Abstract
Background
Aging in males is associated with lower testosterone levels and a decrease in diurnal variation of testosterone secretion. Cross-sectional studies have shown lower than expected testosterone levels among HIV-infected men, but whether age-related changes in serum testosterone differ by HIV serostatus is not known.
Methods
HIV-infected men from the Multicenter AIDS Cohort Study (MACS), age ≥ 45 years at highly active antiretroviral therapy initiation, who had ≥ 2 samples from the subsequent 10 years, were matched to HIV-uninfected men by age, race, MACS site, and calendar time of samples. Linear mixed effects regression models were used to determine whether free testosterone (FT) and its rate of change differed by HIV serostatus.
Results
182 HIV-infected and 267 HIV-uninfected men were included: median age 48.8 years (Interquartile range (IQR); 45.8, 53.4), median numbers of FT measurements per participant 4 (IQR; 3, 5), 65% were drawn in the AM. Mean adjusted FT levels were lower among HIV-infected than HIV-uninfected men in AM samples (−6.1 ng/dL (95% CI: −9.8, −2.4), p=0.001), but not in PM samples (−1.7 ng/dL (−6.0, 2.6), p=0.441). The rate of FT decline with age did not differ by HIV serostatus: 9.2 ng/dL (95% CI: −13.4, −5.0) per 10 years for HIV- infected vs. 7.9 ng/dL (95% CI: −10.2, −5.5) for HIV-uninfected men, p = 0.578.
Conclusion
FT decreased similarly with increasing age regardless of HIV serostatus. The lower AM, but not PM, FT levels among HIV-infected men compared to HIV-uninfected men suggests a loss of diurnal variation in FT among HIV-infected men.
Introduction
In the general male population, testosterone levels decrease with age and may contribute to age-related comorbidities, including sexual dysfunction, sarcopenia, osteoporosis, glucose abnormalities, and cardiovascular disease1,2. In the Third National Health and Nutrition Examination Survey, 12.8% of men between 50–59 years had total testosterone (TT) levels in the hypogonadal range using a cutoff of 300 ng/dL. In men over 70 years, the prevalence of hypogonadism was 24.9%. Age-related changes in the gonadal axis are even more pronounced if free testosterone (FT) levels are examined rather than total testosterone as sex hormone binding globulin (SHBG) increases with aging. More than 30% of men over 70 years have FT concentrations in the hypogonadal range (<4.9 ng/dL).3–5
Hypogonadism has been a commonly recognized condition among HIV-infected men since early in the HIV epidemic with consequences on fat and lean total body mass, muscle strength, bone mineral density and physical function. With effective antiretroviral therapy, TT and FT levels increase6, but hypogonadism remains a common problem among HIV-infected men with prevalence estimates ranging from 21–70%4,7–10. In a previous cross-sectional study conducted in the Multicenter AIDS Cohort Study (MACS) during the era of highly active antiretroviral therapy (HAART)11, we found that hypogonadism (defined as a level of FT or TT below the lower limit of normal or use of testosterone replacement therapy) was more common in HIV-infected men compared to HIV-uninfected participants (24.5% v. 7.8%). Among those not receiving or reporting testosterone use, the lower adjusted FT concentrations among HIV-infected men were equivalent to 13 years of aging12,13. Although FT levels decreased with increasing age in this study, the magnitude of decrease was similar by HIV serostatus and no interaction between HIV-serostatus and age was observed.
There is limited published data on the longitudinal changes in TT or FT levels among older HIV-infected men compared to otherwise similar HIV-uninfected men. We undertook a longitudinal, nested cohort study with in the MACS to determine whether age-related changes in FT differed by HIV serostatus.
Methods
Study Population
The MACS is a prospective study of men who have sex with men (MSM) who are HIV- infected or at risk for HIV-1 infection, ongoing since 1984 at four US sites: Chicago, Baltimore/Washington DC, Pittsburgh and Los Angeles. Details of the study design and methods have been published14. The institutional review boards of each site approved the study protocols and informed consent was obtained from each participant.
Selection Criteria
We identified HIV-infected men who were at least 45-years old at HAART initiation, with at least 2 samples available from the 10 years following HAART initiation. These men were matched to HIV-uninfected men by age (+/−5 years), race, MACS site, and calendar time of samples collections. Men who reported taking exogenous hormones of any kind and/or had FT concentrations > 150 ng/dL suggestive of unreported testosterone use were excluded from the analysis.
Laboratory Methods
All hormone assays were performed using frozen samples in the laboratory of Dr Shalender Bhasin (Boston University, Boston). TT levels were measured from archived serum using liquid chromatographic-tandem mass spectrometry (LC-MS/MS). SHBG was measured using radioimmunoassay. FT was calculated from TT and SHBG measurement using the Vermeulen equation15. For the exploratory analysis described below, serum levels of chemokines and proinflammatory cytokines (IL-1β, IL-2, IL-6, IL-10, IL-12p70, IFN-γ, TNF-α, Eotaxin, IL-8/CXCL8, IP-10/CXCL10, MCP-1/CCL2, MCP-4/CCL13, MIP-1β/CCL4, hsCRP and TARC/CCL17) were assessed using the Meso-Scale Discovery (MSD) Multi-Array® platform (Meso-Scale Diagnostics, LLC, Rockville, MD) in the laboratory of Dr. Jay Bream (Johns Hopkins Bloomberg School of Public Health, Baltimore, MD). Sensitivities for cytokines were typically in the range of 1pg/ml, while chemokine detection ranged from 39 to 158pg/ml. Serum levels of the soluble receptor markers were determined using the multiplexed Luminex xMAP system (Fluorokine® MAP, R & D systems, Minneapolis, MN) Levels of five soluble receptors (sCD14, sgp130, sIL-2Rα, sTNFR2, sIL6R), plus a cytokine (BAFF/BLyS), and the chemokine BLC-BCA1/CXCL13 were measured in one panel (Human Biomarker Custom Premix Kit A). All biomarkers testing were conducted in the laboratory of Dr Oto Martinez-Maza (University of California, Los Angeles, CA). Results for these biomarkers of inflammation and immune activation in a larger MACS cohort have been previously reported16. Serum samples for biomarker measurement were chosen from semi-annual MACS visits closest to the FT study visits (+/− 1 year).
