Abstract
Association of an altered expression of placental mucin 1 (MUC1) with first-trimester spontaneous abortion and its regulation in placenta by an invasion-promoting peptide, adrenomedullin 2 (ADM2), is not known. The objective of this study was to assess 1) the association of MUC1 mRNA expression in the placental villi and decidua with first-trimester spontaneous abortion, 2) the effects of ADM2 on the expression of MUC1 in trophoblast cells in the presence or absence of hypoxia, 3) the effects of ADM2 on expression of MUC1 in decidual stromal cells (DSCs), and 4) if ADM2 regulates the expression of MUC1 and MMP2 protein in trophoblastic spheroids. Data demonstrate that 1) expression of MUC1 mRNA in villous tissue is higher in spontaneous abortion compared to age-matched electively terminated pregnancies (P > 0.05), 2) ADM2 decreases the expression of MUC1 mRNA and protein in trophoblast cells and spheroids with concomitant increases in MMP2 immunoreactivity in the spheroids, 3) ADM2 decreases hypoxia-induced increases in MUC1 immunoreactivity in trophoblast cells, 4) decidual MUC1 mRNA expression is lower in spontaneous compared to elective abortions (P < 0.05), and 5) DSCs express MUC1 mRNA and protein and ADM2 decreases the expression of MUC1 mRNA and protein in DSCs. Taken together, this study demonstrates that first-trimester spontaneous abortion is associated with increases in MUC1 expression in villi and decreases in the decidual tissues, and suggests that ADM2 may contribute to the physiology of embryo implantation and placental growth via increasing MMP2 and decreasing MUC1 expression to facilitate trophoblast invasion.
Keywords: adrenomedullin 2, human placenta, MUC1, trophoblast cells
INTRODUCTION
Mucin 1 (MUC1) is a hypoxia-inducible O-glycosylated transmembrane protein present at the apical surface of epithelial cells in the endometrium throughout the menstrual cycle [1–3]. A recent study shows that MUC1 is expressed in the trophoblast cells of villi, and in trophoblasts that have invaded the decidual segment [4]. In addition, in vitro studies indicate that MUC1 may inhibit trophoblast invasion and promote transendothelial migration [5, 6]. Endometrial MUC1 expression is elevated during the peri-implantation period [7] and MUC1 is suggested to be the first molecule that the embryo encounters before adhering to the endometrium [8, 9]. In the uterus, MUC1 suppresses the interaction between the implanting embryo and maternal endometrial adhesion molecules, thus creating a barrier to implantation in humans [8] and many other mammalian species [10–12]. Removal of MUC1 is necessary for successful implantation in humans [8, 13–15]. It is thought that the paracrine signals originating from the embryo and its local milieu participate in MUC1 clearance from the site of embryo implantation [16].
In human placenta, expression of MUC1 increases with advancing pregnancy, and severe preeclampsia (PE) is associated with further increases in placental MUC1 [17]. In addition, studies show that MUC1 helps to avoid alloreactivity at the maternal-fetal interface [18], restricts proliferation of decidual natural killer (dNK) cells, and regulates their cytotoxic mediator content [19]. An aberrant expression of the factors that regulate trophoblast invasion in early gestation results in pathological pregnancy states, such as intrauterine growth restriction (IUGR), PE, and spontaneous abortion [18, 20–23]. However, it is not known if first-trimester decidual stromal cells (DSCs) express MUC1 or if adrenomedullin 2 (ADM2; an invasion-promoting peptide) regulates the expression of MUC1 at the maternal-fetal interface in human pregnancy.
We recently reported that ADM2 is present in Day 5 human blastocyst secretome and lower serum levels of ADM2 are associated with spontaneous abortion [24]. In addition, ADM2 increases the invasive capacity of first-trimester extravillous trophoblast cells [24]. More importantly, we recently showed that inhibition of endogenous function of ADM2 in early rat pregnancy results in a reduced number of implantation sites accompanied by down-regulation of matrix metalloproteinase (MMP2/MMP9) system [25]. However, the mechanisms of ADM2 action at the maternal-fetal interface in human pregnancy are not known. Therefore, the focus of this study was to assess 1) the expression of MUC1 in villi and decidua obtained from different weeks of first-trimester placenta (6–14 wk), 2) if the expression of MUC1 mRNA is altered in the villous and decidual tissues obtained from spontaneous abortion, 3) the effect of ADM2 on the expression of MUC1 in trophoblast cells in addition to its effects on MUC1 and MMP2 immunoreactivity in trophoblastic spheroids, and 4) if MUC1 mRNA and protein are expressed in the decidual cells, and if ADM2 regulates the expression of MUC1 mRNA and protein in DSCs. Data from this study suggest that MUC1 is differentially regulated in villi and decidua during the first trimester, and that ADM2 inhibits the expression of MUC1 in HTR-8/SVneo cells, DSCs, and trophoblastic spheroids, with concomitant increase in MMP2 immunoreactivity in the spheroids. Further, ADM2 inhibits hypoxia-induced increases in MUC1 immunoreactivity in HTR-8/Svneo cells. Therefore, this study suggests that ADM2 may be a novel regulator of MUC1 expression at the maternal-fetal interface that may contribute to the process of embryo implantation and placental development in the human.
MATERIALS AND METHODS
Human Subjects
This study was approved by the Institutional Review Board at the University of Texas Medical Branch at Galveston (UTMB). The abortion tissues and blood were obtained at 6–14 wk of gestation from consented elective (before administration of any drug) and spontaneous abortion patients at Planned Parenthood Houston and at the on-site clinics of UTMB respectively, within the 30 min of the abortion procedure. The spontaneous abortion tissues were collected from patients diagnosed for fetal death by ultrasound typically within a few hours or days prior to evacuation of the uterine contents. The placental tissues were carefully dissected to exclude any membrane contamination, flash frozen for RNA isolation, embedded in optimum cutting temperature compound (OCT) to make 8-μm-thick sections for immunohistochemical studies, and used for cell isolation. The gestational ages were calculated from date of the last menstrual period with either ultrasound or clinical confirmation. Patients with maternal infection, chromosomal abnormalities, or any kind of physical insult were excluded.
