Abstract
The Y chromosome gene Sry is responsible for sex determination in mammals and initiates a cascade of events that direct differentiation of bipotential genital ridges toward male-specific fate. Sox9 is an autosomal gene and a primary downstream target of SRY. The activation of Sox9 in the absence of Sry is sufficient for initiation of male-specific sex determination. Sry- to -Sox9 replacement has mostly been studied in the context of sex determination during early embryogenesis. Here, we tested whether Sry- to -Sox9 replacement affects male fertility in adulthood. We examined males with the Y chromosome carrying a deletion removing the endogenous Sry, with testes determination driven either by the Sox9 (XY Tdym1Sox9 ) or the Sry (XY Tdym1Sry ) transgenes as well as wild-type males (XY). XY Tdym1Sox9 males had reduced testes size, altered testes shape and vasculature, and increased incidence of defects in seminiferous epithelium underlying the coelomic blood vessel region when compared to XY Tdym1Sry and XY. There were no differences between XY Tdym1Sry and XY Tdym1Sox9 males in respect to sperm number, motility, morphology, and ability to fertilize oocytes in vitro, but for some parameters, transgenic males were impaired when compared to XY. In fecundity trials, XY Tdym1Sry, XY Tdym1Sox9, and XY males yielded similar average numbers of pups and litters. Overall, our findings support that males lacking the testis determinant Sry can be fertile and reinforce the notion that Sry does not play a role in mature gonads. Although transgenic Sox9 overexpression in the absence of Sry results in certain testicular abnormalities, it does not translate into fertility impairment.
Keywords: fertility, sex determination, Sox9, sperm, Sry, testis
INTRODUCTION
In most mammals, including humans, testis determination is regulated by the Y chromosome encoded gene Sry, which acts in fetal gonads and induces development of testes rather than ovaries [1–3]. Identification of Sry as a testis determinant was followed by extensive molecular studies of testis development. The progress in this field was enormous, and much has been learned about the players and pathways involved in this process (reviewed in [4–11]). In short, Sry signaling in XY genital ridges is the first time when the developmental fates of male and female gonads diverge. In the presence of Sry, somatic bipotential precursor cells develop into Sertoli cells, which then support differentiation and development of the male germline. Pre-Sertoli cell differentiation is initiated by the SRY-induced upregulation of autosomally encoded Sox9 (Sry-related HMG box gene 9). Once upregulated, SOX9 is involved in the induction and maintenance of other male specific factors. Together, these factors form a molecular regulatory pathway that helps to antagonize ovarian development while concurrently promoting the male pathway.
Sox9 is a direct target of SRY and has been shown to play a pivotal role in male sexual development. Ablation of Sox9 in humans [12, 13] and mice [14–16] causes male-to-female phenotypic sex reversal while Sox9 gain-of-function, such as duplication of the SOX9 locus in humans [17, 18] and Sry-independent upregulation of Sox9 in the fetal gonadal ridges of Wt1-Sox9 transgenic mice, causes testis formation in XX individuals [19]. The role of Sox9 in sex determination has mostly been studied in the context of two X chromosomes and focused on the period during fetal development when the sex determination takes place. In XX mice transgenic for Wt1-Sox9 (XXSox9), gonadal development follows a male-specific program but the resulting testes are small and display seminiferous tubules with germline development arrested at the early mitotic stage [19]. This is thought to be due to the presence of two X chromosomes and therefore an increased dosage of X-linked gene products [20, 21], and the absence of Y chromosome genes needed to initiate and support normal spermatogenesis [22, 23].
Here, we investigated adult mice carrying the Y chromosome with deleted endogenous Sry (YTdym1) in which male sex determination was driven by transgenic overexpression of Wt1-Sox9. In these mice, Sox9 is expressed from the Wt1 regulatory elements within a yeast artificial chromosome, mimicking gonadal expression of the endogenous Wt1 gene [19]. Thus, transgenic Sox9 is expressed both during the critical sex determination window and during adulthood, allowing SOX9 to participate in testis and germ cell development after the initial steps of sex determination. We examined testis morphology and vasculature, spermatogenesis progression, sperm function in vitro, and male fertility, and demonstrated that although Sox9-driven sex determination results in certain testicular abnormalities in adulthood, they do not translate to fertility and sperm function impairment.
MATERIALS AND METHODS
Chemicals and Media
Mineral oil was purchased from Squibb and Sons; equine chorionic gonadotropin (eCG) and human chorionic gonadotropin (hCG) were purchased from Calbiochem. Unless otherwise stated, all the other chemicals were obtained from Sigma Chemical Co. Medium T6 was used for in vitro fertilization (IVF), Hepes-buffered CZB (Hepes-CZB) for gamete handling [24, 25], and CZB medium [25] for embryo culture. Both CZB and T6 were maintained in an atmosphere of 5% CO2 in air, and HEPES-CZB was maintained in air.
Mice
We used 6- to 12-wk-old B6D2F1 (C57BL/6J × DBA/2) females (NCI) as oocyte donors for IVF and for fecundity testing, and C57BL/6 (NCI) and MF1 females (bred in-house) were used for backcrossing. The mice of interest in this study were males with a Y chromosome with an 11-kb deletion removing the testis determinant Sry (dl1Rlb) [1, 26], designated as YTdym1, complemented either by an autosomally located Sry transgene [Tg(Sry)2Ei] [27] (XYTdym1Sry) or by an autosomally encoded Sox9 transgene driven by Wt1 (Wilms tumor 1) promoter [19] (XYTdym1Sox9).
To produce XYTdym1Sox9 males, XY males transgenic for Wt1-Sox9 (XYSox9) were generated by intracytoplasmic sperm injection with cryopreserved sperm obtained from Andreas Schedl (University of Nice) and oocytes from wild-type females. The XYSox9 males were subsequently bred to XYTdym1 females, which carried an X chromosome and a Y chromosome with deleted Sry, resulting in XYTdym1Sox9 males. The XY males with an intact Y chromosome were used as wild-type controls. Transgenic mice were either on partial MF1 or partial C57BL/6 genetic background (backcrossed for at least four generations), and wild-type controls were full MF1 or C57BL/6. The mice were fed ad libitum with a standard diet and maintained in a temperature and light-controlled room (22°C, 14L:10D) in accordance with the guidelines of the Laboratory Animal Services at the University of Hawaii and guidelines presented in National Research Council's Guide for Care and Use of Laboratory Animals published by Institute for Laboratory Animal Research of the National Academy of Science. The protocol for animal handling and treatment procedures was reviewed and approved by the Animal Care and Use Committee at the University of Hawaii.
Fecundity Testing
Eight-wk-old males were cohabitated for 10 wk with robust and highly fertile B6D2F1 females (one female per male). The number of litters and pups generated by each breeding pair and offspring sex and genotypes were recorded.
IVF
Sperm capacitation and IVF were performed as previously described [28]. Briefly, oocytes were collected from B6D2F1 females induced to superovulate with injections of 5 international units eCG and hCG given 48 h apart. Epididymal sperm were collected by release of cauda epididymis directly into T6 medium and were capacitated for 1 h at 37°C in humidified atmosphere of 5% CO2. Gametes were coincubated for 4 h. After gamete coincubation, the oocytes were washed several times with Hepes-CZB medium, followed by at least one wash with CZB medium. Morphologically normal oocytes were selected for culture.
Sperm Analyses
Cauda epididymal sperm were released into T6 and incubated for 15 min at 37°C. Sperm counts and motility assessments were performed using a hemocytometer. For analysis of sperm morphology, epididymal sperm spreads were stained with silver nitrate as previously described [29]. The slides were scored blind, and 100 sperm per male were examined.
Testes Analyses
The testes were dissected, weighed, and subjected to gross morphological assessment using a stereo microscope. The length and width of each testis were measured, and testis shape was defined as length:width ratio. Testis surface vasculature was scored by quantifying the number of major and minor branches of the coelomic artery. For histology assessment, testes were Bouin-fixed, paraffin-embedded, sectioned (thickness, 5 μm), and stained with hematoxylin (H) and eosin (E) and/or periodic acid Schiff (PAS). We followed a strict protocol for testes sectioning. The testes were first halved along the short axis. The sections were then done from the center toward the pole. We routinely prepared six slides, the first for PAS-H staining, the second for H&E staining, and the third to sixth were unstained. The sections were placed on these six slides in a specific order, which allows for having sections of different depth on the same slide. For the analysis of the coelomic region, we used PAS-H stained slides, and for every male, we scored the same depth sections. Five scored sections were taken from the following depths: 5, 10, 15, 20, and 25 μm.
Isolation of Genital Ridges
Embryos were collected from timed matings of transgenic males with hybrid B6D2F1 strain females, with 1200 h of the day on which the mating plug was observed designated as 0.5 Days Postcoitum (dpc). The embryos were collected at 11.5 dpc into ice-cold diethyl pyrocarbonate-phosphate buffer solution. Genital ridges with mesonephros attached were dissected, immediately frozen in liquid nitrogen, and kept in −80°C until further processing by quantitative RT-PCR (qRT-PCR). The genotypes of the embryos were determined by PCR with Y chromosome markers using genomic DNA extracted from tail tissue. Primers sequences are shown in Supplemental Table S1 (Supplemental Data are available online at www.biolreprod.org).
