Abstract
Word selection allows us to choose words during language production. This is often viewed as a competitive process wherein a lexical representation is retrieved among semantically-related alternatives. The left prefrontal cortex (LPFC) is thought to help overcome competition for word selection through top-down control. However, whether the LPFC is always necessary for word selection remains unclear. We tested 6 LPFC-injured patients and controls in two picture naming paradigms varying in terms of item repetition. Both paradigms elicited the expected semantic interference effects (SIE), reflecting interference caused by semantically-related representations in word selection. However, LPFC patients as a group showed a larger SIE than controls only in the paradigm involving item repetition. We argue that item repetition increases interference caused by semantically-related alternatives, resulting in increased LPFC-dependent cognitive control demands. The remaining network of brain regions associated with word selection appears to be sufficient when items are not repeated.
Keywords: language production, word selection, left prefrontal cortex, semantic interference, proactive control, chronic stroke patients
Introduction
Although seemingly easy, the complex act of producing language relies on a number of distinctly described processes. Among these, lexical selection refers to the act of choosing words as we speak and has been suggested to be the main selection mechanism in the production of single words (Levelt, 1989). It is often thought of as a competitive process wherein a lexical representation is retrieved among semantically-related alternatives (e.g., Levelt, Roelofs, and Meyer, 1999; Levelt, 2001; Roelofs, 2003). The left prefrontal cortex (LPFC), and in particular the left inferior frontal gyrus (LIFG, e.g., Thompson-Schill, D’Esposito, Aguirre, & Farah, 1997; Thompson-Schill et al., 1998; Schnur, Schwartz, Brecher, & Hodgson, 2006; Schnur et al., 2009) is thought to help overcome competition for word selection through top-down control. However, whether the left PFC is always needed or whether its involvement is task specific remains to be investigated. In this study, we directly tested this question by testing stroke survivors with injury to the left PFC as they performed two picture naming tasks known to affect word selection difficulty but varying in terms of item repetition.
Semantic interference as a probe to study word selection
Word selection is often thought of as being a competitive process. This notion is supported by a category of speech errors referred to as semantic errors. Here, the erroneous word produced is semantically-related to the word which should have been produced (i.e, the target word). In the following example, “put the milk in the oven”, the target “refrigerator” was substituted with a semantically-related word, “oven”. Word selection by competition is also supported by experimental findings. Notably, several picture-naming tasks elicit semantic interference effects. These semantic interference effects have traditionally been interpreted as reflecting increased difficulty in lexical selection (e.g., Lupker, 1979; Kroll & Stewart, 1994; Damian, Vigliocco, & Levelt, 2001; Howard, Nickels, Coltheart, & Cole-Virtue, 2006). We note however that there is debate as to whether or not semantic interference occurs directly at the level of lexical selection or prior to lexical selection. Whereas some argue the semantic interference effect reflects increased competition at the level of lexical selection (Howard et al., 2006), others prefer incremental learning accounts in which the semantic interference effect is explained by changes in the connection weights between semantic and lexical representations (Navarrete, Mahon, & Caramazza, 2010; Navarrete, Del Prato, & Mahon, 2012; Navarrete, Del Prato, Peressotti, & Mahon, 2014; Oppenheim, Dell, & Schwartz, 2010). Here we refer to word selection in a broader sense and assume this process to be sensitive to interference caused by semantically-related alternatives.
Paradigms used to study word selection
1. Blocked cyclic picture naming paradigm
In the blocked cyclic picture-naming paradigm, participants name pictures presented within semantically-homogeneous or heterogeneous contexts (Damian et al., 2001; see Kroll & Stewart, 1994, for a first version of the blocked naming paradigm). In this task, pictures are presented within semantically-related versus unrelated blocks and are repeated several times per block (these repetitions are referred to as cycles). For the second cycle onward, performance is worse in homogenous blocks, that is when pictures are of the same semantic category, compared to when they are of different semantic categories, in heterogeneous blocks. This behavioral effect has traditionally been interpreted as reflecting increased competition for word selection caused by the repetitive prior retrieval of words from the same semantic category (Abdel-Rahman & Melinger, 2009; Belke, Meyer, & Damian, 2005; Damian et al., 2001; Kroll & Stewart, 1994; although see Janssen, Carreiras, & Barber, 2011; Janssen, Hernández-Cabrera, van der Meij, & Barber, 2014; Navarrete et al., 2012; 2014; for different interpretations).
Several neuroimaging, electrophysiological, and neuropsychological studies have used this paradigm to target brain regions associated with word selection difficulty and to assess the time-course of this process (Aristei, Melinger, & Abdel Rahman, 2011, Janssen et al., 2011; Janssen et al., 2014; Hocking, McMahon, & de Zubicaray, 2009; Maess, Friederici, Damian, Meyer, & Levelt, 2002; Schnur et al., 2006, Schnur et al., 2009). These have often reported an involvement of the left PFC, and in particular of the LIFG. More specifically, neuropsychological studies have shown that left PFC patients show a larger semantic interference effect than controls (Schnur et al., 2006) and right PFC patients (Ries, Greenhouse, Dronkers, Haaland, & Knight, 2014). In addition, BOLD signal in the LIFG but also in left temporal regions increases in semantically-homogeneous compared to semantically heterogeneous blocks (Hocking et al., 2009; Schnur et al., 2009). Electro- and magneto-encephalographic studies have highlighted a time-window going from approximately 200 to 400 msec post-stimulus presentation as being sensitive to semantic context in this paradigm (Aristei et al., 2011; Janssen et al., 2011; Maess et al., 2002; although see Janssen et al., 2014 for activity in a later time-window: 500 to 750 msec post-stimulus), which is in the time-window typically attributed to lexical selection (Levelt, Roelofs, & Meyer, 1999; Indefrey & Levelt, 2004; Indefrey, 2011; Strijkers & Costa, 2011).
2. Continuous picture naming paradigm
Semantic interference is also experimentally observed without separating pictures in distinct blocks. In the continuous naming paradigm (Howard et al., 2006), pictures from different semantic categories are interleaved with one another. Participants get increasingly slower each time they name a picture from a given semantic category (i.e., there is a monotonic increase in reaction times with ordinal position within categories). Howard et al. (2006) referred to this effect as the cumulative semantic interference effect and has been interpreted as reflecting increasing lexical selection difficulty (although see Navarrete et al., 2010). More specifically, these results have been taken to demonstrate cumulative interference for word selection caused by prior selection of other exemplars of the same semantic category (Howard et al., 2006). Similarly to the blocked picture-naming paradigm, the semantic interference effect in the continuous naming paradigm has been used to probe lexical selection by chronometric, electrophysiological, and neuroimaging studies (Costa, Strijkers, Martin, & Thierry, 2009; Llorens, Trébuchon, Riès, Liégeois-Chauvel, & Alario, 2014; de Zubicaray, McMahon, Howard, 2013).
Electrophysiological results have shown that event-related potentials are sensitive to the number of members of a semantic category named prior to the current one starting around 200 ms after stimulus onset (Costa et al., 2009; see however Llorens et al., 2014, for a non-replication of Costa et al., 2009), similarly as in the blocked cyclic picture-naming paradigm. Using perfusion fMRI, de Zubicaray et al.’s (2013) results suggest the left perirhinal cortex and left middle portion of the middle temporal gyrus (MTG) are involved in word selection using this paradigm as signal in these regions increased monotonically with ordinal position, similarly as for reaction times. Signal changes were also found in the LIFG and were affected by ordinal position. However, this change was not monotonic as in the other areas and signal decrease was observed in three of the five ordinal positions. This makes the association of this region with word selection as involved in this paradigm less clear. Neuroimaging studies looking at brain correlates of semantic interference in the continuous naming paradigm are however scarce. This could be explained by the fact neuroimaging studies typically require a critical number of trials per condition to address signal to noise issues, which is difficult to reach in this particular paradigm.
Role of the left PFC in word selection
While a similar theoretical account has been made for the semantic interference effects elicited by both paradigms (Oppenheim, Dell, & Schwartz, 2010), recently a few studies have suggested that an additional mechanism is likely involved in the blocked versus the continuous paradigm (Belke & Stielow, 2013; Navarrete et al., 2012; Pisoni, Papagno, & Cattaneo, 2012). This argument is based on the fact these tasks differ drastically in terms of design (blocked vs. continuous), item repetition, as well as prior familiarization to the pictures before the experiment. These differences have been shown to affect the electrophysiological signal: event-related potentials associated with these paradigms have different time-courses and topographies (Llorens et al., 2014). Moreover, semantic interference in the blocked cyclic paradigm is generally not cumulative (in the sense that the semantic interference does not increase as a function of the factor cycle), whereas it is in the continuous paradigm (Belke & Stielow, 2013; see however Navarrete et al., 2014). Based on neuropsychological evidence (e.g., Schnur et al., 2006), Belke and Stielow (2013) suggest this additional mechanism present in the blocked cyclic picture naming paradigm may be a top-down control process hosted in the LIFG which biases the level of activation of lexical-semantic representations towards the task relevant items. This knowledge of which items are in the task is not present in the continuous paradigm, not allowing this process to take place. More specifically, the authors argue that in both heterogeneous and homogeneous blocks, this top-down biasing process optimizes lexical access and counters the cumulative cost in the homogeneous blocks. This process, however, does not eliminate the semantic interference effect, which the authors interpret as reflecting increased competition between semantically-related representations in the homogeneous blocks compared to heterogeneous blocks. They make the prediction that this top-down biasing process should not be involved in the continuous naming paradigm given that there, semantic interference is cumulative. If this process is not involved in the continuous paradigm, then patients with LIFG damage should not show a larger cumulative semantic interference effect than controls. The present study directly tests this prediction in patients with left PFC damage involving the LIFG.