HTR-8/SVneo Cell Culture
The HTR-8/SVneo cells (a kind gift from Professor Charles Graham of the Department of Anatomy and Cell Biology at Queen's University, Kingston, ON, Canada) were cultured in RPMI containing 10% fetal bovine serum (FBS) and followed by RPMI containing 2% bovine serum albumin (BSA) overnight prior to any treatment. HTR-8/SVneo cells were cultured at two separate atmosphere-controlled incubators either at 20% O2, 5% CO2 (normoxia) and 37°C or at 2% O2, 5% CO2 (hypoxia) balanced with N2 and at 37°C in the presence or absence of ADM2 (10−9–10−7 M) or ADM2 (10−8 M) in the presence or absence of ADM2 antagonist (ADM2 10−5 M). All treatments were done for 24 h either in 12-well plates for RNA extraction using Qiagen RNA extraction kit (Qiagen Inc.) or lab-Tek chambers (Electron Microscopy Sciences) for immunofluorescent staining.
HTR-8/SVneo Spheroid Generation and Treatments
The HTR-8/SVneo cells were used to generate the spheroids by hanging-drop method [26]. Seven hundred fifty cells were suspended in 7 μl of RPMI containing 2% FBS and 1% methylcellulose, cultured as a hanging drop on the lid of a Petri dish, and incubated in a humidified atmosphere of 95% (vol/vol) air, 5% (vol/vol) CO2 at 37°C for 24 h. Two to four spheroids were transferred to each well in eight-well lab-Tek chambers, treated with or without ADM2 (10−8 M), and cultured as above for 24 h. The spheroids were either used for RNA isolation using a Qiagen RNA extraction kit (Qiagen Inc.) or fixed for immunofluorescent staining with MUC1 or MMP2 antibody.
Isolation and Culture of the DSCs
The samples of decidua from different patients were not mixed, to avoid the induction of cytokine secretion as a result of an allogeneic reaction of leukocytes that initially contaminate DSC cultures. The tissues were extensively washed in PBS and the decidua was carefully freed from the villous tissue. Decidual fragments were finely minced in RPMI 1640 medium with 100 IU/ml penicillin and 50 mg/ml gentamicin, followed by addition of collagenase (10 mg/ml), 0.5% trypsin, and 0.2% EDTA (Sigma), and incubated for 15 min at 37°C. The reaction was stopped by adding an equal amount of cold RPMI containing 20% fetal calf serum (FCS; Gibco). The cell suspension was filtered through a 70-μm sieve and centrifuged at 425 × g for 10 min. The supernatant was discarded and the cell pellet was resuspended in RPMI, layered on Ficoll-Paque (Pharmacia LKB), and centrifuged for 20 min at 600 × g. The cells were collected from the interface, washed in PBS, and resuspended in complete growth medium (RPMI + 10% FCS). The suspension, containing mainly DSCs and leukocytes, was incubated in culture flasks for 1 h in complete growth medium to allow macrophages, granulocytes, and gland cells to adhere to the flask. The supernatant, containing DSCs and lymphocytes, was washed and incubated in complete growth medium. After overnight incubation, DSCs adhered to the flask and lymphocytes present in the supernatant were discarded, leaving the adherent cells comprising of mainly DSCs. The corresponding culture medium was then replaced with fresh complete growth medium. Proliferating DSCs overgrew other possible contaminant cells, thus further guaranteeing the purity of the cultures. Cells were further purified by selection of vimentin-positive cells using the AutoMACS system (Miltenyi Biotec). Cells were tested for purity by immunohistochemical staining with vimentin and cytokeratin antibody (CK7). Purified DSCs were seeded in a 12-well plate for RNA extraction or lab-Tek chambers for immunostaining. Cells were grown to 70% confluency and starved in RPMI + 2% BSA for 24 h prior to treatment with ADM2 (10−9–10−7 M) or a single dose of ADM2 (10−8 M). Cells were harvested after 24 h for RNA isolation using a Qiagen RNA extraction kit. (Qiagen Inc.) or fixed for immunostaining.
Immunofluorescent Staining
OCT-embedded frozen tissue sections, HTR-8/SVneo cells in monolayer, and spheroids were fixed with methanol and acetone mixture (1:1) for 15 min, washed twice for 5 min in PBS containing Tween-20, processed for immunofluorescent staining, and analyzed as reported earlier [27]. Primary antibody staining was done with rabbit anti-MUC1 antibody (gift from D. Carrson, Rice University, Houston, TX) or MMP2 antibody followed by incubation with donkey anti-rabbit IgG Alexa 488 or Texas red. Absence of antibody or rabbit IgG (Ready-to-Use; Dako) served as a negative control. Slides were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Inc.) and viewed by DP70 digital camera (Olympus Optical Co., Ltd.).
Isolation of Total RNA and Quantitative Real-Time PCR
Total RNA was isolated from tissues using TRIzol (Life Technologies) and from cells using an RNA extraction kit (Qiagen). Total RNA was digested with DNase I (cat. 79254; Qiagen), followed by cleanup procedures using a RNeasy minikit (cat. 74104; Qiagen) as per manufacturer's instructions, and cDNA was made as reported earlier [27]. Integrity of RNA was tested by running an aliquot of RNA on 1.1% agarose gel containing 2.2% formaldehyde in addition to testing on an Agilent 2100 Bio-analyzer system (Agilent Technologies). Quantitative real-time PCR was performed using SYBR Green (BioRad) on a CFX96 Real-Time PCR Detection System (cat. 184-5096; Bio-Rad) for MUC1 and glyceraldehyde phosphate dehydrogenase (GAPDH) using gene-specific primer assays (SA Biosciences). Amplification of GAPDH served as an endogenous control. Reactions were incubated at 95°C for 10 min and cycled according to the following parameters: 95°C for 30 sec (melt) and 60°C for 1 min (anneal/extend) for a total of 40 cycles. Negative control without cDNA was performed to test primer specificity. The relative gene expression of MUC1 mRNA was calculated by using the threshold cycle (CT) for GAPDH/CT. For no-RT control, nuclease-free water was used in place of the reverse transcriptase.