RNA Isolation and Real Time RT-PCR
RNA was extracted from mature testes using Trizol and DNase I treatment (Ambion) and purified using RNeasy kit (Qiagen). For genital ridges, RNA was isolated from individual pairs of gonads using Micro-RNA Kit (Qiagen) following the manufacturer's instructions. Reverse transcription of polyadenylated RNA was performed with Superscript Reverse Transcriptase III, according to the manufacturer's guidelines (Invitrogen). Real-time PCR was performed using SYBR Green PCR Master mix on an ABI QuantStudio 12K Flex machine (Applied Biosystems). PCR reactions were incubated at 95°C for 10 min followed by 37 PCR cycles (10 sec at 95°C and 60 sec at 60°C). At least four adult mice or seven embryos per genotype and per background were analyzed. All the reactions were carried out in at least triplicate per assay, and two ubiquitously expressed genes (Actb and Sdha) were used as loading controls. Actin, beta (Actb) was used because it is one of the most commonly used reference genes. Succinate dehydrogenase complex, subunit A, flavoprotein (Sdha) was used before by others working in sex determination field and is considered a suitable reference gene for qRT-PCR expression studies during early gonad development [30]. It has also been reported as one of the most stable reference genes across different tissues (including testis and ovary) in juvenile as well as adult mice [31]. The ΔCt value for each individual sample was calculated by subtracting the average Ct of loading control(s) from the average Ct of a tested gene. The ΔΔCt value was calculated by subtracting the ΔCt of each tested male from the average ΔCt of reference samples (XYRIII males). The data were expressed as a fold value of expression level. Primers sequences are shown in Supplemental Table S1.
Statistics
The Fisher exact test was used to assess the differences between the genotypes for IVF data and to analyze the progeny genotype frequencies. Student t-test was used for all other analyses.
RESULTS
In this study, our goal was to investigate the effects of Sry-to-Sox9 substitution in directing sex determination on spermatogenesis and fertility in mature male mice. To achieve this, we used mice with the Y chromosome carrying a deletion of endogenous Sry in which this loss was counterbalanced either by transgenic addition of Sry (XYTdym1Sry) or transgenic overexpression of Sox9 (XYTdym1Sox9). Mice with an intact Y chromosome (XY) were included in all the analyses as wild-type controls. We investigated these three genotypes on two genetic backgrounds, inbred C57BL/6 and outbred MF1.
Sry and Sox9 Expression
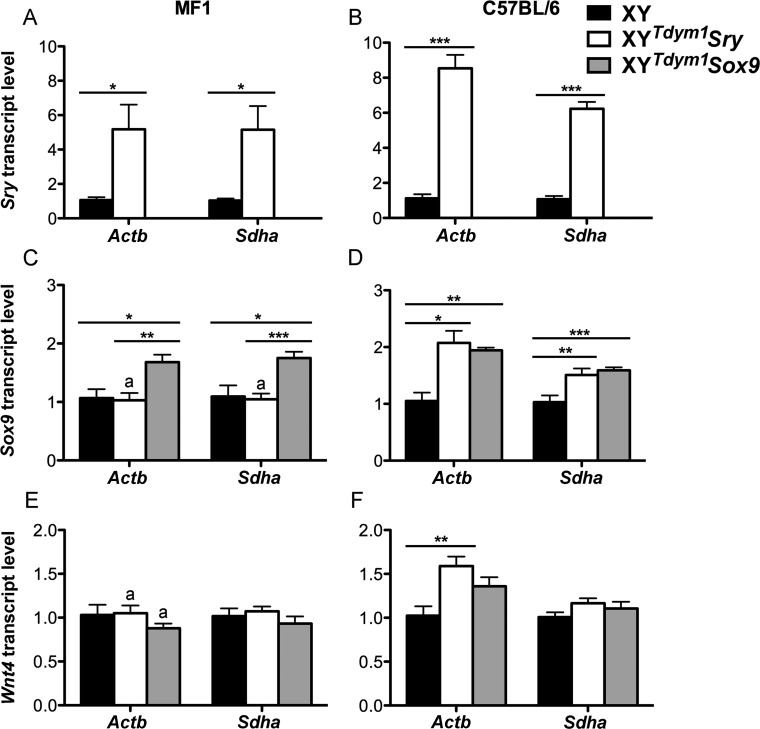
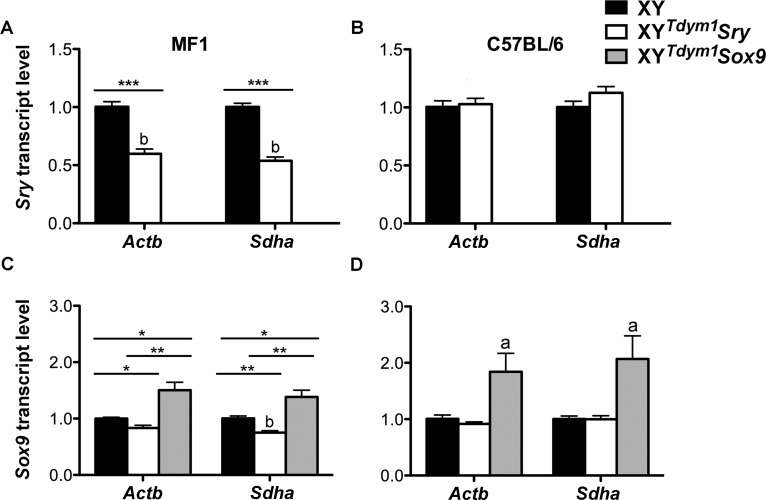
In wild-type males, Sry is first expressed at 10.5 dpc, peaks at 11.5 dpc, and declines after 12.5 dpc [32–34]. When present, it triggers Sox9 expression, which is subsequently maintained throughout life. In the absence of Sry, the female pathway becomes activated, resulting in expression of Wnt4 (and other female factors) (reviewed in [4, 7, 11]). In mature testes, Sry is highly expressed although produced transcripts are considered nonfunctional [35]. We examined transcript levels of Sry, Sox9, and Wnt4 in fetal gonads from 11.5 dpc XYTdym1Sox9 and XYTdym1Sry males using XY males as references (Fig. 1). Sry mRNA was not present in fetal gonads from XYTdym1Sox9 males, as expected. XYTdym1Sry males had Sry transcripts elevated when compared to XY (Fig. 1, A and B). The fold increase was higher on C57BL/6 (∼6- to 9-fold) than on MF1 (∼5-fold) genetic background. Sox9 transcript levels were higher in XYTdym1Sox9 males than in XY males, on both backgrounds. Two types of transgenics had similar levels of Sox9 expression on C57BL/6 background, while on MF1 background, XYTdym1Sox9 males yielded higher transcript levels (Fig. 1, C and D). There were no differences between groups in the expression of female pathway gene Wnt4 except for XYTdym1Sry versus XY comparison on C57BL/6 genetic background, with Actb being used as the reference gene, which showed an increase in the former genotype (Fig. 1, E and F). Next, we examined Sry and Sox9 transcript levels in mature testes (Fig. 2). Sry expression in testes from XYTdym1Sry males was lower than in XY on the MF1 background but similar on the C57BL/6 background. Sox9 expression in XYTdym1Sox9 males was higher than in XY and XYTdym1Sry males. On MF1, but not C57BL/6, background, XYTdym1Sry males had lower Sox9 levels than XY. Altogether, these analyses demonstrated that expression of Sry and Sox9 in transgenic males is not equivalent to that of XY males, with transgenic Sry levels elevated in fetal gonads and Sox9 levels elevated in both fetal and mature gonads.
FIG. 1.
Sry, Sox9, and Wnt4 transcript expression in fetal gonads. Relative Sry (A, B), Sox9 (C, D), and Wnt4 (E, F) mRNA levels in genital ridges from 11 dpc XY, XYTdym1Sry and XYTdym1Sox9 fetuses, on MF1 (A, C, E) and C57BL/6 (B, D, F) genetic backgrounds were obtained by real-time PCR; the levels obtained with XY were arbitrarily set to 1. The loading controls were two ubiquitously expressed genes Actb and Sdha. Values are mean ± SEM, with n = 6 fetuses per group (except Sox9 run where for XYTdym1Sox9 n = 5 fetuses were used). Statistical significance (t-test): *P < 0.05; **P < 0.01; ***P < 0.001; adifferent than the same genotype on C57BL/6 background (P < 0.05). In the pilot experiment, we tested for presence of Sry transcripts in XYTdym1Sox9 males and, as expected, none were found. Therefore, XYTdym1Sox9 males were not included in runs shown in A and B. Primer sequences are shown in Supplemental Table S1.
FIG. 2.
