Abstract
During transit through the female reproductive tract, mammalian spermatozoa are exposed to increasing concentrations of progesterone (P4) released by the cumulus oophorus. P4 triggers massive calcium influx into human sperm through activation of the sperm-specific calcium channel CatSper. These properties of human spermatozoa are thought to be unique since CatSper is not progesterone sensitive in rodent sperm. Here, by performing patch clamp recording from spermatozoa from rhesus macaque for the first time, we report that they express P4-sensitive CatSper channel identically to human sperm and react to P4 by inducing responsiveness to zona pellucida, unlike human sperm, which respond directly to P4. We have also determined the physiologic levels of P4 capable of inducing capacitation-associated changes in macaque sperm. Progesterone (1 μM) induced up to a 3-fold increase in the percentage of sperm undergoing the zona pellucida-induced acrosome reaction with the lowest threshold as low as 10 nM of P4. Submicromolar levels of P4 induced a dose-dependent increase in curvilinear velocity and lateral head displacement, while sperm protein tyrosine phosphorylation was not altered. Macaque spermatozoa exposed to 10 μM of P4 developed fully hyperactivated motility. Similar to human sperm, on approaching cumulus mass and binding to zona pellucida, macaque spermatozoa display hyperactivation and undergo an acrosome reaction that coincides with the rise in the sperm intracellular calcium. Taken together, these data indicate that P4 accelerates the completion of capacitation and provides evidence of spermatozoa “priming” as they move into a gradient of progesterone in search for the oocyte.
Keywords: CatSper ion channel, hyperactivation, progesterone, sperm capacitation, zona-induced acrosome reaction
INTRODUCTION
In order to reach full fertilizing capacity, mammalian spermatozoa must undergo a series of physiological changes that are collectively called capacitation [1–3]. Capacitation can be initiated by changes in lipid composition of the sperm plasma membrane, leading to an increase in intracellular calcium, increased cAMP signaling, changes in protein phosphorylation, and modifications in the sperm glycocalyx [4]. On completion of capacitation, the spermatozoon displays hyperactivated motility, characterized by asymmetrical bending of the flagellum in a whip-like motion [5]. It also can recognize and bind to the zona pellucida (ZP), undergo acrosomal exocytosis, penetrate the ZP, and fuse with the oolemma [3]. This process, as it occurs in vitro, typically unfolds gradually in a fraction of the spermatozoa incubated in defined medium containing NaHCO3 and serum albumin [6–8].
In vivo, however, capacitation likely occurs in a more succinct and coordinated fashion. It has been shown in many mammalian species that spermatozoa are sequestered in the isthmic or proximal region of the oviduct and are maintained in a semiquiescent, non-fully capacitated state until the time of ovulation [9, 10]. Changes in the oviductal milieu in response to mid-cycle or late estrus hormones as well as the presence of the cumulus-oocyte complex in the infundibulum appear to trigger the release of sperm from oviductal reservoirs [11, 12]. The liberation of sperm from reservoirs and their movement toward the ampullar region of the oviduct coincides with their attainment of capacitation [11, 12]. However, the factors that orchestrate this activation of sperm in vivo are not well understood. Evidence supports that changes in oviductal fluid ions, proteins, glycosamino-glycans, and energy substrates, all ultimately resulting from changes in secretory activity of the oviductal epithelium, potentially promote capacitation or subcellular aspects of the process [13] . Similarly, progesterone (P4) secreted by ovarian granulosa cells and the cumulus mass surrounding the ovulated egg is also an important component of oviductal fluid around the time of ovulation [11]. Depending on the concentration and the model species, P4 can have various capacitation-related effects on sperm in vitro, including induction of the acrosome reaction (AR), heightened sperm responsiveness to the ZP, increased calcium influx, alterations in patterns of flagellar movement, and induction of hyperactivated motility [14, 15].
The physiological levels of P4 in the fallopian tubes are hard to determine in vivo, and therefore it is unclear which, if any, of the responses of sperm to P4 is representative of events leading up to fertilization in the oviduct. The concentration of P4 used in many studies has been based roughly on measures of P4 in fluid extracted from preovulatory follicles. In humans, these levels can be as high as 50–100 μM [16, 17]. Some authors have projected that levels of progesterone in the oviductal ampulla experienced by sperm may reach micromolar levels as a consequence of rapid steroid diffusion or as fluid accompanying the cumulus-oocyte complex enters the infundibulum on rupture of the follicle [18]. However, evidence suggests that this is not the case in rodents and rabbits. Fluid collected throughout estrus from the ampullary region of oviducts in hamsters and rabbits contained on average 0.4 and 0.2 μM of P4, respectively [19, 20]. In hamsters, the levels of P4 in ampullary fluid dropped approximately 60% following ovulation due to increased fluid secretion by oviductal epithelium. The final progesterone concentration was over 1000-fold lower in oviductal fluid than in follicular fluid, suggesting that as sperm move into the ampulla, they are exposed to at most 10–100 nanomolar levels of P4. Based on measurements of accumulated P4 secreted by cumulus cells in culture medium, levels of progesterone associated with in vitro-matured cumulus-oocyte complexes has been estimated to range from 0.3 to 3 μM [21], but the P4 concentration in a single cumulus mass has yet to be measured directly. It is known that rodent and human sperm respond differently to P4, which may reflect different concentrations of P4 in female reproductive tract of rodents and primates [14, 16, 22–25] Here, we explored whether submicromolar physiological levels of P4 are capable of inducing capacitation-related changes in rhesus macaque sperm. Until recently, capacitation of macaque sperm required the use of exogenous activators, caffeine, and dibutyryl cyclic adenosine monophosphate (dbcAMP). These activators bypass the normal course of cellular events, bringing sperm to a fully capacitated state within 30 min. In contrast, in the present study, we used modified culture medium that resembles periovulatory oviductal fluid with respect to bicarbonate and glucose content and that allows capacitation to occur spontaneously and gradually [26]. This system is therefore potentially more sensitive for detecting changes in the kinetics of capacitation with P4 treatment than previously described macaque sperm capacitation systems. We evaluated the effects of P4 on various capacitation-associated phenomena that are common to most mammals, including humans. We report that similar to human sperm, P4 triggers macaque spermatozoa to undergo CatSper activation and enhances zona binding and hyperactivation. Tyrosine phosphorylation of sperm proteins were not affected by P4 exposure; however, the ability of sperm to bind to ZP and to undergo the AR seems to be clearly progesterone dependent. The clear similarities between macaque and human sperm point to the possibility of considering rhesus macaque as an alternative animal model for male fertility.
MATERIALS AND METHODS
Chemicals and Reagents
Soybean Trypsin Inhibitor (SBTI)-Alexa Fluor488 Conjugate, Lectin peanut agglutinin from Arachis hypogaea (peanut)-Alexa Fluor 488 Conjugate (Alexa-PNA), Fluo-3 AM, Live-Dead Sperm Viability Kit, Pluronic F-127, and Propidium Iodide (PI) were purchased from Molecular Probes (Life Technologies). Dulbecco phosphate-buffered saline (DPBS) was obtained from Gibco BRL. Tris-HCL minigels (4%–20%) 1.0 mm 10× well and Protein Assay and Hoechst 33258 pentahydrate (bisbenzimide) were obtained from Invitrogen. ECL detection kit was purchased from Amersham (GE Health Care). Antiphosphotyrosine antibody (recombinant 4G10; mouse monoclonal) was obtained from Upstate Biotechnology. Cruz Marker molecular-weight standards (sc-2035) were purchased from Santa Cruz Biotechnology Inc. Affinity-Purified Goat anti-human/mouse COX-4I1 antibody was purchased from R&D Systems Inc. Radio Immuno Precipitation Assay Buffer (RIPA) was obtained from Boston Bioproducts. Protease inhibitor was obtained from Calbiochem. Halt Phosphatase Inhibitor Cocktail was purchased from Thermo Scientific. All other reagents were obtained from Sigma Aldrich Chemical Company.
