Abstract
Xenobiotic estrogens, such as bisphenol A (BPA), disrupt a wide variety of genomic estrogen actions, but their nongenomic estrogen actions remain poorly understood. We investigated nongenomic estrogenic effects of low concentrations of BPA and three related alkylphenols on the inhibition of zebrafish oocye maturation (OM) mediated through a G protein-coupled estrogen receptor 1 (Gper)-dependent epidermal growth factor receptor (Egfr) pathway. BPA (10–100 nM) treatment for 3 h mimicked the effects of estradiol-17beta (E2) and EGF, decreasing spontaneous maturation of defolliculated zebrafish oocytes, an effect not blocked by coincubation with actinomycin D, but blocked by coincubation with a Gper antibody. BPA displayed relatively high binding affinity (15.8% that of E2) for recombinant zebrafish Gper. The inhibitory effects of BPA were attenuated by inhibition of upstream regulators of Egfr, intracellular tyrosine kinase (Src) with PP2, and matrix metalloproteinase with ilomastat. Treatment with an inhibitor of Egfr transactivation, AG1478, and an inhibitor of the mitogen-activated protein kinase (MAPK) 3/1 pathway, U0126, increased spontaneous OM and blocked the inhibitory effects of BPA, E2, and the selective GPER agonist, G-1. Western blot analysis showed that BPA (10–200 nM) mimicked the stimulatory effects of E2 and EGF on Mapk3/1 phosphorylation. Tetrabromobisphenol A, 4-nonylphenol, and tetrachlorobisphenol A (5–100 nM) also inhibited OM, an effect blocked by cotreatment with AG1478, as well as with the GPER antagonist, G-15, and displayed similar binding affinities as BPA to zebrafish Gper. The results suggest that BPA and related alkylphenols disrupt zebrafish OM by a novel nongenomic estrogenic mechanism involving activation of the Gper/Egfr/Mapk3/1 pathway.
Keywords: bisphenol A, EGFR, endocrine disruptors, GPER, GPR30, MAPkinase, meiotic arrest, nonylphenol, oocyte maturation, tetrabromobisphenol A, tetrachorobisphenol A, zebrafish
INTRODUCTION
Extensive research over the past 25 yr has identified a wide variety of genomic estrogen actions in vertebrates that are altered by exposure to anthropogenic chemicals with estrogenic activity (xenoestrogens) through their interactions with nuclear estrogen receptors (ERs) [1–5]. In contrast, although xenoestrogens have also been shown to interfere with nongenomic (nonclassical) estrogen actions [6–8], this mechanism of endocrine disruption has received relatively little attention, and remains poorly understood. Evidence has accumulated that a novel, seven-transmembrane protein, G protein-coupled estrogen receptor 1 (GPER, formerly known as GPR30), is the likely intermediary in a broad range of rapid, cell surface-initiated nongenomic or pregenomic estrogen actions in vertebrate cells and tissues [9–11]. Both wild-type and recombinant human and teleost GPER membrane proteins display high-affinity (dissociation constant = 2.3–3.3 nM), low-capacity, displaceable, specific estradiol-17β (E2) binding characteristic of membrane estrogen receptors [12–14]. Estrogen-induced signal transduction through GPER both in immortalized breast cancer cells and in teleost oocytes involves activation of a stimulatory G protein (Gs) and associated second messenger pathways through cAMP and protein kinase A (PKA), and also through epidermal growth factor receptor (EGFR) transactivation [15–17]. Estrogen activation of these pathways in breast cancer cells causes c-fos expression, proliferation, and migration [18, 19], whereas, in fish oocytes, it prevents the resumption of oocyte meiotic maturation (OM) [13, 17, 20]. The finding that the xenoestrogens, bisphenol A (BPA), 4-nonylphenol (NP), and Kepone (chlordecone), display high binding affinities for human recombinant GPER and act as receptor agonists, mimicking the stimulatory effects of E2 on cAMP production [21], suggests GPER-dependent E2 actions may be susceptible to interference by these compounds.
Although the estrogenic actions of BPA have been known for over 70 yr [22], BPA is still widely used in plastic and epoxy resin manufacture, and is a high-production chemical with a worldwide production of over five million tons/yr [23]. Unbound BPA leaches into the environment from a variety of products, including tin cans and plastic waste in landfills, and is also discharged in sewage, resulting in contamination of surface water, ground water, and sediments [24, 25]. Field surveys from around the world have detected BPA in both urban and rural waterways, suggesting that its potential adverse effects on aquatic organisms may be widespread and not restricted to sites close to the source of BPA pollution [25–28]. BPA concentrations typically are in the range of 3–30 ng/L in the majority of freshwater rivers and streams [29], but much higher concentrations (1–47 μg/L) have been measured at heavily contaminated industrial sites [30]. BPA is moderately bioconcentrated in the tissues of wild fish, with concentrations ranging from 0.2 to 12 μg/kg [31, 32]. Exposure to BPA is nearly ubiquitous in humans, and it has been detected in more than 90% of the persons surveyed in the United States [33]. Moreover, high concentrations of BPA, up to 42 μg/L, have been measured in the blood of workers in BPA-manufacturing plants [34]. However, despite intensive research on the adverse effects of BPA exposure over the last decade, considerable controversy remains over whether BPA, at environmentally realistic concentrations, exerts estrogenic effects and if it poses a human health risk [35–38]. Estrogen exposure of rodents through diet, water bottles, cages, and bedding has likely contributed to the inconsistent results obtained in animal studies with low BPA exposures [39]. In addition, estrogenic actions of BPA often cannot be readily distinguished from other toxic mechanisms, such as its antiandrogenic actions in in vivo studies. Therefore, there is a need to evaluate the estrogenic effects of low BPA concentrations in additional well-characterized animal models of estrogen action, and particularly in those mediated through alternative nonclassical mechanisms.
Estrogen maintenance of meiotic arrest via a nonclassical mechanism has been extensively characterized in Atlantic croaker and zebrafish oocytes [13, 17, 20, 40, 41]. Estrogens, produced by ovarian follicle cells or applied exogenously, activate Gper on the zebrafish oocyte plasma membrane, resulting in rapid Gper-dependent signaling and inhibition of the resumption of OM via a nongenomic mechanism. The results of in vitro OM studies using selective GPER and ER modulators, knockdown of Gper and Esr1 (ERα) expression in zebrafish oocytes by microinjection with morpholino antisense oligonucleotides to these estrogen receptors, or treatment with a specific Gper antibody clearly show an exclusive role for Gper in mediating this inhibitory estrogen action [13, 20]. BPA binds to human GPER with an inhibitory concentration causing a 50% reduction in E2 binding (IC50) of 0.63 × 10−6 M and relative binding affinity (RBA) of 2.83% that of estradiol-17β [21], which is higher than its IC50 reported for human ERα (1 × 10−4 to 4 × 10−5 M) and ERβ (2.5 × 10−5 to 1 × 10 −6 M), with RBAs of 0.001%–0.4% [42, 43]. On the basis of these results, it is hypothesized that BPA activates Gper-dependent signaling in fish during the reproductive cycle to disrupt an important nongenomic estrogenic action regulating the onset of OM.
The aim of this study was to test this hypothesis by investigating possible nongenomic estrogenic effects of BPA mediated through Gper on the maintenance of oocyte meiotic arrest in zebrafish, a well-established vertebrate model of estrogen action through this receptor [17, 20, 40, 41]. The effects of environmentally relevant concentrations of BPA (5–100 nM, equivalent to 1.1–22.8 μg/L) on zebrafish Gper activation and interference with the onset of OM were investigated using an in vitro zebrafish OM bioassay. Interactions of BPA with zebrafish Gper were assessed in a competitive binding assay using zebrafish Gper-transfected HEK293 cells. A potential mechanism of BPA inhibition of OM through activation of an Egfr/Mapk (mitogen-activated protein kinase) 3/1 pathway was investigated using inhibitors of several components of this signaling pathway [17]. In addition, possible activation of Gper-dependent membrane adenylyl cyclase (mAcy) activity was examined by measuring the effects of BPA treatments on cAMP levels in zebrafish oocytes. Finally, the estrogenic actions of the structurally related compounds, the flame retardants tetrachlorobisphenol A (TCBPA) and tetrabromobisphenol A (TBBPA), as well as NP, a degradation product of NP ethoxylate nonionic surfactants (for structures, see Supplemental Fig. S1; supplemental figures are available online at www.biolreprod.org), on zebrafish OM were also investigated to determine if these environmental contaminants exert similar nongenomic estrogenic activities through Gper.