We note that Belke and Stielow’s suggestion is derived from the biased-selection account of the role of the LIFG made by Thompson-Schill and colleagues (e.g., Kan & Thompson-Schill, 2004). The idea is that the LIFG allows biasing response selection towards task-relevant representations in a top-down manner. Interestingly however, this account is largely inspired from studies using the verb generation task (Thompson-Schill et al., 1997; Thompson-Schill et al., 1998), which does not involve the repetition of a small number of stimuli. In the verb generation task, biased-selection is to be understood in the sense that words of the appropriate syntactic category (i.e., verbs) receive biased-selection compared to other words (e.g., nouns). This type of biased-selection is therefore in principle still possible in the continuous naming paradigm, where all the pictures represent objects. Moreover, the role of this brain region has been studied extensively not only in language production but also in language perception (e.g., Hagoort, 2005), and outside of language in the context of working memory and cognitive control processes (e.g., Duncan & Owen, 2000; Jonides & Nee, 2006), in paradigms which do not necessarily entail the repetition of a small set of stimuli (e.g., sentence completion tasks, recent probes test). Thus, whether or not the left PFC is involved in overcoming interference from semantically-related alternatives in the continuous picture naming paradigm remains to be investigated.
Present study
In this study, we tested 14 young controls, 14 aged-matched controls, and 6 left PFC patients as they performed the continuous picture naming paradigm. Our hypothesis was that if the left PFC is not always necessary for word selection and if its involvement is dependent on the knowledge of which items are in the task-set, as suggested by Belke & Stielow, 2013, then left PFC patients should not show a larger cumulative semantic interference effect than the control groups. We tested young controls in this paradigm as well as age-matched controls. This paradigm has rarely been studied in older controls and we wanted to examine whether the results were similar to young controls. In addition, we tested whether the left PFC patients tested in this study showed a larger semantic interference effect in the blocked cyclic picture-naming paradigm, using a subset of the data published in Ries et al. (2014). As shown on a larger group of patients, we predicted the subset of 6 left PFC patients tested here to show a larger semantic interference effect than age-matched controls in this paradigm involving within block item repetition.
Methods
1. Participants
The study was performed in agreement with the Declaration of Helsinki. All subjects gave informed consent approved by the University of California, Berkeley Committee for Protection of Human Subjects or the Department of Veterans Affairs Northern California Health Care System Human Research Protection Program.
The study contained 3 subject groups: a young control group, an age-and-education-matched control group, and a patient group. All participants were right-handed native English speakers with no history of neurological issues, apart from stroke for the patient groups. The young control group contained 14 undergraduate students of the University of California, Berkeley (10 females; age: median = 20 years old, IQR (inter-quartile range) = 19–22). They were recruited through the Department of Psychology’s online research participant recruitment system and participated in the experiment in exchange for course credit.
The age-and-education-matched control group contained 14 participants (3 males, 11 females; age: median = 66 years old, IQR = 63–69; years of education: median = 16 years, IQR = 15–18). The aged-matched control and the patient groups did not differ statistically in terms of age (estimated mean of the difference between groups = 7 years; 95% HDI (highest density interval) =(−7.07, 19.0)1) and age of education (estimated mean of the difference between groups = 1 year; 95% HDI (−4.21, 2.33)), and received remuneration for their participation in the experiment. The age-and-education-matched control group in the blocked-cyclic picture-naming paradigm was the same as the one described in Ries et al. (2014). It contained 14 participants (8 females; age: median = 68 years old, IQR = 62–71; years of education: median = 16 years, IQR = 16–18).
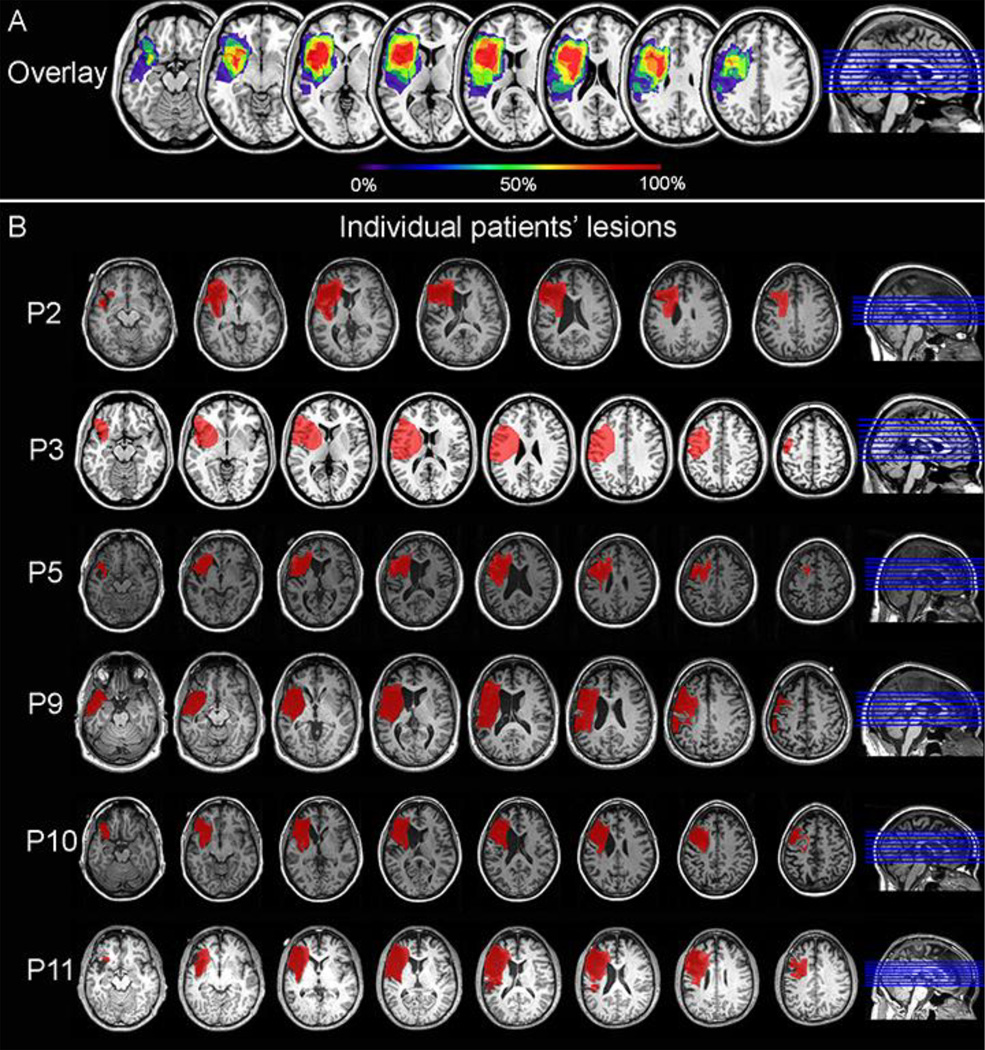
The patient group contained 6 patients with left PFC lesions due to stroke in the left precentral branch of the middle cerebral artery, which provides the major blood supply to the left lateral PFC (3 females; age: median = 56 years old, IQR = 53–61; years of education: median = 18 years, IQR = 16–20). All were chronic stroke patients and were tested on the neuropsychological tests and for the current study at least 6 months post-stroke. Lesions were delineated onto the MRICRO templates by a neurologist (RTK) using input from T1, T2 and Flair scans acquired at least 6 months post-stroke on a Siemens Allegra or Verio 3.0 Tesla MRI scanner. Lesion overlays and individual lesion reconstructions for the 6 left PFC patients are presented in Figure 1. Left PFC patients’ lesions were centered in both the inferior frontal gyrus and the middle frontal gyrus (Figure 1A). All patients were examined on at least 2 subtests of the Western Aphasia Battery (WAB; Kertesz, 1982), measuring spontaneous speech (assessing general conversational speech production abilities; maximum score of 20) and comprehension of sequential commands (assessing general speech comprehension skills; maximum score of 80). The 6 left PFC patients had a median Spontaneous Speech score of 19/20 (IQR = 18–19), reflecting overall good production abilities despite some articulation problems (two patients had a score of 18 reflecting a lack of detail in the picture description or in answering one of the questions). Their median Sequential Command score was 77.5/80 (IQR = 75–80). We note that 3 out of the 6 patients had a perfect score of 80, only one had a relatively low score of 59.5 and the others had a score of 75. The patient with the low comprehension score was nevertheless able to perform the task correctly. Thus, the language production deficits of the patients tested were mild allowing the patients to perform the tasks adequately.
Figure 1.
A. Lesion overlay of the 6 patients. The color coding indicates the amount of overlap between the different patients’ lesions (red corresponds to 100% overlap and purple to 0% overlap). B. Individual patients’ lesions in red. All were reconstructed on the individual patient’s scans and are presented over each patient’s T1 MRI image except for P3 for whom only a CT scan was available. This patient’s lesion is presented on the MNI template.
2. Materials and Design
In this section, we first detail the materials and design for the continuous naming paradigm. We then turn to the blocked cyclic picture naming paradigm which is also described in Ries et al. (2014).
