit cognition: Implications for loss of control over drinking. Alcoholism: Clinical and Experimental Research. 2010;34:1346–1352. doi: 10.1111/j.1530-0277.2010.01218.x. doi:10.1111/j.1530-0277.2010.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore M. T., Blackburn J. S., Harrison E. L. Acute disinhibiting effects of alcohol as a factor in risky driving behavior. Drug and Alcohol Dependence. 2008;95:97–106. doi: 10.1016/j.drugalcdep.2007.12.018. doi:10.1016/j.drugalcdep.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore M. T., Weafer J. Alcohol impairment of behavior in men and women. Addiction. 2004;99:1237–1246. doi: 10.1111/j.1360-0443.2004.00805.x. doi:10.1111/j.1360-0443.2004.00805.x. [DOI] [PubMed] [Google Scholar]
- Friedman T. W., Robinson S. R., Yelland G. W. Impaired perceptual judgment at low blood alcohol concentrations. Alcohol. 2011;45:711–718. doi: 10.1016/j.alcohol.2010.10.007. doi:10.1016/j.alcohol.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Gazzaley A., Cooney J. W., McEvoy K., Knight R. T., D’Esposito M. Top-down enhancement and suppression of the magnitude and speed of neural activity. Journal of Cognitive Neuroscience. 2005a;17:507–517. doi: 10.1162/0898929053279522. doi:10.1162/0898929053279522. [DOI] [PubMed] [Google Scholar]
- Gazzaley A., Cooney J. W., Rissman J., D’Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nature Neuroscience. 2005b;8:1298–1300. doi: 10.1038/nn1543. doi:10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Gilbertson R., Ceballos N. A., Prather R., Nixon S. J. Effects of acute alcohol consumption in older and younger adults: Perceived impairment versus psychomotor performance. Journal of Studies on Alcohol and Drugs. 2009;70:242–252. doi: 10.15288/jsad.2009.70.242. doi:10.15288/jsad.2009.70.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot C. R., Fanning J. R., Bullock J. S., McCloskey M. S., Berman M. E. Effects of alcohol on tests of executive functioning in men and women: A dose response examination. Experimental and Clinical Psychopharmacology. 2010;18:409–417. doi: 10.1037/a0021053. doi:10.1037/a0021053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L. A., Sklar A. L., Nixon S. J. The effects of acute alcohol on psychomotor, set-shifting, and working memory performance in older men and women. Alcohol. 2015;49:185–191. doi: 10.1016/j.alcohol.2015.02.001. doi:10.1016/j. alcohol.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitz J. R., Morgan C. J., Bisby J. A., Rendell P. G., Curran H. V. Global impairment of prospective memory following acute alcohol. Psychopharmacology. 2009;205:379–387. doi: 10.1007/s00213-009-1546-z. doi:10.1007/s00213-009-1546-z. [DOI] [PubMed] [Google Scholar]
- Milani R., Curran H. V. Effects of a low dose of alcohol on recollective experience of illusory memory. Psychopharmacology. 2000;147:397–402. doi: 10.1007/s002130050008. doi:10.1007/s002130050008. [DOI] [PubMed] [Google Scholar]
- Miller M. A., Weafer J., Fillmore M. T. Gender differences in alcohol impairment of simulated driving performance and driving-related skills. Alcohol and Alcoholism. 2009;44:586–593. doi: 10.1093/alcalc/agp051. doi:10.1093/alcalc/agp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz H., Fiorentino D. A review of the literature on the effects of low doses of alcohol on driving-related skills (No. HS-809 028,) Washington, DC: National Highway Traffic Safety Administration; 2000. Retrieved from http://www.nhtsa.gov/people/injury/research/pub/Hs809028/Title.htm. [Google Scholar]
- Nixon S. J., Prather R. A., Lewis B. Sex differences in alcohol-related neurobehavioral consequences. In: Pfefferbaum A., Sullivan E. V., editors. Alcohol and the nervous system (handbook of clinical neurology, 3rd series) Oxford, England: Elsevier; 2014. [DOI] [PubMed] [Google Scholar]
- Reitan R. M., Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. 2nd ed. Tucson, AZ: Neuropsychology Press; 1993. [Google Scholar]
- Robins L. N., Cottler L., Bucholz K. K., Compton W., North C. S., Rourke K. M. The Diagnostic Interview Schedule, Version IV. St. Louis, MO: Washington University; 2000. [Google Scholar]
- Schulte T., Muller-Oehring E. M., Strasburger H., Warzel H., Sabel B. A. Acute effects of alcohol on divided and covert attention in men. Psychopharmacology. 2001;154:61–69. doi: 10.1007/s002130000603. doi:10.1007/s002130000603. [DOI] [PubMed] [Google Scholar]
- Sklar A. L., Boissoneault J., Fillmore M. T., Nixon S. J. Interactions between age and moderate alcohol effects on simulated driving performance. Psychopharmacology. 2014;231:557–566. doi: 10.1007/s00213-013-3269-4. doi:10.1007/s00213-013-3269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar A. L., Gilbertson R., Boissoneault J., Prather R., Nixon S. J. Differential effects of moderate alcohol consumption on performance among older and younger adults. Alcoholism: Clinical and Experimental Research. 2012;36:2150–2156. doi: 10.1111/j.1530-0277.2012.01833.x. doi:10.1111/j.1530-0277.2012.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar A. L., Nixon S. J. Disruption of sensory gating by moderate alcohol doses. Psychopharmacology. 2014;231:4393–4402. doi: 10.1007/s00213-014-3591-5. doi:10.1007/s00213-014-3591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C. D. Manual for State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Detailed tables. 2014 Retrieved from http://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs2013/NSDUH-DetTabs2013.htm. [PubMed]
- Thorne D. R. Throughput: A simple performance index with desirable characteristics. Behavior Research Methods. 2006;38:569–573. doi: 10.3758/bf03193886. doi:10.3758/BF03193886. [DOI] [PubMed] [Google Scholar]
- Tzambazis K., Stough C. Alcohol impairs speed of information processing and simple and choice reaction time and differentially impairs higher-order cognitive abilities. Alcohol and Alcoholism. 2000;35:197–201. doi: 10.1093/alcalc/35.2.197. doi:10.1093/alcalc/35.2.197. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture & U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. Washington, DC: U.S. Government Printing Office; 2010. Retrieved from http://www.health.gov/dietaryguidelines/dga2010/DietaryGuidelines2010.pdf. [Google Scholar]
- Watson P. E., Watson I. D., Batt R. D. Prediction of blood alcohol concentrations in human subjects. Updating the Widmark Equation. Journal of Studies on Alcohol. 1981;42:547–556. doi: 10.15288/jsa.1981.42.547. doi:10.15288/jsa.1981.42.547. [DOI] [PubMed] [Google Scholar]
- Weissenborn R., Duka T. Acute alcohol effects on cognitive function in social drinkers: Their relationship to drinking habits. Psychopharmacology. 2003;165:306–312. doi: 10.1007/s00213-002-1281-1. [DOI] [PubMed] [Google Scholar]