MATERIALS AND METHODS
Chemicals
Ilomastat was purchased from Enzo Life Sciences (Farmington, NY), and U0126 was purchased from Cell Signaling Technology (Danvers, MA). BPA was purchased from City Chemical (West Haven, CT), and TCBPA was purchased from TCI America (Portland, OR). The NP was obtained from Huntsman Corporation (Port Neches, TX). The specific GPER antagonist, G-15, was a gift from Dr. Eric Prossnitz (University of New Mexico Health Science Center, Albuquerque, NM). The tracer used in the estrogen membrane binding assay, [2,4,6,7-3H] estradiol-17β (84Ci/mmol) ([3H]-E2), was purchased from Perkin Elmer (Waltham, MA). The selective antibiotic G418 (geneticin) was purchased from Invitrogen (Grand Island, NY), and actinomycin D was purchased from Biovision (Milpitas, CA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise stated.
Animals
Mature zebrafish (Danio rerio) were purchased from Freshwater Fish (Lewisville, TX) or Seagrest Farms (Gibsonton, FL) and held in 10-gallon tanks with recirculating water (salinity: 0.50/00) at the University of Texas Marine Science Institute. Female zebrafish were maintained in the presence of males at 28°C and subjected to a 14L:10D photoperiod to promote oocyte development. Fish were fed commercial brine shrimp flakes twice daily and live brine shrimp to promote robust oocyte production. Fish were acclimated to laboratory conditions for 1 wk before use in oocyte maturation assays. All the animal care and use protocols were approved by the University of Texas at Austin Animal Care and Use Committee.
Zebrafish Oocyte Maturation Bioassay
For each assay, 10–15 gravid females were killed humanely by immersion in a bath containing tricaine methanesulfonate (MS222) at a concentration of 200 mg/L [20]. Ovarian tissue containing intact follicles was removed and placed in 60% Leibovitz L-15 (L-15) media at 22–24°C in a sterile plastic culture plate. The ovarian tissue was divided into small fragments using fine forceps and scalpel blades under a binocular microscope. The follicle-enclosed oocytes were separated from the ovarian tissue by pipetting the fragments approximately 50 times through a Pasteur pipette. The follicle-enclosed oocytes were then washed three times with L-15 media prior to assay or further treatments.
The follicle layer was removed from the oocytes by incubation with 50 μg/ml collagenase for 45 min [20]. Removal of the follicle layers was confirmed by the absence of 4′,6-diamidino-2-phenylindole-stained nuclei surrounding the oocytes, as described previously [17, 20]. The collagenase was then removed by washing the oocytes three times in L-15 media. Removal of the follicle cells allowed the direct effects of the treatments on oocytes to be investigated.
The in vitro OM bioassay was conducted as described previously with defolliculated oocytes [20]. The inhibitory influence of endogenous E2 on the resumption of meiosis is lost after removal of the follicle layers and the primary site of E2 production [40], which is restored by treatment with E2 and estrogenic compounds [20]. Consequently, vehicle treatment of defolliculated oocytes assesses spontaneous OM in the in vitro bioassay (i.e., nonhormonal, not influenced by E2 secreted by the follicle layer), which can reach 50% of the oocytes after 6 h of incubation, whereas spontaneous maturation of vehicle-treated, follicle-enclosed oocytes is negligible [20]. In the present study, the in vitro OM bioassay was used to determine if BPA and related alkylphenols mimic the inhibitory nongenomic effects of E2 (i.e., are estrogenic) on spontaneous OM and act through the same signaling pathways as those previously identified for E2 and specific GPER agonist, G-1 [17]. Defolliculated oocytes with diameters greater than 450 μm were selected and placed in each well (20–25 oocytes/well) of a 24-well plate with 1 ml of L-15 media. The test compounds for all experiments, with the exception of the OM bioassay with actinomycin D (see Fig. 2A), were dissolved in ethanol and added to the wells (1 μl). The same volume of ethanol was added to the vehicle control wells. dimethyl sulfoxide was used as the solvent for the study with actinomycin D, and 1 μl was added to all wells. The plate was then placed in an incubator at 24°C and meiotic maturation of the oocytes was observed under a dissecting microscope after different periods of incubation, between 3 and 6 h. The oocytes were counted as mature when the germinal vesicle began to disappear and the ooplasm was no longer dense and had become opaque (see Ref. 17 for images of zebrafish OM). Oocyte maturation was recorded for all treatment groups in each assay when >30% of the oocytes in the vehicle control group had undergone germinal vesicle breakdown (GVBD). In most cases, 30% of untreated, denuded oocytes underwent spontaneous GVBD within 3 h. All treatments were replicated three times in an experiment, and all experiments were repeated at least three times. The percent GVBD was calculated as the number of mature oocytes divided by the total number of large 450-μm-diameter oocytes, multiplied by 100. A positive control treatment with 5 nM of the maturation-inducing hormone (MIH) for zebrafish, 17,20β-dihydroxy-4-pregen-3-one (dihydroxyprogesterone [DHP]), was included in all assays to ensure that oocytes were maturationally competent. A negative control treatment with either 100 nM estradiol-17β (E2) or 100 nM of the GPER agonist, G-1 (EMD chemicals, San Diego, CA), was also included in all assays to confirm the inhibitory effects of these estrogens on OM and for comparison with the BPA results. The inhibitory effects of BPA on OM were investigated over the concentration range of 5 nM–100 nM. The effects of blocking several components of the Egfr/Mapk3/1 pathway on the inhibitory effects of 100 nM BPA on OM were examined using specific inhibitors of this signaling pathway at concentrations previously shown to be effective in blocking E2 inhibition of OM [17]. The Src inhibitor, PP2, and the matrix metalloproteinase (MMP) inhibitor, ilomastat, were used at a concentration of 10 μM, whereas the EGFR (ErbB1) inhibitor, AG1478, and the MAPK3/1 inhibitor, U0126, were tested at a concentration of 50 μM. The inhibitory effects of 100 nM BPA on OM were also examined in the presence of 0.1 μg/ml actinomycin D (transcription inhibitor) in order to determine whether BPA is acting through a genomic mechanism [17]. The role of Gper in mediating the inhibitory effects of BPA on OM was investigated by cotreatment with the selective GPER antagonist, G-15, at a concentration of 100 nM [17] and by coincubation with a polyclonal croaker Gper antibody (serum dilution, 1:300) or an equivalent amount of control rabbit IgG (stock dilution, 1:300) [20].
Culture of Gper-Transfected HEK-293 Cells and Plasma Membrane Preparation
HEK293 cells stably expressing zebrafish gper cDNA [40] were selected and maintained with 750 μg/ml G418 in Dulbecco modified Eagle medium/Ham F-12 media without phenol red, supplemented with 5% FBS and cultured as described previously for human GPER [12]. After 7–8 days of culture, cells were harvested by washing three times with ice-cold PBS, followed by the addition of 5 ml ice-cold HAED buffer (25 mM Hepes, 10 mM NaCl, 1 mM dithioerythritol, 1 mM EDTA) and removal of the cells with a cell scraper. Whole cells were either frozen at −80°C or used immediately. The cell suspension was centrifuged for 5 min at 1500 × g. The cells were resuspended in 4 ml HAED with 0.1% protease inhibitor and then centrifuged again at 1500 × g for 5 min. The cells were resuspended in 4 ml HAED and subsequently sonicated for 7 sec. The cell homogenate was then centrifuged for 7 min at 1000 × g to remove the nuclei. The supernatant was centrifuged at 20 000 × g for 25 min to obtain the plasma membrane fraction. The plasma membrane pellet was resuspended in HAED to produce a final protein concentration of 1–1.5 mg/ml.
Competitive Binding of BPA and Related Alkylphenols to Recombinant Zebrafish Gper
The competitive binding assays of BPA, TBBPA, TCBPA, and NP binding to zebrafish Gper on plasma membrane fractions of transfected HEK293 cells were conducted in triplicate, as previously described [12] with minor modifications. Plasma membrane fractions (0.5–0.7 mg/ml) in 150 μl HAED buffer were incubated with E2 or BPA (dissolved in 1 μl ethanol) over the concentration range of 10−10 to 10−5 M together with 4 nM of [3H]-E2 for 30 min at 4°C. The reactions were stopped by rapid filtration through Whatman GF/B filters with a multiwall cell harvester (Brandel, Gaithsburg, MD). Single-point competitive binding assays were conducted in triplicate with 1 μM of the alkylphenols, and with 0.1 μM and 1 μM E2 incubated with [3H]-E2. The radioactivity was measured by scintillation counting. The displacement of [3H]-E2 from the plasma membrane fractions by the range of BPA concentrations was expressed as a percentage of the maximum specific binding of E2.