2.1.Continuous naming paradigm
The stimuli were 149 greyscale photographs of objects presented against a white background. 98 of the pictures were issued from 14 semantic categories with 7 exemplars in each categories, 51 of the pictures were fillers unassociated with the 14 semantic categories. The names of the 98 experimental stimuli and fillers are provided in the Appendix at the end of this paper. The name agreement of experimental pictures was very high (name agreement: median = 100%, IQR = 90–100). The stimuli were selected from the Internet and from the set used by Howard et al. (2006) and were formatted by us to so that each image was 400 pixels high by 400 pixels wide.
Subjects were directed to name the item in each picture as it was presented, responses were recorded by a microphone. Each participant saw 107 items, 5 from each of the 14 categories (70 category items) and 37 filler items. The 107 pictures were randomly inserted into a sequence with the following constraints: Pictures in each category were separated by lags of 2, 4, 5 and 6 intervening items. Fourteen, of 24 (i.e., 4!) possible lag orderings were selected corresponding to the 14 categories (e.g., for furniture items, lag ordering: 4, 5, 2, 6; for fruit, lag ordering: 6, 4, 5, 2; etc.) to control for the number of times each lag ordering was seen per category over participants. The first 3 items of the sequence were filler items. Filler items and the order of the categories in the sequence were randomly assigned. This process was repeated six times following the same constraints and structure, resulting in 7 experimental sequences. In generating the 7 experimental sequences we ensured that a specific category never occupied the same position across the 7 sequences (see Navarrete et al., 2010, for precedent on these constraints). Each category was presented with each of the 14 lag orderings across the 7 experimental lists. In order to avoid item specific effects we adopted two strategies. First, each participant was exposed to 5 of the 7 exemplars per category. Each experimental item was used the same number of times across the 7 sequences (i.e., 5). Second, the experimental items within each category were represented equally at each of the five ordinal positions within a category (i.e., across the 7 sequences each experimental item was presented once in each ordinal position, 1 to 5).
2.2. Blocked-cyclic picture naming paradigm
The stimuli were 252 line-drawings of common objects or animals selected from published collections (Snodgrass & Vanderwart, 1980; Bonin, Peereman, Malardier, Méot, & Chalard, 2003), the Internet, or constructed by us: 216 were used as experimental items and 36 were used as practice items. Their name agreement was also very high (name agreement: median = 93%, IQR = 89–96%). The pictures were colored in green or purple presented on the left or on the right of the fixation cross, for purposes unrelated to the present study. They were issued from 6 semantic categories (e.g. animals, vehicles) and each member (e.g. cat) was represented by 7 different items (e.g. 7 different cats: 6 for the experiment in itself and one for the familiarization to the picture names). There were 6 cycles within each block and 6 members for each category. All items appeared an equal number of times in the homogeneous and heterogeneous blocks.
3. Procedure
Participants were tested in a sound-attenuated dimly-lit environment. The experiments were controlled by the Eprime 2.0 Professional software (Psychology Software Tools, Inc., Pittsburgh, PA), which allows on-line recording of the participants’ verbal responses.
In the continuous naming paradigm, a trial consisted of the following events: (1) a fixation point (“plus” sign at the center of the screen) was presented for 700 ms followed by a blank screen for 500 ms; (2) a picture for 2000 ms (3) a blank screen for 1500 ms. The following trial started automatically. There was a pause every 36 items, during which the participant could rest as long as necessary.
In the blocked-cyclic naming paradigm, a trial consisted of the following events: (1) a fixation point (“plus” sign presented at the center of the screen) for 500 ms; (2) a picture for 2000 ms (3) a blank screen for 2000 ms. The following trial started automatically. Participants were asked to name the picture by saying the name of the picture preceded by the possessive determiner “my” (e.g. “my cat”). The possessive determiner was added to reduce variability in vocal onsets. The task was split into 4 parts of 108 trials each, with two pauses equally spaced within each part. Each part contained 3 blocks. Each block was 36 trials-long (6 cycles within each block and 6 members for each category). Before the task, participants were familiarized with the picture names. We wanted to avoid visual habituation to the experimental stimuli and thus used a set of 36 randomly-presented pictures for familiarization consisting of a seventh exemplar of each member of each category used in the experiment. Importantly, these familiarization items had the same names as the experimental stimuli. During familiarization, the experimenter made verbal corrections when an incorrect or unexpected response was produced. In both tasks, participants were asked to name the pictures as fast and as accurately as possible. Vocal-onset was used as the response-onset measure.
4. Data Processing
Correct responses were defined as answers that matched the canonical name for a given item. We accepted semantically identical names when the participant consistently named the pictures that way (e.g.: airplane for plane, couch for sofa). Any other type of response was defined as an error (e.g.: stutter, semantically different word, hesitation). Correct or incorrect responses made after the offset of the stimulus were marked as “no response” and were excluded from response time averages.
5. Data Analysis
Statistical analysis was performed within R version 3.1.1 (R Core Team, 2012) using the packages “lme4” to compute the mixed effect models (Bates, Maechler, Bolker, & Walker, 2014; Bates, Maechler, Bolker, & Walker, 2014b) and “car” to compute analysis of deviance tables for the fixed effects of the mixed effect models (Fox and Weisberg, 2014). We analyzed the data from the control groups and from the left PFC group using generalized linear (for reaction times) and logistic (for accuracy rates) mixed-effects models (Baayen, Davidson, & Bates, 2008; Jaeger, 2008), which rely on single-trial data rather than on averages over participants or items, and are also free from the assumptions of homogenous variance and sphericity that are inherent to the more classic ANOVA (Pinheiro & Bates, 2000). The individual reaction times (RTs) were log-transformed to reduce skewness and approach a normal distribution. The analyses were performed on log-transformed RTs and accuracy rates.
For the continuous naming paradigm, the analyses were performed on the experimental items (fillers were excluded). We tested for main effects of Position Within Category as a within-participant factor and Group (with the 3 levels: young controls, age-matched controls, and left PFC patients) as a between-participant factor and the interaction between Group and Position Within Category. As random effects, we had intercepts for participants and picture name, as well as by-subject random slopes for Position Within Category. Previous studies have showed that Lag does not modulate the cumulative semantic interference effect (Howard et al., 2006; Navarrete et al. 2010; see also Alario, del Prado Martín, 2010). Hence the factor Lag was not included in the analysis.
For the blocked cyclic picture-naming paradigm, as we were interested in the semantic interference effect, the items corresponding to the first presentation (i.e., first cycle) within blocks were excluded. As explained in Ries et al. (2014), in the blocking naming paradigm the first presentation tends to show, if anything, a semantic facilitation effect as a consequence of semantic priming between related items in homogenous blocks (e.g., Abdel-Rahman & Melinger, 2007; Navarrete et al., 2012; see for further discussion on semantic facilitation effects in the blocking paradigm, Navarrete et al., 2014). We tested for fixed effects of Group (2 levels: age-matched controls, and left PFC patients) as a between-subject factor and Semantic Context (homogeneous vs. heterogeneous), Repetition (from 2 to 6), and stimulus position (i.e., left or right of the fixation cross) as within-subject factors, and interactions between Semantic Context, Repetition, and Group. As random effects, we had intercepts for participants and picture name, as well as by-subject random slopes for within-subject factors.
P-values were obtained using type-II (for the continuous naming paradigm) and type-III (for the blocked naming paradigm, because of the presence of an interaction) analyses-of-deviance tables providing Wald chi-square tests for the fixed effects in the generalized linear mixed-effects models. For all models, we report Wald χ2-values and p-values from the analysis of deviance tables as well as raw β estimates (βraw), 95% confidence intervals around these β estimates (CI), standard errors, t-values for reaction times, and Wald Z and associated p-values for accuracy rates.
Inter-individual variability was also investigated in the continuous naming paradigm. Since this study is the first to report the use of this paradigm in left PFC stroke patients, we wanted to describe the results in more details for this paradigm. For comparison, however, we also report the individual patients’ results for the blocked-cyclic picture naming paradigm in Table 2. For the continuous naming paradigm, we calculated the slope of the linear regression of the mean reaction time values per patient and per aged matched controls. The within-group variance was compared between patient and aged-matched controls using Levene’s test (W) for equality of variance (Levene, 1960), which is more robust to departures from normality of the tested data distributions than the more commonly-used Bartlett test. We then compared the slope of the semantic interference effect of each patient to that of the aged-matched controls by using Crawford and Garthwaite’s method for comparing single cases with a control sample (Crawford and Garthwaite, 2002, see also Crawford, Garthwaite, and Howell, 2009): We calculated modified t-values per patient by adjusting for the age-matched control sample size in each paradigm. For the blocked-cyclic picture naming paradigm, we calculated the mean semantic interference effect per participant by subtracting the mean error rate value in heterogenous blocks to the mean error rate value in homogenous blocks. We then calculated z-scores and modified t-values in the same way as for the continuous naming paradigm.
Table 2.
