Abstract
Introduction
Pulmonary lymphangioleiomyomatosis (LAM) is a rare progressive lung disease affecting almost exclusively women. Neoplastic growth of atypical smooth muscle-like cells in the lung induces destruction of lung parenchyma leading to the formation of lung cysts, rupture of which results in spontaneous pneumothorax. LAM occurs sporadically or in association with inherited hamartoma syndrome tuberous sclerosis complex (TSC). Progression of LAM often results in loss of pulmonary function and death. Increasing understanding of neoplastic LAM cell growth is driving the development of therapeutic approaches targeting the disease progression.
Areas covered
This review provides background to understand the rationale for current treatments used in patients with LAM, to critically appraise the evidence for these treatments, and to discuss future treatment approaches. The literature review includes publications from PubMed and clinicaltrials.gov/.
Expert Opinion
Targeting mTOR activation with rapamycin analogs sirolimus and everolimus are awaiting approval by the FDA for treatment of LAM. A number of other treatment options have been investigated and are currently tested in clinical trials to target LAM cell survival and metastasis. Key remaining and poorly understood areas for development and validation of therapeutic targeting in LAM are destruction of lungs, pathological lymphangiogenesis, and hormonal regulation. Future will reveal whether they could be targeted therapeutically.
Keywords: LAM, sirolimus, doxycycline, simvastatin, autophagy, clinical trials, rare disease, TSC, interstitial lung disease
1. Introduction
Pulmonary lymphangioleiomyomatosis (LAM) is a progressive neoplastic genetic disease affecting predominantly women [1]. The disease is characterized by the infiltration of atypical smooth muscle LAM cells in the lung and lymphatics, and cystic remodeling of lung parenchyma [2]. Obstruction of lymphatics might results in the collection of chylous fluid and fluid-filled lymphangiomyomas in the chest and abdomen [3–5]. The angiomyolipoma, a benign tumors composed of smooth muscle and fat, are also might be present in kidney, and may be detected in liver and spleen of LAM patients [5, 6]. LAM could be sporadic (S-LAM) or associated with hamartoma syndrome tuberous sclerosis complex (TSC-LAM). The disease affects 3–8 women per million in S-LAM [2]. At least 30% of women with TSC develop cystic changes in the lung consistent with LAM [2]. The risk of LAM in TSC patients, however, is age-related. Thus, the prevalence of LAM by age 21 was 27% and 81% in TSC-LAM patients over 41 year old [7]. Importantly, 63% of TSC patients developed LAM, and 13% died from LAM [7]. The mean onset of the disease is about 34–35 years and patients registered with The LAM Foundation show the median survival of 29 years after onset of symptoms.
Growth of atypical immature-looking smooth musclelike cells in the lungs promotes destruction of the lung, formation of air-filled spaces, rupture which leads to spontaneous pneumothoraces and lung collapse [2]. Pneumothorax usually reoccurs after the first incidence. Clinical evidence suggests beneficial effects of pleurodesis after first occurrence of pneumothorax [5, 6]. LAM is also characterized by obstruction of lymphatics and accumulation of chylous fluid either in the thorax or in the abdomen [5]. Lymphangiogenic growth factor VEGF-D, specifically upregulated in blood of patients with LAM but not in other cystic lung diseases [8], is a promising biomarker for diagnosis of LAM. Serum levels of VEGF-D might also serve as prognostic and predictive biomarker of disease severity and response to treatment [9, 10].
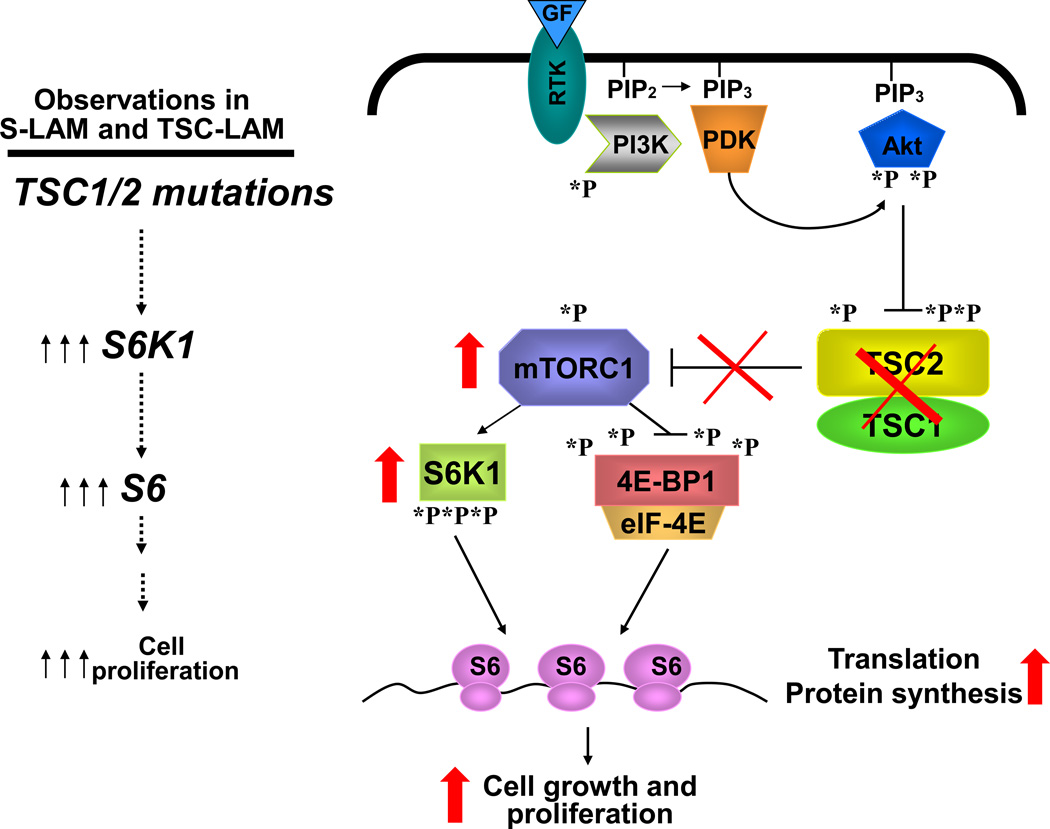
Sporadic or germline mutations in S-LAM or in TSC-LAM, respectively, of tumor suppressor genes tuberous sclerosis complex 1 (TSC1) or TSC2 have been linked with abnormal neoplastic growth of LAM cells in the lung. TSC2 mutations occur more frequently, and are usually associated with more severe manifestations [11]. Loss of TSC1 or TSC2 function results in activation of the mammalian target of rapamycin (mTOR) signaling pathway inducing uncontrolled growth of LAM cells in the lung (Figure 1) [2, 12, 13]. Major advances have been made in inhibiting LAM cell growth by using allosteric inhibitors of mTOR activity such as sirolimus and everolimus, generally named rapalogs [2, 12, 14]. These drugs slow down the disease progression by stabilizing the decline in lung function [15, 16].