Assay of Mapk3/1 Activation
Mapk3/1 activation was assayed by assessing Mapk3/1 phosphorylation. Defolliculated zebrafish oocytes, prepared as described previously for the OM bioassay, were transferred to a 24-well plate containing 1 ml of L-15 media and incubated with 10–200 nM BPA and 100 nM E2 for 15 min at 24°C. The reaction was stopped by placing the plate in an ice bath, the oocytes were pelleted by centrifugation (5–10 sec at 1000 × g), and the L-15 medium was discarded. The oocytes were then washed with 1 ml ice-cold PBS, centrifuged briefly (5–10 sec at 1000 × g), excess PBS was removed, and the oocytes were then stored at −80°C until being analyzed. Oocyte lysates were prepared as described previously [17]. Ice-cold RIPA buffer (100 μl) containing 0.1% protease inhibitor was added to the oocytes, which were then homogenized for 1 min using a glass, handheld homogenizer on ice, vortexed for 30 min at 4°C, followed by centrifugation at 15 000 × g for 5 min to remove the insoluble cell debris. The protein concentration in the supernatant was quantified with a NanoDrop 2000 (Thermo Scientific, Waltham, MA). The samples were boiled with reducing buffer for 5 min and loaded (15 μg protein/lane) on a 10% SDS-polyacrylamide gel and resolved by gel electrophoresis. The protein samples were then transferred for 1 h at 0°C to a nitrocellulose membrane (Bio-Rad, Hercules, CA). Membranes were blocked for 1 h at room temperature in blocking buffer (1.5 g dry milk, 30 ml PBS, 30 μl Tween-20). Membranes were then incubated with the primary rabbit antibody for phosphorylated MAPK3/1 (1:1000; Cell Signaling) and with the primary mouse antibody for total MAPK3/1 (1:1000; Cell Signaling) overnight at 4°C. Membranes were washed and then incubated with secondary antibodies (1:5000), goat anti-rabbit and goat anti-mouse, for 1.5 h. Phosphorylated Mapk3/1 was detected at 800 nm and total Mapk3/1 at 680 nm using a Li-Cor Odyssey Imaging System (Li-Cor, Lincoln, NE). Protein expression was quantified by measuring densitometry using Image J software from the Public Research Centre Henri Tudor (Luxembourg-Kirchberg).
Cyclic AMP Measurement
Measurement of cAMP production by zebrafish oocytes was performed as described previously [20, 40]. Oocytes (30–40 well) were incubated in triplicate with 100 nM E2, 100 nM BPA, or 100 nM G-1 at 24°C for 3 h. The oocytes were lysed, and cAMP concentration in the lysate was subsequently analyzed with a cAMP EIA kit (Cayman Chemical, Ann Arbor, MI) following the manufacturer's instructions.
Statistics
Oocyte maturation results are expressed as means ± SEM of 6–15 observations, and all experiments were repeated at least three times with different batches of oocytes from different donors. Data were analyzed by one-way ANOVA followed by the Bonferroni post hoc test using GraphPad Prism 3.0 software (GraphPad Software, San Diego, CA).
RESULTS
Effects of BPA on OM
Approximately 50% of the vehicle-treated denuded oocytes had undergone spontaneous GVBD by the end of the 3-h incubation period (Fig. 1A). Treatment with 5 nM of the zebrafish MIH, DHP, caused a further marked increase in GVBD to 85% within 3 h (Fig. 1), indicating that the oocytes were maturationally competent. Treatment with 100 nM E2 for 3 h caused a significant decrease in spontaneous OM (∼10% GVBD) compared to that in the vehicle-treated group, confirming the presence of an estrogen-sensitive OM-inhibitory mechanism in the denuded oocytes (Fig. 1A). These inhibitory effects of E2 on OM were mimicked by 100 nM BPA. Treatment with 100 nM EGF caused a similar inhibition of spontaneous OM (Fig. 1A), consistent with previous studies implicating the EGFR pathway in maintenance of meiotic arrest [17]. BPA over the range of concentrations of 10 nM–100 nM inhibited spontaneous OM, mimicking the effects of E2, whereas % GVBD after treatment with 5 nM BPA was not significantly different from vehicle controls (Fig. 1B).
FIG. 1.
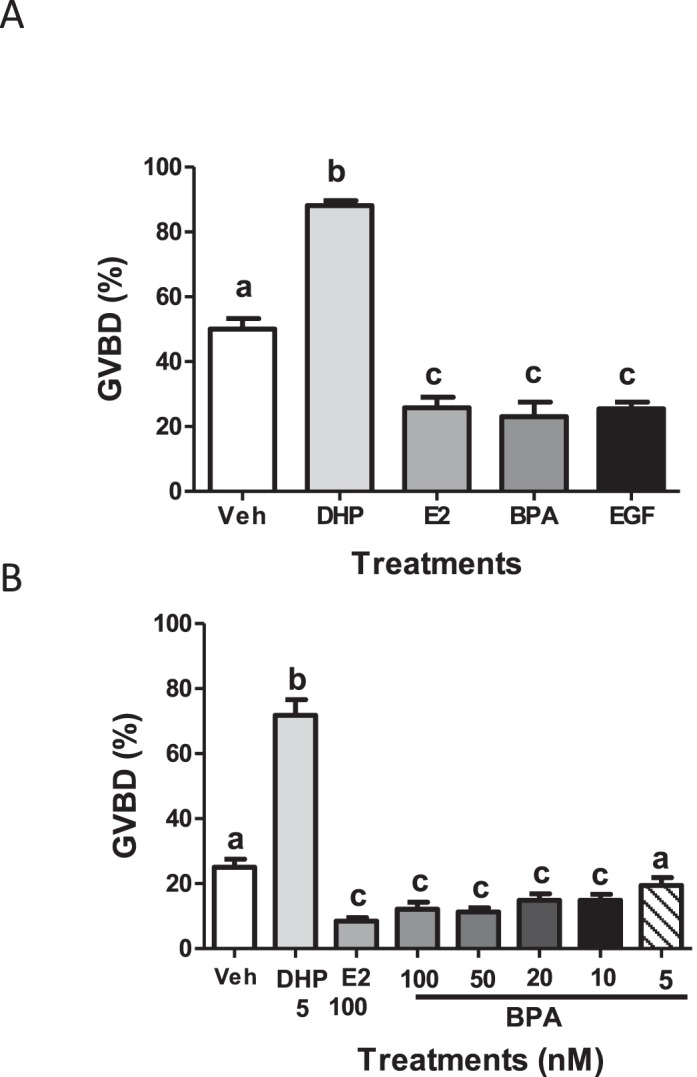
Effects of BPA and EGF on spontaneous maturation of denuded zebrafish oocytes. EGF (100 nM) and BPA (100 nM), and E2 (100 nM) effects on inhibition of OM in an in vitro bioassay (A). BPA effects on OM tested over the concentration range of 5–100 nM (B). BPA, bisphenol-A; DHP, 17,20β-dihydroxy-4-pregen-3-one (dihydroxyprogesterone); EGF, 50 nM EGF; Veh, vehicle ethanol control. Bars denote means ± SEM; n = 6–15. Different letters denote significant differences from each other (P < 0.05, one-way ANOVA and nonparametric Bonferroni test). The entire experiment was repeated three or more times and similar results were obtained each time.
Effects of Cotreatment with Actinomycin D on Inhibition of OM by BPA
To determine whether BPA acts via a genomic mechanism to inhibit OM, denuded oocytes were cotreated with BPA and the transcription inhibitor, actinomycin D (0.1 μg/ml). Cotreatment with actinomycin D did not blunt the inhibitory action of 100 nM BPA on OM (Fig. 2A), indicating that it acts through a nongenomic mechanism to inhibit OM, similar to the action of E2 [17].
FIG. 2.

Mechanism of BPA inhibition of OM. Effects of coincubation with the transcription inhibitor, actinomycin D (A), and the specific Gper antibody (B) on the inhibitory effects of BPA on spontaneous maturation of denuded zebrafish oocytes in the in vitro GVBD bioassay. Anti-GPER, 1:300 GPER antibody; BPA + AD, BPA plus 0.1 μg/ml actinomycin-D; IgG, 1:300 rabbit serum; Veh, vehicle control dimethyl sulfoxide (DMSO). Bars for both graphs denote means ± SEM (n = 7). Different letters denote significant differences from each other (P < 0.05, one-way ANOVA and nonparametric Bonferroni test). All the experiments were repeated three or more times and similar results were obtained each time. Competition curve of BPA binding to cell membranes of HEK293 cells transfected with zebrafish Gper expressed as a percentage of maximum specific [3H]-E2 binding (C). Mean binding results ± SEM (n = 6), plotted from two separate binding assays.