Other Covariates
Variables of interest included race (black, Hispanic, white (reference)), hepatitis C virus (HCV) serostatus (defined by positive antibody or positive HCV-RNA, Yes/No), body mass index (BMI: <20, 20–24.9(reference), 25–29.9, ≥30 kg/m2), tobacco smoking (current, past, never (reference)), history of diabetes mellitus (DM: Fasting glucose≥ 126 mg/dl or self-reported DM and use of DM medications versus no DM (reference)), time of blood draw for FT measurement (morning or afternoon/evening) and cumulative years on HAART for HIV-infected men assessed from data collected at semiannual visits.
Statistical analysis
The first post-HAART visit with a serum sample available for FT testing was defined as the baseline visit. Baseline characteristics among HIV-infected and HIV-uninfected men were compared using non-parametric Wilcoxon test or Chi-square or Fisher exact test as appropriate. A linear mixed effects regression model with t-distributed error (degrees of freedom were estimated in models) was used to determine whether FT and its rate of change with increasing age differed by HIV serostatus. A random intercept assumed to be normally distributed was included in the mixed model.
Factors in the first multivariable linear mixed model included HIV serostatus, age, race (white (reference), black and Hispanic), study center, smoking status, history of DM, BMI, HCV status and time of blood draw for FT measurement (AM vs. PM), and the interaction term of HIV serostatus* age. In a second model, the interaction of HIV serostatus* time of blood draw (AM vs PM) was added.
We carried out two exploratory analyses to better understand our results. First, since there is debate as to whether the decrease in gonadal function with increasing age is a result of the accumulation of age-related comorbid conditions, we adjusted the linear mixed model to include a comorbidity score, which was defined as the total count of the following six comorbid conditions: 1) depression (Centers for Epidemiologic Studies Depression Scale score > 16) [25]; 2) self-reported hypertension or receiving anti -hypertension medications; 3) self-reported DM or receiving diabetes medication); 4) kidney disease (estimated glomerular filtration rate less than 60mL/min/1.73 m2 using the Modification of Diet in Renal Disease equation or a urine protein-to-creatinine ratio greater than or equal to 200mg protein/1g creatinine, or confirmed kidney disease diagnosis with ICD-9 codes 580–591 and 593); 5) liver disease (alanine transaminase or aspartate aminotransferase >150 IU/L, or confirmed liver disease diagnosis with ICD-9 codes 570–573 excluding hepatitis) and; 6) Cancer (diagnosis at or within a year prior to the relevant visit). History of diabetes was excluded from the model that included the comorbidity score. Also, since increased systemic inflammation has been proposed as a mechanism by which age-related decreases in gonadal function-occur and is associated with HIV-infection itself17, we sought to determine whether levels of biomarkers of inflammation and immune activation, using the chemokines and cytokines listed above, were associated with FT levels and whether inclusion of these biomarkers in multivariable models impacted the effects of HIV infection, or time of blood draw (AM vs PM) on FT levels. We evaluated biomarker levels by HIV serostatus using generalized gamma regression models adjusted for age, race, and BMI. We then added each biomarker to the fully adjusted longitudinal mixed model that already included an HIV serostatus* time of blood draw interaction term and the comorbidity score described above in order to examine individual biomarker associations with FT and on point estimates for HIV serostatus, age, and time of blood draw associations with FT. The biomarkers were log2 transformed such that the coefficients represent the effect per doubling of biomarker level. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
Baseline Characteristics
Between October 1, 1995 and September 30, 2009, there were 306 HIV-infected men who met study inclusion criteria. Of those, 271 were matched with 271 HIV-uninfected men. Eighty-nine HIV-infected men and four HIV-uninfected men were excluded from the analysis because they were found to have an excessively high level of FT (>150 ng/dl) at ≥ 1 visit (i.e > 99 percentile of FT in the HIV-uninfected men). Because these levels are unlikely to be physiologically plausible, we assumed that these participants were using exogenous testosterone, but did not report this administration during their MACS visits. The majority of these men (60%) were from the Los Angeles site.
At the baseline visit, our analytic dataset included 449 men (182 HIV-infected and 267 HIV-uninfected men). The HIV-infected men were older, had a lower BMI, and were more likely than the HIV-uninfected men to be current smokers and HCV-infected (Table 1). The prevalence of DM and the racial distribution were similar between groups. The comorbidity score was higher in the HIV-infected men. At the baseline visit, 99% of the HIV-infected participants were HAART-treated (60% with a PI-containing regimen, 37% with a NNRTI-containing regimen) and 58% had received antiretroviral treatment (ART) before HAART initiation. The median CD4 cell count was 371 cells/mm3 and 65% of HIV-infected men had plasma HIV RNA< 400 copies/ml. During the study interval, 267 HIV-uninfected men contributed 1051 person-visits and 182 HIV-infected men contributed 686 person-visits, 72% of which had an associated HIV-RNA levels <400 copies/ml. The median number of FT measurements per participant was 4 (IQR; 3, 5) drawn over a median of 6 years (IQR; 2.9, 9.5). Of these, 65% were drawn in the AM.
Table 1.