Recent developments in identifying the signaling pathways deregulated in pulmonary LAM serve as a background for identifying and development of potential therapeutic target to treat this disease. In this review, I will provide a brief overview of completed clinical trials, ongoing clinical trials, and potential directions for therapeutic interventions for treatment of LAM.
Figure 1. Schematic representation of deregulated cell signaling in LAM.
Mutational inactivation of TSC1/TSC2 uncouples mTORC1 regulation from growth factor control causing uncontrolled protein synthesis and abnormal cell growth in pulmonary lymphangioleiomyomatosis (LAM). mTORC1 is a master regulator of cell growth and metabolism, which mediates three major inputs from growth promoting signals such, for example, growth factors and insulin. mTORC1 directly phosphorylates and activates S6K1 and inhibit 4E-BP1, which leads to protein synthesis, cell growth and proliferation. mTORC1 is negatively regulated by tumor suppressor complex TSC1/ TSC2, which activity is suppressed by inhibitory phosphorylation of TSC2 by PI3K-dependent Akt. PI3K is activated by multiple inputs such as growth factors and insulin, which leads to recruitment of PI3K to the membrane and its binding to RTK. Active PI3K converts phosphatidylinositol-4,5-biphosphate (PIP2) to phosphatidylinositol-3,4,5-trisphosphate (PIP3), which leads to PDK1-dependent phosphorylation of Akt followed by activation of mTOR in the mTORC1 complex.
2. Hormonal therapy for treatment of LAM?
Because LAM predominantly presents in premenopausal women and LAM disease progression also seems to increase during pregnancy targeting hormonal regulation by neutralizing effects of estrogens appears an expected step for therapeutic targeting [2] [[17] and references within]. Preclinical study show a positive effect of estrogens on survival and growth of cells deficient for TSC2 in vivo [18]. However, these data are in odds with clinical evidence demonstrating that progesterone had no positive, and possibly slightly negative, effect on LAM progression.
Currently the efficacy of hormonal therapy with progesterone in slowing the disease progression has not been demonstrated and no clinical trials were performed to determine the effect of hormonal therapy on LAM [19]. The off label use of progesterone therapy have been reported in clinic [6]. While some clinical case reports indicate potentially positive effects, there are also many publications reporting a failure of hormonal therapy in LAM results [[20] and references within]. Retrospective studies summarizing off label use of progestin therapy such as medroxyprogesterone acetate, tamoxifen, luteinizing hormone-releasing hormone (LHRH) or gonadotropin releasing hormone (GnRH) agonists (Lupron), or use of oophorectomy also report conflicting and inconclusive results [[6] and references within]. Further, a retrospective study performed at the National Institutes of Health [20] reported that treatment with progesterone does not improve the decline in lung function in patients with LAM. Among 348 LAM patients evaluated, 139 patients on progesterone were compared to 136 untreated patients. The rate of decline in lung function measured by FEV1 and DLco showed no benefits for LAM patients [20]. Considering that progesterone therapy in LAM patients is associated with adverse effects including venous thromboembolic disease and the development of meningiomas, only carefully designed placebo-controlled clinical trials could provide information about potential use of hormonal therapy in LAM. A trial of Letrozole in LAM (The TRAIL trial #NCT01353209 at http://www.clinicaltrials.gov/) (Table 1) proposes to test a novel approach in hormonal therapy by targeting estrogen secretion with aromatase inhibitor letrozole in postmenopausal women.
Table 1.
Interventional Clinical Trials*
| Trial Number | Title | Purpose | Intervention | Status | Duration | Primary Outcome Measures |
Location |
|---|---|---|---|---|---|---|---|
| NCT01799538 | Trial of Aromatase Inhibition in Lymphangioleiomyomatosis (TRAIL) | To determine whether estrogen suppression by an aromatase inhibitor in postmenopausal women with LAM will prevent or delay progression of lung disease | Letrozole: 2.5 mg daily dose | Unknown | 12 Months | The effect on Forced Expiratory Volume in one second (FEV1) | University of Cincinnati, OH, USA |
| NCT01059318 | A Study to Determine the Effectiveness of Escalating Doses of RAD001 (Everolimus) in Patients with Lymphangioleiomyomatosis | The safety of escalating doses of RAD001 (everolimus) in patients with LAM | Escalating daily doses of Everolimus (RAD001): 2.5mg, 5mg, 10mg | Completed | 26 Weeks | Assess safety, pharmacokinetics of everolimus and pharmacodynamics of serum VEGF-D | Novartis Pharmaceuticals |
| NCT00790400 | Safety Study of Sirolimus and Hydroxychloroquine in Women With Lymphangioleiomyomatosis (SAIL) | The safety and tolerability of the combination of sirolimus and hydroxychloroquine | Combination of sirolimus and escalating daily doses of hydroxychloroquine: 200 mg or 400 mg | Recruiting | 6 Months | Safety of combinational treatment of everolimus and hydroxychloroquine | Brigham and Women's Hospital, Boston, MA, USA |
| NCT01687179 | Safety of Simvastatin in LAM and TSC (SOS) | The safety of simvastatin in the treatment of LAM-S or LAM-TS in patients on a stable (for at least 3 months) dose of sirolimus or everolimus | Combination of sirolimus or everolimus with escalating daily doses of simvastatin: 20 mg and 40 mg | Recruiting | 4 Months | Safety of simvastatin in the treatment of LAM-S and LAM-TS patients on a stable dose of sirolimus or everolimus | University of Pennsylvania, Philadelphia, USA |
| NCT01353209 | Doxycycline In Lymphangioleiomyomatosis (LAM) | Effect of doxycycline on the progression of LAM | Doxycycline: 50mg | Unknown | 24 Months | Mean rate of change of FEV1 | University of Nottigham, UK |
| NCT00414648 | The Tolerability of Saracatinib in Subjects With Lymphangioleiomyomatosis (LAM) (SLAM-1) | Tolerabilty and safety of Saracatinib in patients with LAM | Escalating daily doses of Saracatinib: 50 mg, 125 mg, and 175 mg | Recruiting | 4 Weeks treatment + 8 Weeks follow up | Dose Determination | Baylor College of Medicine, Houston, TX, USA |
| NCT00989742 | Efficacy and Safety of RAD001 in Patients Aged 18 and Over With Angiomyolipoma Associated With Either Tuberous Sclerosis Complex (TSC) or Sporadic Lymphangioleiomyomatosis (LAM) (EXIST-2) | The safety and efficacy of RAD001 in treating patients with Angiomyolipoma associated with TSC or S-LAM | Everolimus (RAD001): 10mg daily dose | Active, not recruiting (has results) | 12 Months | Angiomyolipoma Response Rate | Novartis Pharmaceuticals |