Animal Care
Five adult male rhesus macaques (Macaca mulatta) were housed at the California National Primate Research Center in compliance with standards of the Association for Assessment and Accreditation of Laboratory Animal Care International. Animal protocols were reviewed and approved in advance by the Animal Care and Use Committee of the University of California, Davis, and all studies were conducted in accordance with the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Sperm Collection, Washing, and Capacitation
Semen samples were obtained by electroejaculation from male rhesus macaques under chair restraint as described previously [27]. Following collection, semen samples were diluted in 6 ml of Biggers, Whitten, and Whittingham (BWW) medium modified with HEPES buffer [26, 28], which was maintained at room temperature. Sperm were processed as described by Tollner et al. [26, 29]. Briefly, the semen samples were diluted with an additional 6 ml of BWW medium modified with HEPES buffer containing 0.3% BSA (mBWW) and were washed by centrifugation at 300 × g for 10 min. The resulting pellet was resuspended in 1–2 ml of mBWW medium, layered over 80% Percoll, and centrifuged at 300 × g for 25 min. Following centrifugation, the supernatant was removed and the pellet washed twice by centrifugation (300 × g, 5 min) in BWW (original bicarbonate-buffered formulation) [6] supplemented with 3% BSA. Sperm were resuspended in BWW containing 3% BSA at a concentration of 10–15 × 106/ml and incubated overnight (∼19 h) at room temperature (28°C) with 5% CO2. The next morning, sperm were washed in capacitation medium: BWW with 3% BSA but containing 90 mM bicarbonate and 0.5 mM glucose [30]. Sperm were incubated in capacitation medium for 5 h at 39°C and 5% CO2.
Progesterone Treatment
The effects of progesterone (P4) on sperm capacitation were evaluated at 1, 3, and 5 h of sperm incubation in capacitation medium. P4 or an equal volume of DMSO as a solvent control was added (1% by volume) to suspensions of sperm (final concentration of P4 was 1, 5, or 10 μM) after 0.75, 2.75, and 4.75 h of sperm incubation in capacitation medium. Sperm were further incubated with P4 or DMSO for 15 min, at which point sperm were evaluated for intracellular calcium ([Ca2+i]), AR, tyrosine phosphorylation, and sperm-ZP interaction as described below. The effects of a lower range of P4 doses (0.001, 0.01, 0.1, and 1 μM) on sperm motion and sperm-ZP interaction was evaluated at 3 h of sperm incubation in capacitation medium. For all experiments, the percentage of sperm with progressive motility never dropped below 50%.
Sperm Motion Analysis
For measurements of sperm motion characteristics with computer-assisted sperm analysis (CASA), videomicrography was performed as described previously [20, 21]. Motion characteristics of the recorded sperm were analyzed using the HTM Ceros, version 10.9d (Hamilton Thorne Biosciences, Inc.), visualized with a 20× dark-field phase-contrast objective on an Olympus BH-series phase-contrast microscope (Olympus Corporation) with a heated stage at 37°C. Briefly, following each progesterone treatment, 4-μl drops of sperm suspension were loaded into two MicroCell sperm analysis chambered slides (Vitrolife Inc.) with a 20-μm depth. In each chamber, 8–10 randomly selected microscope fields were recorded, and sperm tracks were digitally captured using a frame rate of 60 Hz and a minimum track time of 0.5 sec. At least 200 sperm per treatment were analyzed for curvilinear velocity (VCL), straight-line velocity (VSL), amplitude of lateral head displacement (ALH), and linearity of sperm trajectory (LIN) [31, 32].
Sperm-Zona Binding and Zona-Induced AR
Real-time zona-induced AR assay was performed as described previously [28]. Briefly, whole ZP were harvested from rhesus macaque ovaries and stored as described previously [33]. Whole ZP were washed in mBWW and mounted in the center of glass slide chambers with a 20-μm depth. Slide chambers were warmed on a microscope stage warmer set at 39°C before sperm were added. Sperm suspensions were adjusted to 3.0 × 106/ml and treated with soybean trypsin inhibitor conjugated to an Alexa-488 fluorochrome (Alexa-SBTI) before being added to the chamber. Alexa-SBTI has been shown to bind to acrosin, an intra-acrosomal serine protease, as sperm undergo the AR [29]. Three minutes following binding of the first sperm to the ZP, the numbers of sperm attached to the ZP were counted as either acrosome reacted (positive for SBTI fluorescence) or acrosome intact (negative for SBTI fluorescence). Two to three zonae per treatment per male were used for all sperm-ZP interaction experiments.
Scanning Laser Confocal Microscopic Imaging
For imaging real-time AR, an Olympus Fluoview 500 scanning laser confocal microscope was used. Alexa488-SBTI (Molecular Probes/Invitrogen) was used as a fluorescent indicator of sperm AR on intact ZP as described [28]. Sperm preparations containing Alexa-SBTI were added to ZP as described above on a Peltier temperature-controlled stage (Brook Industries) at 37°C on an Olympus BX61 upright microscope. Spermatozoa were viewed using both argon laser (488-nm excitation) and transmitted light (interference contrast) to capture fluorescence and light images simultaneously. The confocal microscope was operated in the “free run” mode at 15 frames per second for up to 5 min. Images were recorded through a 20× water immersion fluorescence laser scanning microscope objective. Video imaging of sperm on ZP was initiated just prior to addition of spermatozoa. We focused on the sperm that initially contacted the ZP, keeping the head(s) in the focal plane.
AR of Sperm in Suspension
The AR was also evaluated with Alexa-PNA following treatment of sperm with P4. Hoechst 334258 (0.5 μM) was added as a vitality counterstain to each sample along with progesterone (or DMSO control) and incubated for 15 min. Samples were washed (centrifuged at 735 × g) with DBPS and fixed with 1% paraformaldehyde, washed again with DPBS, and incubated with 95% ethanol. Sperm were dried onto a glass slide and incubated in blocking solution (DPBS with 5% BSA, pH 7.4) overnight at 4°C. Following removal of blocking solution, 10 μl of Alexa-PNA were layered onto the glass slides, and slides were incubated for 15 min at room temperature in the dark. Slides were washed three times with blocking solution. A drop of Vectashield and a coverslip were applied to each slide and then sealed with lacquer-based enamel. Two hundred spermatozoa over 10 microscope fields were scored for acrosomal status.
Tyrosine Phosphorylated Protein and Western Blotting
Protein was extracted from whole sperm as described by Galantino-Homer et al. [34] and Cole et al. [35]. After sperm incubation in capacitation medium or following sperm treatment with dbcAMP and caffeine (positive control for capacitation), protein was extracted from 20 × 106/ml of sperm with RIPA lysis buffer. Solubilizing buffer containing SDS and 2% β-mercaptoethanol was added to sperm protein supernatants. The reduced samples were vortexed for 1 min, followed by boiling for 5 min. Ten microliters of reduced proteins (about 3.26 × 105/ml sperm) were loaded into each lane of a Tris-HCL 4%–20% gradient gel for SDS-PAGE (150 V, 40 mA, 15 W, 15 min; 100 V, 250 mA, 15 W, 60 min) and blotted onto polyvinylidene fluoride (PVDF) membranes (100 V, 250 mA, 15 W, 90 min). Membranes were blocked in 5% gelatin from cold-water fish skin overnight at 4°C or incubated in goat anti-human/mouse COX-4I1 antibody (1:1000). Levels of COX4l1 (a cytochrome c oxidase subunit 4 isoform) on blots were used for estimations of quantity of macaque sperm protein transferred onto Western blot membranes. This approach compared favorably with monoclonal anti-β-tubulin for control of protein blot quantity. Unlike tubulin, which runs at 55 kDa and therefore overlaps with sperm proteins that become phosphorylated with capacitation, COX-4l1 is 21–22 kDa in size and runs below the area of interest on blotting membranes and therefore can be assessed on the same blotting membrane. The membranes were incubated with antiphosphotyrosine recombinant 4G10 mAb (1:1000) in Tween 20 (TTBS) for 1 h on an orbital shaker at room temperature. The blot was washed 3 × 30 min each with TTBS and then incubated in goat anti-mouse IgG conjugated to horseradish peroxidase (HRP; 1:5000) in TTBS on the shaker at room temperature. For COX-4I1 antibody, the blot was incubated in donkey anti-goat IgG HRP (1:2000). After washing, a chemiluminescence signal was developed using ECL-plus reagents and detection on Kodak Biomax MR Film (3- to 5-min exposures). Sperm protein lysates for immunodetection of human and macaque CatSper beta (β) subunits were obtained as described [36], and anti-CatSperβ antibody was used in 1:10,000 dilution [37].