Role of Gper in the Inhibition of OM by BPA
The possible role of Gper in mediating the nongenomic estrogen actions of BPA to inhibit spontaneous maturation of denuded oocytes was investigated using a specific Gper antibody. Coincubation of the specific Gper antibody with BPA completely blocked BPA inhibition of OM, whereas incubation with the control rabbit IgG was ineffective (Fig. 2B), which suggests that BPA acts through Gper to regulate OM, the same ER mechanism regulating OM as that shown previously for E2 [13, 20].
Competitive Binding of BPA to Gper
Binding of BPA to recombinant zebrafish Gper was investigated in a competitive binding assay. BPA caused a concentration-dependent displacement of [3H]-E2 bound to plasma membranes of Gper-transfected HEK293 cells. BPA bound to the zebrafish Gper with a lower affinity than E2 and with an RBA of 15.8% and an IC50 of 53.4 nM (Fig. 2C).
Role of Egfr Signaling in Inhibition of OM by BPA
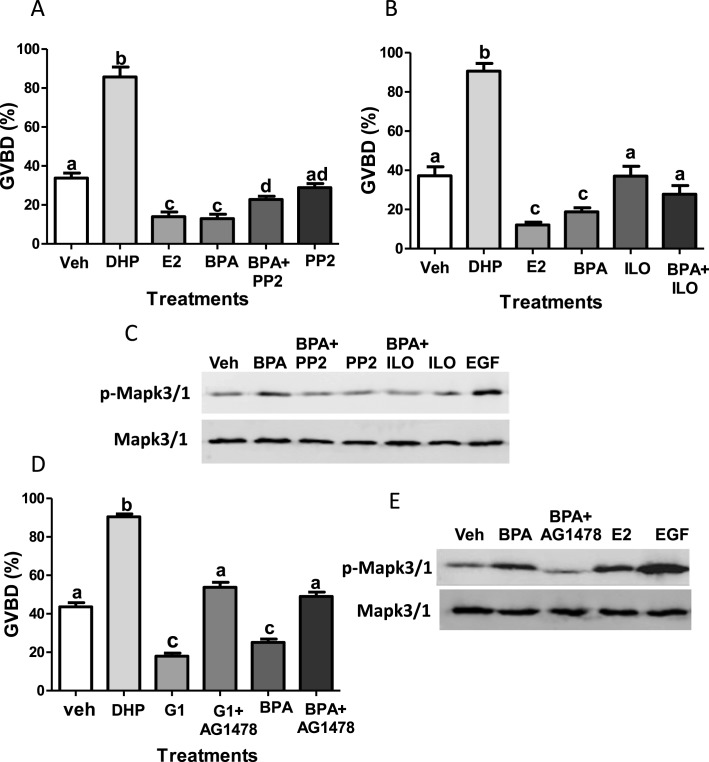
The involvement of the Egfr signaling in the inhibitory effects of BPA on OM was investigated using specific inhibitors of different components of the pathway. Incubation of denuded oocytes with 10 μM of the selective Src kinase inhibitor, PP2, alone did not significantly alter the % spontaneous GVBD compared to that of the vehicle controls (Fig. 3A). Cotreatment with PP2 attenuated the inhibitory effects of BPA, causing a significant increase in OM compared to that induced by BPA alone (Fig. 3A). Similar effects were observed with the MMP inhibitor, ilomastat (Fig. 3B). Treatment with 10 μM ilomastat alone did not significantly alter the % spontaneous GVBD compared to that of the vehicle controls (Fig. 3B), but cotreatment with ilomastat blocked the inhibitory effects of BPA on GVBD (Fig. 3B). Western blot analysis showed that 100-nM BPA treatment increased Mapk3/1 phosphorylation, which was blunted by cotreatment with PP2 or ilomastat (Fig. 3C). These results support a role for BPA in activation of Mapk3/1 in zebrafish oocytes and demonstrate the efficacy of the Src and MMP inhibitors in impairing the downstream Mapk3/1 pathway activated by BPA in this fish model. Coincubation of denuded oocytes with the selective EGFR inhibitor, AG1478 (50 μM), reversed the inhibitory effects of BPA and G-1 on OM (Fig. 3D). The increase in Mapk3/1 phosphorylation by 100-nM BPA treatment was attenuated by cotreatment with AG1478, confirming the effectiveness of the EGFR inhibitor in inhibiting the Mapk3/1 pathway in zebrafish oocytes (Fig. 3E).
FIG. 3.
Role of Egfr and its upstream regulators in BPA inhibition of OM and Mapk3/1 phosphorylation. Effects of cotreatment with the Src kinase inhibitor PP2 (A) and MMP inhibitor ilomastat (B) on the inhibitory effects of BPA on spontaneous maturation of denuded zebrafish oocytes in the in vitro bioassay and BPA phosphorylation of Mapk3/1 (C). Representative Western blot of phosphorylated Mapk3/1 (p-Mapk3/1) and total Mapk3/1 (Mapk3/1) levels after 15-min pretreatment with the inhibitors or vehicle followed by 15-min treatment with BPA or EGF (C). Effects of the Erb1 inhibitor AG1478 (D) on the inhibitory effects of BPA on spontaneous maturation of denuded zebrafish oocytes in the in vitro bioassay and BPA phosphorylation of Mapk3/1 (E). Representative Western blot of p-Mapk3/1 and total Mapk3/1 after 15-min pretreatment with AG1478 or vehicle followed by 15-min treatment with BPA, E2, and EGF (E). Veh, vehicle ethanol control; DHP, 5 nM DHP; E2, 100 nM E2; BPA, 100 nM BPA; PP2, 10 μM PP2; ILO, 10 μM ilomastat; BPA + ILO, 100 nM BPA plus 10 μM ilomastat ; BPA+PP2, 100 nM BPA plus 10 μM PP2; EGF, 50 nM EGF; G1, 100 nM G-1; AG1478 + BPA, 50 μM AG1478 plus 100 nM BPA; G1 + AG1478, 100 nM G-1 + 50 μM AG1478. Error bars denote means ± SEM (n = 8) (A and B); (n = 8) (D). Different letters denote significant differences from each other (P < 0.05, one-way ANOVA and nonparametric Bonferroni test). The GVBD experiments were repeated three or more times and similar results were obtained each time.
Involvement of Mapk3/1 Signaling in Inhibition of OM by BPA
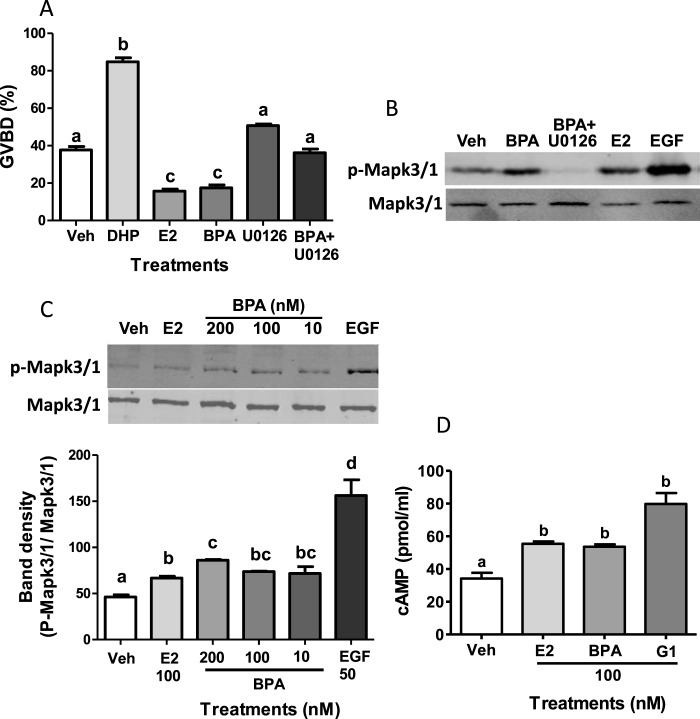
The role of Mapk3/1 signaling in the effects observed with BPA was further examined using the inhibitor, U0126. Treatment with U0126 alone did not significantly alter the % GVBD compared to controls, whereas cotreatment with U0126 blocked the inhibitory effects of BPA on OM, resulting in a significant increase in OM compared to that of the BPA treatment alone (Fig. 4A). Western blot analysis showed that the BPA-induced increase in Mapk3/1 phosphorylation was blocked by 15-min pretreatment with U1026, confirming the effectiveness of this inhibitor in zebrafish oocytes (Fig. 4B). BPA increased Mapk3/1 phosphorylation in denuded zebrafish oocytes in a concentration-dependent manner (Fig. 4C).