| NCT02061397 | RAD001 Therapy of Angiomyolipoma in Patients With TS Complex and Sporadic LAM | Reduction of Angiomyolipoma volume in patients with TSC and LAM | Everolimus (RAD001), 5 and 10 mg/day or 30, 50, 70mg/week | Completed | 24 Months | Patients will be considered responders if their angiomyolipoma volume decreases by thirty percent or more from baseline | Children's Hospital Medical Center, Cincinnati, OH, USA |
| NCT02009241 | Pulmonary Rehabilitation in Lymphangioleiomyomatosis | Determine the effect of pulmonary rehabilitation on exercise capacity, dyspnea, quality of life, muscle force, functional limitation and dynamic hyperiflation | Pulmonary Rehabilitation program consisting of 30 minutes of treadmill aerobic exercise training and 30 minutes of muscle strength training | Recruiting | 12 Weeks | Endurance time during constant work rate cycle ergometry | University of San Paulo, Brasil |
| NCT02116712 | Nebulized or Inhaled Albuterol for Lymphangioleiomyomatosis | Effect of Albuterol given in a metered dose inhaler or with a nebulizer will be compared lung function | Albuterol inhaler (two or four puffs) or Albuterol nebulizer | Recruiting | 3 Days | Improvement in lung function | National Heart, Lung, and Blood Institute (NHLBI), USA |
For more details, see http://clinicaltrials.gov/
Despite the lack of success as a direct approach for hormonal therapy for treatment of LAM, histophatological studies provide some insights about hormonal regulation in LAM pathology. Lung tissue sections from LAM patients demonstrate expression of estrogen and progesterone receptors in smooth muscle α-actin-positive LAM cells of the lesions [21–23]. Generally estradiol levels are higher in women than in men. Importantly, women also uniquely produce progesterone [17], which might contribute to LAM pathology. Interestingly, in other gender-specific disease such as a breast cancer, the levels of estrogen receptors are usually upregulated compared to progesterone receptors. Surprisingly, in LAM lungs the expression of progesterone receptors are significantly higher compared to the level of estrogen receptors [21]. Schuger and colleagues [21] performed analyses of 20 biopsy samples and 24 transplanted lungs from LAM patients and found that expression of progesterone receptor was significantly higher than estrogen receptor in 80% of specimens [21]. Thus LAM appears as an unusual disease in its high ratio between progesterone and estrogen receptor expression compared to other female-specific neoplastic diseases [21]. Little is known about the cellular and molecular mechanisms of estrogen and progesterone signaling in LAM that could shed a light on hormonal regulation and potential molecular targets for therapeutic intervention. Further studies are needed to shed a light on the significance of progesterone receptor upregulation and pathological mechanisms of hormonal signaling in LAM.
3. LAM treatment with rapalogs, sirolimus and everolimus
The major advance in understanding the pathology of LAM which culminated in the identification of the first treatment for LAM disease came with the discovery of the cellular function of the tumor suppressor complex TSC1/TSC2 as a negative regulator of mammalian target of rapamycin (mTOR). Evidence from fruit fly Drosophila, rodent cells and primary human LAM cells showed that mutational inactivation of TSC1/TSC2 are the cause of abnormal growth in LAM [12, 13, 24, 25]. Further, the preclinical study of allosteric inhibitor rapamycin in LAM cells demonstrated that mTOR could be targeted at physiologically relevant doses [12, 13, 24]. These preclinical data paved the way to the phase I–II nonrandomized, open-label 24-month efficacy clinical trial of rapamycin analog sirolimus in patients with TSC and LAM, which was opened for enrollment in 2003 [15]. Patients were treated with escalating doses of sirolimus for 1 year followed by a 12 month observational period. The study included patients with S-LAM and TSC-LAM with diagnosis of pulmonary LAM and at least one angiomyolipoma 1cm or larger. The primary outcome measure was to determine the effect of sirolimus on angiomyolipoma volume at 1 year. The secondary end points included angiomyolipoma volume at 2 years, spirometric measurements, lung volume, diffusing capacity, 6-minute walk test, and the percentage of cyst volume at 1 and 2 years. Patients started on the initial dose of 0.25 mg per square meter of body-surface area of sirolimus that was gradually escalated to achieve a blood level of sirolimus up to 10–15 ng per milliliter. Out of 25 patients enrolled in the study, 20 completed 1 year evaluation, and 18 completed 2-year evaluation. The trial produced very promising results. Thus, the size of angiomyolipoma at 12 months was markedly reduced by about 50% compared to the baseline levels. The significant improvements in lung function were also observed showing an increase in the mean forced expiratory volume in 1 second (FEV1) and the forced vital capacity (FVC) in 1 year of treatment as compared with baseline values [15].
The first open-label sirolimus trial was followed by the international two-stage double-blinded placebo-controlled efficacy and safety of sirolimus trial (MILES trial) for patients with moderate lung impairment [16]. This trial also has two phases – 12-month treatment period followed by 12-month observation period. The primary outcome measure was the rate of change in FEV1 between LAM patients on placebo and sirolimus. The sirolimus levels were maintained at 5–15 ng per milliliter throughout the treatment period. At 1 year on sirolimus, lung functions were stabilized as compared to progressive lung function decline in the placebo group. Sirolimus treatment also improved quality of life and functional performance. Importantly, serum level of lymphangiogenic factor vascular endothelial growth factor D (VEGF-D) was used for the first time as a potential biomarker for LAM and as a secondary outcome in response to treatment. At the baseline, VEGF-D levels were comparable in both groups but were significantly lower in the sirolimus at 6 and 12 months of treatment. The MILES trial demonstrated that sirolimus stabilizes lung functions and improve quality of life of the LAM patients [16].
The open-label and the MILES trials clearly and convincingly demonstrated that sirolimus could stop disease progression and stabilize lung function. The trials also uncovered the limitation of sirolimus as a therapeutic approach. In both trials during the 12-month observation period, lung function decline resumed after sirolimus withdrawal [15, 16]. Further, after sirolimus therapy was stopped for 1 year, growth of angiomyolipoma resumed and caused significant increase of the angiomyolipoma volume [15].