Sperm Electrophysiology
Gigaohm seals were formed at the cytoplasmic droplet that in macaque spermatozoa often was located at the distal part of the midpiece region. Only highly motile capacitated cells were selected. Seals were formed in standard high-saline (HS) buffer containing (in mM) 130 NaCl, 20 HEPES, 10 lactic acid, 5 glucose, 5 KCl, 2 CaCl2, 1 MgSO4, 1 sodium pyruvate, pH 7.4 adjusted with NaOH, and 320 mOsm/L, as reported in [36, 38]. Transition into whole-cell mode was achieved by applying voltage pulses (499–611 mV, 1 msec) and simultaneous suction as described for human spermatozoa [38]. Cells were stimulated every 5 sec, data were sampled at 10 kHz and filtered at 1 kHz, and access resistance was 25–40 MΩ. Pipettes (13–16 MΩ) for whole-cell patch clamp recordings of monovalent CatSper currents were filled with 130 mM Cs-methanesulfonate, 70 mM HEPES, 3 mM EGTA, 2 mM EDTA, and 0.5 mM Tris HCl, pH 7.4, adjusted with CsOH. Divalent-free (DVF) solution for recording of monovalent CatSper currents contained (in mM) 140 Cs-methanesulfonate, 40 HEPES, and 1 EDTA, pH 7.4, adjusted with CsOH. HS solution was used to record baseline current before measuring monovalent CatSper currents. Data were analyzed with Clampfit 10.3 and OriginPro 8.6. Statistical data are presented as mean ± SEM, and n indicates number of experiments. All electrophysiology experiments were performed at 24°C.
Sperm Immunocytochemistry
Macaque sperm cells were seeded onto coverslips in HS solution and processed as described previously for human spermatozoa [37]. Incubation with the primary antibody (rabbit anti-CatSper 4, 1:100 in 0.1% PBS-T and 5% BSA, Alomone lab, #ACC-304) was performed at 4°C overnight. Cells were then washed in PBS-T and incubated with Cy3 conjugated goat anti-rabbit IgG for 45 min at room temperature. After washing, the samples were mounted with ProLong Gold antifade reagent. Images were taken on Olympus IX-71 microscope with Orca-ER camera (Hamamatsu) and processed with HC ImageLive 4.2.033 software.
Statistical Analysis
All percentage data were transformed using the square root of the arcsine before statistical analysis. Differences were examined by using analysis of variance (ANOVA), followed by Tukey all pairwise comparison using Minitab (Minitab, Inc.) or SAS software (SAS Institute Inc.): 1) ZP-AR rate was evaluated for interactive effects of incubation time and P4 treatment by two-way ANOVA, 2) P4 effects on ZP-AR rate both within and across incubation times was evaluated by one-way ANOVA, and 3) the effects of P4 treatment on CASA measures of VCL and ALH were evaluated by two-way ANOVA. Data are reported as mean ± SEM.
RESULTS
P4 Stimulates Zona-Induced AR of Macaque sSpermatozoa
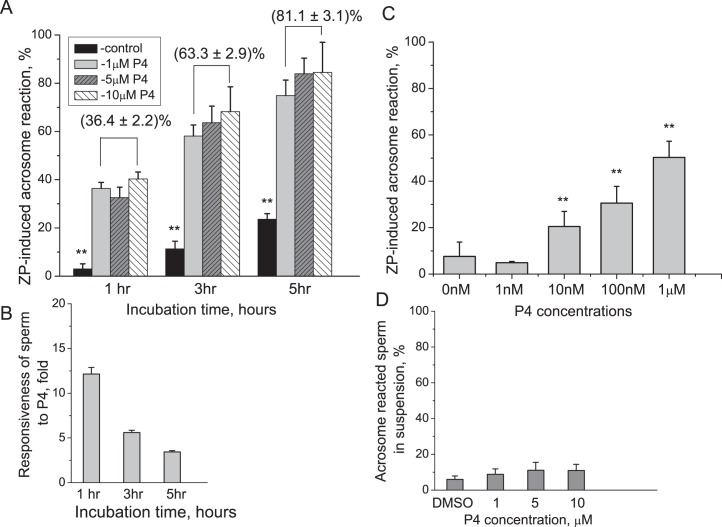
In all zona binding experiments, sperm initiated the AR within 5 sec of attaching to the ZP, as evidenced by visible Alexa-SBTI fluorescence at the point of ZP attachment of the sperm's anterior head. Zona-bound ARs were scored as positive when the anterior head region of sperm corresponding to the acrosomal region displayed bright green fluorescence (see Figure 1 and Supplemental Movie S1 [available online at www.biolreprod.org]). Maximum fluorescence was observed at about 30 sec postattachment (Supplemental Movie S1). P4 treatment increased the percentage of sperm that underwent the ZP-induced AR (Fig. 2A) proportionally to the incubation time prior to P4 exposure. The P4 dose response was 36.4%, 63.3%, and 81.1% for P4 doses of 1, 5, and 10 μM, respectively. The concentration of P4 did not affect the number of sperm cells that underwent the ZP-induced AR with P4 concentrations between 1 and 10 μM. Responsiveness to P4, defined as AR of sperm over control levels, was greatest at 1 h, although P4 continued to promote the zona-induced AR (Fig. 2B). Responsiveness of sperm to ZP increased significantly over 5 h in both control and P4 treatments. By 5 h of incubation, the percentage of control sperm that responded to ZP alone increased nearly 8-fold in comparison to 1 h of incubation (23.6 ± 2.3% vs. 3%; P < 0.05; Fig. 2A). Similarly, sperm treated with P4 demonstrated enhanced responsiveness to the ZP with increased incubation time by doubling on average in the AR rate. Thus, after 1 h of incubation in P4, the percentage of acrosome-reacted cells was 36.4%, 32.6%, and 40.3% for 1, 5, and 10 μM P4, respectively. After 5 h of treatment, the AR rate increased to 74.9%, 83.9%, and 84.5%, respectively (Fig. 2A). In contrast, the numbers of sperm cells that bound to the ZP did not differ with P4 treatment.
FIG. 1.
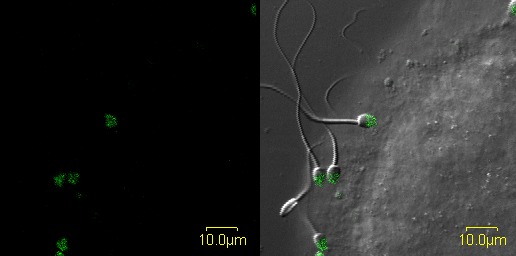
Still image of real-time video (Supplemental Movie S1) of ZP-induced AR of progesterone-treated rhesus macaque sperm. In this confocal video, sperm are shown bound in real time to rhesus ZP and undergoing acrosomal exocytosis characterized by binding of Alexa-488 labeled soybean trypsin inhibitor. Bar = 10 μm.
FIG. 2.
P4 promotes the zona-induced AR in macaque sperm. A) Spermatozoa were capacitated in the presence of either DMSO (control) or P4 (1, 5, and 10 μM) as indicated during different time points. Sperm were introduced into zona-binding chambers, and the percentage of sperm that had undergone the acrosome reaction on intact ZP was determined after 3 min. ** indicates significant differences (P < 0.0001) between treatment or across time intervals. B) Responsiveness of sperm to P4 decreases with incubation time. C) Submicromolar levels of P4 promote the zona-induced AR. Spermatozoa were capacitated for 2.75 h and treated with either DMSO (control) or progesterone (0.001–1 μM). The percentage of ZP-induced AR was determined as in A. ** above columns indicates significant differences (P < 0.01) between progesterone treatments and control. In both A and B, experiments were performed with sperm from four different males, and two zonae per male per treatment were used for each experiment. Data are represented as mean ± SEM of four independent experiments. D) Sperm incubated with different concentration of P4 in suspension undergo very mild AR, which is not dependent on P4 in the ranges between 1 and 10 μM. Only 6.0 ± 1.9% sperm undergo AR during incubation in vehicle control DMSO. The numbers were statistically similar across incubation range in 1, 5, and 10 μM with 8.8 ± 3%, 11.1 ± 4.4%, and 11.0 ± 3.4%, respectively, across all time points.
Next, we evaluated the threshold of sperm responsiveness to P4 by examining the zona-induced AR rate following sperm treatment with a lower dose range of progesterone at ∼3 h of incubation in capacitation medium. The average zona binding-induced AR rate for control, 1 nM, 10 nM, 100 nM, and 1 μM progesterone was 7.6 ± 6.2%, 4.9 ± 0.5%, 20.5 ± 6.5%, 30.6 ± 7.2%, and 50.3 ± 7.0%, respectively. The zona-induced AR rate of sperm treated with 100 nm and 1 μM progesterone was significantly higher (P < 0.05 and P < 0.01, respectively) than AR rates observed with sperm receiving the solvent control (Fig. 2C). Analyses of differences in AR rates between controls and various P4 treatments suggest that the threshold of sperm responsiveness to progesterone approaches 10 nM.