Baseline characteristics of HIV-infected and HIV-uninfected participants
| HIV-infected (n=182) |
HIV-uninfected (n=267) |
p-value* | |
|---|---|---|---|
| Age (years) | 50.2(47.5–54.7) | 48.4(44.5–53.1) | <0.001 |
| Race (%) | 0.429 | ||
| White | 81.9 | 85 | |
| Black | 14.8 | 10.9 | |
| Hispanic | 3.3 | 4.1 | |
| Center (%) | 0.057 | ||
| Baltimore/ Washington DC | 32.4 | 26.2 | |
| Chicago | 19.8 | 20.6 | |
| Pittsburgh | 19.8 | 15.7 | |
| UCLA | 28 | 37.5 | |
| Smoking Status (%) | 0.004 | ||
| Current | 31.3 | 19.5 | |
| Past | 47.8 | 48.3 | |
| Never | 20.9 | 32.2 | |
| BMI (%) | 0.001 | ||
| <20 kg/m2 | 6.6 | 1.9 | |
| 20 – 24.9 kg/m2 | 50.5 | 39.3 | |
| 25 – 29.9 kg/m2 | 33 | 39.7 | |
| ≥ 30 kg/m2 | 9.9 | 19.1 | |
| HCV infection (%) | 9.9 | 3.7 | 0.008 |
| History of diabetes** (%) | 8.8 | 5.2 | 0.139 |
| Depressive Symptoms*** (%) | 25.3 | 19.5 | 0.152 |
| Self-reported hypertension or medication (%) | 22.5 | 20.8 | 0.654 |
| Self-reported diabetes or medication (%) | 6 | 3 | 0.119 |
| Kidney disease (%) | 2.7 | 0.4 | 0.032 |
| Liver disease (%) | 2.7 | 0 | 0.006 |
| Cancer (%) | 3.3 | 0.7 | 0.045 |
| Total number of comorbidities | 0(0–1) | 0(0–1) | 0.081 |
| Morning blood draw (%) | 61.5 | 66.7 | 0.265 |
| Free testosterone (FT, ng/dL) | 63.8(51.4–79.9) | 70.6(58.6–86.5) | 0.001 |
| Number of FT measurements contributed | 4(3–5) | 4(3–5) | 0.141 |
| Years from first FT to last FT measurements | 5.6(2.8–9.5) | 6.7(3–9.4) | 0.150 |
| HIV-infected participants | |||
| Nadir CD4 count (cells/mm3) | 223(149–337) | - | |
| Current CD4 count (cells/mm3) | 371(236–528) | - | |
| HIV RNA <400 copies/ml (%) | 65.2 | - | |
| ART before HAART (%) | 57.7 | - | |
| HAART in last 6 months (%) | 98.9 | - | |
| PI-based regimen (%) | 59.9 | - | |
| NNRTI-based regimen (%) | 36.8 | - | |
| Cumulative years on HAART | 0.29(0.12–0.47) | - | |
| History of AIDS (%) | 9.3 | - |
Data are reported as median (interquartile range) or percentage.
By the non-parametric Wilcoxon test or Chi-square or Fisher exact test, as appropriate.
HCV defined by positive antibodies or HCV-RNA Yes/No
History of DM (DM: Fasting glucose≥ 126 mg/dl or self-reported DM and use of DM medications
Longitudinal changes in free testosterone by HIV-status
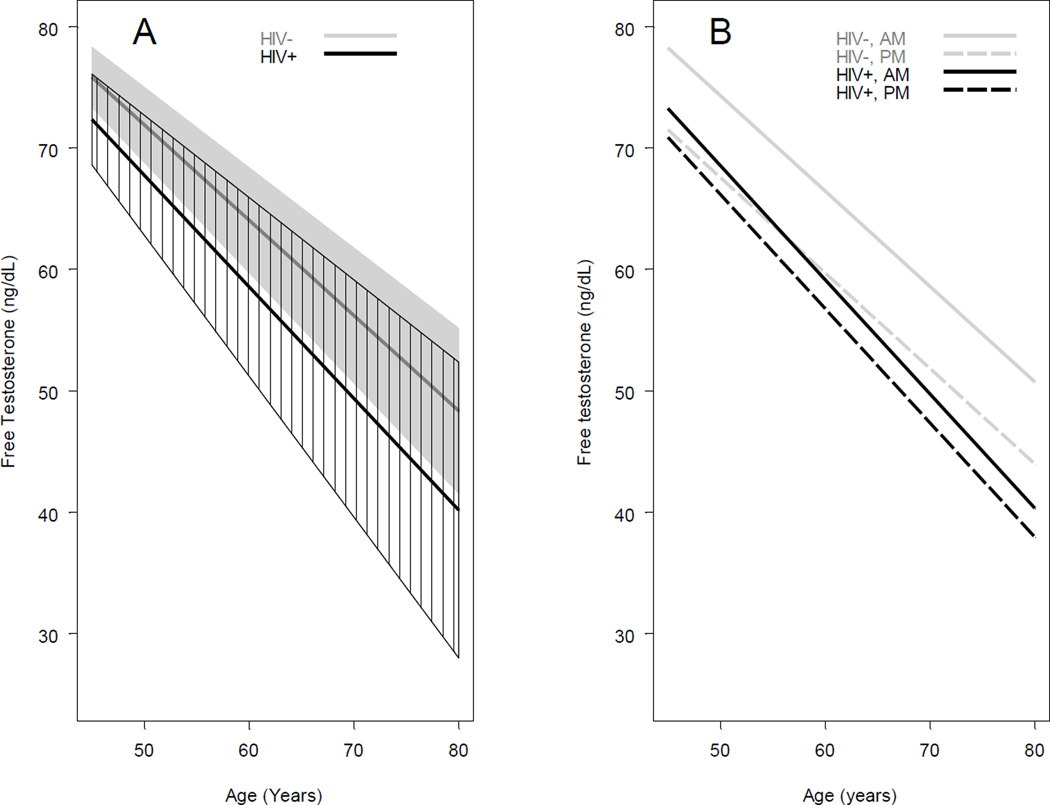
The significant declines in FT by age did not differ by HIV serostatus (p=0.58); mean FT decreases per 10-year increase in age were 9.2 ng/dL (95% CI: −13.4, −5.0) and 7.9 ng/dL (95% CI: −10.2, −5.5) among HIV-infected and HIV-uninfected men, respectively. There was no difference in overall FT level by HIV serostatus (−3.5 ng/dL (95%CI: −8.0, 0.98), p=0.12). Mean FT levels measured from samples drawn in the AM were higher than levels measured in the PM (4.9 ng/dL (95%CI: 2.4, 7.4), p=0.0001) (Fig 1A).
Figure 1. Longitudinal changes in free testosterone levels by HIV-serostatus (A) and by HIV- serostatus and time of blood draw (AM vs PM) (B).