FIG. 4.
Involvement of Mapk3/1 in BPA inhibition of OM and effects of BPA on cAMP production by denuded oocytes. Effects of MAPK3/1 pathway inhibitor U0126 on the inhibitory effects of BPA on spontaneous maturation of denuded zebrafish oocytes in the in vitro GVBD bioassay (A) and BPA phosphorylation of Mapk3/1 (B). Representative Western blot of p-Mapk3/1 and total Mapk3/1 after 15-min pretreatment with U1026 or vehicle followed by 15-min treatment with BPA, E2, and EGF (B). Veh, vehicle ethanol control; BPA, 100 nM BPA; BPA + U0126, 100 nM BPA plus 50 μM U0126; DHP, 5 nM DHP; E2, 100 nM E2; U0126, 50 μM U0126. Effects of BPA on activation of Mapk3/1 (B and C). Representative Western blot of p-Mapk3/1 and total Mapk3/1 levels after 15-min treatment with 10, 100, and 200 nM BPA (C, top) and relative density of p-Mapk3/1 bands relative to total Mapk3/1 determined from densitometry of Western blots (C, bottom). EGF, 50 nM EGF; Veh, vehicle control ethanol. Effects of BPA on cAMP production by denuded oocytes (D). Cyclic AMP levels were measured after 3-h incubation of oocytes with 100 nM BPA, 100 nM E2, and 100 nM G-1 (G1). Error bars denote means ± SEM (n = 8) (A), (n = 6) (C), (n = 3) (D). Different letters denote significant differences from each other (P < 0.05, one-way ANOVA and nonparametric Bonferroni test). The experiments were repeated three or more times and similar results were obtained each time.
Effects of BPA of Oocyte cAMP Levels
Possible activation of Acy through Gper by BPA was examined by measuring cAMP levels in zebrafish oocytes after 3-h BPA treatment. Denuded oocytes treated with either 100 nM E2, 100 nM BPA, or 100 nM G-1 had significant increases in cyclic AMP levels compared to vehicle controls (Fig. 4D). This increase in cAMP levels after BPA treatment was similar to that observed after E2 treatment, which has previously been shown to inhibit OM in zebrafish by maintaining elevated cAMP levels [20].
Effects of the Structurally Related Alkylphenols on OM
The effects of TBBPA, TCBPA, and NP on maturation of denuded oocytes were investigated over the same range of concentrations as those used for BPA (5–100 nM). TBBPA significantly decreased the % GVBD compared to the vehicle controls over the concentration range of 10–100 nM, whereas 5 nM was ineffective (Fig. 5A). NP was effective in inhibiting OM at all concentrations tested, significantly decreasing the % GVBD over the concentration range from 5 to 100 nM (Fig. 5B). Similarly, TCBPA inhibited spontaneous OM over the entire concentration range (5–100 nM; Fig. 5C). However, lower concentrations (2.5 and 1 nM) of NP and TCBPA were ineffective in inhibiting GVBD (Fig. 5D). Similar to the effects of E2, the inhibitory effects of BPA, TCBPA, TBBPA, and NP on the resumption of meiosis were not persistent, and disappeared after longer-term incubation (Supplemental Fig. S2), suggesting that these alkylphenols do not exert nonspecific toxic effects on OM at these concentrations.
FIG. 5.
Effects of alkylphenols structurally related to BPA on inhibition of OM. Effects of tetrabromobisphenol A (TBBPA; A), nonylphenol (NP; B), tetrachlorobisphenol A (TCBPA; C) over the concentration range of 5–100 nM on spontaneous maturation of denuded zebrafish oocytes in the in vitro bioassay. (D) Effects of lower concentrations (1 and 2.5 nM) of NP and TCBPA on OM. Veh, vehicle ethanol control. Error bars denote means ± SEM (n = 6). Different letters denote significant differences from each other (P < 0.05, one-way ANOVA and nonparametric Bonferroni test). The experiments were repeated three or more times and similar results were obtained each time.
Role of Gper and Egfr in Inhibition of OM by TCBPA, TBBPA, and NP
The role of Gper in mediating the inhibitory effects of TCBPA, TBBPA, and NP on OM was investigated using the specific GPER antagonist, G-15. Incubation with G-15 alone did not significantly affect the % GVBD of denuded oocytes, whereas cotreatment of G-15 with the alkylphenol compounds reversed their inhibitory effects on OM, which were not significantly different from those of vehicle controls (Fig. 6A). A single-point competitive binding assay showed that 1 μM BPA, 1 μM NP, 1 μM TBBPA, and 1 μM TCBPA were all effective competitors of [3H]-E2 binding to membranes prepared from cells expressing recombinant zebrafish Gper (Fig. 6B). TCBPA and NP (1 μM) displaced 62% of the bound [3H]-E2, while 1 μM TBBPA and BPA displaced 61% and 60% of the E2 tracer, respectively. One-tenth this concentration of E2 (100 nM) caused a similar displacement of bound [3H]-E2 (62%), suggesting that these alkylphenols have relative binding affinities approximately one-tenth that of E2. The involvement of Egfr signaling in inhibition of OM by these alkylphenols was investigated using the EGFR inhibitor, AG1478. Treatment with AG1478 reversed the inhibitory effects of NP, TBBPA, and TCBPA on OM, significantly increasing the % GVBD (Fig. 6C).
FIG. 6.

Involvement of Gper and Egfr in inhibitory effects of NP, TCBPA, and TBBPA on OM. Effects of the specific GPER antagonist, G-15, on the inhibitory effects of alkylphenols on spontaneous maturation of denuded zebrafish oocytes in the in vitro bioassay (A). G-15, 100 nM G-15; NP, 100 nM NP; NP + G-15, 100 nM NP plus 100 nM G-15; TBB, 100 nM TBBPA; TBB + G-15, 100 nM TBBPA plus 100 nM G-15; TCB, 100 nM TCBPA; TCB + G-15, 100 nM TCBPA plus 100 nM G-15; Veh, vehicle ethanol control; n = 6. Different letters denote significant differences from each other (P < 0.05, one-way ANOVA and nonparametric Bonferroni test). The experiments were repeated three or more times and similar results were obtained each time. Binding of TBBPA, TCBPA, and NP to zebrafish Gper. Displacement of bound [3H]-E2 by 1 μM TCBPA, 1 μM NP, 1 μM BPA, and 1 μM TBBPA compared to that displaced by 100 nM and 1 μM E2 to recombinant zebrafish Gper on transfected HEK293 cell membranes in a single-point competitive binding assay (B). Values are represented as a percent of the total binding (total, membranes incubated with 4 nM [H3]-E2 alone in the absence of any competitor). Error bars denote means ± SEM (n = 4). Different letters denote treatments significantly different from each other (P < 0.05, one-way ANOVA and Dunnett test). Role of Egfr in TBBPA, TCBPA, and NP inhibition of OM (C). Effects of the EGFR inhibitor, AG1478, on the inhibitory effects of alkylphenols on spontaneous maturation of denudedzebrafish oocytes in the in vitro bioassay. +AG1478, plus 50 μM AG1478; NP, 100 nM NP; TBB, 100 nM TBBPA; TCB, 100 nM TCBPA; Veh, ethanol vehicle control. Error bars denote means ± SEM (n = 8). Asterisks denote significant differences between treatments in the presence or absence of AG1478 (P < 0.05, one-way ANOVA and Student t-test).
DISCUSSION
The present results clearly demonstrate that BPA exerts a direct, nongenomic, estrogenic action on zebrafish oocytes to prevent the resumption of meiosis through binding to the membrane estrogen receptor, Gper, and activating a Gper-dependent Egfr/Mapk3/1 pathway. The inhibitory action of BPA on OM was not attenuated by cotreatment with actinomycin D, similar to previous results with E2 [17], indicating that this BPA action is through a nongenomic mechanism. Several lines of evidence indicate the inhibitory estrogenic actions of BPA, and the other alkylphenols on zebrafish OM are mediated via Gper. Consistent with the earlier results with E2 [17, 20], the inhibitory actions of BPA and the other alkylphenols were blocked by coincubation with a teleost Gper antibody or a specific GPER antagonist, G-15. Moreover, BPA and the structurally related alkylphenols, TCBPA, TBBPA, and NP, bound with high affinities to recombinant zebrafish Gper. BPA also mimicked estrogen activation of Gper-dependent signaling pathways in zebrafish oocytes, involving increases in Mapk3/1 phosphorylation and cAMP concentrations [17, 20]. Furthermore, treatment with inhibitors of the Egfr/Mapk3/1 pathway in zebrafish oocytes demonstrated that BPA acts by way of the estrogen-dependent Gper signaling pathway in this species to maintain meiotic arrest [17]. Finally, evidence was also obtained that the other alkylphenols act by the same pathway to inhibit or delay the resumption of meiosis.