Sry and Sox9 transcript expression in mature gonads. Relative Sry (A, B) and Sox9 (C, D) mRNA levels in whole testes from 8- to 11-wk-old XY, XYTdym1Sry, and XYTdym1Sox9 males, on MF1 (A, C) and C57BL/6 (B, D) genetic backgrounds were obtained by real-time PCR; the levels obtained with XY were arbitrarily set to 1. The loading controls were two ubiquitously expressed genes Actb and Sdha. Values are mean ± SEM, with n = 4 males per group. Statistical significance (t-test): adifferent than all the others (P < 0.05); bdifferent than the same genotype on C57BL/6 background (P < 0.05); *P < 0.05; **P < 0.01; ***P < 0.001. XYTdym1Sox9 males do not have Sry and were not included in runs shown in A and B. Primer sequences are shown in Supplemental Table S1.
Testes Analyses
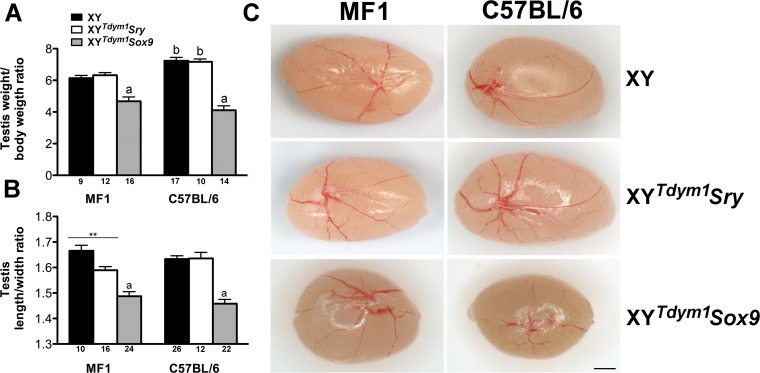
We next carried out the analyses of testes from XY, XYTdym1Sry, and XYTdym1Sox9 males. Testis weight, expressed as a testis weight:body weight ratio, was decreased in XYTdym1Sox9 males when compared to XY and XYTdym1Sry, on both genetic backgrounds (Fig. 3A). Testes from XYTdym1Sox9 males had a distorted shape and appeared more roundish rather than oval typical for XY males (Fig. 3C and Supplemental Fig. S1). This was also evidenced quantitatively, with decreased testis length:width ratio (Fig. 3B). XYTdym1Sry males had similar but milder testis shape distortion noted on MF1, but not C57BL/6 background. Interestingly, testes from ∼75% of XYTdym1Sox9 MF1 males had white patches visible under the tunica albuginea (Supplemental Fig. S2).
FIG. 3.
Testis size and shape. A) Testis:body weight ratio. B) Testis length:width ratio. C) Representative images of testes from all genotypes. Values are mean ± SEM, with the number of testes examined shown under the X axes. Statistical significance (t-test, P < 0.05): adifferent than other genotypes within background; bdifferent than the same genotype on other background; **P < 0.01. Bar = 1 mm. Males were 10–20 wk at testes collection. Testis:body weight ratio was calculated by dividing the combined weight of both testes in milligrams by body weight in grams.
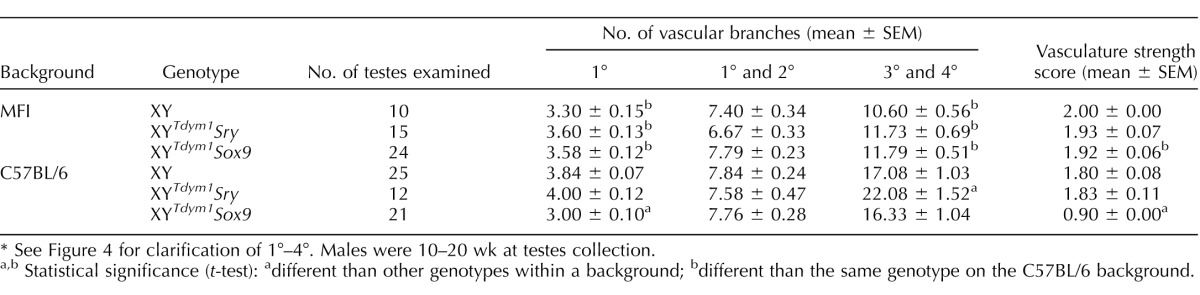
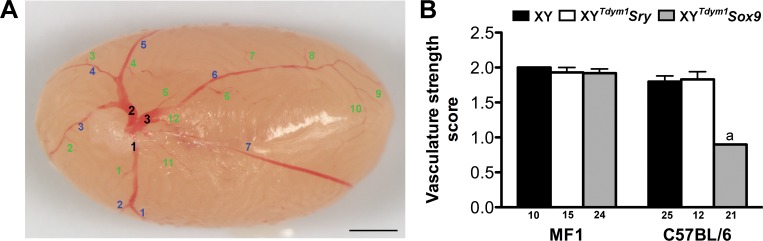
To assess testis vasculature, the numbers of branches of coelomic artery were counted, with distinction between the primary, secondary, and other vascular branches (Table 1 and Fig. 4). This analysis demonstrated that, regardless of the genotype, mice on MF1 background had overall fewer vascular branches than mice on C57BL/6 background (Table 1). This decrease in branch number did not affect the arbitrary vascular strength score except for XYTdym1Sox9 males on an C57BL/6 background, which had vascular development impaired as evidenced by 2-fold decrease in the score (Table 1 and Fig. 4).
TABLE 1.
Vasculature analysis of testes from XYTdym1Sox9, XYTdym1Sry, and XY males.*
See Figure 4 for clarification of 1°–4°. Males were 10–20 wk at testes collection.
Statistical significance (t-test): adifferent than other genotypes within a background; bdifferent than the same genotype on the C57BL/6 background.
FIG. 4.
Testis vasculature. A) Testis vasculature was assessed by quantification of primary (black), secondary (blue), and tertiary/quaternary/threadlike (green) vascular branches. Bar = 2 mm. B) The vasculature strength score was defined as: 2 = good visibility of all blood vessels; 1 = good visibility of primary and secondary vessels and poor visibility of tertiary/quaternary/threadlike vessels; 0 = poor visibility of all the vessels. The values are mean ± SEM, with the number of testes examined shown under the X axis. Statistical significance (t-test, P < 0.05): adifferent than other genotypes within background and the same genotype on MF1 background. Males were 10–20 wk old at testes collection.
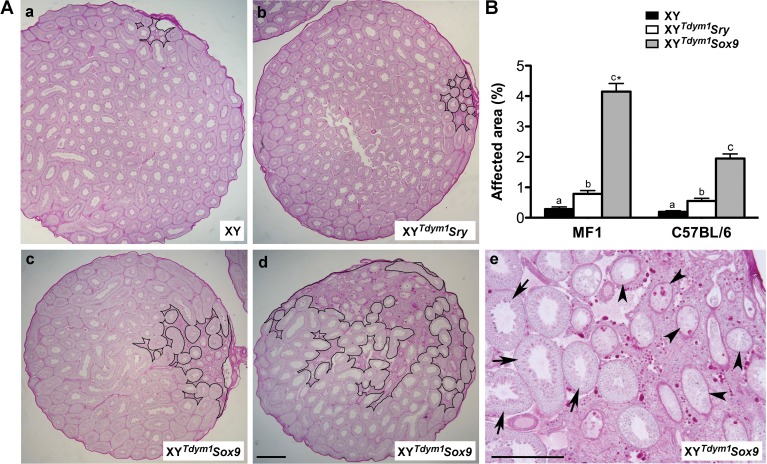
The analysis of testis sections revealed severe defects in the region underlying and adjacent to the coelomic artery in XYTdym1Sox9 males (Fig. 5). In these males, this area had an abundance of interstitial tissue and poor formation of seminiferous tubules (Fig. 5A), and the affected area made up more of the testis, measured as the proportion of the entire cross section (Fig. 5B). In XYTdym1Sry males, milder defects in this region were noted. In both XYTdym1Sry and XYTdym1Sox9 males, the tubules outside affected region were normally formed and contained the expected germ cells, including well-developed elongated spermatids (Fig. 6 and Supplemental Fig. S3).
FIG. 5.
Defects of the area underlying coelomic artery. A) Exemplary panoramics of testes cross sections with normal (a), slightly affected (b), severely affected (c), and extremely severely affected (d, e) area underlying the coelomic vessel. The affected area is outlined with black line in a–d. In e, some of the affected areas are presented at higher magnification to show that both normal tubules (arrows) and abnormal tubules with depleted germ cells (arrowheads) are present. Males were on MF1 genetic background. B) Quantitative analysis of size of the affected region. The area of the entire testicular cross section and the area of the affected region were measured, and the data are expressed as a percentage of the total area. The values are mean ± SEM with n = 15 cross sections (five per male) per group. Male age was 10–11 wk for Aa–Ac, 25 wk for Ad–Ae, and 8–11 wk for B. Statistical significance (t-test, P < 0.05): bars with different letters within background are statistically different; asterisk (*) different than respective genotype on C57BL/6 background. Bar = 500 μm.