Individual patients’ z-scores and t-values calculated with the Crawford & Garthwaite method (Crawford & Garthwaite, 2002) compared to age-matched control samples in each paradigm. The threshold for significance was a t-value of 1.771 given the control sample size of 14 in each paradigm. All significant t-values are in bold. Only two out of six patients had a larger interference effect than age-matched controls in the continuous paradigm whereas five out of six patients had a larger interference effect than age-matched controls in the blocked-cyclic picture naming paradigm.
| Paradigm | Patient | Z-score | T-value (Crawford & Garthwaite) |
|---|---|---|---|
| Continuous | P2 | 2.33 | 2.25 |
| P3 | −0.63 | −0.61 | |
| P5 | −0.96 | −0.93 | |
| P9 | −1.50 | −1.45 | |
| P10 | 2.32 | 2.24 | |
| P11 | 0.14 | 0.13 | |
| Blocked | P2 | 1.08 | 1.04 |
| P3 | 6.34 | 6.12 | |
| P5 | 3.22 | 3.11 | |
| P9 | 2.23 | 2.15 | |
| P10 | 2.07 | 2.00 | |
| P11 | 5.02 | 4.85 | |
The size of the interference effect compared to age-matched controls was directly compared in one paradigm versus the other. Because the interference effect is not calculated in the same way in each paradigm, we calculated z-scores to account for the size of the interference effect in each task in patients versus that of age-matched controls. We then directly compared these z-scores with a one-sided t-test, given the results of the mixed effect models performed in each task independently.
Results
1. Continuous naming paradigm
1.1.Reaction times
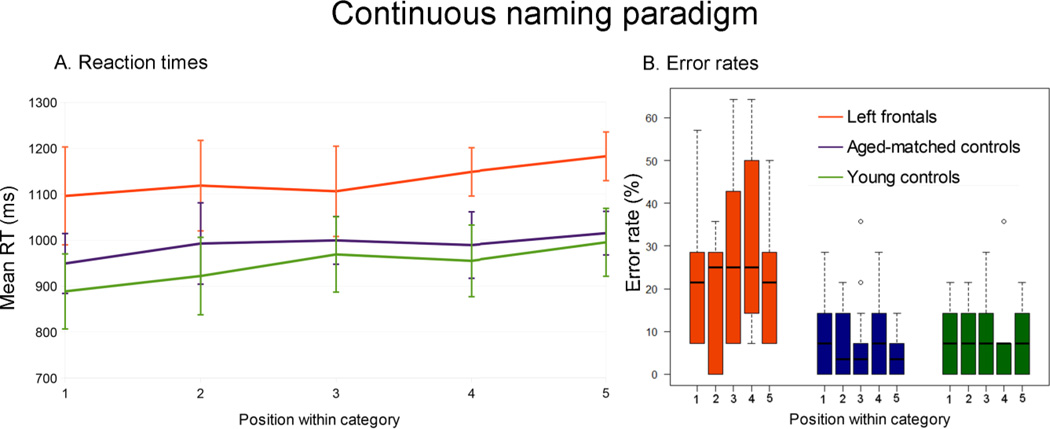
The mean RTs and standard deviations per group and per position-within-category are presented in Table 1. There were main effects of Group (Wald χ2(2) = 11.04, p = .004) and Position within-category (Wald χ2(4) = 30.88, p < .001) on reaction times. Left PFC patients were slower than young controls and aged-matched controls (left PFC patients vs. young controls: βraw = −20.6 × 10−2, CI = [−32.7 × 10−2 −8.4× 10−2], SE = 6.20× 10−2, t=−3.32; left PFC patients vs. aged-matched controls:βraw = −15.8 × 10−2, CI= [−28.0 × 10−2 −3.7 × 10−2], SE = 6.20× 10−2, t=−2.55; Left PFC: mean RT = 1130 ms, σ = 138 ms; Aged-matched controls: mean RT = 989 ms, σ = 107 ms; Young controls: mean RT = 946 ms, σ = 145 ms). A post-hoc t-test determined there was no significant difference between mean RTs for aged-matched vs. young controls (t(24.59)=1.03, p(uncorrected)=.321). For all groups, reaction times were increasingly slower between the first time an item was presented within a category and the last (βraw = 6.5 × 10−2, CI= [0.7 × 10−2 12.4 × 10−2], SE= 2.98× 10−2, t=2.20), revealing a cumulative semantic interference effect. There was no interaction between Group and Position within-category (Wald χ2(8) = 5.07, p = .750), indicating the cumulative semantic interference effect was not larger for one group than for the others (see Figure 2A).
Table 1.
Mean reaction times and median error rates per participant group and per position within category in the continuous naming paradigm. Standard deviations (for reaction times) and interquartile ranges (for error rates) are in brackets.
| Reaction times (in msec) | ||||||
|---|---|---|---|---|---|---|
| Position within category | ||||||
| 1 | 2 | 3 | 4 | 5 | Average | |
| Left PFC patients | 1096 (213) | 1119 (197) | 1106 (197) | 1149 (105) | 1182 (106) | 1130 (138) |
| Aged-matched controls | 949 (130) | 993 (177) | 1000 (104) | 989 (144) | 1015 (95) | 989 (107) |
| Young controls | 889 (163) | 922 (168) | 969 (164) | 955 (156) | 995 (148) | 946 (145) |
| Error rates (in %) | ||||||
| Position within category | ||||||
| 1 | 2 | 3 | 4 | 5 | Median | |
| Left PFC patients | 21 (9–29) | 25 (5–29) | 25 (9–41) | 25 (16–45) | 21(11–27) | 21 (7–34) |
| Aged-matched controls | 7 (0–14) | 4 (0–12.5) | 4 (0–7) | 7 (0–12.5) | 4 (0–7) | 7 (0–14) |
| Young controls | 7 (0–14) | 7 (0–14) | 7 (0–14) | 7 (0–7) | 7 (0–14) | 7 (0–14) |
Figure 2.
Cumulative semantic interference effect per group (left PFC patients, aged-matched controls, young controls) on mean reaction times (A) and median error rates (B). Mean reaction times increase with position-within-category. Left PFC are overall slower and less accurate than controls but do not show a larger semantic interference effect. Medians are indicated by the black horizontal lines in the box-and-whisker plots. Interquartile ranges are represented by the boxes and the total range is depicted by the dotted lines.
1.2.Accuracy rates
The median Error rates and interquartile range per group and per position-within-category are presented in Table 1. There was a main effect of Group (Wald χ2(2) = 14.94, p < .001) but not of Position within-category (Wald χ2(4) = 1.93, p = .748) on accuracy rates (see Figure 2B). Left PFC patients were less accurate than young controls and aged-matched controls (left PFC patients vs. young controls: βraw = 1.59, CI = [.67 2.52], SE = 0.47, Z=3.37, p < .001; left PFC patients vs. aged-matched controls: βraw = 1.81, CI = [.88 2.75], SE = 0.48, Z=3.79, p < .001; Left PFC: median error rate = 21 %, IQR = 7–34 %; Aged-matched controls: median error rate = 7 %, IQR = 0–14 %; Young controls: median error rate = 7 %, IQR = 0–14 %). A post-hoc Wilcoxon rank sum test determined there was no significant difference between mean error rates for aged-matched vs. young controls (W = 2646.5, p(uncorrected)=389). There was again no interaction between Group and Position-within-category (Wald χ2 (8) = 4.50, p = 809).
1.2.3. Individual variability
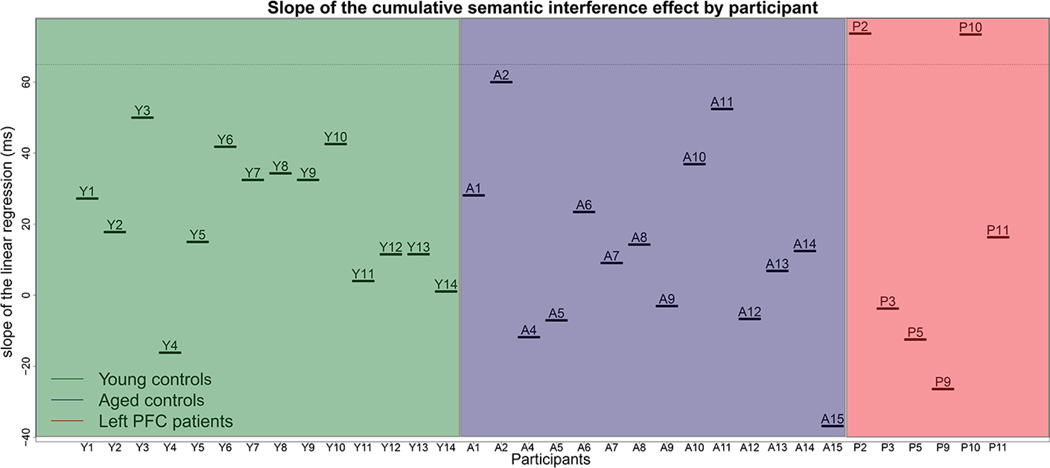
We tested whether a greater semantic interference effect in left PFC patients vs. aged-matched and young controls was observed on a patient-by-patient basis. The slopes of the semantic interference effect on mean reaction times differed between individuals in all groups (Figure 3). This within-group variance was marginally different between the groups (W(2)=2.86, p=072; left PFC patients: mean slope: 20 ms, σ = 44 ms; aged-matched controls: mean slope 13 ms, , σ = 26 ms; young controls: mean slope 22 ms, , σ = 19 ms). Post-hoc Levene’s tests suggested this marginal group effect is explained by the fact left PFC patients’ slopes are more variable than that of young controls (W(1)=5.67, p(uncorrected) = 0.029) but not of that of aged-matched controls (W(1)=2.27, p(uncorrected)=149). We also compared the slope of the semantic interference effect of each patient to that of the aged-matched controls using Crawford & Garthwaite method (Crawford & Garthwaite, 2002) for comparing single cases with a control sample. Two left PFC patients (P2 and P10) were found to have a slope that was steeper than that of the aged-matched controls (modified t-values were superior to 1.771, see Table 2). It is unclear at this point what is at the origin of this larger semantic interference effect as no clear difference in lesion location, lesion size, years post-onset, scores on neuropsychological tests, or demographic factors could be seen between these two patients compared to the others.