Xenoestrogens, such as octylphenol, have previously been shown to block FSH-induced resumption of meiosis in mouse oocytes and maturation of bovine follicle-enclosed oocytes in vitro at concentrations of 0.01–1 μg/ml [44, 45]. An estrogenic action of NP to prevent the resumption of meiosis in vitro has also been demonstrated in tripletooth goby oocytes [46], but the site and mechanism of xenoestrogen action were not identified in any of these studies. BPA also causes a delay in meiosis of denuded mouse oocytes [47, 48], and inhibits progestin-induced GVBD of Atlantic croaker oocytes [49]. However, the concentrations of BPA causing significant inhibition in these studies were 1000-fold higher than those shown to be effective (10−100 nM) in the present study. BPA disrupts multiple stages of oocyte meiosis in Rhesus monkeys and mice, including meiotic entry and meiotic prophase, as well as the final stages of meiosis, metaphase I and II [50, 51]. High concentrations of BPA also inhibit the final maturation stages in human oocytes in vitro [52, 53]. In contrast, acute effects of BPA and other alkylphenols on an earlier stage of meiosis, the completion of the first meiotic division and GVBD, were observed on denuded zebrafish oocytes in the current study. Moreover, the inhibitory estrogenic effects of low concentrations of BPA and the other alkyphenols, like those of E2, on spontaneous maturation of denuded oocytes were not permanent, and disappeared after longer-term incubation (Supplemental Fig. S2). These results are consistent with activation of Gper/Gs signaling causing short-term activation of Egfr- and mAc-dependent pathways and transient increases in second messengers, such as cAMP [20]. Collectively, these results indicate that the effects of BPA and the related alkylphenols on maturation of zebrafish oocytes described in the present study and their mechanism of action are fundamentally different from those reported previously for BPA on vertebrate oocytes.
A major finding of this study is that BPA activates the same intracellular signaling pathways as those activated by E2 to maintain meiotic arrest of zebrafish oocytes [17, 20]. The results show that BPA inhibition of OM is blocked by the same inhibitors of the EGFR/MAPK3/1 pathway as those used previously to investigate E2 signaling in zebrafish oocytes. Inhibition of upstream regulators of Egfr transactivation, Src with PP2 and MMP with ilomastat, blocked BPA inhibition of OM. An inhibitor of Egfr transactivation, AG1478, as well as an inhibitor of MAPK3/1 activation, U01206, also attenuated BPA inhibition of OM. The finding that BPA also activates the other Gper-dependent estrogen signaling pathway in zebrafish oocytes, resulting in increased cAMP production, presumably due to increases in mAcy activity, was not unexpected, since GPER in human cancer cells as well as in croaker and zebrafish oocytes is coupled to a Gs [12, 13, 20], and BPA increases cAMP concentrations in HEK293 cells stably transfected with human GPER [21]. BPA has also been shown to activate the PKA pathway in human testicular seminoma cells, although the receptor mediating this signaling was not identified [54]. In addition BPA activates Gs βγ subunit-dependent signaling in ER-negative human breast cancer SKBR3 cells, resulting in rapid MAPK3/1 phosphorylation [55], similar to our results in zebrafish. In agreement with our findings in zebrafish oocytes, recent results with the EGFR inhibitor, AG1478, in breast and lung cancer cells suggest that BPA phosphorylation of MAPK3/1 is mediated through EGFR [56, 57]. Moreover, in agreement with signaling results in zebrafish, BPA stimulation of proliferation and migration of breast cancer cells and transcriptional regulation of c-fos through GPER are dependent on EGFR and MAPK activation, because these BPA actions are blocked by cotreatment AG1478 or MAPK3/1 inhibitors [55, 56]. Taken together, these results suggest that BPA acts as a GPER agonist to activate EGFR/MAPK3/1 and PKA pathways in a variety of reproductive cells from both fish and mammals, leading to disruption of their physiological functions.
Arguably, the most interesting finding of the present study was the very high binding affinity BPA has for zebrafish GPER and its high potency in the GVBD bioassay. The IC50 of BPA for human GPER (0.63 μM) is 8- to 150-fold lower than that reported for human ERα (4.9–100 μM) and 1.5- to 40-fold lower than that for ERβ (0.96–36 μM) [21, 42, 43, 58]. However, BPA's affinity for zebrafish Gper is even greater, with a 5-fold-lower IC50 of 53.4 nM. Whereas BPA has a higher RBA of 2.83% for human GPER compared to ERα (0.001–0.2) and ERβ (0.004–1.2) [21, 42, 43, 58], its RBA for zebrafish Gper is even higher (15.8%). Limited data from single-point assays with the other alkyl phenols suggest that they have RBAs similar to that of BPA for zebrafish Gper. These high binding affinities are consistent with the high potencies of the alkyl phenols (5–10 nM) in preventing the resumption of meiosis, similar to that reported for E2 (5 nM) [20]. However, the effectiveness of lower concentrations (<5 nM) of E2 in inhibiting OM have not been investigated. The finding that the estrogenic activities of the flame retardants, TCBPA and TBBPA, are similar to that of BPA in the GVBD assay suggests that halogenation of BPA does not diminish Gper activity, unlike ER activity, which is dramatically reduced upon substitution of the phenolic moieties of BPA with bromines [59–61]. Collectively, these results suggest that nongenomic estrogen functions mediated by Gper in zebrafish and related teleost species may be particularly susceptible to interference with low concentrations of these alkylphenols.
The endocrine-disrupting effects of BPA and halogenated alkylphenols are likely very complex, since they also involve binding to other receptors, including the peroxisome proliferator-activated receptor γ [61], the human pregnane X receptor [62], the estrogen-related receptor γ [63], the human androgen receptor [62], and the thyroid receptor [64], and alter actions of brain aromatase activity [65]. A diverse range of reproductive effects of BPA have been identified in aquatic and mammalian species [29, 66]. Moreover, BPA acts at multiple sites on the hypothalamus-pituitary-gonad axis to alter reproductive function [66]. Therefore, it is difficult to discern the principal mechanism of BPA toxicity on an individual reproductive function. Here, we explore one potential mechanism of endocrine disruption by BPA in a well-characterized primary cell model of estrogen maintenance of oocyte meiotic arrest using denuded zebrafish oocytes. Zebrafish release a batch of eggs that have completed OM and are capable of being fertilized around dawn every day. Thus, the timing and synchronization of OM is critical for reproductive success, and delayed ovulation and decreased production of fertilized eggs has been reported after exposure of zebrafish and other teleosts to BPA and related alkylphenols [67–69]. Multiple mechanisms have been identified in vertebrates that maintain oocyte meiotic arrest in order to prevent precocious OM and the production of infertile eggs [20, 70, 71]. We show here that low concentrations of BPA block OM by a novel, nongenomic, estrogenic mechanism of endocrine disruption through binding to the Gper, transactivation of Egfr, and activation of Mapk3/1. Moreover, this action through Gper is shared by three chemicals that are structurally similar to BPA: TCBPA, TBBPA, and NP. Thus, the results from this study support the hypothesis that BPA can act through a nongenomic estrogen signaling pathway via Gper to disrupt a critical cellular function in zebrafish: oocyte maturation.
Footnotes
Supported by National Institutes of Health grant ESO 12961 to P.T.