Thus, inhibition of mTOR with sirolimus (Bissler et al., 2008; McCormack et al., 2011) or everolimus, another rapamycin derivative inhibiting mTOR, used in phase III clinical studies for treatment of angiomyolipoma in patients with TSC and LAM [26], show promising results suggesting sirolimus and everolimus could be drugs for treatment for S-LAM and TSC-LAM. Because sirolimus and everolimus are not yet approved by FDA for treatment of LAM, these drugs might be considered on an individual basis at the specialized LAM and TSC clinics after careful evaluation of risk/benefit ratio as suggested by the European Respiratory Society guidelines [6].
4. Therapeutic strategies to eradicate LAM cells
Progressive growth of LAM lesions causes the destruction of lung parenchyma, obstruction of lymphatics, accumulation of chylous effusions and lung collapse. Clinical studies suggest that therapeutic use of sirolimus and everolimus might require life-long treatment (although this have to be clinically proven) because drug withdrawal results in further disease progression [5, 27, 28]. Why did mTOR inhibitors sirolimus and everolimus fail to have a long-lasting effect in LAM? One of the major limitations of mTOR inhibitors of rapamycin group, including sirolimus and everolimus, is their cytostatic but not cytotoxic effect [29]. Rapamycin only partially inhibits mTOR activity because it induces allosteric inhibition of mTOR without affecting mTOR catalytic activity. Further, rapamycin inhibition of S6K1, which activity is regulated by mTOR, releases a negative feedback loop on PI3K signaling that induces activation of the pathway and supports cell survival [14]. Rapamycin also transiently and partially inhibits phosphorylation of 4E-BP1, another molecule regulated by mTOR, thus having only modest inhibitory effect on protein translation [29] (See Figure 1). To overcome the limitation of rapamycin, the second generation of mTOR inhibitors targeting the catalytic activity of mTOR have been developed and are currently being tested in pre-clinical studies and clinical trials in the treatment of cancer [14]. This group of drugs has not been considered or pre-clinically tested for LAM.
The cytostatic effects of rapalogs sirolimus and everolimus have motivated the search for novel or additional therapeutic targets for LAM, drug targeting of which results in LAM cell death. Two clinical trials currently testing this approach are listed on ClinicalTrials.gov: The Safety Study of Sirolimus and Hydroxychloroquine in Women with Lymphangioleiomyomatosis (SAIL trial), and Safety of Simvastatin in the Treatment of LAM-S and LAM-TS patients (SOS trial). Both trials were preceded by preclinical studies discovering novel molecular targets such as increased autophagy [30] and RhoA GTPase upregulation [31, 32] in cells deficient for TSC2 gene, targeting of which with chloroquine or simvastatin, respectively, caused cell death and abrogation of tumors.
Chloroquine, a class of anti-malarial drugs, is used to prevent and treat acute attacks of malaria. It is also now in clinical trials to treat discoid or systemic lupus erythematosus and rheumatoid arthritis. Chloroquine has been considered as an agent which might enhance cancer therapy and has been currently clinically tested for safety [33]. A potential treatment of LAM with chloroquine is based on findings that TSC2 loss upregulates autophagy, and inhibition of autophagy with chloroquine sensitizes TSC2-null cells to growth-inhibitory effect of rapamycin inducing cell death [30]. In vivo data also show decreased tumor burden in mice treated with a combination of chloroquine and rapamycin. Importantly, chloroquine alone does not kill TSC2-null cells, and requires the presence of rapamycin to induce a synergistic effect [30]. These preclinical data provided supportive evidence for the SAIL trial (Table 1). The goal of the SAIL trial is to determine whether the combination of rapamycin analog sirolimus and hydroxychloroquine is safe and well tolerated. LAM patients will be on the combination therapy with sirolimus (blood levels between 5–15 ng/ml) and escalating doses of hydroxychloroquine (200 mg or 400 mg). The safety of drug combination will be monitored up to 6 months. Serum levels of VEGF-D will be monitored to determine its potential role in predicting rates of disease progression and determine responsiveness to combination therapy.
Identification that RhoA GTPase is activated in LAM [34, 35] and is required for LAM cell survival [32] led to pre-clinical testing of statins, 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductase inhibitors, and pleiotropic agents that might contribute to the prevention of human cancers [36]. Statins, that modulate the lipid metabolism, regulate the geranylgeranylation of Rho GTPases that is required for their membrane anchoring and activation. Initial pre-clinical studies using synthetic atorvastatin (Lipitor) did not improve the outcome of syngeneic growth of TSC2-null tumors in nude mice formed by mouse embryonic fibroblast immortalized by deletion of the tumor suppressor p53 [37], and renal and liver tumors in TSC2+/− mice [38] that do not develop lung tumors. In contrast, a simvastatin (Zocor) not only inhibited xenographic tumor growth of TSC2-null SM-like cells derived from uterine leiomyoma by promoting apoptosis, but also prevented tumor recurrence after treatment withdrawal [32]. Further, early treatment with a combination of simvastatin and rapamycin prevents growth of lung lesions and lung destruction in mouse model of LAM [31]. Side-by-side comparison demonstrated higher potency of simvastatin compared with atorvastatin on inhibition of TSC2-null cell growth and promoting cell death [39]. Collectively, preclinical studies suggest that simvastatin (Zocor) and atorvastatin (Lipitor) have differential effects on TSC2-null tumors. Retrospective analysis [40] of clinical cases to evaluate whether simvastatin and atorvastatin have differential effects in the clinic might provide important information for clinical use of statins for combinational treatment of LAM. Currently, the safety of simvastatin has been tested in phase I-II clinical trial (Table 1). The goal of the SOS trial is to determine safety and any potential benefits to S-LAM and TSC-LAM patients taking simvastatin and everolimus to be monitored for 4 months. Patients will start on 20 mg simvastatin for 2 months followed by increase of simvatstain dose to 40 mg daily. Safety (including any major changes or deterioration in patient health) will be measured by reductions from baselines in physical examination, pulmonary function tests and/or blood values, and in the reporting of new or aggravated adverse events. Patients will be monitored for the effect of combinational therapy on pulmonary function, quality of life, and serum level of VEGF-D.
5. Targeting lung destruction in LAM?
Progressive destruction of lung parenchyma is the most prominent and debilitating pathological manifestation of LAM. The prevailing hypothesis is that the lung destruction and cystic space formation in patients with LAM is mediated by degradation of extracellular matrix as a result of an imbalance between matrix degrading proteases (MMPs) and their endogenous inhibitors TIMPs [2, 41]. Indeed, elastic fibers in remodeled alveoli in lungs from LAM patients are scant, and those that remain are often disrupted [42]. Increased MMP-1, MMP-2, MMP-9, MMP-11, and MMP-19 levels have been reported in lungs from patients with LAM [43], and mTORC1-independent upregulation of MMP-2 expression was shown in TSC2-deficient cells and cells from LAM patients [44–46]. In vivo study demonstrates that growth of TSC2-null lesions in murine lung is associated with an increase in MMP-2, MMP-3, MMP-7, MMP-9 and MMP-12 levels [31]. The extent to which altered amounts of TIMPs are involved in lung destruction in LAM remains to be determined.