Statistical Analysis
Data sets were analyzed by Prism GraphPad Software. The means of the various groups were analyzed by unpaired t-test or one-way ANOVA and P ≤ 0.05 was considered statistically significant.
RESULTS
Correlation of MUC1 Expression in First-Trimester Villous Tissue with Spontaneous Abortion
MUC1 mRNA levels were assessed in villous tissue collected from women undergoing elective and spontaneous abortions at different weeks of the first trimester. Figure 1A shows that MUC1 mRNA expression was significantly higher in villous tissues from spontaneous abortion compared to age-matched elective abortion (*P < 0.05). Similar to the expression of MUC1 mRNA in villous tissue from spontaneous abortion, MUC1 immunoreactivity was also greater in villous tissue of spontaneous abortion at Gestational Weeks 7 and 10 compared to the age-matched control (Fig. 1B; magnification 400×).
FIG. 1.
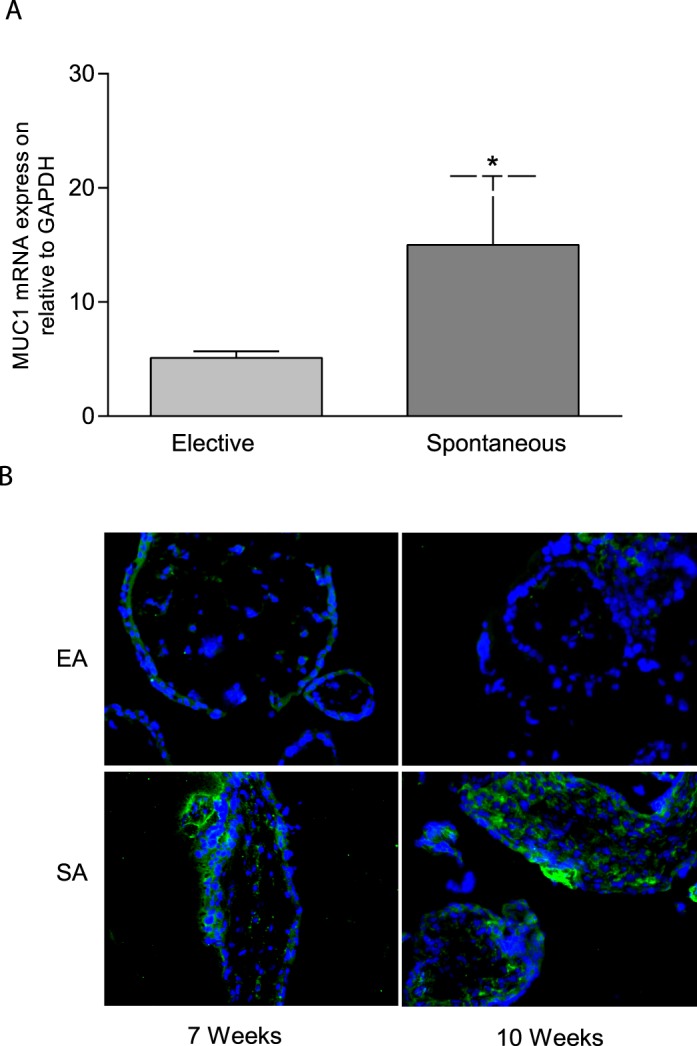
Expression of MUC1 mRNA and protein in villous tissue from first-trimester elective and spontaneous abortions. A) Quantitative PCR analysis showing comparison of MUC1 mRNA expression in villous tissue from spontaneous abortion with age-matched electively terminated pregnancies. The figure shows higher mRNA levels in spontaneously aborted villous tissue compared to elective abortions (*P < 0.05; n = 8–13), B) Immunofluorescent staining showing MUC1 immunoreactivity (green) in elective (EA) and spontaneously aborted (SA) villous tissues collected from 7 and 10 wk of gestation. IgG staining was used as negative control and DAPI was used for blue nuclear staining (original magnification ×400). Asterisk (*) indicates a significant difference between the groups.
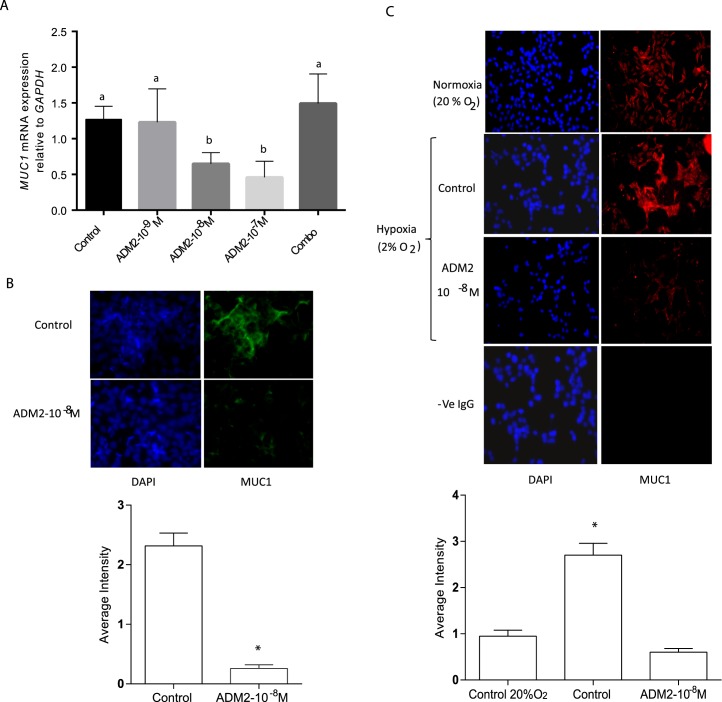
Effect of ADM2 on the Expression of MUC1 mRNA in HTR-8/SVneo Cell Monolayers
As shown in Figure 2, ADM2 dose-dependently decreased the expression of MUC1 mRNA (Fig. 2A; P < 0.05; n = 3) in HTR-8/SVneo cells, and these effects were blocked in presence of ADM2 antagonist (ADM217-47). Similarly, MUC1 protein immunoreactivity (Fig. 2B; P < 0.05; n = 3) was also decreased in the presence of ADM2 in HTR-8/SVneo cells. Further, Figure 2C shows that MUC1 immunoreactivity was higher in HTR-8/SVneo cells exposed to hypoxia, and these hypoxia-induced increases were inhibited in the presence of ADM2 (10−8 M) (magnification 400×).