Panel A shows adjusted mean free testosterone (FT) by age from multiple linear mixed model 1 with random intercept and T-distributed errors (estimated degree of freedom = 5) by HIV-serostatus. The gray line in the middle of the gray polygon represents the adjusted mean FT by age for the HIV−, and the gray shading represents its 95% confidence interval. The black line in the middle of the polygon with black shading lines is the adjusted mean FT for the HIV+, and the shaded area represents its 95% confidence interval. Model 1 included factors of HIV-status, age, race, study center, smoking status, BMI, HCV, history of diabetes, cumulative year on HAART for HIV+, time of blood drawn for FT measurement, and the interaction of HIV-status * age. Panel B shows adjusted mean FT by age from multiple linear mixed model 2 by HIV-status and time of blood drawn. Gray solid line represents the adjusted morning (AM) FT for the HIV−, gray dashed line represents the adjusted afternoon (PM) FT for the HIV−, black solid line represents the adjusted AM FT for the HIV+, and black dashed line represents the adjusted PM FT for the HIV+. Model 2 included all factors in model 1 and also the interaction of HIV-status * time of blood drawn.
In a second model, the interaction of HIV serostatus* time of blood drawn (AM vs. PM), approached statistical significance (p=0.06), providing some evidence that the relationship between HIV serostatus and FT depended on the time of the blood draw (Fig 1B). In this model, HIV-infected men had lower FT than HIV-uninfected men (−5.0 ng/dL (95%CI: −9.8, −0.29) at age 45, p=0.037). The adjusted mean FT level in AM samples was significantly lower among HIV-infected compared to HIV-uninfected men (−6.1 ng/dL (95% CI: −9.8, −2.4); p=0.001), but a significant difference was not observed in the PM samples (−1.7 ng/dL (95% CI: −6.0, −2.6); p= 0.441). The AM and PM FT levels did not differ significantly among the HIV-infected men (73.3 ng/dL (95% CI: 69.3, 77.2) ng/dL vs 70.9 ng/dL (95% CI: 66.5, 75.3), p=0.193), but, among the HIV-uninfected men, the AM levels of FT were significantly higher 78.3 ng/dL (95% CI: 75.6, 80.9) vs 71.5 ng/dL (95% CI: 68.3, 74.7) p<0.001). Similar to the previous model, the rate of FT decline with advancing age in this model which included an HIV serostatus* time of blood drawn interaction term did not differ significantly by HIV serostatus. In an exploratory analysis, the addition of comorbidity score to the fully adjusted model did not appreciably change our results (data not shown).
Exploratory Analyses: Effect of Inflammatory Biomarkers on Free Testosterone
Of 449 men included in the primary analysis, 224 men (715 person-visits) with both FT and serum biomarker measurements available were included in the analysis of the association between biomarkers and FT (Table 2). At the baseline visit, men with biomarkers were more likely to be older, non-Caucasian, HIV-infected, HCV-infected, and current smokers compared to men for whom biomarkers levels were not available.
Table 2.
Baseline characteristics by ARRA1 biomarker availability
| With biomarker (n=224) |
Without biomarker (n=225) |
p-value* | |
|---|---|---|---|
| Age (years) | 49.7(46.9–54.4) | 48.4(44.9–53.2) | 0.002 |
| Race (%) | 0.016 | ||
| White | 79.5 | 88 | |
| Black | 17 | 8 | |
| Hispanic | 3.6 | 4 | |
| Center (%) | <0.001 | ||
| Baltimore | 30.8 | 26.7 | |
| Chicago | 25.9 | 14.7 | |
| Pittsburgh | 18.3 | 16.4 | |
| UCLA | 25 | 42.2 | |
| Smoking Status (%) | 0.002 | ||
| Current | 29.9 | 18.7 | |
| Past | 49.1 | 47.1 | |
| Never | 21 | 34.2 | |
| BMI (%) | 0.400 | ||
| <20 kg/m2 | 4.5 | 3.1 | |
| 20 – 24.9 kg/m2 | 46.9 | 40.9 | |
| 25 – 29.9 kg/m2 | 35.3 | 38.7 | |
| ≥ 30 kg/m2 | 13.4 | 17.3 | |
| HCV infection (%) | 11.6 | 0.9 | <0.001 |
| History of diabetes (%) | 8 | 5.3 | 0.252 |
| Morning blood draw (%) | 62.5 | 66.7 | 0.356 |
| Free testosterone (FT, ng/dL) | 66.1(52.4–81.5) | 70.3(58.5–87.3) | 0.011 |
| Number of FT measurements contributed | 4(3–5) | 4(3–5) | 0.331 |
| Years from first FT to last FT measurements | 5.5(2.9–9.4) | 7.5(3–9.5) | 0.155 |
| HIV-positive (%) n | 77.7 (n=174) | 3.6 (n=8) | <0.001 |
| Nadir CD4 count (cells/mm3) | 223(149–336) | 242(129–375) | 0.806 |
| Current CD4 count (cells/mm3) | 372(236–528) | 307(235–589) | 0.847 |
| HIV RNA < 400 copies/ml (%) | 64.2 | 87.5 | 0.265 |
| Cumulative years on HAART | 0.29(0.12–0.47) | 0.35(0.19–0.49) | 0.655 |
| History of AIDS (n) | 16 | 1 | 0.753 |
Data are reported as median (interquartile range) or percentage.
By the non-parametric Wilcoxon test or Chi-square or Fisher exact test, as appropriate.
After adjusting for age, race and BMI, levels of multiple biomarkers were significantly associated with HIV infection: BAFF (relative percentile (RP) HIV-infected vs. HIV-uninfected 1.17 (95% CI: 1.07,1.29), sCD14 (RP=1.26 (95% CI: 1.16, 1.38)), sCD27 (RP=1.33 (95% CI: 1.20, 1.48)), sIL-2Rα (RP=1.12 (95% CI: 1.02, 1.24)), sTNFR2 (RP=1.24 (95% CI: 1.23, 1.38)), TNF-α (RP=1.17 (95% CI: 1.05, 1.30)), IP-10 (RP=1.67 (95% CI: 1.41, 2.00)), MCP-4 (RP=1.25 (95% CI: 1.08, 1.46)) MCP-1 (RP=1.22 (95% CI: 1.08, 1.38)), hsCRP (RP=1.53 (95% CI: 1.08, 2.13)),IL-10 (RP=0.79 (95% CI: 0.63, 0.98)), IL-12 p70 (RP=0.68 (95% CI: 0.45, 0.97)), and GM-CSF (RP=0.68 (95% CI: 0.49, 0.98)). No differences by HIV serostatus were found for levels of BLC-BCA1, gp130, sIL6R, IL-6, IL-8, eotaxin, MIP-1β, TARC, IFN-γ, IL1B and IL-2.