REFERENCES
- Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans Environ Health Perspect 1993. 101 5: 378– 384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan JA. Functional toxicology: a new approach to detect biologically active xenobiotics Environ Health Perspect 1993. 101 5: 386– 388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis AK, Thomas P. Binding characteristics of estrogen receptor (ER) in Atlantic croaker (Micropogonias undulatus) testis: different affinity for estrogens and xenobiotics from that of hepatic ER Biol Reprod 1999. 61 51– 60 [DOI] [PubMed] [Google Scholar]
- Fucic A, Gamulin M, Ferencic Z, Katic J, von Krauss KM, Bartonova A, Merlo DF. Environmental exposure to xenoestrogens and oestrogen related cancers: reproductive system, breast, lung, kidney, pancreas, brain. Environ Health. 2012;11:S8. doi: 10.1186/1476-069X-11-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RM, Fang H, Branham WS, Hass BS, Dial SL, Moland CL, Tong W, Shi L, Perkins R, Sheehan DM. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands Toxicol Sci 2000. 54 1: 138– 153 [DOI] [PubMed] [Google Scholar]
- Watson CS, Bulayeva NN, Wozniak AL, Finnerty CC. Signaling from the membrane via membrane estrogen receptor-alpha: estrogens, xenoestrogens, and phytoestrogens Steroids 2005. 70 364– 371 [DOI] [PubMed] [Google Scholar]
- Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B. Nongenomic actions of estrogens and xenoestrogens by binding a plasma membrane receptor unrelated to estrogen receptor alpha and estrogen receptor beta Proc Natl Acad Sci U S A 2000. 97 11603– 11608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis AK, Thomas P. Effects of estrogens and xenoestrogens on androgen production by Atlantic croaker testes in vitro: evidence for a nongenomic action mediated by an estrogen membrane receptor Biol Reprod 2000. 62 995– 1004 [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease Nat Rev Endocrinol 2011. 7 715– 726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P. Minireview: G-protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and physiological role in female reproductive cancer and renal physiology Endocrinology 2012. 153 2953– 2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggiolini M, Picard D. The unfolding stories of GPR30, a new membrane bound estrogen receptor J Endocrinol 2010. 204 2: 105– 14 [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells Endocrinology 2005. 146 2: 624– 632 [DOI] [PubMed] [Google Scholar]
- Pang YF, Dong J, Thomas P. Estrogen signaling characteristics of Atlantic croaker GPR30 and evidence it is involved in maintenance of oocyte meiotic arrest Endocrinology 2008. 149 3410– 3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhu P, Sham KW, Yuen JM, Xie C, Zhang Y, Liu Y, Li S, Huang X, Cheng CH, Lin H. Identification of a membrane estrogen receptor in zebrafish with homology to mammalian GPER and its high expression in early germ cells of the testis Biol Reprod 2009. 80 1253– 1261 [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF Mol Endocrinol 2000. 14 10: 1649– 1660 [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P. GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release Trends Endocrinol Metab 2005. 16 362– 367 [DOI] [PubMed] [Google Scholar]
- Peyton C, Thomas P. Involvement of epidermal growth factor receptor signaling in estrogen inhibition of oocyte maturation mediated through the G protein-coupled estrogen receptor (Gper) in zebrafish (Danio rerio) Biol Reprod 2011. 85 1: 42– 50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaling AL, Prossnitz ER, Hathaway HJ. GPER mediates estrogen-induced signaling and proliferation in human breast epithelial cells and normal and malignant breast Horm Canc 2014. 5 146– 160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggiolini M, Vivacqua S, Fasanella G, Recchia AG, Sisci D, Pezzi V, Monataro D, Musti AM, Picard D, Ando S. The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17β-estradiol and phytoestrogens in breast cancer cell J Biol Chem 2004. 279 27008– 27016 [DOI] [PubMed] [Google Scholar]
- Pang Y, Thomas P. Role of G protein-coupled estrogen receptor 1, GPER, in inhibition of oocyte maturation by endogenous estrogens in zebrafish Dev Biol 2010. 342 2: 194– 206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption J Steroid Biochem Mol Biol 2006. 102 (1-5): 175– 179 [DOI] [PubMed] [Google Scholar]
- Dodds EC, Lawson W. Molecular structure in relation to oestrogenic activity: compounds without a phenanthrene ring Proc R Soc Lond B Biol Sci 1938. 125 839: 222– 232 [Google Scholar]
- Bailin PD, Byrne M, Lewis S, Liroff R. Public Awareness Drives Market for Safer Alternatives: Bisphenol A Market Analysis Report Investor Environmental Health Network; September 15 2008. http://www.iehn.org/publications.reports.bpa.php. [Google Scholar]
- Coors A, Jones PD, Giesy JP, Ratte HT. Removal of estrogenic activity from municipal waste landfill leachate assessed with a bioassay based on reporter gene expression Environ Sci Technol 2003. 37 15: 3430– 3434 [DOI] [PubMed] [Google Scholar]
- Zhang X, Li Q, Li G, Wang Z, Yan C. Levels of estrogenic compounds in Xiamen Bay sediment, China Mar Pollut Bull 2009. 58 8: 1210– 1216 [DOI] [PubMed] [Google Scholar]
- Bolz U, Hagenmaier H, Korner W. Phenolic xenoestrogens in surface water, sediments, and sewage sludge from Baden-Wurttemberg, south-west Germany Environ Pollut 2001. 115 2: 291– 301 [DOI] [PubMed] [Google Scholar]
- Kawahata H, Ohta H, Inoue M, Suzuki A. Endocrine disrupter nonylphenol and bisphenol A contamination in Okinawa and Ishigaki Islands, Japan—within coral reefs and adjacent river mouths Chemosphere 2004. 55 11: 1519– 1527 [DOI] [PubMed] [Google Scholar]
- Ribeiro C, Pardal MA, Tiritan ME, Rocha E, Margalho RM, Rocha MJ. Spatial distribution and quantification of endocrine-disrupting chemicals in Sado River estuary, Portugal Environ Monit Assess 2009. 159 (1–4): 415– 427 [DOI] [PubMed] [Google Scholar]
- Bhandari RK, Deem SL, Holliday DK, Jandegian CM, Kassotis CD, Nagel SC, Tillit DE. Vom Saal FS, Rosenfeld CS. Effects of the environmental estrogenic contaminants bisphenol A and 17α-ethinyl estradiol on sexual development and adult behaviors in aquatic wildlife species Gen Comp Endocrinol 2015. 214 195– 219 [DOI] [PubMed] [Google Scholar]
- Lee C-C, Jiang L-Y, Kuo Y-L, Hsieh C-Y, Chen CS, Tien C-J. The potential role of water quality parameters on occurrence of nonylphenol and bisphenol A and identification of their discharge sources in the river ecosystems Chemosphere 2013. 91 904– 911 [DOI] [PubMed] [Google Scholar]
- Renz L, Volz C, Michanowicz D, Ferrar K, Christian C, Lenzner D, El-Hefnawy T. A study of parabens and bisphenol A in surface water and fish brain tissue from the Greater Pittsburgh area Ecotoxicol 2013. 22 4: 632– 641 [DOI] [PubMed] [Google Scholar]
- Lee C-C, Jiang L-Y, Kuo Y-L, Chen C-Y, Hsieh C-Y, Hung C-F, Tien C-J. Characteristics of nonylphenol and bisphenol A accumulation by fish and implications for ecological and human health Sci Tot Environ 2015. 502 417– 425 [DOI] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population Environ Health Perspect 2005. 113 391– 395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, He XB, Li FH, Liu C. A serological survey of bisphenol A in Shenzhen City Pract Prev Med 2005. 12 241– 242 [Google Scholar]
- Bolt HM, Stewart JD. Highlight report: the bisphenol A controversy Arch Toxicol 2011. 85 1491– 1492 [DOI] [PubMed] [Google Scholar]
- Sharpe RM. Is it time to end concerns over the estrogenic effects of bisphenol A? Toxicol Sci 2010. 114 1– 4 [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Sonneschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of the controversies in the field of endocrine disruption Endoc Rev 2009. 30 75– 95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thigpen JE, Setchell KDR, Kissling GE, Locklear J, Caviness GF, Whiteside T, Belcher SM, Brown NM, Collins BJ, Lih FR, Tomer KB, Padilla-Banks E, et al. The estrogenic content of rodent diets, bedding, cages, and water bottles and its effects on bisphenol A studies J Am Assoc Lab Anim Sci 2013. 52 130– 141 [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment Environ Health Perspect 2005. 113 8: 926– 933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourasia T, Pang Y, Thomas P. The catecholestrogen, 2-hydroxyestradiol-17beta, acts as a G protein-coupled estrogen receptor 1 (GPER) antagonist to promote the resumption of meiosis in zebrafish oocytes Biol Reprod 2015. 92 1– 13 [DOI] [PubMed] [Google Scholar]