FIG. 2.
Effects of ADM2 treatment on the expression of MUC1 mRNA and protein in HTR-8/SVneo cell monolayer. A) Effect of ADM2 on the expression of MUC1 mRNA in HTR-8/SVneo cell monolayer in presence (Combo) or absence of ADM2 antagonist (10−5 M). The bar graph represents the mean ± SEM from 3 replicates. Different letters on the error bars indicate significant difference between the groups (P < 0.05, n = 3). B) Colored pictures of immunofluorescent staining show ADM2 (10−8 M)-induced decreases in MUC1-specific immunoreactivity (green) in HTR-8/SVneo monolayer, and bar graph represents the mean ± SEM of the average staining intensity (*P < 0.05; n = 3; original magnification ×400). C) Immunofluorescent staining of HTR-8SV/neo cells exposed to hypoxia (2% O2) in the presence or absence of ADM2 (10−8 M). Bar graph represents the average staining intensity expressed as the mean ± SEM (*P < 0.05; n = 3). IgG was used as negative control and DAPI for blue nuclear staining. Original magnification ×400.
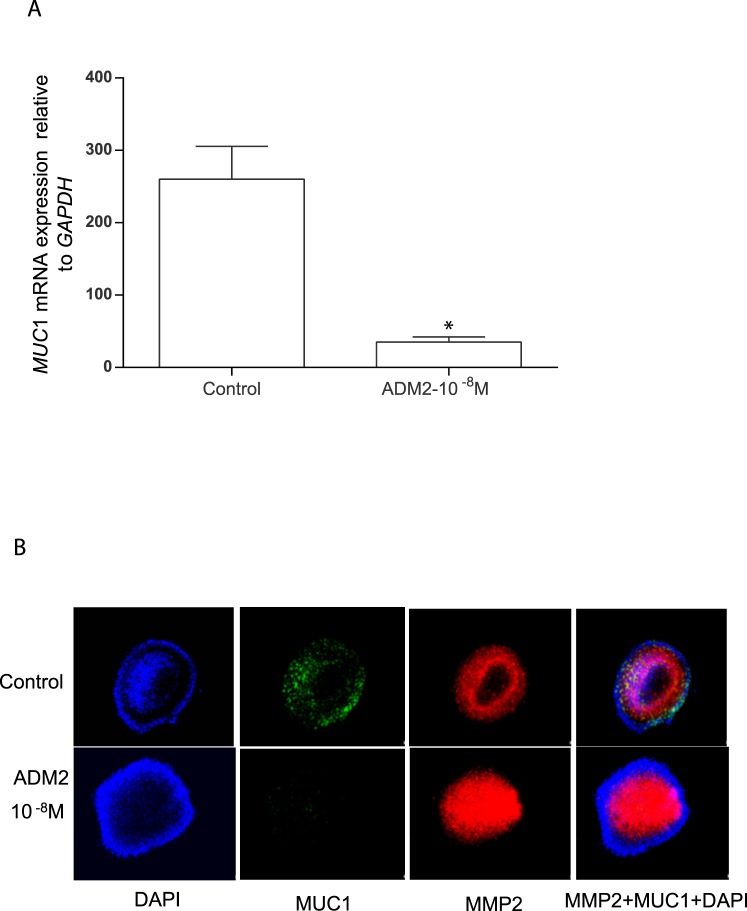
Effect of ADM2 on the Expression of MUC1 mRNA in HTR-8/SVneo Spheroids
The blastocyst trophectoderm cells express MUC1 [15] and ADM2 has been shown to be secreted by Day 5 human blastocyst [24]. Therefore, using spheroids of HTR-8/SVneo cells (as a model to mimic the blastocyst trophoblast), this study assessed the effects of ADM2 (10−8 M) on the expression of MUC1 mRNA. Figure 3 demonstrates that similar to the HTR-8/SVneo monolayer, these trophoblastic spheroids also respond to ADM2 (10−8 M) with a significant decrease in the expression of MUC1 mRNA (Fig. 3A; P < 0.05, n = 3) and immunoreactivity (Fig. 3B). In addition, concomitant to the ADM2-induced decreases in MUC1 expression, ADM2 stimulated an increase in immunoreactivity for MMP2 in the trophoblastic spheroids (magnification 400×).
FIG. 3.
Effect of ADM2 on the expression of MUC1 and MMP2 immunoreactivity in HTR- 8/SVneo spheroids. A) Quantitative PCR analysis showing the effect of ADM2 on the expression of MUC1 mRNA in HTR-8/SVneo spheroids. Bar graph represents the mean ± SEM from three replicates (*P < 0.05; n = 3). B) Immunofluorescent staining showing ADM2 (10−8 M)-induced decreases in MUC1 staining (green) and increases in MMP2-specific immunoreactivity (red) in HTR-8/SVneo spheroids. IgG staining was used as the negative control and DAPI for blue nuclear staining. Original magnification ×400.