Figure 3.
Slope of the cumulative semantic interference effect on mean reaction times per participant in the young controls, aged-matched controls, and patient groups. Two patients (P2 and P10) had a slope which was steeper than the average slope of the aged-matched control group plus 2 standard deviations from this mean.
2. Blocked-cyclic picture naming paradigm
The results in this section are extracted from data collected and partly presented in a recent publication (Ries et al, 2014). One patient included in the present analyses was rejected from the analyses in Ries et al. (2014) because of a very high error rate (59%). We included this patient in the present analyses so that the same exact patients would be present in the analyses of both naming paradigms. The data from the same 14 aged-matched controls as reported in (Ries et al, 2014) are used here.
2.1.Reaction times
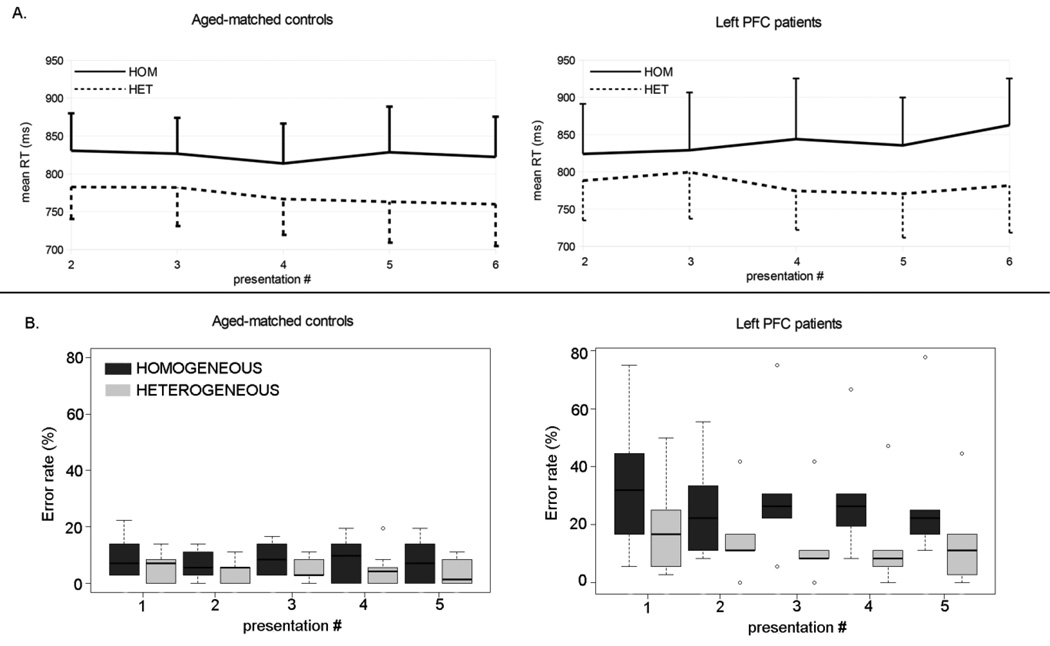
There was a main effect of Semantic Context on reaction times (Wald χ2(1) = 48.19, p < .001): RTs were longer in semantically homogeneous versus heterogeneous blocks (βraw = 3.31 × 10−2, CI = [2.38 × 10−2 4.25 × 10−2], SE = 4.77× 10−3, t=6.94; HOM: mean RT = 828 ms, σ = 111 ms; HET: mean RT = 774 ms, σ = 100 ms). There was also a marginal effect of Repetition (Wald χ2(4) = 9.18, p = .057), reaction times tended to decrease with increasing repetition (βraw = −2.02 × 10−2, CI = [−3.80 × 10−2 −0.24 × 10−2], SE = 9.09× 10−3, t =−2.22, see Figure 4A and Table 3). There was no interaction between Repetition and Semantic Context (Wald χ2(4) = 4.68, p = .322). None of the other relevant comparisons reached statistical significance.
Figure 4.
Semantic context effect in the Naming task on mean reaction times (A) and median error rates (B) for aged-matched controls (left), and left PFC patients (right). Values for homogeneous blocks (HOM) are depicted by the solid lines and values for heterogeneous blocks (HET) are depicted by the dotted lines for the reaction times (A) and by the dark grey and light grey boxes respectively for the error rates (B). Values for repetitions 2 to 6 are presented. For reaction times (A), standard deviations are represented by the horizontal lines (only positive values are presented for the homogeneous condition and only negative values are presented for the heterogeneous condition for visual clarity). For error rates (B), medians are indicated by the black horizontal lines in the box-and-whisker plots. Interquartile ranges are represented by the boxes and the total range is depicted by the dotted lines.
Table 3.
Mean reaction times and median error rates per participant group and per presentation number within category in the blocked cyclic picture naming paradigm. Standard deviations (for reaction times) and interquartile ranges (for error rates) are in brackets.
| Reaction times (in msec) | |||||||
|---|---|---|---|---|---|---|---|
| Presentation # | |||||||
| 2 | 3 | 4 | 5 | 6 | Average | ||
| Left PFC patients | HOM | 824 (67) | 829 (77) | 844 (81) | 836 (64) | 863 (63) | 838 (140) |
| HET | 788 (53) | 800 (63) | 774 (52) | 771 (59) | 782 (63) | 783 (115) | |
| Aged-matched controls |
HOM | 831 (49) | 827 (47) | 814 (53) | 828 (60) | 822 (53) | 824 (102) |
| HET | 783 (42) | 782 (51) | 767 (47) | 763 (54) | 760 (55) | 771 (97) | |
| Error rates (in %) | |||||||
| Presentation # | |||||||
| 2 | 3 | 4 | 5 | 6 | Median | ||
| Left PFC patients | HOM | 32 (19–42) |
22 (13–32) |
26 (23–30) |
26 (20–31) |
22 (18–24) |
25 (17–33) |
| HET | 17 (6–25) |
11 (11–15) |
8 (8–10) |
8 (6–10) |
11 (4–16) |
10 (6–17) |
|
| Aged-matched controls |
HOM | 7 (3–13) | 6 (3–10) | 8 (3–13) | 10 (1–14) | 7 (1–13) | 8 (3–14) |
| HET | 7 (1–8) | 6 (1–6) | 3 (3–8) | 4 (1–6) | 1 (0–8) | 4 (0–8) | |
2.2.Accuracy rates
There was a main effect of Semantic Context on error rates (Wald χ2(1) = 16.00, p < .001): Overall, participants were less accurate in semantically homogeneous versus heterogeneous blocks (βraw = −3.39 × 10−1, CI = [−5.05 × 10−1 1.73 × 10−1], SE = 8.48 × 10−2, Wald Z =−4.00, p < .001, Figure 2 B and Table 3). There was no main effect of Repetition on accuracy rates (Wald χ2(4) = 2.59, p = 628), and no interaction of Repetition with Semantic Context (Wald χ2(4) = 1.47, p =.832). There was a main effect of Group (Wald χ2(1) = 10.67, p = .001), left PFC patients were less accurate than controls (βraw = −15.86× 10−1, CI = [−25.37× 10−1 −6.35× 10−1], SE = 4.85 × 10−1, Wald Z =−3.27, p = 001). Critically, there was an interaction between Group and Semantic Context (Wald χ2(2) = 5.69, p =.017), left PFC patients had a larger semantic interference effect than controls (βraw = −2.78 × 10−1, CI = [−5.06 × 10−1 −4.96 × 10−2], SE = 1.17 × 10−1, Wald Z =−239, p = 017: Left PFC patients: HOM median error rate = 25%, IQR = 17–33%; HET median error rate = 10%, IQR = 6–17%, Controls: HOM: median error rate = 8%, IQR = 3–14%; HET: median error rate = 4%, σ = 0–8%, Figure 4B). There was no other significant effect in the other comparisons under analysis. Individual patients’ results were also compared to the age-matched control sample using Crawford & Garthwaite method (Crawford & Garthwaite, 2002) for comparing single cases with a control sample. The results show that five out of six patients had a larger interference effect than the age-matched control sample in this paradigm (see Table 2).
3. Direct comparison of the interference effect in the two paradigms
The size of the interference effect compared to age-matched controls was directly compared in one paradigm versus the other. As predicted based on the mixed effect models performed in each paradigm, the difference in the size of the interference effect between left PFC patients and controls was greater in the blocked-cyclic picture naming paradigm than in the continuous naming paradigm (t(5)=−2.18, p=.040, a one-sided t-test was used given the expected direction of the effect).
Discussion
We tested the performance of chronic stroke patients with lesions in the left PFC and aged-matched and young controls in the continuous naming paradigm (Howard et al., 2006). A main effect of Position within-category was found overall participants thus replicating the cumulative semantic interference effect (i.e., naming latencies increased with each within-category item that is named in a sequence). Left PFC patients were overall slower and less accurate than both control groups. Critically when analyzed as a group, left PFC patients did not show a larger cumulative semantic interference effect than the control groups neither on the RTs nor in the error rates. These results contrast with those obtained in the blocked cyclic picture-naming paradigm in the same patients. In this paradigm, left PFC patients were less accurate than controls and, critically, they showed a larger semantic interference effect than controls on error rates. Taken together, these group results suggest that the left PFC may not always be necessary to overcome interference caused by previous retrieval of semantically-related alternatives in word selection. We discuss the implications of these findings in more details below.