While the real-time zona-binding assay enables direct observation of the AR, it is possible that P4 induces the AR directly and that some sperm bind to the ZP shortly after initiating the AR in suspension. We determined that P4 does not directly induce the AR in sperm in solution in the absence of ZP by measuring AR rates using Alexa-PNA staining. No differences in the percentage of acrosome-reacted sperm in suspension were observed between solvent control (Fig. 2D) and millimolar concentrations of P4. AR rates remained relatively constant with duration of incubation prior to adding P4.
P4 Changes Macaque Sperm Motility and Triggers Hyperactivation
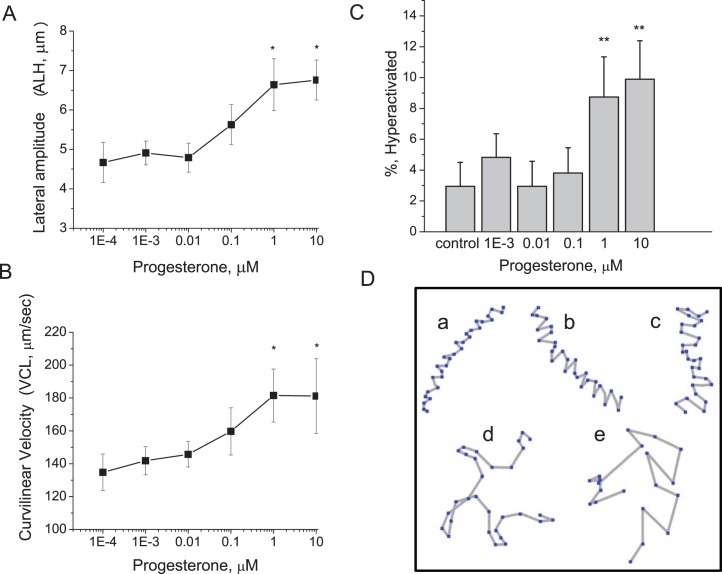
The velocity and pattern of macaque sperm motion changed with P4 concentration. The ALH increased proportionally with increased P4 concentrations, with maximum responsiveness achieved at 10 μM P4, corresponding to a 44% increase in ALH compared to controls (Fig. 3A). Similarly, sperm appeared to exhibit a dose-dependent increase in VCL, although treatments could not be shown to differ using Tukey pairwise comparisons. At 1 μM progesterone, sperm VCL was on average 26% greater than that of controls (Fig. 3B). Changes in VSL (μm/sec) and LIN with P4 treatment were not significant. An increase in the percentage of hyperactivated sperm was seen after treatment with 1 and 10 μM P4 (Fig. 3C). Figure 3D demonstrates representative tracks of motility patterns of sperm undergoing hyperactivated motility from low ALH (Fig. 3D, a–c) to increasingly elevated ALH (Fig. 3D, d and e). We have demonstrated previously that sperm hyperactivation is characterized by extremes in both ALH and VCL [32, 39, 40]. The percentage of sperm that exceeded these threshold values increased with dose of P4. Maximal responses of 8.7 ± 2.6% and 9.9 ± 2.5% of sperm exhibiting hyperactivated motility were observed following treatment with 1 and 10 μM P4, respectively (Fig. 3C).
FIG. 3.
P4 induces changes in sperm motion characteristics. Sperm were incubated in capacitation medium for 2.75 h and then treated with either DMSO (control) or P4 (0.001–10 μM). Changes in sperm ALH (A) and VCL (B) and the percentage of sperm exhibiting hyperactivated motility (C) were evaluated with CASA. Experiments were performed with sperm from five different males. For each experiment, a minimum of 200 sperm tracks per treatment were analyzed. Asterisks above data points and columns indicate significant differences (P < 0.05) between progesterone treatments and control. Data are represented as mean ± SEM of four independent experiments. Responses at 1 and 10 μM P4 were significantly different from responses of sperm receiving solvent control (P < 0.05). D) Representative tracks of sperm generated by CASA demonstrate range of motility patterns exhibited by sperm following treatment with progesterone. Increased progesterone levels resulted in higher proportions of sperm with tracks resembling b and c compared to sperm with tracks resembling a, a typical motility pattern for controls. Tracks d and e reflect fully hyperactivated sperm, which approached 10% of sperm treated with 1 and 10 μM progesterone, respectively.
P4 Did Not Affect Levels of Tyrosine Phosphorylation of Sperm Proteins
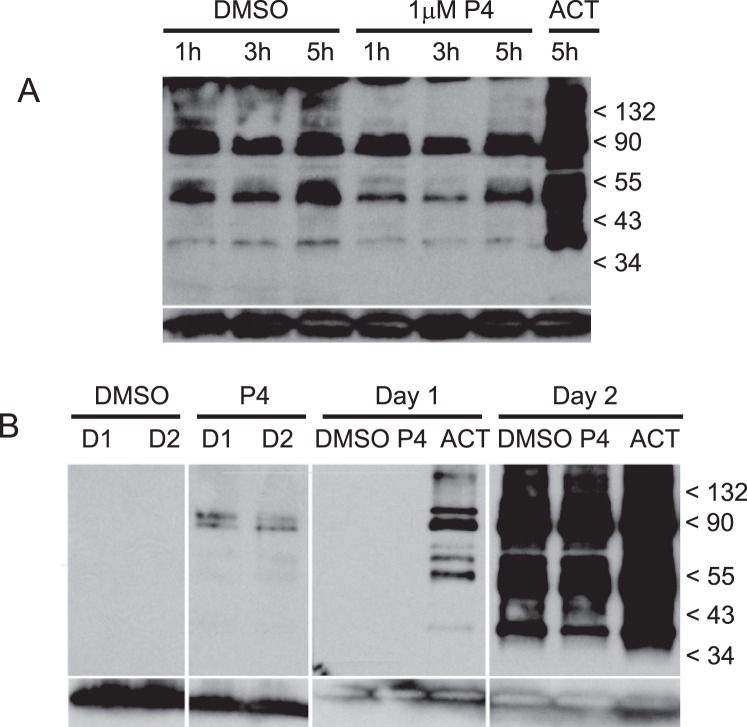
Tyrosine phosphorylation activity in sperm was determined by Western blotting with type-specific antiphosphorylated tyrosine protein antibodies (4G10). Levels of tyrosine phosphorylation of sperm proteins did not change with P4 treatment (Fig. 4A), nor did levels of phosphorylation differ with incubation time in capacitation medium (following overnight incubation in BWW) as determined at 1, 3, and 5 h (Fig. 4A, lanes 1–6). When sperm are incubated in capacitation medium on the day of semen collection, no protein tyrosine phosphorylation was apparent, even after treatment with P4 (Fig. 4B, lanes 1 and 2). Tyrosine phosphorylation could be detected at very reduced levels shortly after sperm were transferred into capacitation medium following overnight incubation (Fig. 4B, lane 3), suggesting that the majority phosphorylation occurred within the first hour of incubation in capacitation medium. In both same-day and overnight experiments, treatment of sperm with activator compounds, dbcAMP, and caffeine induced phosphorylation activity (Fig. 4A, lane 7; Fig. 4B, lane 10).
FIG. 4.
Tyrosine-specific phosphorylation of sperm proteins. Following incubation in capacitation medium, which follows the standard overnight incubation protocol (second day of incubation), sperm were treated with progesterone and then solubilized for Western blots (A). Whole sperm protein was separated by PAGE, blotted onto PVDF membranes, and probed with antiphosphotyrosine recombinant 4G10 mAb. Blot A contains the following sperm treatments; DMSO at 1, 3, and 5 h (lanes 1–3); 1 μM progesterone at 1, 3, and 5 h (lanes 4–6); and activator at 5 h (lane 7). Protein phosphorylation activity was compared between sperm incubated in capacitation medium on the same day as sample collection (first day) and following overnight incubation and subsequent transfer into capacitation medium (second day) (B). Blot B contains the following sperm treatments: DMSO at 0.25 h the first day and 0.25 h the second day (lanes 1 and 3, respectively); 1 μM progesterone at 0.25 h the first day and 0.25 h the second day (lanes 2 and 4, respectively); DMSO, 1 μM progesterone and activator at 5 h the first day (lanes 5–7, respectively); and DMSO, 1 μM progesterone and activator at 5 h the second day (lanes 8–10). Anti-human/mouse COX-4I1 antibody was used as a control for quantities of sperm proteins loaded on gels and blotted onto membranes (bottom row, A and B). A protein of approximately 20–22 kDa corresponding to COX-4I1 is shown at the bottom each lane.