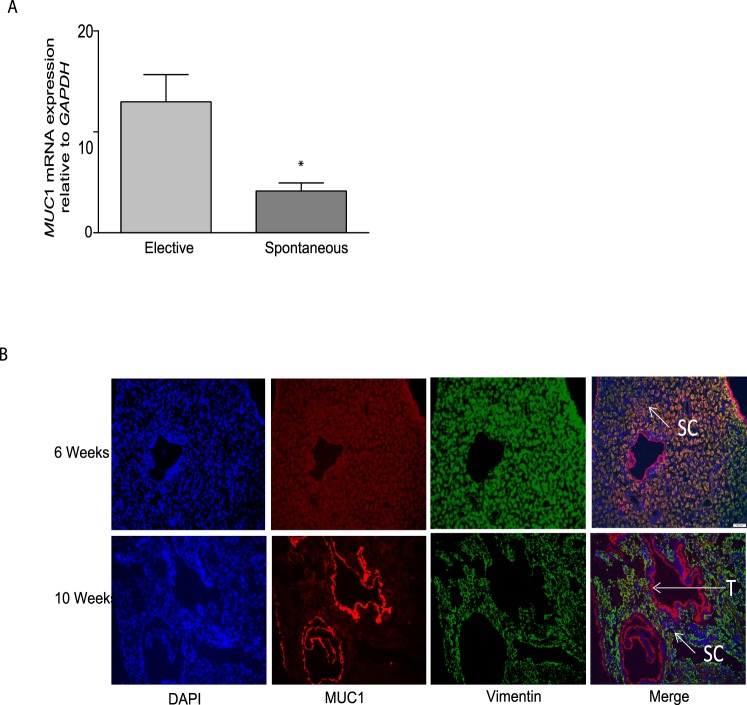
Expression of MUC1 in First-Trimester Decidual Tissue and Its Correlation with Spontaneous Abortion
The expression of MUC1 mRNA was also assessed in decidual tissues collected at different weeks of the first trimester from women undergoing elective and spontaneous abortion. As shown in Figure 4A, the expression of MUC1 mRNA levels was significantly lower in the decidual tissues from spontaneous abortion when compared to age-matched controls (Fig. 4A, n = 8–13, P < 0.05). Further, immunofluorescent studies shown in Figure 4B were performed to assess the localization of MUC1 immunoreactivity in decidual tissues obtained from elective terminations during the sixth and 10th weeks of gestation. Figure 4B shows that MUC1 immunoreactivity was localized to the DSCs and to the trophoblast cells that had invaded the decidua in the first trimester. However, interestingly, the expression of MUC1 immunoreactivity was more prominent in vimentin-positive stromal cells compared to the trophoblast cells in decidua from 6-wk elective abortion, whereas in decidua from 10-wk elective abortion, MUC1 immunoreactivity was more prominent in the trophoblast cells compared to the stromal cells (Fig. 4B).
FIG. 4.
Expression of MUC1 in decidual tissue obtained from elective abortions during the first trimester of human pregnancy. A) Quantitative PCR analysis showing comparison of MUC1 mRNA expression in first-trimester decidual tissue from spontaneous abortions with the levels in age-matched electively terminated pregnancies. The figure demonstrates a significant decrease in MUC1 levels in tissues obtained from spontaneous abortions. The bar graph represents the mean ± SEM of each group (*P < 0.05; n = 8–13). B) MUC1 immunoreactivity in the decidual tissues collected from patients undergoing elective abortion during 6 and 10 wk of gestation. The figure demonstrates localization of MUC1 immunoreactivity in decidual stromal (SC) and trophoblast cells (T). As shown, MUC1 immunoreactivity is stronger in SC compared to T in 6-wk decidua, whereas in 10-wk decidua, MUC1 immunoreactivity is more prominent in T compared to SC. IgG staining was used as negative control and DAPI for blue nuclear staining. Original magnification ×400.
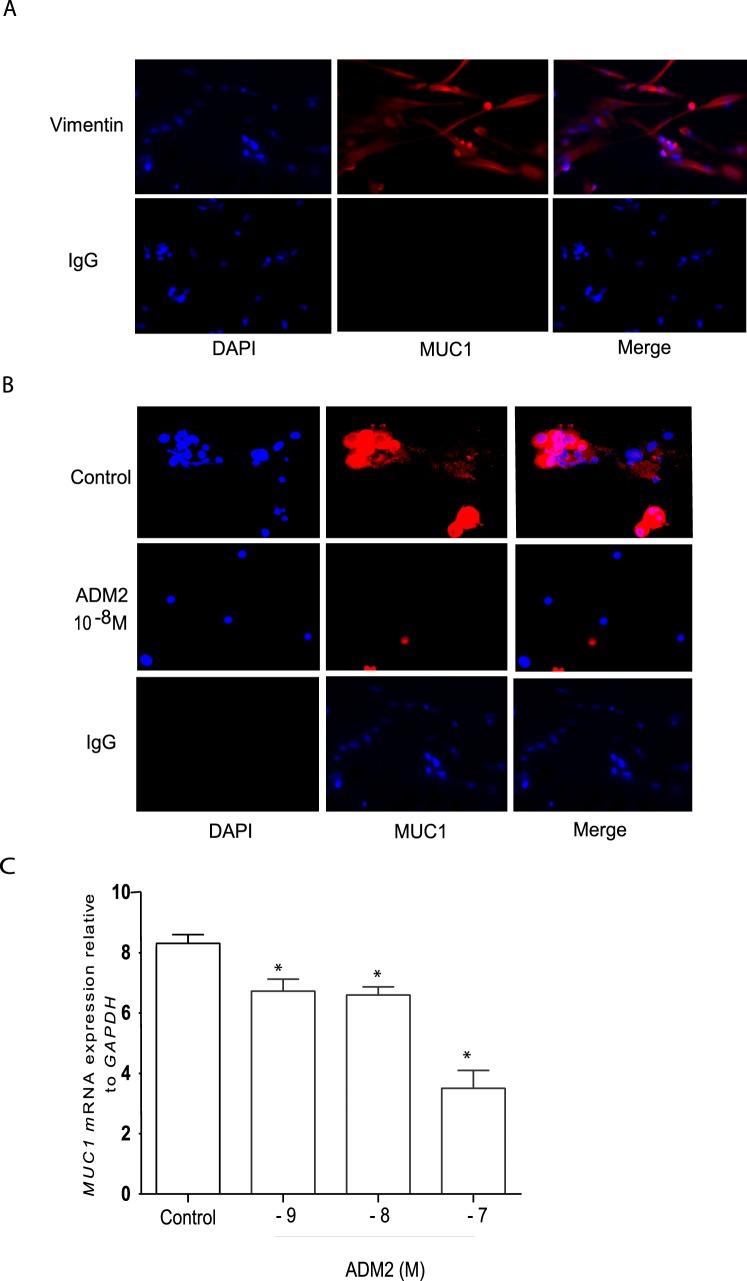
Effect of ADM2 on the Expression of MUC1 mRNA and Protein in Primary DSCs
The stromal cells were isolated from the decidual tissues obtained from the elective abortions at 6 and 11 wk of gestation to confirm RNA and protein expression of MUC1 in DSCs. As shown in Figure 5A, DSCs isolated were >98% pure. Further, Figure 5B demonstrates that ADM2 (10−8 M) causes a decrease in MUC1 immunoreactivity in DSCs isolated from decidua at 11 wk (magnification 400×). In addition, similar to the effect on MUC1 immunoreactivity in DSCs, ADM2 (10−9–10−7 M) dose-dependently decreased the expression of MUC1 mRNA in the isolated DSCs (Fig. 5C; P < 0.05; n = 3).