Role of the left PFC in word selection: a conditional boosting mechanism?
As reviewed in the Introduction, the left PFC and especially the LIFG has been identified by several neuroimaging and neuropsychological studies as playing an important role in the act of choosing words as we speak (Ries et al., 2014; Schnur et al., 2006; Schnur et al., 2009; Thompson-Schill et al., 1997; Thompson-Schill et al., 1998). Specifically, it has been suggested that the LIFG may be involved in helping to overcome interference caused by semantically-related alternatives in word selection. This is suggested from studies using the verb generation task where BOLD signal is increased when many verbs can be generated for a given noun (i.e., high selection items) in comparison with when only one or few verbs can be generated (i.e., low selection items, Thompson-Schill et al., 1997). In addition, patients with lesions in the LIFG are particularly impaired for high versus low selection items (Thompson-Schill et al., 1998). Moreover, using the blocked-cyclic picture naming paradigm introduced by Damian et al. (2001), it has been shown that in comparison to control groups, patients with lesions in the left PFC, and particularly in the LIFG suffer more interference. That is, these patients are more impaired when they have to name pictures within a semantically-homogeneous context compared to when they are named in a semantically-heterogeneous context (Ries et al., 2014; Schnur et al., 2006; Schnur et al., 2009).
More recently, lexical selection has been investigated using another type of picture naming paradigm, which we referred to as the continuous naming paradigm (Howard et al., 2006), and which generates a cumulative semantic interference effect. The cumulative semantic interference effect has also been interpreted as reflecting increased word selection difficulty. As mentioned in the introduction, whether or not this effect occurs directly from the level of lexical selection or prior to lexical selection is a matter of current debate. Whereas some argue the semantic interference effect reflects increased competition at the level of lexical selection (Howard et al., 2006), others prefer incremental learning accounts in which the semantic interference effect is explained by changes in the connection weights between semantic and lexical representations (Navarrete et al., 2010; 2012; 2014; Oppenheim et al., 2010). Nevertheless, these accounts converge in the idea that each naming event, each time a word is accessed for speech, hampers the subsequent selection of words of the same semantic category.
The semantic interference effects observed in the blocked-cyclic and in the continuous picture-naming paradigm have been interpreted within the same theoretical framework (Oppenheim et al., 2010). The more words of a given semantic category that have been accessed, the more activation a given word representation of this semantic category will need so that its activation can exceed the activation levels of its previously named neighbors for successful selection. A boosting mechanism comes into play to multiply the activation levels of lexical representations until the target’s activation level is discernible from that of its neighbors. This computational model does not rely on strong physiological assumptions. The authors nevertheless speak of a potential role of the left PFC in this boosting mechanism when selection is difficult. This boosting mechanism resembles the top-down control account made by the above-mentioned studies (Ries et al., 2014; Schnur et al., 2006; Schnur et al., 2009; Thompson-Schill et al., 1997; Thompson-Schill et al., 1998). However, this model does assume this boosting mechanism to be involved in both the cumulative and the blocked-cyclic picture-naming paradigm. The overall pattern of results we report are not fully in support of this assumption if the boosting mechanism is indeed always hosted in the LIFG. Yet, whether or not this boosting mechanism or top-down control mechanism is uniquely hosted in the LIFG remains an empirical question. We return to this point in the following section about the other regions associated with word selection in language production.
As mentioned in the introduction, a few studies have suggested that an additional mechanism is likely involved in the blocked versus continuous paradigm (Belke & Stielow, 2013; Navarrete et al., 2012; 2014; Pisoni et al., 2012). Belke & Stielow (2013) suggest this additional mechanism present in the blocked cyclic picture naming paradigm may be a top-down control process hosted in the LIFG which allows biasing the level of activation of lexical-semantic representations towards the items that are present in the task. This knowledge of which items are in the task is not present in the continuous paradigm, not allowing this proactive control process to take place. Our results seem to be, at first view, directly in line with Belke and Stielow (2013)’s prediction. However, two points deserve further mention. First, as mentioned above, the LIFG has been found to play a role in word selection and memory retrieval in tasks which do not involve the repetition of a small set of stimuli (i.e., verb generation, Thompson-Schill et al., 1997, 1998; recent probes test, Jonides & Nee, 2006). In fact, Belke and Stielow’s suggestion is directly derived from Thompson-Schill and colleagues biased-selection account (Kan & Thompson-Schill, 2004), which is mainly based on studies using the verb generation task. In the verb generation task, biased-selection is to be understood in the sense that words of the appropriate syntactic category (i.e., verbs) will be favored compared to other words (e.g., nouns). This type of biased-selection is therefore still possible in the continuous naming paradigm, where all the pictures represent objects. Second, the individual patients results we report show that some patients with left PFC lesions can have a greater cumulative semantic effect than controls. It is not clear at this point what differentiates these patients’ lesions from that of the other patients as no obvious difference can be seen in the structural neuroimaging data or in the patients’ demographics and scores on the neuropsychological tests. A possibility could be that a specific cortical subregion of the left PFC or fiber tracts linking regions involved in word selection are more affected in these patients than in the others but this remains to be investigated in future studies. Thus, Belke and Stielow (2013)’s suggestion for the role of the LIFG in word selection may need to be nuanced in light of these two points. We therefore suggest that the difference between the continuous and blocked-cyclic picture naming paradigms would be better understood in terms of how much biased-selection can occur rather than in an all-or-none fashion.
One point that deserves mention is the large inter-individual variability in the size but also in the presence or absence of the cumulative semantic interference effect in aged controls and in left PFC patients. Indeed, 36% of the aged controls and 50% of the left PFC patients did not show a cumulative semantic interference effect. In contrast, only one young control participant (7%) did not show this effect. We note that the mean slope of the cumulative semantic interference effect in young controls in our study (22 ms) is in the range of what has been reported in studies using the same paradigm (30 ms in Howard et al., 2006; 9 ms in Navarrete et al., 2010), suggesting the increased variability we observe is not due to specific parameters of the design used in the present study. At first view, these results suggest older participants may have more variable performance in this paradigm than younger participants. Aging studies have shown that the size of the cumulative semantic interference effect does not differ from that of older participants compared to younger controls (Gordon and Cheimariou, 2014; Mulatti, Calia, De Caro, and Della Sala, 2014). This is in agreement with the group results we report. However, looking at individual participants’ performance shows a more nuanced pattern. We are not aware of studies looking at the inter-individual variability in this effect and can only speculate on an explanation. As shown by Mulatti et al. (2014), pathological aging such as in the case of Mild Cognitive Impairment can lead to the absence of cumulative semantic interference effect. The authors interpreted this result in terms of inefficient semantic access in these patients. In our study, we did not test for cognitive impairment in the aged-matched controls and it is possible that some participants may have been been diagnosed as having such impairment had they been tested for it. Mulatti et al.’s (2014) result may also be informative in terms of the inter-individual variability found in the left PFC patients for the cumulative semantic interference effect. Indeed, the patients who did not show a cumulative semantic interference effect may have also been suffering from impaired semantic access, similarly to MCI patients. We note however that this type of interpretation has previously not been proposed concerning left PFC patients’ word finding deficits. Indeed, left PFC patients often know what they want to say but have trouble narrowing their search on the specific word, perhaps reflecting their difficulty in overcoming interference from related alternatives. Further investigations are needed to disentangle these hypotheses and to explain why certain patients with left PFC injuries show an increased cumulative semantic interference effect while others show an absence of cumulative semantic interference effect in the continuous picture naming paradigm. Nevertheless, we note that if the left PFC were critical in overcoming interference from semantically-related alternatives in this task, this should have been reflected in the group results, similar to the blocked-naming paradigm.
Other regions involved in word selection
The involvement of the LIFG in word selection does not preclude the possible involvement of other brain regions in helping to solve ambiguity between semantically-related lexical alternatives. As mentioned in the introduction, the left PFC is not the only region associated with word selection. Indeed, medial frontal regions (i.e., the anterior cingulate cortex, e.g., Piai, Roelofs, Acheson, & Takashima, 2013, and the pre-supplementary motor area, pre-SMA, Alario, Chainay, Lehericy, & Cohen, 2006; Tremblay & Gracco, 2009; Tremblay & Gracco, 2010), as well as different regions of the left temporal cortex, including the left middle (e.g., Baldo, Arévalo, Patterson, & Dronkers, 2013; Indefrey, 2011; de Zubicaray et al., 2013) and inferior temporal gyri (e.g., Trebuchon-Da Fonseca et al., 2009), have also been associated with word selection.
In particular, the posterior middle and inferior temporal cortex has been associated with initial lexical access (e.g., Trebuchon-Da Fonseca et al., 2009; Dronkers, Wilkins, Van Valin, Redfern, & Jaeger, 2004). Critically, patients with lesions encompassing the posterior half of the middle temporal gyrus (MTG), often lose the ability to retrieve words completely (Dronkers et al., 2004). These patients differ markedly from patients with left inferior frontal lesions. In addition, BOLD signal in medial frontal regions such as the ACC and pre-SMA has been found to increase with response selection difficulty in language production (Alario et al., 2006; Piai et al., 2013; Tremblay & Gracco, 2009; Tremblay & Gracco, 2010) and in actions in general (see Ridderinkhof, Forstmann, Wylie, Burle, & van den Wildenberg, 2011, for a review). Stimulation of the pre-SMA in humans has been associated with motor actions (including verbal actions) suppression (e.g., Chauvel, Rey, Buser, & Bancaud, 1996). Thus, it is possible that the remaining parts of the network may be sufficient for word selection in the continuous naming paradigm when the left PFC is damaged. One can imagine that the left temporal cortex alone or that connections between the temporal cortex and the medial frontal cortex may be sufficient to solve word selection in this paradigm. Whether one of these alternatives is valid or if additional regions may be involved remains to be investigated.