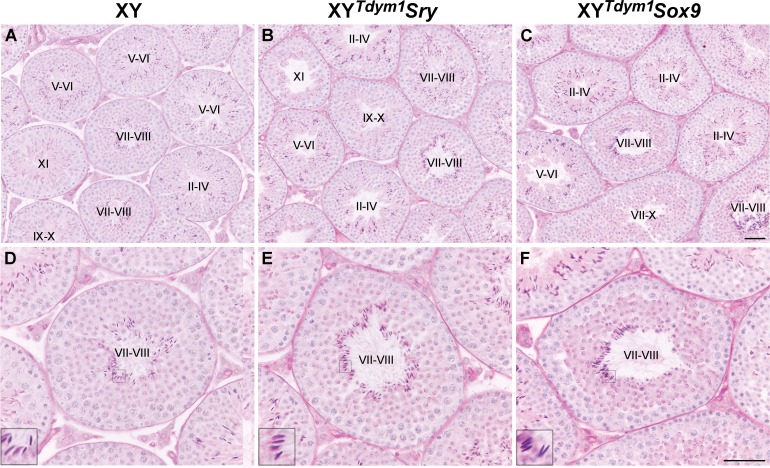
FIG. 6.
Testes histology and normalcy of spermatogenesis progression. Exemplary cross sections of testes from 8- to 11-wk-old XY (A, D), XYTdym1Sry (B, E), and XYTdym1Sox9 (C, F) on C57BL/6 genetic background. Several seminiferous tubules are shown under lower magnification (A–C) to emphasize that spermatogenesis is ongoing in all the tubules. In D–F, single tubules are shown at higher magnification to emphasize that all the expected germ cells are present, with elongated spermatids shown in insets. Tubule stages are shown in roman numerals. Bar = 50 μm. Inset = 3× magnification. The same analysis was performed for mice on MF1, and the images are shown in Supplemental Figure S3.
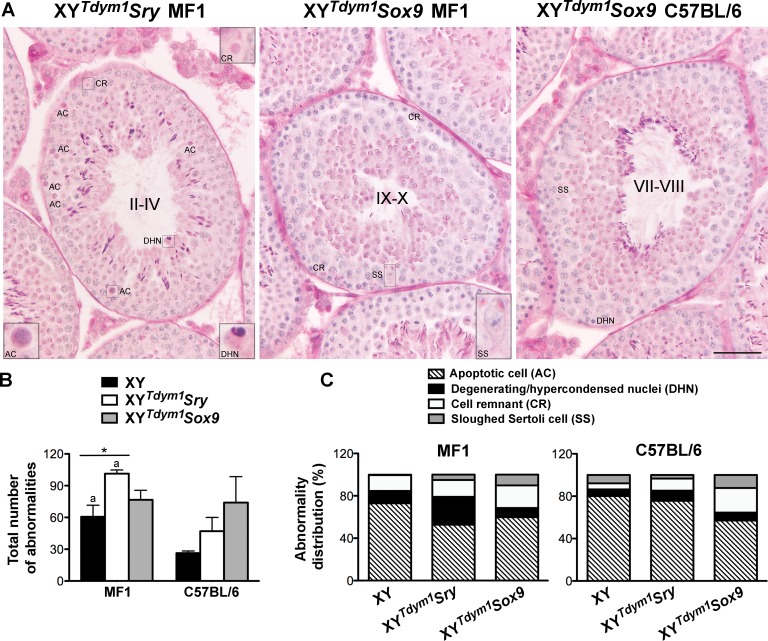
Occasional seminiferous epithelium abnormalities, such as apoptotic cells, cells with hypercondensed/degenerating nuclei, cell remnants, and sloughed Sertoli cells (Fig. 7A and Supplemental Fig. S4) were observed in all the genotypes. When these abnormalities were quantified, XYTdym1Sry had more defects than XY on MF1 background, and both XY and XYTdym1Sry were more affected on MF1 background when compared to C57BL/6. XYTdym1Sox9 males did not differ from other genotypes on either background (Fig. 7B). The distribution of abnormality types expressed as a percentage of all defects varied between the genotypes (Fig. 7C). On an MF1 background, XYTdym1Sry males had less apoptotic cells and more cells with hypercondensed/degenerating nuclei, and both XYTdym1Sry and XYTdym1Sox9 had more sloughed Sertoli cells when compared to XY (P < 0.05, t-test). On C57BL/6 background, XYTdym1Sox9 had less apoptotic cells than the other genotypes (P < 0.05, t-test). When the respective genotypes were compared across backgrounds, XYTdym1Sry and XY had more cells with hypercondensed/degenerating nuclei, XYTdym1Sry had less apoptotic cells, and XY had less sloughed Sertoli cells on an MF1 background. XYTdym1Sox9 yielded similar results on both backgrounds. The number of Sertoli cells was similar across genotypes and backgrounds (Supplemental Fig. S5).
FIG. 7.
Testis histology and abnormalities. A) Cross sections of testicular tubules from XYTdym1Sry and XYTdym1Sox9 males showing exemplary abnormalities of seminiferous epithelium. Four types of defects were differentiated: apoptotic cell (AC), degenerating/hypercondensed nucleus (DHN), cell remnant (CR), and sloughed Sertoli cell (SS). B) Total number of abnormalities in XY, XYTdym1Sry, and XYTdym1Sox9 males. C) Distribution of abnormality types in XY, XYTdym1Sry, and XYTdym1Sox9 males. Data shown in B and C are from the analysis of three males per genotype on each genetic background. For each male, five tubules per each stage cluster (XII–I, II–IV, V–VI, VII–VIII, IX–X, XI) were scored, adding to 30 tubules examined per male and 90 per genotype on a given background. In B, bars are mean and SEM of n = 3 males. Statistical significance (t-test, P < 0.05): adifferent than respective genotype on C57BL/6 background; *P < 0.05. Statistical differences between groups for data shown in C were assessed after percentages were transformed to angles and are discussed in the text. Tubule stages in A are shown in roman numerals. Bar = 50 μm. Insets = 3× magnification. Males were 8–11 wk old at testes collection.
Altogether, the analyses of testes revealed presence of some defects in XYTdym1Sox9 males, which affected testis weight, shape, vasculature, and the area within the testes underlying the coelomic vessel. XYTdym1Sry males had remnants of this testicular phenotype, with mild impairment in testis shape and area underlying coelomic artery. In both genotypes, these defects did not translate into spermatogenic deficiency because the majority of tubules had efficiently developing germ cells. The cellular abnormalities were infrequent and observable in all genotypes, including XY controls, and thus not specific to transgenic males.
Sperm Analyses In Vitro
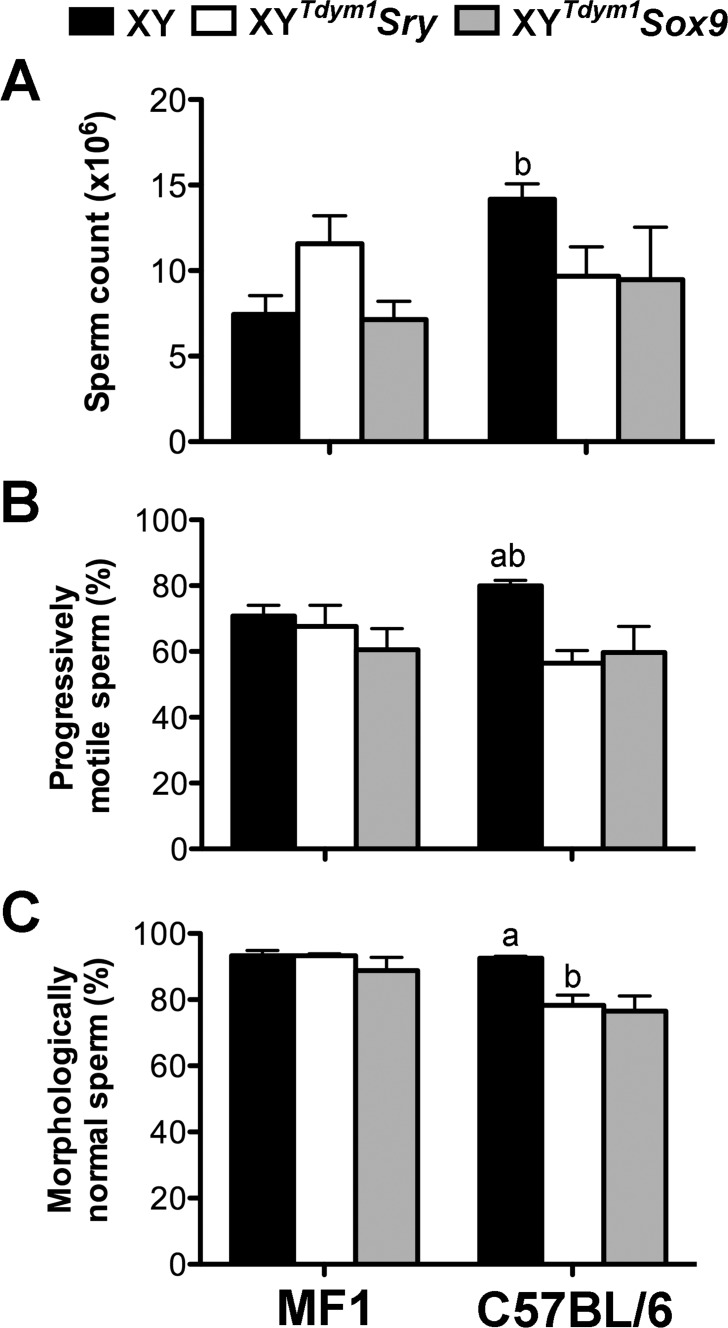
Because histological assessment of testes indicated that spermatogenesis was ongoing and that elongated spermatids were produced, we next carried out analyses of epididymal sperm. Both types of transgenic males produced high numbers of sperm (Fig. 8A), at least 50% of which were progressively motile (Fig. 8B), and more than 75% with morphologically normal head shape (Fig. 8C). There were no differences between XY, XYTdym1Sry, and XYTdym1Sox9 MF1 males in sperm number and proportion of motile and morphologically normal sperm. On the C57BL/6 background, however, both types of transgenic males scored lower for motility and morphology than XY males. When sperm function was assessed in vitro, sperm from both types of transgenic males were less able to fertilize oocytes than sperm from XY with a difference more pronounced on MF1 background, but did not differ from each other (Table 2). Males on MF1 genetic background yielded lower fertilization rates than C57BL/6 males, regardless the genotype. For all the tested genotypes, most of the two-cell embryos developed to the blastocyst stage (Table 2). To summarize, sperm analyses support that there are no differences between males with sex determination driven by transgenic Sry and by transgenic Sox9, and that in both genotypes sperm are functional in vitro.