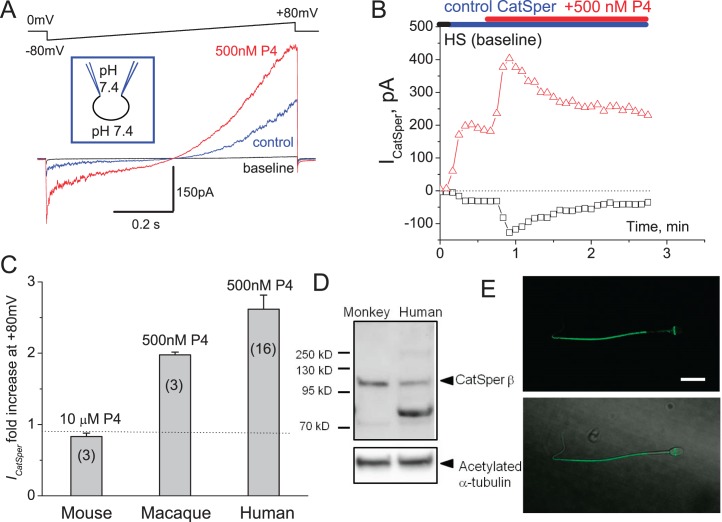
Whole-Cell Recording from Macaque Sperm Revealed That CatSper Channel Is Expressed in Macaque Sperm and Is P4 Sensitive
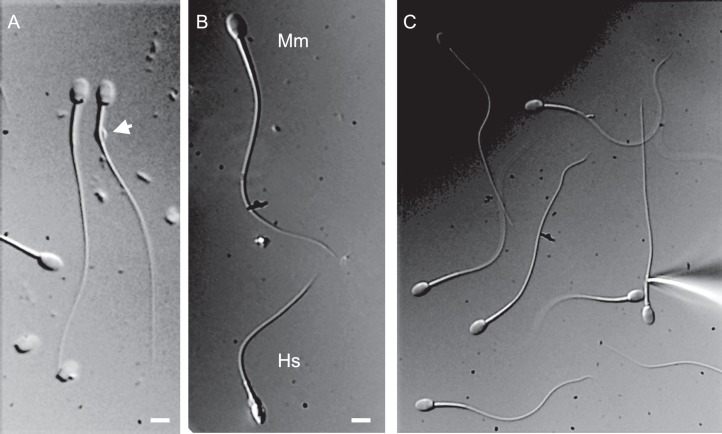
P4-activated calcium channel CatSper has been shown to be present in human spermatozoa and is crucial for male fertility [37]. However, P4 activation of CatSper was unique for human sperm since mouse spermatozoa have CatSper, which is progesterone insensitive [24]. In order to determine whether macaque sperm resemble human spermatozoa in their ion channel repertoire and to determine whether P4 triggers macaque sperm hyperactivation by activating CatSper channel, as it does in humans, we performed whole-cell recording from capacitated macaque spermatozoa. Figure 5 shows that a portion of macaque sperm retains cytoplasmic droplets, enabling the formation of a tight gigaohm seal between cytoplasmic droplets and the recording pipette. Macaque sperm are larger than human sperm (Fig. 5B), and their relative capacitance is 1.6 ± 0.03 pF (n = 5) in comparison with human sperm, which have a capacitance of 0.9 pF [36]. Whole-cell patch clamp recording revealed that monkey sperm possess CatSper channel, which is also progesterone sensitive, in the same manner as human sperm (Fig. 6, A and B). Immunoblotting and immunocytochemistry analyses further confirmed CatSper channel expression in primate spermatozoa (Fig. 6, C and D) and its strict flagellar localization primarily in the principal piece (Fig. 6D). Therefore, macaque spermatozoa indeed resemble human sperm in their regulation and display similar regulatory elements, such as the P4-sensitive flagellar calcium channel CatSper. We have recorded CatSper current from three monkey sperm cells, and all three cells were progesterone sensitive (Fig. 6A). However, macaque CatSper quickly desensitized on progesterone exposure, which was different from human CatSper behavior (Fig. 6B). The average response of macaque outward CatSper in the absence of progesterone was 107.6 ± 47.4 pA, which was increased on 500 nM P4 exposure to 216.1 ± 96.8 pA. The average response of macaque inward CatSper in the absence of progesterone was −24.9 ± 9.7 pA, which was increased on 500 nM P4 exposure to −104 ± 66.7 pA. This corresponds to an average 2-fold increase of the outward macaque CatSper, which is comparable to the 2.7-fold increase of human outward CatSper current (Fig. 6C). Mouse CatSper is known to be insensitive to progesterone, and indeed we have not seen any upregulation of murine CatSper channel activity upon exposure to even 10 μM P4 (Fig. 6C).
FIG. 5.
DIC images of macaque and human sperm. A) Some macaque sperm retain a cytoplasmic droplet (arrow), which makes the sperm patch clamp technique applicable. B) Comparison between spermatozoon from M. mulatta (Mm) and human fertile donor (Hs). Bar = 5 μm. C) Patch clamp pipette attached to the monkey sperm.
FIG. 6.
CatSper channel is present in macaque sperm and is progesterone sensitive. A) CatSper currents (ICatSper) recorded from macaque spermatozoon in response to an indicated voltage ramp. Representative monovalent whole-cell ICatSper in the absence (blue) and presence (red) of 500 nM progesterone (P4). Currents were elicited by voltage ramps from a holding potential of 0 mV. Ramps were applied from −80 to +80 mV. The bath solution (DVF) contained 140 mM CsMeSO3, 1 mM EDTA, and 40 mM Hepes, pH adjusted with CsOH to 7.4. Pipettes were filled with 130 mM CsMeSO3, 60 mM Hepes, 1 mM EDTA, and 5 mM EGTA, pH 7.4. Shown are representative data out of three similar recordings; three different macaque spermatozoa were tested. B) Time dependence of primate ICatSper sensitivity to P4. Baseline (no CatSper activity) was recorded in HS solution, after which it was substituted with DVF solution as mentioned above; 500 nM P4 were added in DVF solution. C) Comparison between sensitivity of human, mouse, and primate CatSpers to indicated concentrations of P4; n = indicates number of sperm cells tested. D) Western blot of primate and human isolated spermatozoa shows CatSperβ subunit being expressed in sperm of both species. Acetylated tubulin was used as loading control. E) CatSper immunocytochemistry staining. Anti-CatSper4 antibody (Alomone labs; 1:100 dilution) recognize principal piece of monkey sperm. Upper panel shows immunofluorescent signal for CatSper4 from monkey spermatozoon, while the lower panel shows DIC image of the same cell overlaid with the immunofluorescent signal shown above.
DISCUSSION
Our experiments establish four novel findings: 1) P4 induces changes in macaque sperm motion, leading to fully hyperactivated motility; 2) P4 promotes the zona-induced AR, increasing AR rates by over 3-fold; 3) P4 has no effect on capacitation-associated tyrosine phosphorylation of sperm proteins; and 4) macaque sperm express P4-sensitive CatSper channels. While previous reports indicate a modest change in motility and intracellular calcium [Ca++i] in macaque sperm [15, 40], the responsiveness of sperm to P4 in these earlier studies does correlate with observations described in our report. We believe these differences stem from the use of fundamentally different capacitation systems.
Until recently, efficient capacitation of macaque sperm required the use of the exogenous activators, caffeine, and dbcAMP. Events that normally occur over several hours in vitro in sperm of other mammalian species (development of hyperactivated motility, elevation of intracellular Ca2+, phosphorylation/dephosphorylation of sperm proteins, loss of surface proteins, etc.) are completed or optimized within as little as 30 min of chemical activation in the macaque [15, 41, 42]. Alternatively, culture medium modified to resemble periovulatory oviductal fluid with respect to bicarbonate and glucose content has been shown to support macaque sperm capacitation without the use of chemical stimulants [26]. In the present study, rhesus monkey sperm incubated in this high-bicarbonate/low-glucose medium undergoes capacitation spontaneously. At 5 h of incubation, nearly 25% of sperm were able to undergo the zona-induced AR, while over 80% were capacitated following progesterone treatment. On the other hand, with respect to sperm-ZP interaction, macaque sperm capacitated with activators are not responsive to progesterone. Tyrosine phosphorylation of sperm proteins, shown in Figure 4 (lanes 8–10), exemplifies the overstimulatory effects of activator. One hour following treatment with activator, levels of tyrosine phosphorylation greatly exceed levels observed in sperm following 5 h of incubation in capacitation medium, a condition that is consistent with capacitation in over 80% of sperm. We suspect that the lack of reports evaluating the effects of P4 in sperm from this common primate species is a reflection of the tendency of activator compounds to override or bypass the normal sequence of events associated with capacitation.