In addition, we also point to the increasing literature showing the importance of the white matter pathways underlying brain function in general and language in particular (e.g., Duffau, Moritz-Gasser, & Mandonnet, 2014; Hope, Seghier, Leff, & Price, 2013; Saur et al., 2008; Turken & Dronkers, 2011). Duffau and colleagues recently proposed a different type of model of speech production in which different pathways and brain regions can subserve similar functions in picture naming. The authors refer to this model as being hodotopical in the sense that it is both topological (from the greek, topos = place), pointing to the importance of specific cortical areas to subserve brain function, and hodological (from the greek, hodos = path), pointing to the importance of white matter pathways linking these brain regions. In particular, three parallel ventral semantic routes have been proposed: one direct route linking the posterior inferior occipito-temporal area to the dorso-lateral frontal cortex and the inferior frontal gyrus through the inferior frontal-occipital fascicle; one indirect route with a relay at the level of a temporal pole and constituted by the anterior part of the inferior longitudinal fascicle and the uncinate fascicle; and a third pathway connecting the angular gyrus to the superior temporal gyrus up to the temporal pole through the middle longitudinal fascicle (see Duffau et al., 2014 for a review). Thus, it is possible that slightly different pathways may be involved in the continuous versus the blocked-cyclic picture naming paradigm, contributing to the overall different pattern of results observed in our group of patients. In addition, such pathways may also be differently affected in the left PFC patients we tested and could underlie the inter-individual variability we report concerning the cumulative semantic interference effect.
Conclusion
In sum, our group results suggest the left PFC is not always necessary for word selection. In particular, left PFC engagement is reduced when top-down biasing towards task-relevant representations is less salient. We suggest the remaining network of brain regions associated with word selection may be sufficient to overcome interference caused by semantically-related alternatives when left PFC-dependent proactive cognitive control demands are reduced. In addition, the large inter-individual variability observed in the left PFC patients concerning the cumulative semantic interference effect indicates that variability in the deficits following left PFC lesion should also be taken into account in order to further the understanding of the role of this group of brain regions in word selection.
Highlights.
We investigate the role of the left PFC in resolving interference in word selection.
Left PFC-injured patients and controls performed two picture naming tasks.
Left PFC patients are differentially impaired depending on task manipulations.
The left PFC is not always necessary for word selection.
Its involvement is reduced when proactive cognitive control demands are reduced.
Acknowledgements
This research was supported by a post-doctoral grant from the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under Award Number F32DC013245 to S.K.R., NINDS grant 2R37NS21135 and the Nielsen Corporation to R.T.K., grants 10F–RCS-006 and CX000254 from the US Department of Veterans Affairs Clinical Sciences Research and Development Program to N.F.D‥ The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Department of Veterans Affairs, or the United States government. This article was also prepared within the framework of the Basic Research Program at the National Research University Higher School of Economics (HSE) and supported within the framework of a subsidy granted to the HSE by the Government of the Russian Federation for the implementation of the Global Competitiveness Program. We would like to thank Brian Curran and Donatella Scabini, for patient delineation, Brian Curran and Clay Clayworth for lesion reconstruction, and the members of the Center for Aphasia and Related Disorders at the VA Health Care System in Martinez, CA, for neuropsychological testing and for their useful comments on earlier versions of this manuscript. Finally, we are very thankful to the research volunteers who took part in this study.
Appendix
Table 4.
Set of experimental item names, category names in bold, and filler item names for the continuous paradigm.
| Experimental items: | ||||||
|---|---|---|---|---|---|---|
| Body Parts | Clothes | Farm Animals | Furniture | Music | Tableware | Transport |
| legs | bra | sheep | bench | harp | spoon | helicopter |
| neck | sweater | rabbit | drawer | trumpet | fork | truck |
| foot | shoe | cow | library | violin | pot | bus |
| nose | shirt | pig | sofa | organ | knife | train |
| finger | pants | cat | chair | drum | bottle | plane |
| eye | hat | horse | table | guitar | cup | ship |
| hand | dress | dog | bed | piano | glass | car |
| Vegetables | Tools | Marine Animals | Buildings | Appliances | Accessories | Toys |
| garlic | axe | jellyfish | barn | teapot | earring | kite |
| eggplant | scissors | octopus | windmill | blender | sunglasses | balloon |
| carrot | shovel | dolphin | lighthouse | dishwasher | cane | dice |
| onion | hammer | lobster | castle | toaster | necklace | blocks |
| pepper | screwdriver | whale | garage | microwave | purse | doll |
| potato | brush | seal | church | refrigerator | ring | ball |
| corn | saw | shark | house | oven | watch | robot |
| Fillers: | ||||||
| anchor | bell | dart | hose | mouse | rose | waterfall |
| ashtray | book | easel | key | ocean | scales | whistle |
| baby | box | fan | keyboard | painting | sword | yarn |
| barrel | bread | field | knot | paper | television | |
| basket | camera | flag | laptop | pen | toilet | |
| bathtub | chain | flashlight | match | pencil | toothbrush | |
| beach | cigar | hanger | medal | phone | towel | |
| beer | clock | hinge | mountain | razor | tree | |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We used Bayesian estimation as in Kruschke (2013) to compare groups given the distributions of the variables were not normal. Here the estimates were built from 20,000 simulated replications. The fact the HDI crosses zero does not allow us to say there is a credible difference between the two groups. Thus, statistically, there is no difference in age between the two groups.
Conflict of Interest statement
We declare that the research reported here was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Alario F-X, Chainay H, Lehericy S, Cohen L. The role of the supplementary motor area (SMA) in word production. Brain Research. 2006;1076(1):129–143. doi: 10.1016/j.brainres.2005.11.104. [DOI] [PubMed] [Google Scholar]
- Alario FX, del Prado Martín FM. On the origin of the “cumulative semantic inhibition” effect. Memory & cognition. 2010;38(1):57–66. doi: 10.3758/MC.38.1.57. [DOI] [PubMed] [Google Scholar]
- Aristei S, Melinger A, Abdel Rahman R. Electrophysiological chronometry of semantic context effects in language production. Journal of Cognitive Neuroscience. 2011;23(7):1567–1586. doi: 10.1162/jocn.2010.21474. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. Journal of Memory and Language. 2008;59(4):390–412. [Google Scholar]
- Baldo JV, Arévalo A, Patterson JP, Dronkers NF. Grey and white matter correlates of picture naming: Evidence from a voxel-based lesion analysis of the Boston Naming Test. Cortex. 2013;49(3):658–667. doi: 10.1016/j.cortex.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. lme4: Linear mixed-effects models using Eigen and S4. R Package Version 1. 2014a:1–7. Retrieved from http://CRAN.R-project.org/package=lme4. [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. lme4: Linear mixed-effects models using Eigen and S4. ArXiv E-Print; Submitted to Journal of Statistical Software. 2014b Retrieved from http://arxiv.org/abs/1406.5823.