Because the cystic lung destruction in LAM has been attributed to increased expression and activity of MMPs, use of pharmacological MMP inhibitors appears as a potential approach. However, drug development to target MMPs has been unsuccessful, and currently, there are no MMP inhibitors approved for clinical use. In preclinical testing doxycycline, which clinically used as an antibiotic, demonstrated inhibitory effect on MMPs in TSC2-null cells [45, 47]. Doxycycline, inexpensive and well tolerated drug, appeared as an attractive candidate for clinical use. Thus, in 2006 a sensational clinical case reported use of escalating dose of doxycycline by a single LAM patient showing improvement in lung oxygenation [47]. This study prompted a clinical trial to determine the effect of doxycycline in LAM. A randomized, double blind, placebo controlled trial of doxycycline in LAM opened in the United Kingdom in 2009 (Table 1). Currently, trial is closed for enrollment and results were reported by Johnson and colleagues [48]. Another doxycycline trial has been completed in Brasil [49]. In this open label trial, 31 LAM patients were treated with 100 mg daily doxycycline for 12 months. No significant differences in serum levels of MMP-9 and VEGF-D have been detected between pre- and post-doxycycline treatment. Patients with mild symptoms appeared to have better response to therapy. Although, as noted by authors, these effects seemed not related to doxycycline treatment [50].
6. Targeting metastatic spread of LAM cells?
Pulmonary LAM is a low grade metastasizing neoplastic disease [1]. LAM cells were detected in blood, chyle, and urine [51], and LAM cell shedding was detected in the lymph [52]. Genetic link has been established between cell forming angiomyolipoma and LAM lesions in the lung [53]. Recurrent LAM was reported after lung transplantation and confirmed genetic mutations of the host in transplanted lung [54]. In primary culture human LAM cells behave like typical cancer cells – they do not require nutrients for exponential growth [12, 55], show uncontrolled migration and invasiveness [35]. Importantly, invasiveness of LAM-derived cells in cell culture is not responsive to rapamycin, suggesting that other therapeutic approaches may be needed.
Pro-oncogene Src is a non-receptor protein tyrosine kinase with a well-established role in cancer and metastasis [56]. A study by Eissa and colleagues demonstrates Src activation in TSC2-null cells [57]. Src activation induces migration and invasiveness by downregulating expression of E-cadherin, a hallmark of epithelial to mesenchymal transition, in TSC2-null rat embryonic fibroblasts. Inhibition of Src attenuates TSC2-null cell migration and invasiveness. Pretreatment of TSC2-null cells in culture with Src inhibitor saracatinib attenuates their colonization of the lungs suggesting a role of Src in experimental metastatic model [57]. This preclinical evidence lead to initiation of the phase I study of Tolerability of Saracatinib in Subjects with Lymphangioleiomyomatosis (SLAM trial) (Table 1). Saracatinib is a Src family kinase inhibitor currently in phase II clinical trials in patients including those with breast cancer, colorectal cancer, prostate cancer, and melanoma. The goal of the SLAM trial is to determine a safety profile in patients treated with escalating doses of saracatinib. Patients will be given three daily doses (50 mg, 125 mg, and 175 mg) of saracatinib for 4 weeks followed by 4 weeks observation. The SLAM trial is open for enrolment.
7. Using bronchodilators in LAM?
Airflow obstruction is one of the abnormalities of pulmonary function in LAM, but the underlying mechanism is not well understood. Typically, in asthma, airway obstruction results from chronic or irreversible contraction of airway smooth muscle. Inhaled β2-adrenergic receptor agonists cause airway relaxation, dilation of airways, is a common therapeutic approach in treatment of patients with asthma to improve airway obstruction [58]. Retrospective and prospective study of lung function of 143 patients and lung biopsies of 74 demonstrated a correlation between a positive response to bronchodilators with more severe airflow obstruction and a predominantly solid pattern of LAM lesions in the lung biopsy [59]. This study also showed an increase in FEV1 by 12% in approximately 30% LAM patients on β2-adrenergic receptor agonists albuterol [59]. A recent pilot study using other β2-adrenergic receptor agonist salbutamol produced a slight improvement in airway obstruction without improvement in dynamic hyperinflation and in exercise tolerance [50]. The goal of the current trial (see Table 1) is to determine the most effective route to deliver albuterol, either as a metered dose inhaler or a nebulizer.
8. Expert Opinion
Sirolimus is the only treatment of LAM validated in clinical trials approach [15, 16], and is currently under review at the FDA for approval as a LAM treatment. Sirolimus stabilizes lung function and improves some measures of quality of life and functional performance in patients with moderately severe LAM with FEV1 after bronchodilation of 70% or less of predicted value [5]. The drug has several adverse effects after one year of treatment, most of which are typical for this class of drugs. The drug withdrawal follows the disease progression with worsening of lung function and decline in lung capacity. Because of sirolimus side effects, the reduction of sirolimus daily dose might be an option. A retrospective, observational 6-month study of 15 patients of blood level of sirolimus performed in Japan reported promising findings about a potential effect of low doses of sirolimus on lung function [60]. Thus, the annual rates of change in FEV1 and FVC were improved in 9 patients. The chylous effusions resolved in 6 out of 7 patients. The clinical trial to validate this pilot data is needed to determine whether a reduction in daily dose of sirolimus might be an option for LAM patients.
Because sirolimus does not provide complete and sustain response to treatment, the development of new generation of mTOR inhibitors is underway for treatment of cancer [14]. Rapalogs are the first-generation of mTOR inhibitors and do not completely inhibit mTOR activity, thus drug withdrawal resumes cells or tumor growth. Rapamycin is a naturally-derived antibiotic from a soil bacterium Streptomyces hydroscopius. In mammalian cells rapamycin forms a complex with another protein named FKBP12, which then binds to specific rapamycin-binding domain of mTOR without direct effect on catalytic site of mTOR. In vitro and in vivo studies uncovered an underlying mechanism limiting rapalog effects on mTOR activity as a drug and its cytostatic effects on cell and tumor growth [14, 32, 61–65].
The second-generation of mTOR inhibitors that directly target the mTOR catalytic site have been developed, and data show that they can completely inhibit cell growth as compared to rapamycin which only reducing cell proliferation [14]. This new generation of ATP-competitive drugs shows potent mTOR inhibition and are currently in early clinical trials for treatment of cancer [66]. In the future, promising second generation of mTOR inhibitors might find their way for treatment of LAM after vigorous pre-clinical and clinical testing to determine their relevance and safety for LAM patients.