We then evaluated whether: 1) these biomarkers were associated with FT and 2) whether the inclusion of each biomarker in the fully adjusted regression model affected the point estimates assessing the relationship between HIV serostatus, age and timing of blood draw and FT. Of the biomarkers examined, gp130, MCP-1, sCD14, sTNFR2, BAFF, IL-6, TARC, and hsCRP levels were each significantly associated with lower FT (p<0.05, for each) (Table 3). When each of these markers was added individually to the regression model, the effect estimates for HIV serostatus stratified by time of blood draw or age stratified by HIV serostatus did not change appreciably. For example, at age 52 years, FT level drawn in the AM was 10.3 ng/dL lower among HIV-infected than HIV-uninfected men (Column 1, Table 3). After the inclusion of gp130 in the same statistical model, the effect of HIV serostatus on AM FT was very close to the previous model (−9.8 ng/dL). Similarly, the effects of age on FT among both HIV-infected and HIV-uninfected men did not change with the addition of gp130 or any of the other biomarkers that were associated with lower FT into the regression model.
Table 3. Relationship between Biomarkers of Inflammation/ Immune Activation with Free Testosterone.
The first column in the table shows the effects of HIV-status and time of blood draw in a fully adjusted mixed effects regression model†. Each subsequent column shows the same effect estimates with the inclusion of a biomarker in the regression model. The effect estimates of the biomarker on FT (per doubling of biomarker) are also given.
| Models† | No biomarkers Estimate (95% CI) |
With gp130 Estimate (95% CI) |
With MCP1 Estimate (95% CI) |
With CD14 Estimate (95% CI) |
With sTNFr2 Estimate (95% CI) |
With BAFF Estimate (95% CI) |
With IL6 Estimate (95% CI) |
With TARC Estimate (95% CI) |
With CRP Estimate (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Effects | |||||||||
| HIV+ vs. HIV− in AM samples (at mean age 52) | −10.3* (−17.6, −3.0) |
−9.8* (−17.1, −2.5) |
−8.5* (−15.9, −1.1) |
−8.6* (−16.0, −1.1) |
−8.4* (−15.9, −1.0) |
−9.0* (−16.5 −1.6) |
−9.7* (−17.1, −2.3) |
−9.6* (−17.0, −2.3) |
−9.5* (−16.9, −2.1) |
| HIV+ vs. HIV− in PM samples (at mean age 52) | −1.6 (−10.1, 6.8) |
−0.9 (−9.4, 7.6) |
−0.5 (−8.9, 8.0) |
−0.4 (−8.9, 8.2) |
1.2 (−7.4, 9.8) |
−0.4 (−9.0, 8.1) |
−1.3 (−9.7, 7.2) |
−0.9 (−9.3, 7.6) |
−1.2 (−9.8, 7.4) |
| HIV−, age (per 10-year) | −10.9 * (−19.3, −2.6) |
−11.3* (−19.6, −2.9) |
−10.8* (−19.1, −2.4) |
−10.9* (−19.3, 2.6) |
−11.2* (−19.6, −2.9) |
−10.9* (−19.2, −2.5) |
−10.2* (−18.6, −1.9) |
−11.4* (−19.7, −3.0) |
−10.8* (−19.2, 2.5) |
| HIV+, age (per 10-year) | −9.1* (−13.3, −4.9) |
−9.0* (−13.2, −4.9) |
−9.2* (−13.4, 5.0) |
−9.1* (−13.3, −4.9) |
−8.5* (−12.7, −4.2) |
−9.0* (−13.2, −4.7) |
−8.1* (−12.3, −3.8) |
−9.0* (−13.2, −4.8) |
−8.5* (−12.8, −4.2) |
| HIV−, PM (vs. AM) | −10.4* (−20.4; −0.5) |
−10.4* (−20.3, −0.5) |
−9.9* (−19.8, −0.1) |
−10.0* (−19.9, −0.1) |
−11.4* (−21.3, −1.5) |
−10.3* (−20.2, −0.4) |
−10.5* (−20.5, −0.6) |
−10.8* (−20.7, −0.8) |
−10.3* (−20.4, −0.3) |
| HIV+, PM (vs. AM) | −1.8 (−5.9, 2.4) |
−1.5 (−5.7, 2.6) |
−1.9 (−6.0, 2.2) |
−1.8 (−5.9, 2.3) |
−1.8 (−5.9, 2.3) |
−1.7 (−5.8, 2.5) |
−2.1 (−6.1, 2.0) |
−2.0 (−6.1, 2.2) |
−2.0 (−6.1, 2.2) |
| Biomarkers (per 1 log2) | |||||||||
| gp130 | - | −5.5* (−10.6; −0.3) |
- | - | - | - | - | - | - |
| MCP1 | - | - | −4.9* (−8.3; −1.5) |
- | - | - | - | - | - |
| CD14 | −4.6* (−8.9; −0.2) |
- | |||||||
| sTNFr2 | - | - | - | - | −4.5* (−7.4, −1.6) |
- | - | - | |
| BAFF | - | - | - | −3.3 * (−6.3, −0.3) |
|||||
| IL6 | - | - | - | - | - | - | −2.5* (−3.8; −1.2) |
- | - |
| TARC | - | - | - | - | - | - | - | −1.9* (−3.7, −0.03) |
- |
| CRP | - | - | - | - | - | - | - | - | −1.1* (−2.1; −0.05) |
p<0.05
All models included following factors: HIV-serostatus, age, race, MACS center, smoking status, BMI, HCV serostatus, cumulative years on HAART among HIV-infected men, time of day of blood draw, total number of comorbidities, the interaction of HIV-status * age, and the interaction of HIV-status * time of blood drawn.