FIG. 5.
Isolation of DSCs from first-trimester decidual tissues and the effects of ADM2 on the expression of MUC1 protein and mRNA in the DSCs. A) The primary DSCs isolated from 11-wk elective abortion were stained with vimentin. More than 98% of DSCs were vimentin positive (red). B) Immunofluorescent staining of MUC1 (red) in DSCs isolated from 11-wk decidua and treated with ADM2 (10−8 M). The figure shows ADM2-induced decreases in MUC1-specific immunoreactivity (red). IgG staining was used as negative control and DAPI for blue nuclear staining (original magnification ×400). C) Quantitative PCR analysis showing ADM2-induced dose-dependent decreases in the expression of MUC1 mRNA in primary DSCs. The bar graph represents the mean ± SEM of 3 replicates (*P < 0.05).
DISCUSSION
Trophoblast invasion is the hallmark of first-trimester placental development and depends immensely on the invasion-promoting and inhibitory factors expressed at the maternal-fetal interface. The current study demonstrates that the expression of MUC1 is higher in placental villi and lower in the deciduae of human pregnancies undergoing spontaneous abortion in the first trimester compared to age-matched electively terminated pregnancies. Further, ADM2, an invasion-promoting peptide, caused decreases in the expression of MUC1 in the trophoblast cells as well as in the DSCs from first-trimester human placenta and in trophoblastic spheroids with concomitant increase in the expression of MMP2 in the spheroids. Taken together, this study demonstrates an association of altered expression of MUC1 with spontaneous abortion and that ADM2 regulates the expression of MUC1 in trophoblast cells and in DSCs to support early stages of placental formation in human pregnancy.
Previous reports indicate that MUC1 expression is elevated in carcinoma tumors and by hypoxia [1, 2]. In addition, the expression of MUC1 is reported to be higher in placenta from severely preeclamptic compared to normal pregnant women [5, 6, 28, 29], and PE is also known to be associated with placental hypoxia [5, 6, 28, 29]. Thus, the inhibitory effect of MUC1 on trophoblast invasion together with the elevated MUC1 levels in PE allows us to speculate that the pregnancies that develop into PE could have higher MUC1 levels in the placenta during early weeks of placental formation, which then may result in inefficient remodeling of spiral arteries and shallow trophoblast invasion [5, 6]. This speculation, to an extent, is supported by the elevated levels of MUC1 mRNA and protein in the villous tissue of first-trimester spontaneous abortions compared to those of age-matched controls (Fig. 1, A and B; P < 0.05). Moreover, the elevated levels of MUC1 in spontaneous abortion could be the cause or a consequence of the hypoxic state of pregnancies undergoing spontaneous abortion in the first trimester. Therefore, it is possible that those pregnancies with severe pathological hypoxia could elevate MUC1 levels during early pregnancy, resulting in spontaneous abortion in the first trimester. However, mild pathological hypoxia may still elevate MUC1 levels, perhaps not as highly as in spontaneous abortion, allowing the pregnancy to continue, but with complications such as PE and IUGR.
Recently it was reported that inhibition of the endogenous ADM2 function in early rat pregnancy resulted in impaired embryo implantation and restricted feto-placental growth [25, 30], and that ADM2 promoted the invasive capacity of first-trimester trophoblast cells in the human placenta [24]. The current study demonstrates that ADM2 causes a significant decline in the expression of MUC1 mRNA (Fig. 2A) and protein (Fig. 2B) in HTR-8/SVneo cells, and that addition of ADM2 to trophoblast cells exposed to hypoxia inhibits the hypoxia-induced increase in MUC1 immunoreactivity in trophoblast cells (Fig. 2C). Therefore, these data suggest ADM2 is a novel regulator of MUC1 expression in the trophoblast cells, and that ADM2 may have a protective role in gestational hypoxia that accompanies pregnancy complications such as PE, IUGR, and gestational diabetes by regulating MUC1 expression.
MUC1 is an extremely potent inhibitor of embryo attachment to the uterine epithelia [13, 31]. However, in vitro implantation models indicate that MUC1 is lost only at the site of embryo attachment in humans [8, 15, 32], suggesting that factors expressed on the blastocyst surface or released from blastocyst trigger the loss of MUC1 at the point of contact. Matrix metalloprotease 2 (MMP2) is expressed consistently during development from one-cell to blastocyst stage and MT1-MMP that activates pro-MMP2 protein is suggested to have a sheddase activity on MUC1 [33]. Because we showed earlier that ADM2 regulates the expression and activity of MMP2/9 during implantation and early placentation in rat pregnancy [25], we hypothesized that ADM2 would increase the expression of MMP2 and decrease the expression of MUC1 in human trophectoderm trophoblasts to support implantation and trophoblast invasion. Because of ethical reasons associated with the use of human blastocysts and dissimilarities in human and rodent embryo implantation and early placental formation, we used trophoblastic spheroids of HTR-8/SVneo cells as a 3-D model mimicking blastocysts to test this hypothesis. As shown in Figure 3, ADM2 causes an increase in MMP2 immunoreactivity, which is concomitant to a decrease in the expression of MUC1 protein in the trophoblastic spheroids.