FIG. 8.
Sperm analyses. Sperm analyses were performed for 8- to 11-wk-old XY, XYTdym1Sry, and XYTdym1Sox9 males. A) Sperm number. B) Progressive sperm motility. C) Normal sperm head shape. Values are mean ± SEM, with n = 4 males per group. Statistical significance (t-test, P < 0.05): adifferent than other genotypes within background; bdifferent than the same genotype on other background.
TABLE 2.
The results of in vitro fertilization (IVF) with sperm from XYTdym1Sox9, XYTdym1Sry, and XY males.*
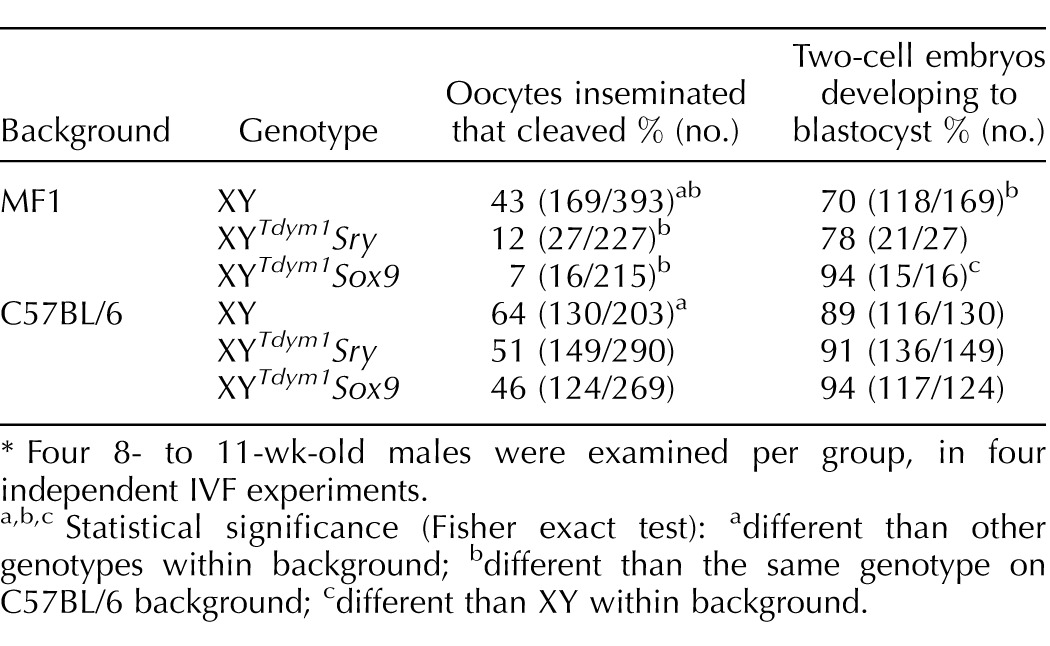
Four 8- to 11-wk-old males were examined per group, in four independent IVF experiments.
Statistical significance (Fisher exact test): adifferent than other genotypes within background; bdifferent than the same genotype on C57BL/6 background; cdifferent than XY within background.
Fecundity
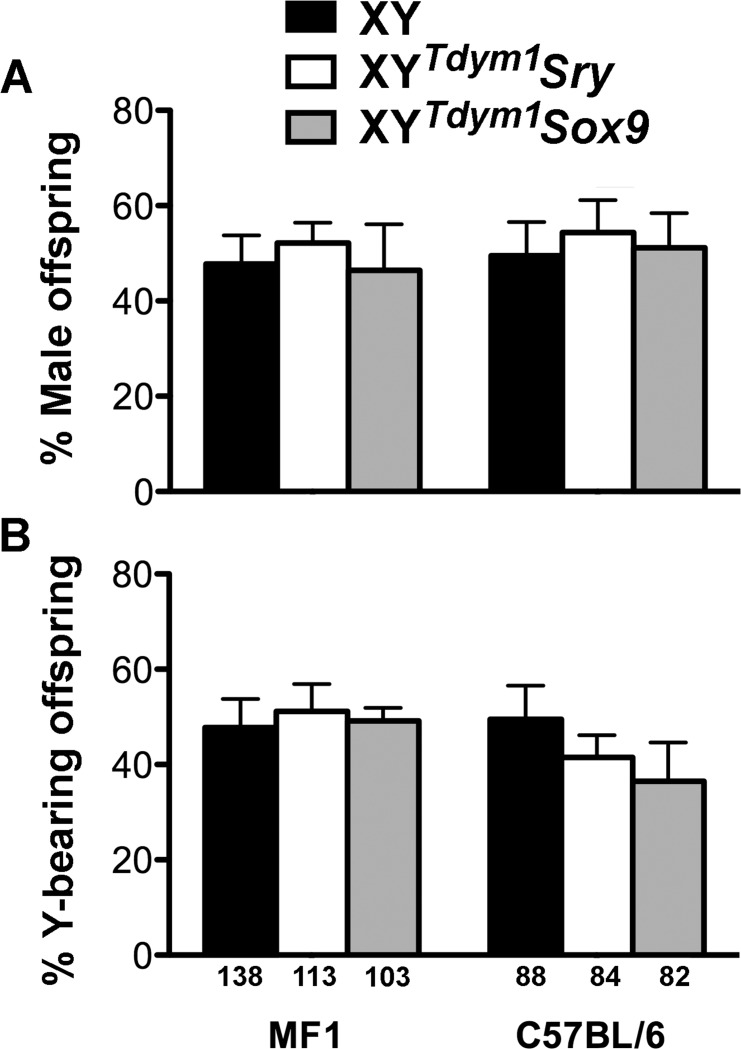
As a final check for the differences between XYTdym1Sox9 and XYTdym1Sry males, we performed fecundity trials. No differences were observed between the examined groups (XY, XYTdym1Sox9, and XYTdym1Sry) in the number of litters and pups per male (Table 3). Males on the MF1 genetic background were overall more efficient breeders than C57BL/6 males, although the difference reached statistical significance only in the comparison of XY males. Because the transgenic sex determinant (Sry and Sox9) is transmitted independently from the Y chromosome, transgenic males are expected to yield four progeny types: XX and XYTdym1 females and XY and XXSry/Sox9 males. XY, XYTdym1Sox9, and XYTdym1Sry males all yielded a similar, and expected, sex ratio (Fig. 9A) and Y chromosome transmission (Fig. 9B).
TABLE 3.
The analysis of fecundity of XYTdym1Sox9, XYTdym1Sry, and XY males.*
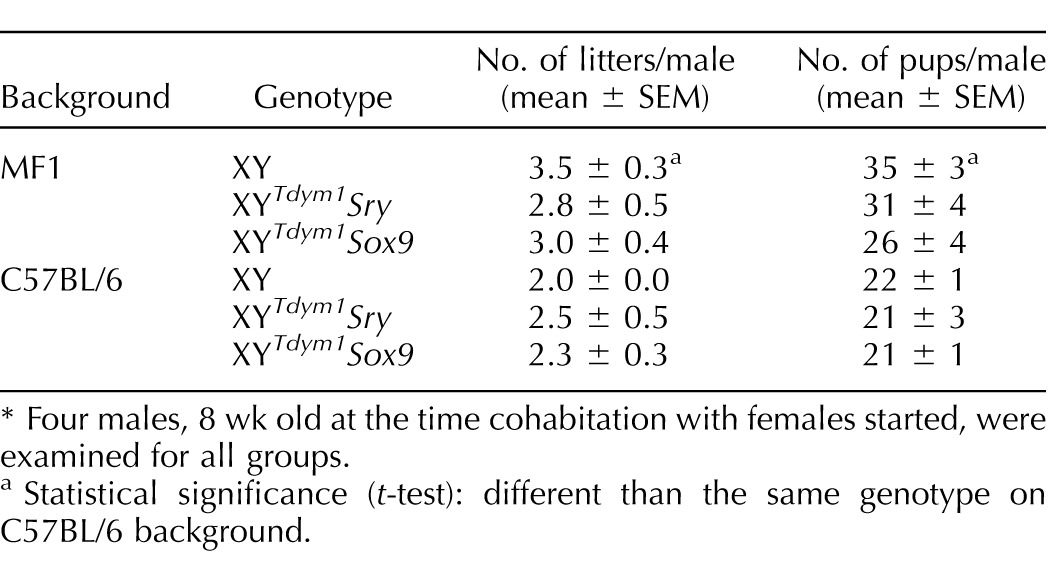
Four males, 8 wk old at the time cohabitation with females started, were examined for all groups.