- Belke E, Meyer AS, Damian MF. Refractory effects in picture naming as assessed in a semantic blocking paradigm. The Quarterly Journal of Experimental Psychology Section A. 2005;58(4):667–692. doi: 10.1080/02724980443000142. [DOI] [PubMed] [Google Scholar]
- Belke E, Stielow A. Cumulative and non-cumulative semantic interference in object naming: evidence from blocked and continuous manipulations of semantic context. Quarterly Journal of Experimental Psychology (2006) 2013;66(11):2135–2160. doi: 10.1080/17470218.2013.775318. [DOI] [PubMed] [Google Scholar]
- Bonin P, Peereman R, Malardier N, Méot A, Chalard M. A new set of 299 pictures for psycholinguistic studies: French norms for name agreement, image agreement, conceptual familiarity, visual complexity, image variability, age of acquisition, and naming latencies. Behavior Research Methods, Instruments, & Computers: A Journal of the Psychonomic Society, Inc. 2003;35(1):158–167. doi: 10.3758/bf03195507. [DOI] [PubMed] [Google Scholar]
- Brown AS. Inhibition in cued retrieval. Journal of Experimental Psychology: Human Learning and Memory. 1981;7(3):204–215. [Google Scholar]
- Howard D, Nickels L, Coltheart M, Cole-Virtue J. Cumulative semantic inhibition in picture naming: experimental and computational studies. Cognition. 2006;100(3):464–482. doi: 10.1016/j.cognition.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Chauvel PY, Rey M, Buser P, Bancaud J. What stimulation of the supplementary motor area in humans tells about its functional organization. Advances in Neurology. 1996;70:199–209. [PubMed] [Google Scholar]
- Costa A, Strijkers K, Martin C, Thierry G. The time course of word retrieval revealed by event-related brain potentials during overt speech. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(50):21442–21446. doi: 10.1073/pnas.0908921106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford JR, Garthwaite PH. Investigation of the single case in neuropsychology: confidence limits on the abnormality of test scores and test score differences. Neuropsychologia. 2002;40(8):1196–1208. doi: 10.1016/s0028-3932(01)00224-x. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Garthwaite PH, Howell DC. On comparing a single case with a control sample: an alternative perspective. Neuropsychologia. 2009;47(13):2690–2695. doi: 10.1016/j.neuropsychologia.2009.04.011. http://doi.org/10.1016/j.neuropsychologia.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Damian MF, Vigliocco G, Levelt WJM. Effects of semantic context in the naming of pictures and words. Cognition. 2001;81(3):B77–B86. doi: 10.1016/s0010-0277(01)00135-4. [DOI] [PubMed] [Google Scholar]
- De Zubicaray GI, McMahon KL, Howard D. Perfusion fMRI evidence for priming of shared feature-to-lexical connections during cumulative semantic interference in spoken word production. Language, Cognition and Neuroscience. 2013;30(3):261–272. [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92(1–2):145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Duffau H, Moritz-Gasser S, Mandonnet E. A re-examination of neural basis of language processing: proposal of a dynamic hodotopical model from data provided by brain stimulation mapping during picture naming. Brain and Language. 2014;131:1–10. doi: 10.1016/j.bandl.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23(10):475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Gordon JK, Cheimariou S. Semantic interference in a randomized naming task: effects of age, order, and category. Cognitive Neuropsychology. 2013;30(7–8):476–94. doi: 10.1080/02643294.2013.877437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P. On Broca, brain, and binding: a new framework. Trends in Cognitive Sciences. 2005;9(9):416–423. doi: 10.1016/j.tics.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Hocking J, McMahon KL, de Zubicaray GI. Semantic context and visual feature effects in object naming: an fMRI study using arterial spin labeling. Journal of Cognitive Neuroscience. 2009;21(8):1571–1583. doi: 10.1162/jocn.2009.21114. [DOI] [PubMed] [Google Scholar]
- Hope TMH, Seghier ML, Leff AP, Price CJ. Predicting outcome and recovery after stroke with lesions extracted from MRI images. NeuroImage: Clinical. 2013;2:424–433. doi: 10.1016/j.nicl.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D, Nickels L, Coltheart M, Cole-Virtue J. Cumulative semantic inhibition in picture naming: experimental and computational studies. Cognition. 2006;100(3):464–482. doi: 10.1016/j.cognition.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Indefrey P. The spatial and temporal signatures of word production components: a critical update. Frontiers in Psychology. 2011;2:255. doi: 10.3389/fpsyg.2011.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004;92(1–2):101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Jaeger TF. Categorical Data Analysis: Away from ANOVAs (transformation or not) and towards Logit Mixed Models. Journal of Memory and Language. 2008;59(4):434–446. doi: 10.1016/j.jml.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen N, Carreiras M, Barber HA. Electrophysiological effects of semantic context in picture and word naming. NeuroImage. 2011;57(3):1243–1250. doi: 10.1016/j.neuroimage.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Janssen N, Hernández-Cabrera JA, van der Meij M, Barber HA. Tracking the Time Course of Competition During Word Production: Evidence for a Post-Retrieval Mechanism of Conflict Resolution. Cerebral Cortex (New York, N.Y.: 1991) 2014 doi: 10.1093/cercor/bhu092. [DOI] [PubMed] [Google Scholar]
- Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neuroscience. 2006;139(1):181–193. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Kan IP, Thompson-Schill SL. Selection from perceptual and conceptual representations. Cognitive, Affective & Behavioral Neuroscience. 2004;4(4):466–482. doi: 10.3758/cabn.4.4.466. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Western Aphasia Battery test manual. Grune & Stratton; 1982. [Google Scholar]
- Kroll JF, Stewart E. Category Interference in Translation and Picture Naming: Evidence for Asymmetric Connections Between Bilingual Memory Representations. Journal of Memory and Language. 1994;33(2):149–174. [Google Scholar]
- Kruschke JK. Bayesian estimation supersedes the t test. Journal of Experimental Psychology: General. 2013;142:573–603. doi: 10.1037/a0029146. [DOI] [PubMed] [Google Scholar]
- Levelt WJ, Roelofs A, Meyer AS. A theory of lexical access in speech production. The Behavioral and Brain Sciences. 1999;22(1):1–38. doi: 10.1017/s0140525x99001776. discussion 38–75. [DOI] [PubMed] [Google Scholar]
- Levene H. Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling. Stanford University Press; 1960. Robust tests for equality of variances; pp. 278–292. [Google Scholar]
- Llorens A, Trébuchon A, Riès S, Liégeois-Chauvel C, Alario F-X. How familiarization and repetition modulate the picture naming network. Brain and Language. 2014;133:47–58. doi: 10.1016/j.bandl.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupker SJ. The semantic nature of response competition in the picture-word interference task. Memory & Cognition. 1979;7(6):485–495. [Google Scholar]
- Maess B, Friederici AD, Damian M, Meyer AS, Levelt WJM. Semantic category interference in overt picture naming: sharpening current density localization by PCA. Journal of Cognitive Neuroscience. 2002;14(3):455–462. doi: 10.1162/089892902317361967. [DOI] [PubMed] [Google Scholar]
- Mulatti C, Calia C, Fara DeCaro C, Della Sala S. The cumulative semantic interference effect in normal and pathological ageing. Neuropsychologia. 2014;65:125–130. doi: 10.1016/j.neuropsychologia.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Navarrete E, Del Prato P, Peressotti F, Mahon BZ. Lexical Retrieval is not by Competition: Evidence from the Blocked Naming Paradigm. Journal of Memory and Language. 2014;76:253–272. doi: 10.1016/j.jml.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete E, Del Prato P, Mahon BZ. Factors determining semantic facilitation and interference in the cyclic naming paradigm. Frontiers in Psychology. 2012;3:38. doi: 10.3389/fpsyg.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete E, Mahon BZ, Caramazza A. The cumulative semantic cost does not reflect lexical selection by competition. Acta Psychologica. 2010;134(3):279–289. doi: 10.1016/j.actpsy.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim GM, Dell GS, Schwartz MF. The dark side of incremental learning: a model of cumulative semantic interference during lexical access in speech production. Cognition. 2010;114(2):227–252. doi: 10.1016/j.cognition.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piai V, Roelofs A, Acheson DJ, Takashima A. Attention for speaking: domain-general control from the anterior cingulate cortex in spoken word production. Frontiers in Human Neuroscience. 2013;7:832. doi: 10.3389/fnhum.2013.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-PLUS. Springer Science & Business Media; 2000. [Google Scholar]
- Pisoni A, Papagno C, Cattaneo Z. Neural correlates of the semantic interference effect: new evidence from transcranial direct current stimulation. Neuroscience. 2012;223:56–67. doi: 10.1016/j.neuroscience.2012.07.046. [DOI] [PubMed] [Google Scholar]
- Rahman RA, Melinger A. When bees hamper the production of honey: Lexical interference from associates in speech production. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2007;33(3):604–614. doi: 10.1037/0278-7393.33.3.604. [DOI] [PubMed] [Google Scholar]
- Rahman RA, Melinger A. Semantic context effects in language production: A swinging lexical network proposal and a review. Language and Cognitive Processes. 2009;24(5):713–734. [Google Scholar]
- Richard Ridderinkhof K, Forstmann BU, Wylie SA, Burle B, van den Wildenberg WPM. Neurocognitive mechanisms of action control: resisting the call of the Sirens. Wiley Interdisciplinary Reviews: Cognitive Science. 2011;2(2):174–192. doi: 10.1002/wcs.99. [DOI] [PubMed] [Google Scholar]
- Ries SK, Greenhouse I, Dronkers NF, Haaland KY, Knight RT. Double dissociation of the roles of the left and right prefrontal cortices in anticipatory regulation of action. Neuropsychologia. 2014;63:215–225. doi: 10.1016/j.neuropsychologia.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kümmerer D, Kellmeyer P, Vry M-S, … Weiller C. Ventral and dorsal pathways for language. Proceedings of the National Academy of Sciences. 2008;105(46):18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnur TT, Schwartz MF, Brecher A, Hodgson C. Semantic interference during blocked-cyclic naming: Evidence from aphasia. Journal of Memory and Language. 2006;54(2):199–227. [Google Scholar]
- Schnur TT, Schwartz MF, Kimberg DY, Hirshorn E, Coslett HB, Thompson-Schill SL. Localizing interference during naming: convergent neuroimaging and neuropsychological evidence for the function of Broca’s area. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(1):322–327. doi: 10.1073/pnas.0805874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology. Human Learning and Memory. 1980;6(2):174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Strijkers K, Costa A. Riding the lexical speedway: a critical review on the time course of lexical selection in speech production. Language Sciences. 2011;2:356. doi: 10.3389/fpsyg.2011.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(26):14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, D’Esposito M, Kan IP, Knight RT. Verb generation in patients with focal frontal lesions: a neuropsychological test of neuroimaging findings. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(26):15855–15860. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebuchon-Da Fonseca A, Guedj E, Alario F-X, Laguitton V, Mundler O, Chauvel P, Liegeois-Chauvel C. Brain regions underlying word finding difficulties in temporal lobe epilepsy. Brain: A Journal of Neurology. 2009;132(Pt 10):2772–2784. doi: 10.1093/brain/awp083. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Gracco VL. Contribution of the pre-SMA to the production of words and non-speech oral motor gestures, as revealed by repetitive transcranial magnetic stimulation (rTMS) Brain Research. 2009;1268:112–124. doi: 10.1016/j.brainres.2009.02.076. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Gracco VL. On the selection of words and oral motor responses: evidence of a response-independent fronto-parietal network. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2010;46(1):15–28. doi: 10.1016/j.cortex.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Frontiers in Systems Neuroscience. 2011;5:1. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]