Discussion
To our knowledge, this is the largest and longest study, which has compared longitudinal changes in gonadal function among HIV-infected and HIV-uninfected men. We found that FT decreased similarly over a 10-year interval in both group. However, AM FT levels were lower among HIV-infected men compared to the HIV-uninfected men, but not PM levels. These findings may suggest a loss of diurnal variation in FT among HIV-infected men, a commonly recognized phenomenon among aging men.
In the general male population, multiple cross-sectional and longitudinal studies have shown that gonadal function decreases with age, an occurrence that has been termed “andropause”18–24. For example, in the 10-year follow-up Massachusetts Male Aging study20, a large cross-sectional study of men 39 to 70 years old, each additional year of age was associated with an approximate 1.2% decrease in FT. These cross-sectional results have been confirmed in longitudinal studies18,20,24. The present study found very similar results: FT declined at approximately 1.2% per year in both HIV-infected and HIV-uninfected men. This result is also consistent with our previous cross-sectional study in the MACS11.
Mechanisms underlying reductions in testosterone with aging are not entirely clear. Both testicular dysfunction and inadequate gonadotropin production have been implicated25,26. It has also been suggested that the decrease in testosterone among older men may represent aggregative long-term effects of multiple age-related co-morbid conditions on the hypothalamic-pituitary-gonadal (HPG) axis, rather than aging per se11. However, our data argue against his explanation because when we adjusted for multiple comorbidities27, including smoking status, HCV-co-infection, history of diabetes, cumulative years on HAART for HIV-infected participants, diabetes mellitus, depression, hypertension, kidney disease, liver disease and cancer within a year, the effect of age on changes in FT was unaltered.
Our finding that FT decreases at a similar rate among HIV-infected and HIV-uninfected men suggests that “andropause” is not accelerated among ART-treated HIV-infected men. It should be noted however that our analysis excluded men who reported testosterone use or had FT levels in the super physiologic range, suggestive of exogenous testosterone use. Since these men may have more pronounced HPG dysfunction and such men are more likely to be HIV-infected11, our analysis may have underestimated the differences in the longitudinal changes in FT between HIV-infected and –uninfected men. In addition, the age distribution of studied men was relatively narrow. Longer-term studies are required to better understand the changes in HPG function among HIV-infected men in their seventh and eighth decade.
Although changes in FT over the study interval were similar by HIV serostatus, HIV-infected men on average had lower FT compared to HIV-uninfected men. These results are consistent with our previous cross-sectional sub-study in the MACS in which FT was significantly lower among HIV-infected than HIV-uninfected men11. Other cross-sectional studies of HIV-infected persons have shown similar findings, but most were done in the early pre-HAART era, examined HIV-infected persons with wasting, or did not include an internal HIV-uninfected comparison group6,10,11,28.
Differences in FT by HIV serostatus in our study were apparent only for FT samples collected in the AM rather than the PM. In men with a normal sleep/wake cycle, there is diurnal variation of testosterone secretion with a peak between 6–9 AM with nadir levels at approximately 6 PM29–31. The magnitude of the diurnal variation decreases with age. For example, in a study which measured FT every 30 minutes for 24 hours in 10 young (23–33yr) and 8 older (55–64yr) men, diurnal variation was approximately 40% in the younger men and 30% in the older men32. Our finding that FT differed by time of blood draw in the HIV-uninfected but not the HIV-infected men may suggest that the AM rise in testosterone is blunted in men with HIV-infection and the diurnal variation is decreased, similar to the effect of normal aging. Prospective studies with multiple blood draws over the course of the day among HIV-infected men and matched controls are needed to test this hypothesis.
One potential mechanism for the loss of diurnal variation with aging is the effect of systemic inflammation on gonadal function. Markers of systemic inflammation increase with aging33and have been shown to be inversely correlated with serum testosterone. While it has been hypothesized that lower testosterone production may lead to systemic inflammation34, another possible explanation is that systemic inflammation directly affects gonadal function. Administration of IL-6 or TNF to healthy male volunteers has been shown to decrease testosterone in a dose-dependent manner, suggesting a direct effect on gonadal function35,36. In a pre-clinical model, TNF infusion also inhibited the secretion of gonadotropin releasing hormone (GnRH)37. More recently, the activation of the IKK-β/NFΚB pathway in the hypothalamus of mice led to a decline of GnRH secretion. Thus systemic immune activation may also have detrimental effects on GnRH secretion17. In our study their inclusion did not attenuate the difference in AM FT by HIV serostatus or the effect of aging on FT in our regression models. Therefore, inflammation or immune activation did not explain the differences in FT between the HIV-infected and HIV-uninfected men.
The clinical impact of lower FT among HIV-infected men is unclear. The effect of HIV serostatus in our regression models was equivalent to approximately 6 years of aging. In the general population, the decline in gonadal function with aging has been associated with multiple adverse health outcomes, including cardiovascular diseases, diabetes mellitus, osteoporosis, bone fractures and metabolic syndrome1,2, although it is unclear if these relationships are causal19,21–23. Ongoing clinical trials of testosterone replacement in older men will help answer this important question38.
Our study has several limitations. First, the age range of studied men was narrow and the follow-up time relatively short. Second, we did not correlate FT changes with clinical outcomes in this analysis, an area of emphasis for future MACS work. Third, the vast majority of our population was Caucasian, so may not be generalizable to other racial groups. Fourth, specific ART regimens have been associated with differential changes in FT6 and use of testosterone replacement39. The relationship between ART and FT is an important topic but beyond the scope of this analysis. Finally, only 50% of our population had biomarkers of inflammation/immune activation biomarkers available and although differences by HIV infection observed in the larger cohort16 were also observed here. The study may have been underpowered to observe smaller effect sizes.
In conclusion, while HIV-infected and HIV-uninfected men experienced similar declines in FT over the 10-year study interval, FT was lower and had smaller diurnal variation among the HIV-infected men. Taken together, our findings may suggest a premature aging of the HPG axis in HIV-infected men based on blunting of the normal diurnal variation, but further studies are needed to test this hypothesis.