Most notable of our current findings, P4 promotes or primes sperm for the zona-induced AR but does not induce the AR directly, at least not at levels we evaluated. First described by Tollner and coworkers [28, 42], the use of SBTI for detection of sperm AR as it occurs in “real time” enabled us to observe the AR after sperm bind to the ZP. Over 80% of macaque sperm that underwent the ZP-induced AR did so within 1 min of sperm binding to the intact ZP and exhibited a similar time course of “tight binding” and SBTI labeling as described for sperm from the cynomolgus macaque [26, 42]. Treatment of sperm with P4 did not appear to change the kinetics of tight binding and SBTI labeling at earlier time points (1 and 3 h of incubation in capacitation medium) but significantly enhanced in a dose-dependent manner the number of sperm that could undergo the AR after binding to the ZP. At later time points (5 h of incubation in capacitation medium), progesterone treatment appeared to enhance the kinetics of the zona-induced AR, with many sperm exhibiting SBTI-associated fluorescence over the acrosome within seconds of binding to the ZP. Progesterone treatment had no effect on the acrosomal status of sperm in suspension. The binding of viable sperm that were already acrosome reacted (SBTI positive) to intact ZP was a rare event, and these sperm were not scored.
P4 has been shown to prime sperm for the AR in response to the ZP in other mammalian species [43]. First demonstrated in the mouse, P4 treatment of sperm under capacitating conditions enhanced the ZP-induced AR rate by approximately 33% [43]. Similarly in humans, treatment of sperm with P4 increased the rate of ZP-induced AR by 22% [44]. At the threshold of P4 sensitivity of the zona-induced AR in our study, we observed a significant enhancement of the AR rate of approximately 73%. Progesterone promotes a 6.6-fold increase in the zona-induced AR in the macaque, four to five times the response observed in mouse and human sperm for higher doses. In short, our data suggest that macaque sperm under the conditions described here are uniquely sensitive to the priming effects of this steroid hormone. Further supporting the species differences in AR requirements, we note that capacitated mouse sperm readily complete the AR in response to progesterone [45] and that, in transgenic male mice whose spermatozoa express enhanced green fluorescence protein, the vast majority of sperm can be seen to undergo the AR as they transit through the cumulus and before binding to the ZP [46]. Similarly, in humans, progesterone has been shown to directly induce both capacitation and the AR [14]. While real-time observations of acrosomal exocytosis in cumulus are lacking in humans, studies demonstrate that some 16%–17% of human sperm that interact with the cumulus mass undergo the AR [47, 48]. In contrast, the lack of response of macaque sperm, even at arguably supraphysiologic levels of P4 with respect to the AR, and the near instantaneous response following binding to the ZP suggest that the ZP is an important trigger of the AR and not merely a redundant mechanism [49, 50].
P4 induces hyperactivation in primate sperm [15]. Our study shows that both VCL and ALH steadily increase in response to increasing concentrations of progesterone. As these motion parameters increase, the percentage of sperm that exhibit hyperactivated motility also increases, with maximal effects observed at 1 and 10 μM progesterone. Hyperactivation in response to 1 μM progesterone has been observed in activated cynomolgus monkey sperm, although the threshold CASA measures for sorting hyperactivated sperm (VCL > 150 μm/sec and ALH > 8 μm) [15] were much lower than those used in this study. Since the sperm of rhesus and cynomolgus macaques are very similar and both studies used the same CASA system with nearly identical settings, we consider the results to be comparable. When we apply the lower sorting values to sperm tracks analyzed in our study, nearly 60% of sperm treated with 1 μM progesterone would be considered hyperactivated. Yet most sperm that possess motion characteristics near these threshold values have tracks similar to those shown in Figure 3, B and C (but not Fig. 3, D and E), which show motion patterns more consistent with the sorting values we selected. Using the cynomolgous macaque model [15], the authors report a 20%–25% increase in the number of sperm that achieve hyperactivated motility with progesterone, compared with a 4-fold, or 400%, increase in numbers of hyperactivated sperm reported here. Based on similar sorting procedures used in the study with cynomolgus sperm, treatment of human sperm with 1 and 3 μM progesterone induced a modest increase in the percentage of sperm with hyperactivated motility in normal [51] and infertile men [52], respectively.
The effects of P4 on both the macaque sperm AR and motility are likely due to the well-characterized ability of P4 to stimulate an increase in sperm [Ca2+i]. Intracellular [Ca2+] elevation resulting from both influx and release from intracellular stores is of pivotal importance in sperm function, leading to changes in sperm motility and the remodeling of lipid membranes that primes the sperm for zona-binding and the ZP-induced AR [23, 53]. Recent studies demonstrate that the primary and direct effect of P4 on sperm motility is through mobilization of Ca2+ via the CatSper channel [37, 54]. The CatSper channel is located in the principal piece of sperm and is necessary for hyperactivated motility [55–57]. P4-dependent increases in ion currents through the CatSper cation channel induce hyperactivation [24, 37]. Progesterone has also been shown to induce Ca2+ mobilization in the sperm head, working through a gamma aminobutyric acid-like receptor in the plasma membrane [58], although it appears possible that a rise in Ca2+ in the head of sperm is also due to tail-to-head propagation of Ca2+ following activation of CatSper channels [59, 60]. Whatever the source of Ca2+, P4 stimulates an intracellular surge of this cation into the sperm head, either leading directly to the AR [16, 58] or enhancing the zona-induced AR [43, 44].
In the macaque, P4 treatment of sperm results in a dose-dependent increase in sperm [Ca2+i] following 1 h of incubation in capacitation medium. An increase in [Ca2+i] reflective of P4 dose was observed at later time points, but the changes in Ca2+ were no longer significantly different from those observed in the controls. The lack of significant effect appears to be due to a sizable increase in [Ca2+i] observed in control sperm by 3 and 5 h of incubation, a phenomenon that potentially explains why sperm were relatively less responsive to P4 at later time points with respect to ZP-induced AR.
P4 does not appear to induce tyrosine phosphorylation changes in macaque sperm. In agreement with observations in mouse and human, conditions that inhibit generation of cAMP and activation of PKA had no effect on the P4-induced ion influx [24], nor did P4 treatment of capacitated sperm result in increased cAMP [54].
Our data suggest that as primate sperm experience increasing concentrations of P4, they are readied to interact with the oocyte and its vestments. We propose that primate sperm enter the ampulla of the oviduct, perhaps not fully capacitated but able to respond to nanomolar and picomolar concentrations of progesterone. Picomolar levels of P4 attract sperm and create pulsatile changes or waves of [Ca2+i] that stimulate an exaggerated flagellar “kick” that rights sperm into the path of the P4 gradient [61, 62]. As sperm approach the cumulus-oocyte complex and nanomolar concentrations of progesterone, the activity of CatSper channels increase, with Ca2+ currents greatly enhanced by alkaline conditions [24, 54] known to be present in the periovulatory oviductal milieu in macaques and women [13, 63, 64]. The rising flagellar Ca2+ serves to propel sperm with greater velocity and vigor toward the oocyte. On reaching the cumulus mass, sperm potentially experience micromolar levels of progesterone [21], which produces a surge of [Ca2+i] of several hundred nanomolar [65–67] levels, resulting in repeated sweeping, asymmetric deflections of the flagellum characteristic of hyperactivation [68]. Hyperactivated motility potentially assists sperm in penetrating the viscoelastic hyaluronic acid extracellular matrix of the cumulus mass [69, 70]. Our studies suggest that as progesterone-primed sperm reach the ZP, they bind and readily, possibly within seconds, undergo the acrosome reaction. These sperm, having been hyperactivated for only a few moments, are able to sustain the flagellar torque necessary to penetrate the ZP [71] and reach the oolemma. Future studies involving “single sperm” [Ca2+i] measurements will be necessary to link progesterone-induced changes in Ca2+ mobilization with specific sperm behaviors.
In conclusion, the capacitation conditions described in this study are consistent with periovulatory oviductal fluid conditions in women and nonhuman primates with respect to pH, bicarbonate concentration, and levels of glucose [13, 26]. Only under these conditions have the majority of macaque sperm been shown to capacitate spontaneously [26] and, in the present study, are found to be highly responsive to P4. While many sperm are able to capacitate without P4 over extended incubation times [26], the addition of P4 increases the number of sperm that can complete the process at earlier time points. In short, our results demonstrate that P4 accelerates the completion of capacitation of macaque sperm, as evidenced by the ZP-induced AP and hyperactivated motility, and suggest that as sperm move into a gradient of P4 in vivo, they become fully primed to interact with the oocyte.