Recent study uncovers intriguing and surprising number of patients with LAM neither TSC1 nor TSC2 gene mutations have been identified [67]. This evidence suggests that other factors, yet to be discovered, could cause LAM disease. Thus, discovery of new genes, gene modifiers or epigenetics changes might hold a promise for novel therapeutic target(s) for drug development and treatment of S-LAM and TSC-LAM.
Rapalogs and future generation of mTOR inhibitors predominantly target LAM cell growth. Mutation of TSC1/TSC2 in addition to unleashing the cell growth also deregulates cellular metabolism [68–72]. In the past 10 years, targeting cancer metabolism has emerged as very promising direction for development of targeted therapies [73]. Some of the pharmacological agents which have been used in clinic and a few which have been under development might provide a potential approach for combination therapies for LAM in a future.
Over the last decade, substantial progress has been made in identification of molecular targets and in their clinical validation for treatment of LAM. LAM disease progression could be slowed down, but the goal for the near future is to find an answer as to whether lung destruction can be prevented or can lung function be restored in LAM patients. Therapeutic approaches to prevent destruction of lung parenchyma and development of abnormal lymphatics have not been developed and are urgently needed. Future directions and molecular targets for LAM treatment (Table 2) might include inhibitory antibody against VEGF-D or VEGFR3 inhibitors to prevent VEGF-D cellular signaling, development of novel immunotherapy [74], drugs to enhance stress in endoplasmic reticulum [75] and induce cell death, and repurposed or novel drug developed by the pharmaceutical companies. LAM is a fatal lung disease and we are closing in on finding the best treatment. The goal is to treat and control this disease completely. I would like to see this happen in my lifetime.
Table 2.
Therapeutic Targeting in LAM
| LAM Pathology/Target | Potential Drug |
|---|---|
| LAM Cell Growth | Sirolimus |
| Everolimus | |
| New Generation mTOR inhibitors* | |
| LAM Cell Survival | Simvastatin |
| Hydroxychloroquine | |
| LAM Cell Migration | Simvastatin |
| Saracatinib | |
| Estrogen | Letrozole |
| Fulvestrant (Faslodex)* | |
| Lymphangiogenesis | Pazpanib/axitinib* |
| Soluble VEGFR-3* | |
| Anti-VEGF-D antibody* | |
| Matrix Degradation | Doxycycline |
| Metalloproteinase inhibitors* | |
| Cathepsin inhibitors* | |
| Endoplasmic Reticulum (ER) Stress | Bortezomib (Velcade)* |
| Metabolic Reprogramming | Glucose/glutamine depravation* |
| Epigallocatechin gallate (EGCG)* | |
Potential drugs of interest and therapeutic approaches which have not yet been tested
Footnotes
Declaration of interest
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Cited literature
- 1.McCormack FX, et al. Lymphangioleiomyomatosis. American Journal of Respiratory and Critical Care Medicine. 2012;186(12):1210–1212. doi: 10.1164/rccm.201205-0848OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henske EP, McCormack FX. Lymphangioleiomyomatosis — a wolf in sheep’s clothing. J Clin Invest. 2012;122(11):3807–3816. doi: 10.1172/JCI58709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glasgow CG, et al. Lymphangioleiomyomatosis (LAM): Molecular insights lead to targeted therapies. Respiratory Medicine. 2010;104(Supplement 1):S45–S58. doi: 10.1016/j.rmed.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsui K, et al. Extrapulmonary lymphangioleiomyomatosis (LAM): Clinicopathologic features in 22 cases. Human Pathology. 2000;31(10):1242–1248. doi: 10.1053/hupa.2000.18500. [DOI] [PubMed] [Google Scholar]
- 5.Meraj R, et al. Lymphangioleiomyomatosis: New Concepts in Pathogenesis, Diagnosis, and Treatment. Semin Respir Crit Care Med. 2012;33(05):486–497. doi: 10.1055/s-0032-1325159. [DOI] [PubMed] [Google Scholar]
- 6.Johnson SR, et al. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. European Respiratory Journal. 2010;35(1):14–26. doi: 10.1183/09031936.00076209. [DOI] [PubMed] [Google Scholar]
- 7.Cudzilo CJ, et al. Lymphangioleiomyomatosis screening in women with tuberous sclerosis. CHEST Journal. 2013;144(2):578–585. doi: 10.1378/chest.12-2813. [DOI] [PubMed] [Google Scholar]
- 8.Young LR, et al. Serum Vascular Endothelial Growth Factor-D Prospectively Distinguishes Lymphangioleiomyomatosis From Other Diseases. Chest. 2010;138(3):674–681. doi: 10.1378/chest.10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young LR, Inoue Y, McCormack FX. Diagnostic Potential of Serum VEGF-D for Lymphangioleiomyomatosis. New England Journal of Medicine. 2008;358(2):199–200. doi: 10.1056/NEJMc0707517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young LR, et al. Serum VEGF-D concentration as a biomarker of lymphangioleiomyomatosis severity and treatment response: a prospective analysis of the Multicenter International Lymphangioleiomyomatosis Efficacy of Sirolimus (MILES) trial. Lancet. 2013 doi: 10.1016/S2213-2600(13)70090-0. On line. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dabora SL, et al. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. American Journal of Human Genetics. 2001;68:64–80. doi: 10.1086/316951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goncharova EA, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation: a role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis. Journal of Biological Chemistry. 2002;277:30958–30967. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- 13.Kwiatkowski DJ, et al. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Human Molecular Genetics. 2002;11:525–534. doi: 10.1093/hmg/11.5.525. [DOI] [PubMed] [Google Scholar]
- 14.Guertin DA, Sabatini DM. The Pharmacology of mTOR Inhibition. Sci. Signal. 2009;2(67):1–6. doi: 10.1126/scisignal.267pe24. pe24. [DOI] [PubMed] [Google Scholar]