# Multicenter AIDS Cohort Study
The Multicenter AIDS Cohort Study (MACS) includes the following: Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with centers at Baltimore (U01-AI35042): The Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (PI), Jay Bream, Todd Brown, Barbara Crain, Adrian Dobs, Richard Elion, Richard Elion, Michelle Estrella, Lisette Johnson-Hill, Sean Leng, Anne Monroe, Cynthia Munro, Michael W. Plankey, Wendy Post, Ned Sacktor, Jennifer Schrack, Chloe Thio; Chicago (U01-AI35039): Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: Steven M. Wolinsky (PI), John P. Phair, Sheila Badri, Dana Gabuzda, Frank J. Palella, Jr., Sudhir Penugonda, Susheel Reddy, Matthew Stephens, Linda Teplin; Los Angeles (U01-AI35040): University of California, UCLA Schools of Public Health and Medicine: Roger Detels (PI), Otoniel Martínez-Maza (Co-P I), Aaron Aronow, Peter Anton, Robert Bolan, Elizabeth Breen, Anthony Butch, Shehnaz Hussain, Beth Jamieson, Eric N. Miller, John Oishi, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang; Pittsburgh (U01-AI35041): University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (PI), Lawrence A. Kingsley (Co-PI), James T. Becker, Phalguni Gupta, Kenneth Ho, Susan Koletar, Jeremy J. Martinson, John W. Mellors, Anthony J. Silvestre, Ronald D. Stall; Data Coordinating Center (UM1-AI35043): The Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (PI), Gypsyamber D’Souza (Co-PI), Alison, Abraham, Keri Althoff, Jennifer Deal, Priya Duggal, Sabina Haberlen, Alvaro Muoz, Derek Ng, Janet Schollenberger, Eric C. Seaberg, Sol Su, Pamela Surkan. Institute of Allergy and Infectious Diseases: Robin E. Huebner; National Cancer Institute: Geraldina Dominguez. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1-TR001079 (JHU ICTR) from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH), Johns Hopkins ICTR, or NCATS. The MACS website is located at http://www.statepi.jhsph.edu/macs/macs.html.
References
- 1.Yeap BB. Are declining testosterone levels a major risk factor for ill-health in aging men? International journal of impotence research. 2009 Jan-Feb;21(1):24–36. doi: 10.1038/ijir.2008.60. [DOI] [PubMed] [Google Scholar]
- 2.Travison TG, Araujo AB, Hall SA, McKinlay JB. Temporal trends in testosterone levels and treatment in older men. Current opinion in endocrinology, diabetes, and obesity. 2009 Jun;16(3):211–217. doi: 10.1097/med.0b013e32832b6348. [DOI] [PubMed] [Google Scholar]
- 3.Rohrmann S, Platz EA, Selvin E, et al. The prevalence of low sex steroid hormone concentrations in men in the Third National Health and Nutrition Examination Survey (NHANES III) Clinical endocrinology. 2011 Aug;75(2):232–239. doi: 10.1111/j.1365-2265.2011.04043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobs AS, Dempsey MA, Ladenson PW, Polk BF. Endocrine disorders in men infected with human immunodeficiency virus. The American journal of medicine. 1988 Mar;84(3 Pt 2):611–616. doi: 10.1016/0002-9343(88)90144-1. [DOI] [PubMed] [Google Scholar]
- 5.Tancredi A, Reginster JY, Luyckx F, Legros JJ. No major month to month variation in free testosterone levels in aging males. Minor impact on the biological diagnosis of 'andropause'. Psychoneuroendocrinology. 2005 Aug;30(7):638–646. doi: 10.1016/j.psyneuen.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Dube MP, Parker RA, Mulligan K, et al. Effects of potent antiretroviral therapy on free testosterone levels and fat-free mass in men in a prospective, randomized trial: A5005s, a substudy of AIDS Clinical Trials Group Study 384. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007 Jul 1;45(1):120–126. doi: 10.1086/518620. [DOI] [PubMed] [Google Scholar]
- 7.Klein RS, Lo Y, Santoro N, Dobs AS. Androgen levels in older men who have or who are at risk of acquiring HIV infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005 Dec 15;41(12):1794–1803. doi: 10.1086/498311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raffi F, Brisseau JM, Planchon B, Remi JP, Barrier JH, Grolleau JY. Endocrine function in 98 HIV-infected patients: a prospective study. Aids. 1991 Jun;5(6):729–733. doi: 10.1097/00002030-199106000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Rietschel P, Corcoran C, Stanley T, Basgoz N, Klibanski A, Grinspoon S. Prevalence of hypogonadism among men with weight loss related to human immunodeficiency virus infection who were receiving highly active antiretroviral therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2000 Nov;31(5):1240–1244. doi: 10.1086/317457. [DOI] [PubMed] [Google Scholar]
- 10.Wunder DM, Bersinger NA, Fux CA, et al. Hypogonadism in HIV-1-infected men is common and does not resolve during antiretroviral therapy. Antiviral therapy. 2007;12(2):261–265. [PubMed] [Google Scholar]
- 11.Monroe AK, Dobs AS, Xu X, et al. Sex hormones, insulin resistance, and diabetes mellitus among men with or at risk for HIV infection. Journal of acquired immune deficiency syndromes. 2011 Oct 1;58(2):173–180. doi: 10.1097/QAI.0b013e3182278c09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monroe AK, Dobs AS, Xu X, et al. Low free testosterone in HIV-infected men is not associated with subclinical cardiovascular disease. HIV medicine. 2012 Jul;13(6):358–366. doi: 10.1111/j.1468-1293.2011.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monroe AKDA, Palella FJ, Kingsley LA, Witt MD, Brown TT. 14th International Workshop on Adverse Drug Reactions and Co-morbidities in HIV, 2012. Washington, DC: 2012. Free Testosterone Levels Should be Used Routinely to Diagnose Hypogonadism in HIV-Infected Men. [Google Scholar]