Footnotes
This work was supported by the National Institutes of Health, Office of Research Infrastructure Programs (formerly National Center for Research Resources), Division of Comparative Medicine grant no. R01RR016581 (S.A.M.), and the March of Dimes Basil O'Connor Award and Hellman Fellowship (P.V.L.).
These authors contributed equally to this work.
These are authors are considered joint senior authors.
REFERENCES
- Austin CR. Observations on the penetration of the sperm in the mammalian egg Aust J Sci Res B 1951. 4 581– 596 [DOI] [PubMed] [Google Scholar]
- Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes Nature 1951. 168 697– 698 [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. Mammalian fertilization In Knobil ENJ. (ed.), The Physiology of Reproduction New York: Raven Press; 1994. 189– 317 [Google Scholar]
- Jaiswal BS, Eisenbach M. Capacitation San Diego: Academic Press; 2002. 57– 117 [Google Scholar]
- Chang MC. The meaning of sperm capacitation. A historical perspective J Androl 1984. 5 45– 50 [DOI] [PubMed] [Google Scholar]
- Overstreet JW, Yanagimachi R, Katz DF, Hayashi K, Hanson FW. Penetration of human spermatozoa into the human zona pellucida and the zona-free hamster egg: a study of fertile donors and infertile patients Fertil Steril 1980. 33 534– 542 [DOI] [PubMed] [Google Scholar]
- Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation Development 1995. 121 1129– 1137 [DOI] [PubMed] [Google Scholar]
- Visconti PE, Stewart-Savage J, Blasco A, Battaglia L, Miranda P, Kopf GS, Tezon JG. Roles of bicarbonate, cAMP, and protein tyrosine phosphorylation on capacitation and the spontaneous acrosome reaction of hamster sperm Biol Reprod 1999. 61 76– 84 [DOI] [PubMed] [Google Scholar]
- Smith TT. The modulation of sperm function by the oviductal epithelium Biol Reprod 1998. 58 1102– 1104 [DOI] [PubMed] [Google Scholar]
- Topfer-Petersen E, Wagner A, Friedrich J, Petrunkina A, Ekhlasi-Hundrieser M, Waberski D, Drommer W. Function of the mammalian oviductal sperm reservoir J Exp Zool 2002. 292 210– 215 [DOI] [PubMed] [Google Scholar]
- Hunter RH, Rodriguez-Martinez H. Capacitation of mammalian spermatozoa in vivo, with a specific focus on events in the Fallopian tubes Mol Reprod Dev 2004. 67 243– 250 [DOI] [PubMed] [Google Scholar]
- Suarez SS, Pacey AA. Sperm transport in the female reproductive tract Hum Reprod Update 2006. 12 23– 37 [DOI] [PubMed] [Google Scholar]
- Leese HJ, Tay JI, Reischl J, Downing SJ. Formation of Fallopian tubal fluid: role of a neglected epithelium Reproduction 2001. 121 339– 346 [DOI] [PubMed] [Google Scholar]
- Baldi E, Luconi M, Muratori M, Marchiani S, Tamburrino L, Forti G. Nongenomic activation of spermatozoa by steroid hormones: facts and fictions Mol Cell Endocrinol 2009. 308 39– 46 [DOI] [PubMed] [Google Scholar]
- Gwathmey T, Blackmore PF, Mahony MC. Progesterone-induced calcium influx in cynomolgus monkey (Macaca fascicularis) spermatozoa J Androl 2000. 21 534– 540 [PubMed] [Google Scholar]
- Osman RA, Andria ML, Jones AD, Meizel S. Steroid induced exocytosis: the human sperm acrosome reaction Biochem Biophys Res Commun 1989. 160 828– 833 [DOI] [PubMed] [Google Scholar]
- Wen X, Li D, Tozer AJ, Docherty SM, Iles RK. Estradiol, progesterone, testosterone profiles in human follicular fluid and cultured granulosa cells from luteinized pre-ovulatory follicles. Reprod Biol Endocrinol. 2010;8:117. doi: 10.1186/1477-7827-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CV, Barratt CL, Publicover SJ. Stimulation of human spermatozoa with progesterone gradients to simulate approach to the oocyte. Induction of [Ca(2+)](i) oscillations and cyclical transitions in flagellar beating J Biol Chem 2004. 279 46315– 46325 [DOI] [PubMed] [Google Scholar]
- Libersky EA, Boatman DE. Progesterone concentrations in serum, follicular fluid, and oviductal fluid of the golden hamster during the periovulatory period Biol Reprod 1995. 53 477– 482 [DOI] [PubMed] [Google Scholar]
- Richardson LL, Oliphant G. Steroid concentrations in rabbit oviductal fluid during oestrus and pseudopregnancy J Reprod Fertil 1981. 62 427– 431 [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Kawashima I, Gunji Y, Hishinuma M, Shimada M. Progesterone is essential for maintenance of Tace/Adam17 mRNA expression, but not EGF-like factor, in cumulus cells, which enhances the EGF receptor signaling pathway during in vitro maturation of porcine COCs J Reprod Dev 2010. 56 315– 323 [DOI] [PubMed] [Google Scholar]
- Baldi E, Casano R, Falsetti C, Krausz C, Maggi M, Forti G. Intracellular calcium accumulation and responsiveness to progesterone in capacitating human spermatozoa J Androl 1991. 12 323– 330 [PubMed] [Google Scholar]
- Blackmore PF, Beebe SJ, Danforth DR, Progesterone Alexander N. and 17 alpha-hydroxyprogesterone. Novel stimulators of calcium influx in human sperm J Biol Chem 1990. 265 1376– 1380 [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm Nature 2011. 471 387– 391 [DOI] [PubMed] [Google Scholar]
- Pietrobon EO, Soria M, Dominguez LA. Monclus Mde L, Fornes MW. Simultaneous activation of PLA2 and PLC are required to promote acrosomal reaction stimulated by progesterone via G-proteins Mol Reprod Dev 2005. 70 58– 63 [DOI] [PubMed] [Google Scholar]
- Tollner TL, Vandevoort CA, Yudin AI, Treece CA, Overstreet JW, Cherr GN. Release of DEFB126 from macaque sperm and completion of capacitation are triggered by conditions that simulate periovulatory oviductal fluid Mol Reprod Dev 2009. 76 431– 443 [DOI] [PubMed] [Google Scholar]
- Sarason RL, VandeVoort CA, Mader DR, Overstreet JW. The use of nonmetal electrodes in electroejaculation of restrained but unanesthetized macaques J Med Primatol 1991. 20 122– 125 [PubMed] [Google Scholar]
- Tollner TL, Yudin AI, Cherr GN, Overstreet JW. Real-time observations of individual macaque sperm undergoing tight binding and the acrosome reaction on the zona pellucida Biol Reprod 2003. 68 664– 672 [DOI] [PubMed] [Google Scholar]
- Tollner TL, Yudin AI, Cherr GN, Overstreet JW. Soybean trypsin inhibitor as a probe for the acrosome reaction in motile cynomolgus macaque sperm Zygote 2000. 8 127– 137 [DOI] [PubMed] [Google Scholar]
- Aardema H, Vos PL, Lolicato F, Roelen BA, Knijn HM, Vaandrager AB, Helms JB, Gadella BM. Oleic acid prevents detrimental effects of saturated fatty acids on bovine oocyte developmental competence Biol Reprod 2011. 85 62– 69 [DOI] [PubMed] [Google Scholar]
- Tollner TL, Dong Q, VandeVoort CA. Frozen-thawed rhesus sperm retain normal morphology and highly progressive motility but exhibit sharply reduced efficiency in penetrating cervical mucus and hyaluronic acid gel Cryobiology 2011. 62 15– 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumber J, Meyers SA. Hyperactivated motility in rhesus macaque (Macaca mulatta) spermatozoa J Androl 2006. 27 459– 468 [DOI] [PubMed] [Google Scholar]
- VandeVoort CA, Tollner TL, Overstreet JW. Sperm-zona pellucida interaction in cynomolgus and rhesus macaques J Androl 1992. 13 428– 432 [PubMed] [Google Scholar]
- Galantino-Homer HL, Visconti PE, Kopf GS. Regulation of protein tyrosine phosphorylation during bovine sperm capacitation by a cyclic adenosine 3′5′-monophosphate-dependent pathway Biol Reprod 1997. 56 707– 719 [DOI] [PubMed] [Google Scholar]
- Cole JA, Meyers SA. Osmotic stress stimulates phosphorylation and cellular expression of heat shock proteins in rhesus macaque sperm J Androl 2011. 32 402– 410 [DOI] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Fedorenko A, Kirichok Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel Cell 2010. 140 327– 337 [DOI] [PubMed] [Google Scholar]