Statistical significance (t-test): different than the same genotype on C57BL/6 background.
FIG. 9.
Progeny genotype frequencies. Offspring obtained after mating of XY, XYTdym1Sry, and XYTdym1Sox9 males with wild-type females were sexed and genotyped. A) Proportion of males. B) Proportion of offspring carrying Y chromosome. No differences between groups were observed (t-test, after transformation of percentage to angles). The number of genotyped offspring is shown under the X axis.
Age Effect
The analyses of testis weight, shape, and vasculature were performed on males ranging in age from 10 to 20 wk, while all other analyses were done on younger males (8–11 wk of age). We therefore wondered whether the testicular defects were due to an increase in age. To address that, we performed a regression analysis to correlate the age of mice with these three specific testicular phenotypes. No correlations were observed for transgenic males (Supplemental Figs. S6–S8). In fecundity trials, when we extended breeding time (data not shown), no decline was observed in older males. However, when the analysis of the region underlying the coelomic vessels (see Fig. 5 for data obtained with younger males) was performed for three old XYTdym1Sox9 MF1 males, the percentage of affected area increased ∼3-fold in 25- to 38-wk-old males as compared to 8- to 11-wk-old males of the same genotype. Moreover, patchy testes (Supplemental Fig. S2) were observed more frequently in old (19–38 wk) than in young (9 wk) XYTdym1Sox9 MF1 males. Altogether these data suggest that while the age has no impact on testis weight, shape, vascular patterning, and fertility, some internal testicular defects are enhanced in older XYTdym1Sox9 MF1 males.
DISCUSSION
We demonstrated that males devoid of the Y chromosome-encoded sex-determining gene Sry and with sex determination driven by SRY direct target, Sox9, produce functional spermatozoa and are fertile.
We are aware of only one study describing spermatogenesis and fertility in mice lacking Sry [36]. In this study, the investigated mice carried the Ods (Odsex; ocular degeneration with sex reversal)-dominant insertional mutation, which upregulates Sox9 by altering the upstream enhancer elements [37]. When the Ods mutation was added to mice with a Y chromosome lacking the endogenous Sry (YTdym1), the resulting heterozygous males were transiently fertile and exhibited abnormal testicular vasculature. These defects were rescued in homozygous males that were long-term fertile. The rescue of the phenotype was likely due to an increase in Sox9 expression because the testicular SOX9 level in homozygotes was ∼3-fold higher than in heterozygotes [36]. The Odsex model has been lost and is no longer available to the scientific community (Richard Behringer, University of Texas, MD Anderson Cancer Center, personal communication).
Here, we examined males with transgenic overexpression of Sox9. The Wt1-Sox9 transgene we used drives an upregulation of Sox9 in the developing gonads at the appropriate time (10.5 dpc). The Wt1 promoter also maintains transgenic Sox9 expression at later stages and during adulthood, allowing transgenic SOX9 to participate in testis and germ cell development after the initial steps of sex determination. The presence of the Wt1-Sox9 transgene increased global Sox9 expression above that noted in wild-type males in both fetal and mature gonads. When XYTdym1Sox9 males were bred between 2 and 4 mo of age, they were fertile at the level comparable to XYTdym1Sry and XY controls. When we continued breeding for the subsequent 2.5 mo (data not shown), no decline was observed. This stands in contrast to what was observed with heterozygous Odsex mutant males, which at the age of 5–6 mo were completely sterile [36], and suggests that Sox9 levels in XYTdym1Sox9 males were sufficient and compatible with stable fertility.
Similarly, as in Odsex mice, we observed some testicular defects in XYTdym1Sox9 males. The testicular phenotype included decreased testis weight, abnormal testis shape, and impairment in testis vasculature and in the region underlying the coelomic vessel (Figs. 3–5). Decreased testis weight is often associated with depleted germ cell counts [23]. Although a quantitative analysis of spermatogenesis progression on testes sections was not performed, the epididymal sperm counts in XYTdym1Sox9 males were normal. Decreased testes weights were also observed in Odsex mutants although in this model it was linked to germ cell degeneration [36].
Testis vasculature in adult XYTdym1Sox9 males on a C57BL/6 background was impaired, with a poorly resolved coelomic artery and collateral vessels. It has been shown previously that testis morphogenesis is at least partially dependent on both internal and external vascularization [38–40]. Perturbation of the developing coelomic artery and transient vascular plexus that sits between the mesonephros and gonad may not only be linked to gross anatomy abnormality of XYTdym1Sox9 testes but may also be the cause of the poorly resolved external vasculature observed. Vasculature disorganization similar to that noted in XYTdym1Sox9 males was reported for heterozygous, but not homozygous, Odsex mice in both fetal and mature gonads [36]. Although we did not examine the vasculature of fetal gonads from XYTdym1Sox9 males, when the expression of Wnt4, a signaling molecule for proper pattern of gonadal vascularization, was quantified with qRT-PCR in 11.5 dpc genital ridges, we observed no differences between XYTdym1Sox9 and wild-type XY males. Wnt4 misexpression in male fetal gonads has been linked to disruption of vascular patterning [41, 42], and in the Odsex model, Wnt4 was deregulated in heterozygous but not homozygous males [36].
The testicular phenotype observed in mature males can be a consequence of disturbances taking place during the sex determination window (10.5–12.5 dpc), events occurring during later stages of gonadal formation, and/or mechanisms operating in the mature gonad. There is ample published evidence showing that deregulation of Sox9 expression during sex determination can have profound effects on testis formation, with a potential to induce partial or complete sex reversal [12–19]. In mature testes Sox9 is expressed in Sertoli cells [13, 43, 44]. The presence of SOX9 at puberty suggests that this protein is needed for Sertoli cell maturation and their acquisition of the ability to support spermatogenesis. When Sox9 was conditionally turned off after sex determination had taken place (from 14.5 dpc), testis cords developed normally in spite of decreased transcript levels of anti-Mullerian hormone (Amh), the SOX9 target gene responsible for Mullerian duct regression [45]. Null mutants showed normal embryonic development and fertility when young but suffered from severe spermatogenesis defects starting from about 3 mo of age, and eventually became sterile due to progressive depletion in germ cell number [45]. By 1 yr of age, testes from these Sox9-null mice were dominated by Sertoli cell only and hypospermatogenic seminiferous tubules, but interestingly, the coelomic artery was normally developed and resolved [45]. Together, these published findings suggest that testis cord differentiation is independent of Sox9 but reinforces that Sox9 is essential for maintenance of testicular function in adults. Because testosterone levels were unaffected, spermatogenic failure was not due to a repression of steroidogenesis. Rather, the lack of SOX9 seemed to impair functional interaction between Sertoli and germ cells, possibly involving deregulation of genes involved in stress response and inflammation in Sertoli cells [46]. Here, we did not observe Sox9 depletion. On the contrary, Sox9 transcript levels were elevated in both fetal and mature gonad of XYTdym1Sox9 males. This is probably why the deregulation of Sox9 in XYTdym1Sox9 males was compatible with fertility and the observed testicular phenotype was not linked to sperm dysfunction.
Previous studies have shown that Sox9 is involved in stem cell/progenitor cell maintenance and/or regulating differentiation in adult neural, mammary, intestinal, dermal, and hepatic tissues [47–51]. Given that Sertoli cells form the blood-testis barrier, which encapsulates proliferating and differentiating spermatogonia, a similar function for Sox9 in the adult testis seems plausible. The age- and stage-specific differences in the level of Sox9 expression in the seminiferous tubules of the adult suggest that Sox9 may have a pivotal role in germ cell differentiation as well. Indeed, it has been shown that in the adult rat, the Sertoli cells of most regions of the seminiferous tubules were positive for SOX9 but the strongest reaction was found in the dark zone of seminiferous tubules containing preleptotene spermatocytes, the cells representing the first stage of meiosis I that will traverse through the blood-testis barrier to enter the luminal compartment [52]. Thus, elevated levels of testicular Sox9, as we see in XYTdym1Sox9 males, may actually be beneficial for spermatogenesis progression.