- 14.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. American journal of epidemiology. 1987 Aug;126(2):310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 15.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. The Journal of clinical endocrinology and metabolism. 1999 Oct;84(10):3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 16.Wada NI, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. Aids. 2015 Feb 20;29(4):463–471. doi: 10.1097/QAD.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang G, Li J, Purkayastha S, et al. Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature. 2013 May 9;497(7448):211–216. doi: 10.1038/nature12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson JM, Chen JJ, Crapo L, Gray GD, Greenleaf WJ, Catania JA. Hormonal changes and sexual function in aging men. The Journal of clinical endocrinology and metabolism. 1983 Jul;57(1):71–77. doi: 10.1210/jcem-57-1-71. [DOI] [PubMed] [Google Scholar]
- 19.Ferrini RL, Barrett-Connor E. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. American journal of epidemiology. 1998 Apr 15;147(8):750–754. doi: 10.1093/oxfordjournals.aje.a009519. [DOI] [PubMed] [Google Scholar]
- 20.Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. The Journal of clinical endocrinology and metabolism. 1991 Nov;73(5):1016–1025. doi: 10.1210/jcem-73-5-1016. [DOI] [PubMed] [Google Scholar]
- 21.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR Baltimore Longitudinal Study of A. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. The Journal of clinical endocrinology and metabolism. 2001 Feb;86(2):724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 22.Morley JE, Kaiser FE, Perry HM, 3rd, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism: clinical and experimental. 1997 Apr;46(4):410–413. doi: 10.1016/s0026-0495(97)90057-3. [DOI] [PubMed] [Google Scholar]
- 23.Zmuda JM, Cauley JA, Kriska A, Glynn NW, Gutai JP, Kuller LH. Longitudinal relation between endogenous testosterone and cardiovascular disease risk factors in middle-aged men. A 13-year follow-up of former Multiple Risk Factor Intervention Trial participants. American journal of epidemiology. 1997 Oct 15;146(8):609–617. doi: 10.1093/oxfordjournals.aje.a009326. [DOI] [PubMed] [Google Scholar]
- 24.Zumoff B, Strain GW, Kream J, et al. Age variation of the 24-hour mean plasma concentrations of androgens, estrogens, and gonadotropins in normal adult men. The Journal of clinical endocrinology and metabolism. 1982 Mar;54(3):534–538. doi: 10.1210/jcem-54-3-534. [DOI] [PubMed] [Google Scholar]
- 25.Crum NF, Furtek KJ, Olson PE, Amling CL, Wallace MR. A review of hypogonadism and erectile dysfunction among HIV-infected men during the pre- and post-HAART eras: diagnosis, pathogenesis, and management. AIDS patient care and STDs. 2005 Oct;19(10):655–671. doi: 10.1089/apc.2005.19.655. [DOI] [PubMed] [Google Scholar]
- 26.Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. The Journal of clinical endocrinology and metabolism. 2002 Feb;87(2):589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 27.Basaria S. Need for standardising adverse event reporting in testosterone trials. Evidence-based medicine. 2013 Sep 13; doi: 10.1136/eb-2013-101402. [DOI] [PubMed] [Google Scholar]
- 28.Rochira V, Zirilli L, Orlando G, et al. Premature decline of serum total testosterone in HIV-infected men in the HAART-era. PloS one. 2011;6(12):e28512. doi: 10.1371/journal.pone.0028512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. The Journal of clinical endocrinology and metabolism. 1983 Jun;56(6):1278–1281. doi: 10.1210/jcem-56-6-1278. [DOI] [PubMed] [Google Scholar]
- 30.Plymate SR, Tenover JS, Bremner WJ. Circadian variation in testosterone, sex hormone-binding globulin, and calculated non-sex hormone-binding globulin bound testosterone in healthy young and elderly men. Journal of andrology. 1989 Sep-Oct;10(5):366–371. doi: 10.1002/j.1939-4640.1989.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 31.Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. The Journal of clinical endocrinology and metabolism. 2009 Mar;94(3):907–913. doi: 10.1210/jc.2008-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diver MJ, Imtiaz KE, Ahmad AM, Vora JP, Fraser WD. Diurnal rhythms of serum total, free and bioavailable testosterone and of SHBG in middle-aged men compared with those in young men. Clinical endocrinology. 2003 Jun;58(6):710–717. doi: 10.1046/j.1365-2265.2003.01772.x. [DOI] [PubMed] [Google Scholar]
- 33.Ferrucci L, Corsi A, Lauretani F, et al. The origins of age-related proinflammatory state. Blood. 2005 Mar 15;105(6):2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khosla S, Atkinson EJ, Dunstan CR, O'Fallon WM. Effect of estrogen versus testosterone on circulating osteoprotegerin and other cytokine levels in normal elderly men. The Journal of clinical endocrinology and metabolism. 2002 Apr;87(4):1550–1554. doi: 10.1210/jcem.87.4.8397. [DOI] [PubMed] [Google Scholar]
- 35.Tsigos C, Papanicolaou DA, Kyrou I, Raptis SA, Chrousos GP. Dose-dependent effects of recombinant human interleukin-6 on the pituitary-testicular axis. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 1999 Nov;19(11):1271–1276. doi: 10.1089/107999099312948. [DOI] [PubMed] [Google Scholar]
- 36.van der Poll T, Romijn JA, Endert E, Sauerwein HP. Effects of tumor necrosis factor on the hypothalamic-pituitary-testicular axis in healthy men. Metabolism: clinical and experimental. 1993 Mar;42(3):303–307. doi: 10.1016/0026-0495(93)90078-3. [DOI] [PubMed] [Google Scholar]
- 37.Gaillard RC, Turnill D, Sappino P, Muller AF. Tumor necrosis factor alpha inhibits the hormonal response of the pituitary gland to hypothalamic releasing factors. Endocrinology. 1990 Jul;127(1):101–106. doi: 10.1210/endo-127-1-101. [DOI] [PubMed] [Google Scholar]
- 38.Snyder PJ, Ellenberg SS, Cunningham GR, et al. The Testosterone Trials: Seven coordinated trials of testosterone treatment in elderly men. Clinical trials. 2014 Mar 31;11(3):362–375. doi: 10.1177/1740774514524032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhatia R, Murphy AB, Raper JL, et al. Testosterone replacement therapy among HIV-infected men in the CFAR Network of Integrated Clinical Systems. Aids. 2015 Jan 2;29(1):77–81. doi: 10.1097/QAD.0000000000000521. [DOI] [PMC free article] [PubMed] [Google Scholar]