- Smith JF, Syritsyna O, Fellous M, Serres C, Mannowetz N, Kirichok Y, Lishko PV. Disruption of the principal, progesterone-activated sperm Ca2+ channel in a CatSper2-deficient infertile patient Proc Natl Acad Sci U S A 2013. 110 6823– 6828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lishko P, Clapham DE, Navarro B, Kirichok Y. Sperm patch-clamp Methods Enzymol 2013. 525 59– 83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudin AI, Tollner TL, Treece CA, Kays R, Cherr GN, Overstreet JW, Bevins CL. Beta-defensin 22 is a major component of the mouse sperm glycocalyx Reproduction 2008. 136 753– 765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahony MC, Gwathmey T. Protein tyrosine phosphorylation during hyperactivated motility of cynomolgus monkey (Macaca fascicularis) spermatozoa Biol Reprod 1999. 60 1239– 1243 [DOI] [PubMed] [Google Scholar]
- Boatman DE, Bavister BD. Stimulation of rhesus monkey sperm capacitation by cyclic nucleotide mediators J Reprod Fertil 1984. 71 357– 366 [DOI] [PubMed] [Google Scholar]
- Tollner TL, Yudin AI, Treece CA, Overstreet JW, Cherr GN. Macaque sperm release ESP13.2 and PSP94 during capacitation: the absence of ESP13.2 is linked to sperm-zona recognition and binding Mol Reprod Dev 2004. 69 325– 337 [DOI] [PubMed] [Google Scholar]
- Roldan ER, Murase T, Shi QX. Exocytosis in spermatozoa in response to progesterone and zona pellucida Science 1994. 266 1578– 1581 [DOI] [PubMed] [Google Scholar]
- Schuffner AA, Bastiaan HS, Duran HE, Lin ZY, Morshedi M, Franken DR, Oehninger S. Zona pellucida-induced acrosome reaction in human sperm: dependency on activation of pertussis toxin-sensitive G(i) protein and extracellular calcium, and priming effect of progesterone and follicular fluid Mol Hum Reprod 2002. 8 722– 727 [DOI] [PubMed] [Google Scholar]
- Hector FH, Paola DC. Mora Gustavo G, Arturo BG, Ivone CR. Inhibition of the acrosome reaction (AR) and fertilization capacity of mouse spermatozoa by norethisterone A-ring reduced metabolite (5alpha-NET) Andrologia 2005. 37 135– 142 [DOI] [PubMed] [Google Scholar]
- Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Chiba K, Hirohashi N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization Proc Natl Acad Sci U S A 2011. 108 4892– 4896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SJ, Chiu PC, Lee KF, Tse JM, Ho PC, Yeung WS. Establishment of a capillary-cumulus model to study the selection of sperm for fertilization by the cumulus oophorus Hum Reprod 2004. 19 1562– 1569 [DOI] [PubMed] [Google Scholar]
- Stock CE, Bates R, Lindsay KS, Edmonds DK, Fraser LR. Human oocyte-cumulus complexes stimulate the human acrosome reaction J Reprod Fertil 1989. 86 723– 730 [DOI] [PubMed] [Google Scholar]
- Hirohashi N, Spina FA, Romarowski A, Buffone MG. Redistribution of the intra-acrosomal EGFP before acrosomal exocytosis in mouse spermatozoa Reproduction 2015. 149 657– 663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffone MG, Foster JA, Gerton GL. The role of the acrosomal matrix in fertilization Int J Dev Biol 2008. 52 511– 522 [DOI] [PubMed] [Google Scholar]
- Uhler ML, Leung A, Chan SY, Wang C. Direct effects of progesterone and antiprogesterone on human sperm hyperactivated motility and acrosome reaction Fertil Steril 1992. 58 1191– 1198 [PubMed] [Google Scholar]
- Oehninger S, Sueldo C, Lanzendorf S, Mahony M, Burkman LJ, Alexander NJ, Hodgen GD. A sequential analysis of the effect of progesterone on specific sperm functions crucial to fertilization in vitro in infertile patients Hum Reprod 1994. 9 1322– 1327 [DOI] [PubMed] [Google Scholar]
- O'Toole CM, Arnoult C, Darszon A, Steinhardt RA, Florman HM. Ca(2+) entry through store-operated channels in mouse sperm is initiated by egg ZP3 and drives the acrosome reaction Mol Biol Cell 2000. 11 1571– 1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm Nature 2011. 471 382– 386 [DOI] [PubMed] [Google Scholar]
- Carlson AE, Burnett LA, del Camino D, Quill TA, Hille B, Chong JA, Moran MM, Babcock DF. Pharmacological targeting of native CatSper channels reveals a required role in maintenance of sperm hyperactivation. PLoS One. 2009;4:e6844. doi: 10.1371/journal.pone.0006844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Moran MM, Navarro B, Chong JA, Krapivinsky G, Krapivinsky L, Kirichok Y, Ramsey IS, Quill TA, Clapham DE. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility Proc Natl Acad Sci U S A 2007. 104 1219– 1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, Tilly JL, Clapham DE. A sperm ion channel required for sperm motility and male fertility Nature 2001. 413 603– 609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meizel S, Turner KO, Nuccitelli R. Progesterone triggers a wave of increased free calcium during the human sperm acrosome reaction Dev Biol 1997. 182 67– 75 [DOI] [PubMed] [Google Scholar]
- Xia J, Reigada D, Mitchell CH, Ren D. CATSPER channel-mediated Ca2+ entry into mouse sperm triggers a tail-to-head propagation Biol Reprod 2007. 77 551– 559 [DOI] [PubMed] [Google Scholar]
- Xia J, Ren D. The BSA-induced Ca2+ influx during sperm capacitation is CATSPER channel-dependent. Reprod Biol Endocrinol. 2009;7:119. doi: 10.1186/1477-7827-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidobaldi HA, Teves ME, Unates DR, Anastasia A, Giojalas LC. Progesterone from the cumulus cells is the sperm chemoattractant secreted by the rabbit oocyte cumulus complex. PLoS One. 2008;3:e3040. doi: 10.1371/journal.pone.0003040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren-Benaroya R, Orvieto R, Gakamsky A, Pinchasov M, Eisenbach M. The sperm chemoattractant secreted from human cumulus cells is progesterone Hum Reprod 2008. 23 2339– 2345 [DOI] [PubMed] [Google Scholar]
- Maas DH, Storey BT, Mastroianni L., Jr Hydrogen ion and carbon dioxide content of the oviductal fluid of the rhesus monkey (Macaca mulatta) Fertil Steril 1977. 28 981– 985 [PubMed] [Google Scholar]
- Gardner DK, Lane M, Calderon I, Leeton J. Environment of the preimplantation human embryo in vivo: metabolite analysis of oviduct and uterine fluids and metabolism of cumulus cells Fertil Steril 1996. 65 349– 353 [DOI] [PubMed] [Google Scholar]
- Kobori H, Miyazaki S, Kuwabara Y. Characterization of intracellular Ca(2+) increase in response to progesterone and cyclic nucleotides in mouse spermatozoa Biol Reprod 2000. 63 113– 120 [DOI] [PubMed] [Google Scholar]
- Kirkman-Brown JC, Bray C, Stewart PM, Barratt CL, Publicover SJ. Biphasic elevation of [Ca(2+)](i) in individual human spermatozoa exposed to progesterone Dev Biol 2000. 222 326– 335 [DOI] [PubMed] [Google Scholar]
- Bedu-Addo K, Barratt CL, Kirkman-Brown JC, Publicover SJ. Patterns of [Ca2+](i) mobilization and cell response in human spermatozoa exposed to progesterone Dev Biol 2007. 302 324– 332 [DOI] [PubMed] [Google Scholar]
- Olson SD, Suarez SS, Fauci LJ. Coupling biochemistry and hydrodynamics captures hyperactivated sperm motility in a simple flagellar model J Theor Biol 2011. 283 203– 216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez SS, Katz DF, Overstreet JW. Movement characteristics and acrosomal status of rabbit spermatozoa recovered at the site and time of fertilization Biol Reprod 1983. 29 1277– 1287 [DOI] [PubMed] [Google Scholar]
- Drobnis EZ, Yudin AI, Cherr GN, Katz DF. Kinematics of hamster sperm during penetration of the cumulus cell matrix Gamete Res 1988. 21 367– 383 [DOI] [PubMed] [Google Scholar]
- Drobnis EZ, Yudin AI, Cherr GN, Katz DF. Hamster sperm penetration of the zona pellucida: kinematic analysis and mechanical implications Dev Biol 1988. 130 311– 323 [DOI] [PubMed] [Google Scholar]