One possibility that cannot be disregarded is that the testicular phenotype in XYTdym1Sox9 males can be due to the lack of Sry, rather than to changes in the level of Sox9 transcripts. This is particularly important considering that it has recently been elegantly shown that SRY with its downstream targets is capable of guiding of Sertoli cell differentiation in a SOX9-independent manner [53]. We observed that XYTdym1Sry males, in which sex determination is driven by transgenic overexpression of Sry, had remnants of the testicular phenotype noted in XYTdym1Sox9 males. The Sry transcript level in fetal gonads from these males was ∼5- to 8-fold higher than in wild-type XY. Because the Sry transgene is present in the genome in 12–14 copies [54], high overexpression was expected. Indeed, it has recently been shown that the same transgene resulted in enhanced Sry expression in the brain [55]. The Sry transcripts in adult mouse testes are circular and thought to be aberrant and nontranslatable [35]. However, they have been shown to serve as sponges for at least one type of microRNAs, miR-138 [56], so their potential role as epigenetic regulator(s) in testes cannot be disregarded. Contrary to what we have seen in fetal gonads, transgenic Sry expression in testes from mature XYTdym1Sry males was similar to or lower than in XY. The lack of overexpression is probably due to transgene-silencing mechanisms, the presence of which has been previously reported [57]. The Sox9 expression in XYTdym1Sry males was elevated in fetal and mature gonads, although in fetal gonads only on a C57BL/6 background. The interdependence of Sry and Sox9 makes it difficult to conclude which of these two factors, when deregulated, contribute to the testicular phenotype in XYTdym1Sry males.
We used mice on two genetic backgrounds, outbred MF1 and inbred C57BL/6, and observed some background-specific phenotypes. Several studies suggested that the C57BL/6 background is sensitized to disruptions in testis determination due to the relatively delayed expression of testis-determining genes and higher levels of expression of ovary-determining genes [11, 58, 59]. Here, testis vasculature was weak in XYTdym1Sox9 males on the C57BL/6 but not on the MF1 background (Table 1 and Fig. 4B), but the defects in the region underlying the coelomic vessels were more pronounced in XYTdym1Sox9 MF1 males (Fig. 5B); in addition, the patchy testis appearance (Supplemental Fig. S2) was present exclusively on the MF1 background. There were also background-specific differences affecting XYTdym1Sry males, which had higher Sry expression on the C57BL/6 background and elevated incidence of abnormalities of seminiferous epithelium on the MF1 background. We conclude that observed background-specific differences are not unusual and relate to the genetic differences between strains, likely affecting spatiotemporal regulation of expression of sex determination-specific genes. The genetic background influenced sex determination also in the Odsex model, but only when the Sox9 levels were not sufficient. Heterozygous XXOds mice developed as males, or females, or intersex, depending on the background, while homozygous XXOds/Ods mice developed always as males [60].
Our work provided evidence that the Sry role in male sex determination can be dispensed by manipulating its downstream target Sox9 and that the resulting males are fertile. These males represent the only currently available model in which sex determination is driven by Sox9 upregulation in the absence of Sry. Although we observed some testicular defects, these did not affect sperm development and function. Our findings support the notion that Sry does not play an essential role in the mature testis. We recently reported that in the mouse only two Y chromosome genes, the testis determinant Sry and the spermatogonial proliferation factor Eif2s3y, are needed for a male to be able to reproduce with the help of assisted-reproduction technologies [23]. The demonstration that Sry function can be substituted by Sox9 overexpression opens the possibility to further reduce the Y chromosome contribution and produce males with only one Y chromosome gene, Eif2s3y, followed by testing if these males generate haploid gametes and can sire assisted-reproduction technology offspring.
ACKNOWLEDGMENT
Histological sections were prepared by JABSOM Histology Core supported by NIH grants NNCRR 5 G12 RR003061-26 and NIMHHD 8 G12 MD007601-26. The authors thank Yasuhiro Yamauchi and Jonathan Riel, postdoctoral fellows in the laboratory, for advice and help during the duration of the project, as well as numerous undergraduate students who assisted with mouse genotyping. The authors are also grateful to Andreas Schedl (University of Nice, France) for providing sperm samples from XYSox9 transgenic males.
Footnotes
This material is based on work supported by NIH HD072380 and HCF 14ADVC-64546 to M.A.W. Presented in part at the 2014 Annual Meeting of the American Society for Reproductive Medicine, October 18–22, 2014, Honolulu, Hawaii, the 7th International Symposium on Vertebrate Sex Determination, April 13–17, 2015, Kona, Hawaii, and the 48th Annual Meeting of the Society for the Study of Reproduction, June 18–22, 2015, San Juan, Puerto Rico.
REFERENCES
- Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Munsterberg A, Vivian N, Goodfellow P, Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes Nature 1990. 346 245– 250 [DOI] [PubMed] [Google Scholar]
- Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif Nature 1990. 346 240– 244 [DOI] [PubMed] [Google Scholar]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry Nature 1991. 351 117– 121 [DOI] [PubMed] [Google Scholar]
- Eggers S, Ohnesorg T, Sinclair A. Genetic regulation of mammalian gonad development Nat Rev Endocrinol 2014. 10 673– 683 [DOI] [PubMed] [Google Scholar]
- Larney C, Bailey TL, Koopman P. Switching on sex: transcriptional regulation of the testis-determining gene Sry Development 2014. 141 2195– 2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YT, Capel B. Cell fate commitment during mammalian sex determination Curr Opin Genet Dev 2015. 32 144– 152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland K, Bowles J, Koopman P. Male sex determination: insights into molecular mechanisms Asian J Androl 2012. 14 164– 171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Harley VR. Disorders of sex development: new genes, new concepts Nat Rev Endocrinol 2013. 9 79– 91 [DOI] [PubMed] [Google Scholar]
- Quinn A, Koopman P. The molecular genetics of sex determination and sex reversal in mammals Semin Reprod Med 2012. 30 351– 363 [DOI] [PubMed] [Google Scholar]
- Sekido R, Lovell-Badge R. Sex determination and SRY: down to a wink and a nudge? Trends Genet 2009. 25 19– 29 [DOI] [PubMed] [Google Scholar]
- Warr N, Greenfield A. The molecular and cellular basis of gonadal sex reversal in mice and humans Wiley Interdiscip Rev Dev Biol 2012. 1 559– 577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JW, Dominguez-Steglich MA, Guioli S, Kwok C, Weller PA, Stevanovic M, Weissenbach J, Mansour S, Young ID, Goodfellow PN, Brook JD, Schafer AJ. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene Nature 1994. 372 525– 530 [DOI] [PubMed] [Google Scholar]
- Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, Wolf U, Tommerup N, et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9 Cell 1994. 79 1111– 1120 [DOI] [PubMed] [Google Scholar]
- Barrionuevo F, Bagheri-Fam S, Klattig J, Kist R, Taketo MM, Englert C, Scherer G. Homozygous inactivation of Sox9 causes complete XY sex reversal in mice Biol Reprod 2006. 74 195– 201 [DOI] [PubMed] [Google Scholar]
- Chaboissier MC, Kobayashi A, Vidal VI, Lutzkendorf S, van de Kant HJ, Wegner M, de Rooij DG, Behringer RR, Schedl A. Functional analysis of Sox8 and Sox9 during sex determination in the mouse Development 2004. 131 1891– 1901 [DOI] [PubMed] [Google Scholar]
- Lavery R, Lardenois A, Ranc-Jianmotamedi F, Pauper E, Gregoire EP, Vigier C, Moreilhon C, Primig M, Chaboissier MCXY. Sox9 embryonic loss-of-function mouse mutants show complete sex reversal and produce partially fertile XY oocytes Dev Biol 2011. 354 111– 122 [DOI] [PubMed] [Google Scholar]
- Huang B, Wang S, Ning Y, Lamb AN, Bartley J. Autosomal XX sex reversal caused by duplication of SOX9 Am J Med Genet 1999. 87 349– 353 [DOI] [PubMed] [Google Scholar]
- Lee GM, Ko JM, Shin CH, Yang SWA. Korean boy with 46,XX testicular disorder of sex development caused by SOX9 duplication Ann Pediatr Endocrinol Metab 2014. 19 108– 112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal VP, Chaboissier MC, de Rooij DG, Schedl A. Sox9 induces testis development in XX transgenic mice Nat Genet 2001. 28 216– 217 [DOI] [PubMed] [Google Scholar]
- Hunt PA, Worthman C, Levinson H, Stallings J, LeMaire R, Mroz K, Park C, Handel MA. Germ cell loss in the XXY male mouse: altered X-chromosome dosage affects prenatal development Mol Reprod Dev 1998. 49 101– 111 [DOI] [PubMed] [Google Scholar]