- 15.Bissler JJ, et al. Sirolimus for Angiomyolipoma in Tuberous Sclerosis Complex or Lymphangioleiomyomatosis. N. Engl. J. Med. 2008;358(2):140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCormack FX, et al. Efficacy and Safety of Sirolimus in Lymphangioleiomyomatosis. New England Journal of Medicine. 2011;364:1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammes SR, Krymskaya VP. Targeted Approaches Toward Understanding and Treating Pulmonary Lymphangioleiomyomatosis (LAM) Hormones and Cancer. 2013;4:70–77. doi: 10.1007/s12672-012-0128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu JJ, et al. Estrogen promotes the survival and pulmonary metastasis of tuberin-null cells. Proc. Natl. Acad. Sci. 2009;106(8):2635–2640. doi: 10.1073/pnas.0810790106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryu JH, et al. The NHLBI Lymphangioleiomyomatosis Registry: Characteristics of 230 Patients at Enrollment. Am. J. Respir. Crit. Care Med. 2006;173(1):105–111. doi: 10.1164/rccm.200409-1298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taveira-DaSilva AM, et al. Decline in Lung Function in Patients With Lymphangioleiomyomatosis Treated With or Without Progesterone. Chest. 2004;126(6):1867–1874. doi: 10.1378/chest.126.6.1867. [DOI] [PubMed] [Google Scholar]
- 21.Gao L, et al. In pulmonary lymphangioleiomyomatosis expression of progesterone receptor is frequently higher than that of estrogen receptor. Virchows Archiv. 2014;464(4):495–503. doi: 10.1007/s00428-014-1559-9. [DOI] [PubMed] [Google Scholar]
- 22.Pan L-H, et al. Pulmonary Lymphangioleiomyomatosis: A Case Report with Immunohistochemical Details and DNA Analysis. The Tohoku Journal of Experimental Medicine. 2003;199(2):119–126. doi: 10.1620/tjem.199.119. [DOI] [PubMed] [Google Scholar]
- 23.Flavin RJ, et al. β-Catenin Is a Useful Adjunct Immunohistochemical Marker for the Diagnosis of Pulmonary Lymphangioleiomyomatosis. American Journal of Clinical Pathology. 2011;135(5):776–782. doi: 10.1309/AJCPPC9EX1ZHMRMA. [DOI] [PubMed] [Google Scholar]
- 24.Inoki K, et al. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signaling. Nature Cell Biology. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 25.Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nature Cell Biology. 2002;4:658–665. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- 26.Bissler JJ, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2013 doi: 10.1016/S0140-6736(12)61767-X. [DOI] [PubMed] [Google Scholar]
- 27.Krymskaya VP. Treatment Option(s) for Pulmonary Lymphangioleiomyomatosis: Progress and Current Challenges. American Journal of Respiratory Cell and Molecular Biology. 2012;46(5):563–565. doi: 10.1165/rcmb.2011-0381ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Chemaly S, Henske EP. Towards personalised therapy for lymphangioleiomyomatosis: lessons from cancer. European Respiratory Review. 2014;23(131):30–35. doi: 10.1183/09059180.00008813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell. Biol. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parkhitko A, et al. Tumorigenesis in tuberous sclerosis complex is autophagy and p62/sequestosome 1 (SQSTM1)-dependent. Proceedings of the National Academy of Sciences. 2011;108(30):12455–12460. doi: 10.1073/pnas.1104361108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goncharova EA, et al. Prevention of Alveolar Destruction and Airspace Enlargement in a Mouse Model of Pulmonary Lymphangioleiomyomatosis (LAM) Science Translational Medicine. 2012;4(154):154ra134. doi: 10.1126/scitranslmed.3003840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goncharova EA, et al. mTORC2 Is Required for Proliferation and Survival of TSC2-Null Cells. Molecular and Cellular Biology. 2011;31(12):2484–2498. doi: 10.1128/MCB.01061-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solomon VR, Lee H. Chloroquine and its analogs: A new promise of an old drug for effective and safe cancer therapies. European Journal of Pharmacology. 2009;625(1–3):220–233. doi: 10.1016/j.ejphar.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 34.Goncharova E, et al. TSC2 modulates actin cytoskeleton and focal adhesion through TSC1-binding domain and the Rac1 GTPase. Journal of Cell Biology. 2004;167(6):1171–1182. doi: 10.1083/jcb.200405130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goncharova EA, et al. Modulation of cell migration and invasiveness by tumor suppressor TSC2 in Lymphangioleiomyomatosis. American Journal of Respiratory Cell and Molecular Biology. 2006;34:473–480. doi: 10.1165/rcmb.2005-0374OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demierre M-F, et al. Statins and cancer prevention. Nature Reviews Cancer. 2005;5(12):930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 37.Lee N, et al. Rapamycin weekly maintenance dosing and the potential efficacy of combination sorafenib plus rapamycin but not atorvastatin or doxycycline in tuberous sclerosis preclinical models. BMC Pharmacol. 2009;9(1):8. doi: 10.1186/1471-2210-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finlay G, et al. Renal and liver tumors in Tsc2(+/−) mice, a model of tuberous sclerosis complex, do not respond to treatment with atorvastatin, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Molecular Cancer Therapeutics. 2009;8:1799–1807. doi: 10.1158/1535-7163.MCT-09-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atochina-Vasserman EN, et al. Statins in Lymphangioleiomyomatosis. Simvastatin and Atorvastatin Induce Differential Effects on tuberous sclerosis complex 2–Null Cell Growth and Signaling. American Journal of Respiratory Cell and Molecular Biology. 2013;49(5):704–709. doi: 10.1165/rcmb.2013-0203RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Chemaly S, et al. Statins in lymphangioleiomyomatosis: a word of caution. European Respiratory Journal. 2009;34(2):513–514. doi: 10.1183/09031936.00012709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krymskaya VP, Shipley JM. Lymphangioleiomyomatosis: A Complex Tale of Serum Response Factor-Mediated TTIMP-3 Regulation. American Journal of Respiratory Cell and Molecular Biology. 2003;28(5):546–550. doi: 10.1165/rcmb.F267. [DOI] [PubMed] [Google Scholar]
- 42.Fukuda Y, et al. Role of elastic fiber degradation in emphysema-like lesions of pulmonary lymphangiomyomatosis. Human Pathology. 1990;21(12):1252–1261. doi: 10.1016/s0046-8177(06)80039-0. [DOI] [PubMed] [Google Scholar]
- 43.Hayashi T, et al. Immunohistochemical study of MMPs and TIMPs in pulmonary lymphangioleiomyomatosis. Human Pathology. 1997;28:1071–1078. doi: 10.1016/s0046-8177(97)90061-7. [DOI] [PubMed] [Google Scholar]
- 44.Lee P-S, et al. Rapamycin-insensitive Up-regulation of MMP2 and Other Genes in TSC2-deficient LAM-like Cells. American Journal of Respiratory Cell and Molecular Biology. 2009;42:227–234. doi: 10.1165/rcmb.2009-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang WYC, Clements D, Johnson SR. Effect of doxycycline on proliferation, MMP production and adhesion in LAM related cells. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2010;299:L393–L400. doi: 10.1152/ajplung.00437.2009. [DOI] [PubMed] [Google Scholar]