In contrast to the villous tissue, expression of MUC1 mRNA in the decidua is significantly lower in spontaneous abortion compared to that in age-matched elective abortions, suggesting an association of lower MUC1 mRNA with first-trimester spontaneous abortion (Fig. 4A; P < 0.05). Early phases of human pregnancy are associated with the accumulation of a unique subset of natural killer (NK) cells in the maternal decidua. Decidual NK cells that are devoid of cytotoxicity play a pivotal role in successful pregnancy. By secreting large amounts of cytokines/chemokines and angiogenic factors, dNK cells participate in all steps of placentation, including trophoblast invasion into the maternal endometrium and vascular remodeling.
The villous-independent phase of spiral artery remodeling at the onset of pregnancy is thought to be mediated, at least in part, by uterine NK cells, which accumulate around spiral arteries [34]. Functionally in decidua, MUC1 is shown to restrict proliferation of NK cells and regulate their content of cytotoxic mediators [19]. Thus, an abnormal level of decidual MUC1 is likely to have an adverse effect on the establishment of a successful pregnancy, and decreases in the decidual MUC1 in spontaneous abortion could be the cause or consequence of a pathological condition. However, although these data suggest that first-trimester spontaneous abortion is associated with decreases in the decidual MUC1 mRNA, they should be interpreted with caution because of the heterogeneous nature of the decidual tissue, which comprises different cell types, such as leukocytes, dNK cells, stromal cells, and trophoblast cells, that could contribute to the expression of MUC1 mRNA in the decidual tissue.
On examining the localization of MUC1 protein in the decidua obtained during the different weeks of first-trimester elective abortions, intense MUC1 immunoreactivity was found localized to the stromal cells as well as to the trophoblast cells that had invaded the decidua. Interestingly, the stromal cells expressed relatively higher amount of MUC1 immunoreactivity compared to the trophoblast cells in the decidual tissue from elective abortion at 6 wk, whereas at 10 wk decidua MUC1 immunoreactivity was decreased in the stromal cells with concomitant increase in the trophoblast cells (Fig. 4B). This suggests that the MUC1 expression is differentially regulated with time and space in decidua during the first trimester of human pregnancy. The expression of MUC1 was further confirmed in primary DSCs isolated (>98% pure; Fig. 5A) from pregnancies that were terminated electively during 6 and 11 wk of gestation as described in Materials and Methods. The MUC1 protein and mRNA were expressed at both the sixth and the 11th week of gestation (data not shown), and ADM2 (10−8 M) caused significant decreases in the expression of MUC1 protein (Fig. 5B) as well as mRNA in DSCs (Fig. 5C; P < 0.05). The current study suggests that the DSCs could be a potential source of MUC1 acting on dNK cells, and that ADM2 may be involved in regulating MUC1 expression in decidua to facilitate implantation and early placental formation.
In summary, the current study demonstrates that ADM2 causes decreases in the expression of MUC1 mRNA and immunoreactivity in trophoblast cells and DSCs from first-trimester human placenta. Moreover, ADM2 also causes decreases in MUC1 mRNA and immunoreactivity along with a concomitant increase in MMP2 immunoreactivity in the trophoblastic spheroids. We therefore suggest that higher expression of ADM2 in the maternal-fetal interface during early gestation may have a physiological role in maintaining optimal MUC1 expression to promote implantation and facilitate efficient trophoblast invasion to support the progression of a healthy pregnancy. Future studies utilizing cocultures of 1) trophoblast cells and DSCs and 2) trophoblast cells and endometrial epithelial cells with knockdown and overexpression of MUC1 will help to further dissect the ADM2-MUC1-specific pathways in trophoblast invasion.
ACKNOWLEDGMENT
The authors would like to thank Ms. Sandra Garcia Dale for her incredible administrative assistance.
Footnotes
Supported by National Institutes of Health grants HD054867 (to M.C.) and HL58144 (to C.Y.).
REFERENCES
- Aubert S, Fauquette V, Hemon B, Lepoivre R, Briez N, Bernard D, Van S. I, Leroy X, Perrais M. MUC1, a new hypoxia inducible factor target gene, is an actor in clear renal cell carcinoma tumor progression Cancer Res 2009. 69 5707– 5715 [DOI] [PubMed] [Google Scholar]
- Mikami Y, Hisatsune A, Tashiro T, Isohama Y, Katsuki H. Hypoxia enhances MUC1 expression in a lung adenocarcinoma cell line Biochem Biophys Res Commun 2009. 379 1060– 1065 [DOI] [PubMed] [Google Scholar]
- Carson DD. The cytoplasmic tail of MUC1: a very busy place. Sci Signal. 2008;1:e35. doi: 10.1126/scisignal.127pe35. [DOI] [PubMed] [Google Scholar]
- Shyu MK, Lin MC, Liu CH, Fu YR, Shih JC, Lee CN, Chen HY, Huang J, Huang MC, Hsieh FJ. MUC1 expression is increased during human placental development and suppresses trophoblast-like cell invasion in vitro Biol Reprod 2008. 79 233– 239 [DOI] [PubMed] [Google Scholar]
- Rahn JJ, Chow JW, Horne GJ, Mah BK, Emerman JT, Hoffman P, Hugh JC. MUC1 mediates transendothelial migration in vitro by ligating endothelial cell ICAM-1 Clin Exp Metastasis 2005. 22 475– 483 [DOI] [PubMed] [Google Scholar]
- Thirkill TL, Cao T, Stout M, Blankenship TN, Barakat A, Douglas GC. MUC1 is involved in trophoblast transendothelial migration Biochim Biophys Acta 2007. 1773 1007– 1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins Annu Rev Physiol 2008. 70 431– 457 [DOI] [PubMed] [Google Scholar]
- Aplin JD. Embryo implantation: the molecular mechanism remains elusive Reprod Biomed Online 2007. 14 spec no 1 49–55 [DOI] [PubMed] [Google Scholar]