- Lue Y, Rao PN, Sinha Hikim AP, Im M, Salameh WA, Yen PH, Wang C, Swerdloff RS. XXY male mice: an experimental model for Klinefelter syndrome Endocrinology 2001. 142 1461– 1470 [DOI] [PubMed] [Google Scholar]
- Mazeyrat S, Saut N, Grigoriev V, Mahadevaiah SK, Ojarikre OA, Rattigan A, Bishop C, Eicher EM, Mitchell MJ, Burgoyne PSA. Y-encoded subunit of the translation initiation factor Eif2 is essential for mouse spermatogenesis Nat Genet 2001. 29 49– 53 [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Riel JM, Stoytcheva Z, Ward MA. Two Y genes can replace the entire Y chromosome for assisted reproduction in the mouse Science 2014. 343 69– 72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P, Barros C, Whittingham DG. Preservation of hamster oocytes to assay the fertilizing capacity of human spermatozoa J Reprod Fertil 1982. 66 161– 168 [DOI] [PubMed] [Google Scholar]
- Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro J Reprod Fertil 1989. 86 679– 688 [DOI] [PubMed] [Google Scholar]
- Gubbay J, Vivian N, Economou A, Jackson D, Goodfellow P, Lovell-Badge R. Inverted repeat structure of the Sry locus in mice Proc Natl Acad Sci U S A 1992. 89 7953– 7957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevaiah SK, Odorisio T, Elliott DJ, Rattigan A, Szot M, Laval SH, Washburn LL, McCarrey JR, Cattanach BM, Lovell-Badge R, Burgoyne PS. Mouse homologues of the human AZF candidate gene RBM are expressed in spermatogonia and spermatids, and map to a Y chromosome deletion interval associated with a high incidence of sperm abnormalities Hum Mol Genet 1998. 7 715– 727 [DOI] [PubMed] [Google Scholar]
- Ajduk A, Yamauchi Y, Ward MA. Sperm chromatin remodeling after intracytoplasmic sperm injection differs from that of in vitro fertilization Biol Reprod 2006. 75 442– 451 [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Riel JM, Wong SJ, Ojarikre OA, Burgoyne PS, Ward MA. Live offspring from mice lacking the Y chromosome long arm gene complement Biol Reprod 2009. 81 353– 361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svingen T, Spiller CM, Kashimada K, Harley VR, Koopman P. Identification of suitable normalizing genes for quantitative real-time RT-PCR analysis of gene expression in fetal mouse gonads Sex Dev 2009. 3 194– 204 [DOI] [PubMed] [Google Scholar]
- Svingen T, Letting H, Hadrup N, Hass U, Vinggaard AM. Selection of reference genes for quantitative RT-PCR (RT-qPCR) analysis of rat tissues under physiological and toxicological conditions. PeerJ. 2015;3:e855. doi: 10.7717/peerj.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker A, Capel B, Goodfellow P, Lovell-Badge R. Expression of Sry, the mouse sex determining gene Development 1995. 121 1603– 1614 [DOI] [PubMed] [Google Scholar]
- Jeske YW, Bowles J, Greenfield A, Koopman P. Expression of a linear Sry transcript in the mouse genital ridge Nat Genet 1995. 10 480– 482 [DOI] [PubMed] [Google Scholar]
- Koopman P, Munsterberg A, Capel B, Vivian N, Lovell-Badge R. Expression of a candidate sex-determining gene during mouse testis differentiation Nature 1990. 348 450– 452 [DOI] [PubMed] [Google Scholar]
- Capel B, Swain A, Nicolis S, Hacker A, Walter M, Koopman P, Goodfellow P, Lovell-Badge R. Circular transcripts of the testis-determining gene Sry in adult mouse testis Cell 1993. 73 1019– 1030 [DOI] [PubMed] [Google Scholar]
- Qin Y, Bishop CE. Sox9 is sufficient for functional testis development producing fertile male mice in the absence of Sry Hum Mol Genet 2005. 14 1221– 1229 [DOI] [PubMed] [Google Scholar]
- Bishop CE, Whitworth DJ, Qin Y, Agoulnik AI, Agoulnik IU, Harrison WR, Behringer RR, Overbeek PA. A transgenic insertion upstream of sox9 is associated with dominant XX sex reversal in the mouse Nat Genet 2000. 26 490– 494 [DOI] [PubMed] [Google Scholar]
- Cool J, DeFalco TJ, Capel B. Vascular-mesenchymal cross-talk through Vegf and Pdgf drives organ patterning Proc Natl Acad Sci U S A 2011. 108 167– 172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFalco T, Bhattacharya I, Williams AV, Sams DM, Capel B. Yolk-sac-derived macrophages regulate fetal testis vascularization and morphogenesis Proc Natl Acad Sci U S A 2014. 111 E2384– E2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coveney D, Cool J, Oliver T, Capel B. Four-dimensional analysis of vascularization during primary development of an organ, the gonad Proc Natl Acad Sci U S A 2008. 105 7212– 7217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeays-Ward K, Hoyle C, Brennan J, Dandonneau M, Alldus G, Capel B, Swain A. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad Development 2003. 130 3663– 3670 [DOI] [PubMed] [Google Scholar]
- Jordan BK, Shen JH, Olaso R, Ingraham HA, Vilain E. Wnt4 overexpression disrupts normal testicular vasculature and inhibits testosterone synthesis by repressing steroidogenic factor 1/beta-catenin synergy Proc Natl Acad Sci U S A 2003. 100 10866– 10871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais da Silva S, Hacker A, Harley V, Goodfellow P, Swain A, Lovell-Badge R. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds Nat Genet 1996. 14 62– 68 [DOI] [PubMed] [Google Scholar]
- Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P. A male-specific role for SOX9 in vertebrate sex determination Development 1996. 122 2813– 2822 [DOI] [PubMed] [Google Scholar]
- Barrionuevo F, Georg I, Scherthan H, Lecureuil C, Guillou F, Wegner M, Scherer G. Testis cord differentiation after the sex determination stage is independent of Sox9 but fails in the combined absence of Sox9 and Sox8 Dev Biol 2009. 327 301– 312 [DOI] [PubMed] [Google Scholar]
- Lardenois A, Chalmel F, Barrionuevo F, Demougin P, Scherer G, Primig M. Profiling spermatogenic failure in adult testes bearing Sox9-deficient Sertoli cells identifies genes involved in feminization, inflammation and stress. Reprod Biol Endocrinol. 2010;8:154. doi: 10.1186/1477-7827-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadaja M, Keyes BE, Lin M, Pasolli HA, Genander M, Polak L, Stokes N, Zheng D, Fuchs E. SOX9: a stem cell transcriptional regulator of secreted niche signaling factors Genes Dev 2014. 28 328– 341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Keckesova Z, Donaher JL, Shibue T, Tischler V, Reinhardt F, Itzkovitz S, Noske A, Zurrer-Hardi U, Bell G, Tam WL, Mani SA, et al. Slug and Sox9 cooperatively determine the mammary stem cell state Cell 2012. 148 1015– 1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, Masui T, Kawaguchi M, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine Nat Genet 2011. 43 34– 41 [DOI] [PubMed] [Google Scholar]
- Scott CE, Wynn SL, Sesay A, Cruz C, Cheung M, Gomez Gaviro MV, Booth S, Gao B, Cheah KS, Lovell-Badge R, Briscoe J. SOX9 induces and maintains neural stem cells Nat Neurosci 2010. 13 1181– 1189 [DOI] [PubMed] [Google Scholar]
- Passeron T, Valencia JC, Bertolotto C, Hoashi T, Le Pape E, Takahashi K, Ballotti R, Hearing VJ. SOX9 is a key player in ultraviolet B-induced melanocyte differentiation and pigmentation Proc Natl Acad Sci U S A 2007. 104 13984– 13989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frojdman K, Harley VR, Pelliniemi LJ. Sox9 protein in rat Sertoli cells is age and stage dependent Histochem Cell Biol 2000. 113 31– 36 [DOI] [PubMed] [Google Scholar]
- Nicol B, Yao HH. Gonadal identity in the absence of pro-testis factor SOX9 and pro-ovary factor beta-catenin in mice. Biol Reprod. 2015;93:35. doi: 10.1095/biolreprod.115.131276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Mackie R, Kampf K, Domadia S, Brown JD, O'Neill R, Arnold AP. Four core genotypes mouse model: localization of the Sry transgene and bioassay for testicular hormone levels. BMC Res Notes. 2015;8:69. doi: 10.1186/s13104-015-0986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwani B, Bentivegna K, Benashski SE, Venna VR, Xu Y, Arnold AP, McCullough LD. Sex differences in ischemic stroke sensitivity are influenced by gonadal hormones, not by sex chromosome complement J Cereb Blood Flow Metab 2015. 35 221– 229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges Nature 2013. 495 384– 388 [DOI] [PubMed] [Google Scholar]
- Calero-Nieto FJ, Bert AG, Cockerill PN. Transcription-dependent silencing of inducible convergent transgenes in transgenic mice. Epigenetics Chromatin. 2010;3:3. doi: 10.1186/1756-8935-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger SC, Aylor DL, Syed HA, Magwene PM, Threadgill DW, Capel B. Elucidation of the transcription network governing mammalian sex determination by exploiting strain-specific susceptibility to sex reversal Genes Dev 2009. 23 2521– 2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa SM, Washburn LL, Kahlon RS, Musson MC, Bouma GJ, Eicher EM, Albrecht KH. Sex reversal in C57BL/6J XY mice caused by increased expression of ovarian genes and insufficient activation of the testis determining pathway. PLoS Genet. 2012;8:e1002569. doi: 10.1371/journal.pgen.1002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Poirier C, Truong C, Schumacher A, Agoulnik AI, Bishop CE. A major locus on mouse chromosome 18 controls XX sex reversal in Odd Sex (Ods) mice Hum Mol Genet 2003. 12 509– 515 [DOI] [PubMed] [Google Scholar]