- 46.Moir LM, et al. Doxycycline inhibits MMP-2 secretion from TSC2-null MEFs and lymphangioleiomyomatosis cells. British Journal of Pharmacology. 2011;164:83–92. doi: 10.1111/j.1476-5381.2011.01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moses MA, Harper J, Folkman J. Doxycycline Treatment for Lymphangioleiomyomatosis with Urinary Monitoring for MMPs. New England Journal of Medicine. 2006;354(24):2621–2622. doi: 10.1056/NEJMc053410. [DOI] [PubMed] [Google Scholar]
- 48.Chang WYC, et al. A 2-year randomised placebo-controlled trial of doxycycline for lymphangioleiomyomatosis. European Respiratory Journal. 2014;43(4):1114–1123. doi: 10.1183/09031936.00167413. [DOI] [PubMed] [Google Scholar]
- 49.Pimenta SP, et al. Doxiciclina em pacientes com linfangioleiomiomatose: biomarcadores e resposta funcional pulmonar. Jornal Brasileiro de Pneumologia. 2013;39:5–15. [Google Scholar]
- 50.Baldi BG, et al. A pilot study assessing the effect of bronchodilator on dynamic hyperinflation in LAM. Respiratory Medicine. 2013;107(11):1773–1780. doi: 10.1016/j.rmed.2013.08.045. [DOI] [PubMed] [Google Scholar]
- 51.Crooks DM, et al. Molecular and genetic analysis of disseminated neoplastic cells in lymphangioleiomyomatosis. Proceedings of National Academy of Sciences USA. 2004;101(50):17462–17467. doi: 10.1073/pnas.0407971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumasaka T, et al. Lymphangiogenesis-Mediated Shedding of LAM Cell Clusters as a Mechanism for Dissemination in Lymphangioleiomyomatosis. Am. J. Surg. Pathol. 2005;29:1356–1366. doi: 10.1097/01.pas.0000172192.25295.45. [DOI] [PubMed] [Google Scholar]
- 53.Henske EP. Metastasis of benign tumor cells in tuberous sclerosis complex. Genes, Chromosomes & Cancer. 2003;38:376–381. doi: 10.1002/gcc.10252. [DOI] [PubMed] [Google Scholar]
- 54.Karbowniczek M, et al. Recurrent lymphangiomyomatosis after transplantation: genetic analyses reveal a metastatic mechanism. American Journal of Respiratory and Critical Care Medicine. 2003;167(7):976–982. doi: 10.1164/rccm.200208-969OC. [DOI] [PubMed] [Google Scholar]
- 55.Goncharova EA, et al. Abnormal smooth muscle cell growth in LAM: role for tumor suppressor TSC2. American Journal of Respiratory Cell and Molecular Biology. 2006;34:561–572. doi: 10.1165/rcmb.2005-0300OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeatman TJ. A renaissance for Src. Nature Reviews Cancer. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 57.Tyryshkin A, Bhattacharya A, Eissa NT. Src Kinase Is a Novel Therapeutic Target in Lymphangioleiomyomatosis. Cancer Research. 2014;74(7):1996–2005. doi: 10.1158/0008-5472.CAN-13-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8(3):218–230. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- 59.Taveira-DaSilva AM, et al. Reversible airflow obstruction, proliferation of abnormal smooth muscle cells, and impairment of gas exchange as predictors of outcome of Lymphangioleiomyomatosis. American Journal of Respiratory and Critical Care Medicine. 2001;164:1072–1076. doi: 10.1164/ajrccm.164.6.2102125. [DOI] [PubMed] [Google Scholar]
- 60.Ando K, et al. The efficacy and safety of low-dose sirolimus for treatment of lymphangioleiomyomatosis. Respiratory Investigation. 2013;51(3):175–183. doi: 10.1016/j.resinv.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 61.Choo AY, Blenis J. TORgeting oncogene addiction for cancer therapy. Cancer Cell. 2006;9(2):77–79. doi: 10.1016/j.ccr.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 62.Choo AY, et al. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proceedings of the National Academy of Sciences. 2008;105(45):17414–17419. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thoreen CC, et al. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 2012;485(7396):109–113. doi: 10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dorrello NV, et al. S6K1- and βTRCP-Mediated Degradation of PDCD4 Promotes Protein Translation and Cell Growth. Science. 2006;314(5798):467–471. doi: 10.1126/science.1130276. [DOI] [PubMed] [Google Scholar]
- 65.Holz MK, et al. mTOR and S6K1 Mediate Assembly of the Translation Preinitiation Complex through Dynamic Protein Interchange and Ordered Phosphorylation Events. Cell. 2005;123(4):569–580. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 66.Benjamin D, et al. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat. Rev. Drug Discov. 2011;10(11):868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 67.Badri KR, et al. Exonic Mutations of TSC2/TSC1 Are Common but Not Seen in All Sporadic Pulmonary Lymphangioleiomyomatosis. American Journal of Respiratory and Critical Care Medicine. 2013;187(6):663–665. doi: 10.1164/ajrccm.187.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Düvel K, et al. Activation of a Metabolic Gene Regulatory Network Downstream of mTOR Complex 1. Molecular Cell. 2010;39(2):171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ben-Sahra I, et al. Stimulation of de Novo Pyrimidine Synthesis by Growth Signaling Through mTOR and S6K1. Science. 2013;339:1323–1328. doi: 10.1126/science.1228792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dibble CC, Manning BD. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat Cell Biol. 2013;15(6):555–564. doi: 10.1038/ncb2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Menon S, et al. Spatial Control of the TSC Complex Integrates Insulin and Nutrient Regulation of mTORC1 at the Lysosome. Cell. 2014;156(4):771–785. doi: 10.1016/j.cell.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Csibi A, et al. The mTORC1 Pathway Stimulates Glutamine Metabolism and Cell Proliferation by Repressing SIRT4. Cell. 2013;153(4):840–854. doi: 10.1016/j.cell.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Galluzzi L, et al. Metabolic targets for cancer therapy. Nat Rev Drug Discov. 2013;12(11):829–846. doi: 10.1038/nrd4145. [DOI] [PubMed] [Google Scholar]
- 74.Dilling DF, et al. A current viewpoint of Lymphangioleimyomatosis supporting immunotherapeutic treatment options. American Journal of Respiratory Cell and Molecular Biology. 2012;46:1–5. doi: 10.1165/rcmb.2011-0215TR. [DOI] [PubMed] [Google Scholar]
- 75.De Raedt T, et al. Exploiting Cancer Cell Vulnerabilities to Develop a Combination Therapy for Ras-Driven Tumors. Cancer Cell. 2011;20(3):400–413. doi: 10.1016/j.ccr.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]