- Refaat B, Simpson H, Britton E, Biswas J, Wells M, Aplin JD, Ledger W. Why does the fallopian tube fail in ectopic pregnancy? The role of activins, inducible nitric oxide synthase, and MUC1 in ectopic implantation Fertil Steril 2012. 97 1115– 1123 [DOI] [PubMed] [Google Scholar]
- Brayman M, Thathiah A, Carson DD. MUC1: a multifunctional cell surface component of reproductive tissue epithelia. Reprod Biol Endocrinol. 2004;2:4. doi: 10.1186/1477-7827-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmaraj N, Gendler SJ, Carson DD. Expression of human MUC1 during early pregnancy in the human MUC1 transgenic mouse model Biol Reprod 2009. 81 1182– 1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacunza E, Ferretti V, Barbeito C, Segal-Eiras A, Croce MV. Immunohistochemical evidence of Muc1 expression during rat embryonic development. Eur J Histochem. 2010;54:e49. doi: 10.4081/ejh.2010.e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplin JD. MUC-1 glycosylation in endometrium: possible roles of the apical glycocalyx at implantation Hum Reprod 1999. 14 (suppl 2): 17– 25 [DOI] [PubMed] [Google Scholar]
- Dharmaraj N, Wang P, Carson DD. Cytokine and progesterone receptor interplay in the regulation of MUC1 gene expression Mol Endocrinol 2010. 24 2253– 2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meseguer M, Aplin JD, Caballero-Campo P, O'Connor JE, Martin JC, Remohi J, Pellicer A, Simon C. Human endometrial mucin MUC1 is up-regulated by progesterone and down-regulated in vitro by the human blastocyst Biol Reprod 2001. 64 590– 601 [DOI] [PubMed] [Google Scholar]
- Sharma A, Kumar P. Understanding implantation window, a crucial phenomenon J Hum Reprod Sci 2012. 5 2– 6 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Shyu MK, Chen CW, Lin NY, Liao WC, Chen CH, Lin CJ, Huang HC, Lee JJ, Huang MJ, Tseng GF, Shih JC, Lee CN, et al. MUC1 expression is elevated in severe preeclamptic placentas and suppresses trophoblast cell invasion via beta1-integrin signaling J Clin Endocrinol Metab 2011. 96 3759– 3767 [DOI] [PubMed] [Google Scholar]
- Redzovic A, Laskarin G, Dominovic M, Haller H, Rukavina D. Mucins help to avoid alloreactivity at the maternal fetal interface. Clin Dev Immunol. 2013;2013:542152. doi: 10.1155/2013/542152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskarin G, Medancic SS, Redzovic A, Duric D, Rukavina D. Specific decidual CD14(+) cells hamper cognate NK cell proliferation and cytolytic mediator expression after mucin 1 treatment in vitro J Reprod Immunol 2012. 95 36– 45 [DOI] [PubMed] [Google Scholar]
- Bose P, Kadyrov M, Goldin R, Hahn S, Backos M, Regan L, Huppertz B. Aberrations of early trophoblast differentiation predispose to pregnancy failure: lessons from the anti-phospholipid syndrome Placenta 2006. 27 869– 875 [DOI] [PubMed] [Google Scholar]
- Brosens I, Dixon HG, Robertson WB. Fetal growth retardation and the arteries of the placental bed Br J Obstet Gynaecol 1977. 84 656– 663 [DOI] [PubMed] [Google Scholar]
- Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of pre-eclampsia J Pathol 1970. 101:vi. [PubMed] [Google Scholar]
- Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia Biol Reprod 2003. 69 1– 7 [DOI] [PubMed] [Google Scholar]
- Havemann D, Balakrishnan M, Borahay M, Theiler R, Jennings K, Endsley J, Phelps J, Hankins GD, Yallampalli C, Chauhan M. Intermedin/adrenomedullin 2 is associated with implantation and placentation via trophoblast invasion in human pregnancy J Clin Endocrinol Metab 2012. 98 695– 703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan M, Elkins R, Balakrishnan M, Yallampalli C. Potential role of intermedin/adrenomedullin 2 in early embryonic development in rats Regul Pept 2011. 170 65– 71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Knoefler I, Schleussner E, Markert UR, Fitzgerald JS. HTR8/SVneo cells display trophoblast progenitor cell-like characteristics indicative of self-renewal, repopulation activity, and expression of “stemness-” associated transcription factors. Biomed Res Int. 2013;2013:243649. doi: 10.1155/2013/243649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan M, Yallampalli U, Dong YL, Hankins GD, Yallampalli C. Expression of adrenomedullin 2 (ADM2)/intermedin (IMD) in human placenta: role in trophoblast invasion and migration Biol Reprod 2009. 81 777– 783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright JE, Tse WK, Whitley GS. Hepatocyte growth factor induced human trophoblast motility involves phosphatidylinositol-3-kinase, mitogen-activated protein kinase, and inducible nitric oxide synthase Exp Cell Res 2002. 279 219– 226 [DOI] [PubMed] [Google Scholar]
- Verlohren S, Geusens N, Morton J, Verhaegen I, Hering L, Herse F, Dudenhausen JW, Muller DN, Luft FC, Cartwright JE, Davidge ST, Pijnenborg R, et al. Inhibition of trophoblast-induced spiral artery remodeling reduces placental perfusion in rat pregnancy Hypertension 2010. 56 304– 310 [DOI] [PubMed] [Google Scholar]
- Chauhan M, Yallampalli U, Reed L, Yallampalli C. Adrenomedullin 2 antagonist infusion to rats during midgestation causes fetoplacental growth restriction through apoptosis Biol Reprod 2006. 75 940– 947 [DOI] [PubMed] [Google Scholar]
- Aplin JD, Seif MW, Graham RA, Hey NA, Behzad F, Campbell S. The endometrial cell surface and implantation. Expression of the polymorphic mucin MUC-1 and adhesion molecules during the endometrial cycle Ann N Y Acad Sci 1994. 734 103– 121 [DOI] [PubMed] [Google Scholar]
- Meseguer M, Pellicer A, Simon C. MUC1 and endometrial receptivity Mol Hum Reprod 1998. 4 1089– 1098 [DOI] [PubMed] [Google Scholar]
- Wang H, Wen Y, Mooney S, Li H, Behr B, Polan ML. Matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase expression in human preimplantation embryos Fertil Steril 2003. 80 (suppl 2): 736– 742 [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies Placenta 2006. 27 939– 958 [DOI] [PubMed] [Google Scholar]