- Aizen Y, Thomas P. Involvement of progesterone membrane component 1 (PGRMC1) in estrogen maintenance of meiotic arrest in zebrafish oocytes through regulation of epidermal growth factor receptor expression on oocyte membranes J Endocrinol 2015. 225 59– 68 [DOI] [PubMed] [Google Scholar]
- Mathews JB, Twomey K, Zacharewski TR. In vitro and In vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors α and β Chem Res Toxicol 2001. 14 149– 157 [DOI] [PubMed] [Google Scholar]
- Takemura H, Ma J, Sayama K, Terao Y, Zhu BT, Shimoi K. In vitro and in vivo estrogenic activity of chlorinated derivatives of bisphenol A Toxicology 2005. 2007 215– 221 [DOI] [PubMed] [Google Scholar]
- Hafne AL, Byskov AG, Ynding-Larsen C, Tcerdal A. Response on spontaneous and induced maturation in vitro from estradiol, diethylstilbestrol and octylphenol in mouse oocytes. Theriogenology. 2000;53:454. [Google Scholar]
- Pocar P, Augustin R, Gandolfi F, Fischer B. Toxic effects of in vitro exposure to p-tert-octylphenol on bovine oocyte maturation and developmental competence Biol Reprod 2003. 69 462– 468 [DOI] [PubMed] [Google Scholar]
- Hwang IJ, Kim HW, Kim JK, Lee YD, Baek HJ. Estrogenicity of 4-nonylphenol and diethylstilbestrol on in vitro oocyte maturation of the dusky tripletooth goby, Tridentiger obscurus Animal Cells Syst 2010. 14 3: 161– 167 [Google Scholar]
- Can A, Semiz O, Cinar O. Bisphenol-A induces cell cycle delay and alters centrosome and spindle microtubular organization in oocytes during meiosis Mol Hum Reprod 2005. 11 389– 396 [DOI] [PubMed] [Google Scholar]
- Eichenlaub-Ritter U, Vogt E, Cukurcam S, Sun F, Pacchierotti F, Parry J. Exposure of mouse oocytes to bisphenol A causes meiotic arrest but not aneuploidy Mut Res 2008. 651 82– 92 [DOI] [PubMed] [Google Scholar]
- Thomas P, Sweatman J. Interference by atrazine and bisphenol A with progestin binding to the ovarian membrane progestin receptor and induction of oocyte maturation in Atlantic croaker Mar Environ Res 2008. 66 1– 2 [DOI] [PubMed] [Google Scholar]
- Hunt PA, Lawson C, Gieske M, Murdoch B, Smith H, Marre A, Hassold T, VandeVoor CA. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey Proc Natl Acad Sci USA 2012. 109 17525– 17530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges CA, Ilagan A, Jennings D, Keri R, Nilson J, Hunt PA. Experimental evidence that changes in oocyte growth influence meiotic chromosome segregation Hum Reprod 2002. 17 1171– 1180 [DOI] [PubMed] [Google Scholar]
- Machtinger R, Combelles CMH, Missmer SA, Correia KF, Williams P, Hauser R, Racowsky C. Bisphenol-A and human oocyte maturation in vitro Hum Reprod 2013. 28 2735– 2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machtinger R, Orvieto R, Bisphenol A. oocyte maturation, implantation and IVF outcome: review of animal and human data Reprod Biomed Online 2014. 29 404– 410 [DOI] [PubMed] [Google Scholar]
- Bouskine A, Nebout M, Brucker-Davis F, Benahmed M, Fenichel P. Low doses of bisphenol A promote human seminoma cell proliferation by activating PKA and PKG via a membrane G-protein-coupled estrogen receptor Environ Health Perspect 2009. 117 1053– 1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Terasak S, Kiyama R. Bipshenol A induces rapid activation of Erk1/2 through GPR30 in human breast cancer cells Environ Pollut 2011. 159 212– 218 [DOI] [PubMed] [Google Scholar]
- Pupo M, Pisano A, Lappano R, Santolla MF, De Francesco EM, Abonante S, Rosano C, Maggiolini M. Bisphenol A induces gene expression changes and proliferative effects through GPER in breast cancer cells and cancer-associated fibroblasts Environ Health Perspect 2012. 120 1177– 1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K-S, Chen H-Q, Chen Y-S, Qui K-F, Zheng X-B, Li G-C. Yang h-D, Wen C-J. Bisphenol A stimulates lung cancer migration via upregulation of matrix metalloproteinase by GPER/EGFF/Erk1/2 signal pathway Biomed Pharmacol 2014. 68 1037– 1043 [DOI] [PubMed] [Google Scholar]
- Mueller SO, Kling M, Arifin Firzani P, Mecky A, Duranti E, Shields-Botella J, Delansorne R, Broschard T, Kramer P. Activation of estrogen receptor α and ERβ by 4-methylbenzylidene-camphor in human and rat cells: comparison with phyto- and xeno-estrogens Toxicol Lett 2003. 142 89– 101 [DOI] [PubMed] [Google Scholar]
- Meerts IA, Letcher RJ, Hoving S, Marsh G, Bergman A, Lemmen JG, van der Burg B, Brouwer A. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PDBEs, and polybrominated bisphenol A compounds Environ Health Perspect 2001. 109 4: 399– 407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CM, Meussen-Elholm E, Samuelsen M, Holme JA, Hongslo JK. Effects of the environmental oestrogens bisphenol A, tetrachlorobisphenol A, tetrabromobisphenol A, 4-hydroxybiphenyl and 4, 4′-dihydroxybiphenyl on oestrogen receptor binding, cell proliferation and regulation of oestrogen sensitive proteins in the human breast cancer cell line MCF-7 Pharmacol Toxicol 2003. 92 4: 180– 188 [DOI] [PubMed] [Google Scholar]
- Riu A, le Maire A, Grimaldi M, Audebert M, Hillenweck A, Bourguet W, Blaguer P, Zalko D. Characterization of novel ligands of ERα, ERβ, and PPARγ: the case of halogenated bisphenol A and their conjugated metabolites Toxicol Sci 2011. 122 372– 382 [DOI] [PubMed] [Google Scholar]
- Molina-Molina J-M, Amaya E, Grimaldi M, Sáenz J-M, Real M, Fernández MF, Balaguer P, Olea N. In vitro study of agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors Toxicol Applied Pharmacol 2013. 271 127– 135 [DOI] [PubMed] [Google Scholar]
- Takayanagi S, Tokunaga T, Liu X, Okada H, Matsushima A, Shimohigashi Y. Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor gamma (ERRgamma) with high constitutive activity Toxicol Lett 2006. 167 2: 95– 105 [DOI] [PubMed] [Google Scholar]
- Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, Hataya Y, Shimatsu A, Kuzuya H, Nakao K. Thyroid hormone action is disrupted by bisphenol A as an antagonist J Clin Endocrinol Metab 2002. 87 5185– 5190 [DOI] [PubMed] [Google Scholar]
- Kishida M, McLellan M, Miranda JA, Callard GV. Estrogen and xenoestrogens upregulate the brain aromatase isoform (P450aromB) and perturb markers of early development in zebrafish (Danio rerio) Comp Biochem Physiol B Biochem Mol Biol 2001. 129 (2-3): 261– 268 [DOI] [PubMed] [Google Scholar]
- Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects J Steroid Biochem Mol Biol 2011. 127 27– 34 [DOI] [PubMed] [Google Scholar]
- Giesy JP, Pierens SL, Snyder EM, Miles-Richardson S, Kramer VJ, Snyder SA, Nichols KM, Villeneuve DL. Effects of 4-nonylphenol on fecundity and biomarkers of estrogenicity in fathead minnows (Pimephales promelas) Environ Toxicol Chem 2000. 19 1368– 1377 [Google Scholar]
- Segner H, Caroll K, Fenske M, Janssen CR, Maack G, Pascoe D, Schäfers C, Vandenbergh GF, Watts M, Wenzel A. Identification of endocrine-disrupting effects in aquatic vertebrates and invertebrates-report from the European IDEA project Ecotox Environ Safety 2003. 54 312– 314 [DOI] [PubMed] [Google Scholar]
- Naderi M, Wong MYL, Gholami F. Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults Aquatic Toxicol 2014. 148 195– 203 [DOI] [PubMed] [Google Scholar]
- Solc P, Schultz RM, Motlik J. Prophase 1 arrest and progression to metaphase 1 in mouse oocytes: comparison of resumption of meiosis and recovery from G2-arrest in somatic cells Mol Hum Reprod 2010. 16 654– 664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C-R, Wei Y, Qi S-T, Chen L, Zhang Q-H, Ma J-Y, Luo Y-B, Wang Y-P, Hou Y, Schatten H, Liu Z-H, Sun Q-Y. The G protein coupled receptor 3 is involved in cAMP and cGMP signaling and maintenance of meiotic arrest in porcine oocytes. PLoS One. 2012;7:e38807. doi: 10.1371/journal.pone.0038807. [DOI] [PMC free article] [PubMed] [Google Scholar]