Abstract
Objective
Preliminary test of a manualized, measurement-guided treatment for depression for adolescents and young adults in care at four sites of the Adolescent Trials Network for HIV/AIDS Interventions (ATN).
Design
The U.S. sites were randomly assigned to either a 24-week, combination cognitive behavioral therapy and medication management algorithm (COMB) tailored for youth living with HIV (YLWH) or to treatment as usual (TAU).
Methods
Youth at TAU sites had access to therapists and medication management as needed. COMB site clinicians were trained in the manualized intervention and participated in supervision calls to monitor intervention fidelity.
Results
Over the course of the study with 44 participants, those in COMB, compared to those in TAU, reported fewer depressive symptoms, p<0.01 (as measured by the Quick Inventory for Depression Symptomatology) and were more likely to be in remission, p<0.001, (65% vs.10% at week 24 end of treatment, and 71% vs. 7% at week 48 final follow-up). A greater proportion of COMB participants received psychotherapy (95% vs. 45%, p<0.001) and attended more sessions (12.6 vs. 5, p<0.001) than those in TAU. Viral load decreased in both groups and was associated (p<0.05) with reduction in depressive symptoms.
Conclusions
A 24-week manualized, measurement-guided psychotherapy and medication management algorithm tailored for YLWH was more effective in achieving and sustaining remission from depression than treatment as usual at HIV care clinic sites. Given observed treatment efficacy, this structured combination treatment could be disseminated to medical clinics in order to successfully treat YLWH, who are at particular risk for depression.
Keywords: Major Depressive Disorder (MDD), Cognitive Behavioral Therapy (CBT), Antidepressants, Youth, Young Adults, Human Immunodeficiency Virus (HIV)
Introduction
Depression is prevalent among youth living with HIV (YLWH) [1]. In a study of 2,032 YLWH in medical care, 21% reported depression, which is higher than the general prevalence [2]. Other studies report depression rates in YLWH as high as 50% [3,4]. Depression increases morbidity and mortality for YLWH. It interferes with adherence to HIV treatment, increases caregiver burden, increases healthcare costs, and decreases quality of life [5,6]. Thus, treatment of depressive disorders is essential for improving both psychiatric and medical outcomes for YLWH, especially since effective antiretroviral treatments have dramatically increased life expectancy [5].
Prior reports indicate serotonin reuptake inhibitors (SSRIs) are generally safe and effective in the treatment of depression in adults with HIV [7,8], and that these treatments not only improve depression outcomes, but also are associated with greater adherence and increased CD4 T-cell counts [9]. No studies have addressed the efficacy of SSRIs in YLWH, how to proceed after no or minimal response to treatment, or examined the effectiveness of treatment guided by measures of symptomatology.
Several psychotherapies have been shown to be effective in treating depression. However, YLWH present unique issues, including chronic illness, loss, stigmatization, and cultural and sexual diversities. Motivational interviewing (MI) and Cognitive Behavior Therapy (CBT) have been shown to improve adherence to antiretroviral medications, as well as to improve depressive symptoms and life satisfaction [10]. CBT has been used successfully with diverse populations [11,12], including lesbian, gay and bisexual clients and adults living with HIV [13,14]. Despite its perceived effectiveness, there has not been a controlled trial of CBT in depressed adolescents or young adults living with HIV.
HIV clinic prescribers commonly use SSRIs, although many are not psychiatrists, and clinic therapists use a wide variety of therapies such as CBT, interpersonal therapy, general supportive therapy, mindfulness and relation relaxation training. Because of their training and work in HIV-care settings, their treatment is tailored for the needs of individual patients, although there is no systematic description of the treatment options, decision points, or measurement that guides treatment. Practice guidelines suggest that a combination of medication management and an evidenced-based psychotherapy, such as CBT, show a more rapid reduction in depressive symptoms than a single treatment modality (i.e., either psychotropic medication or psychotherapy) for depression [15,16,17]. Additionally, other studies show that measured-care treatment (care decisions are guided by measures of symptomatology) is even more effective [18]. Therefore, in this study, a combined, measured-care treatment approach was chosen, within which both CBT and the medication management algorithm (MMA) were adapted for use in YLWH to be compared with treatment as usual (TAU) at participating Adolescent Trials Network for HIV/AIDS Interventions (ATN) sites [19,20].
STUDY AIMS
This is the first study to adapt and preliminarily test a measured-care combined treatment strategy that included psychopharmacology and psychotherapy for YLWH with depression in the medical care setting. Investigators predicted that, based on previous research, participants in the 24 week combined treatment (COMB) would have fewer depressive symptoms, a greater rate of response to treatment, and a greater rate of remission from depression than those receiving treatment as usual (TAU), despite the HIV experience of TAU providers and their current use of SSRIs and CBT. In addition, it was hypothesized that COMB participants would show, over the course of the study, less hopelessness and greater life satisfaction, constructs frequently associated with depression [21,22]. Also, it was predicted that COMB would be associated with greater use and better adherence to antiretroviral treatment, and better HIV health status outcomes (CD4+ T-cell count (CD4) and HIV RNA viral load (VL)) because of the known association of depression and these health indicators.
Method
PARTICIPANTS
Participants were ages 18–24 years, engaged in care at participating U.S. ATN sites, with documented HIV confirmed by medical records, and a diagnosis of nonpsychotic depression, either Major Depressive Disorder (MDD), Depression NOS, or Dysthymia as defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), with significant symptomatology at entry (as defined by a Quick Inventory of Depressive Symptomatology-Self-Report (QIDS-SR) scores ≥ 7). Participants were excluded from the study if they had a history of any psychosis/psychotic disorders or Bipolar I or II Disorder, had a first degree relative diagnosed with Bipolar I Disorder, had a diagnosis of alcohol or substance dependence according to DSM-IV in the last 6 months, and/or if they were in imminent danger to themselves or others.
PROCEDURES
Four ATN sites were randomly assigned to either COMB or TAU so that TAU clinicians were not exposed to the manualized CBT and/or medication management algorithm. Sites were chosen by the ATN Site Selection Committee based on their capacity to enroll at least ten eligible participants within the period of study, the availability of staff, and ATN work load. Study procedures were approved by Institutional Review Boards at all participating organizations. YLWH who were referred to a mental health professional for evaluation for depression, or who were in treatment but still symptomatic, were approached for participation in the study. Informed consent was obtained per local institutional guidelines. A project-trained mental health clinician confirmed that participants had QIDS-SR scores ≥ 7, and no exclusionary condition.
Combined CBT and MMA (COMB) [24-week intervention]
The Medication Management Algorithm (MMA) was developed by incorporating current practice parameters for depression [15,23,24] and HIV treatment in adults [23,24], recent data from adult and adolescent depression trials [25,26], and previously used algorithmic designs [27,28]. Modifications included rescue treatments for the onset of new disorders and symptoms specific to chronic illness or HIV (e.g., sleep disturbance, weight loss). The MMA includes strategies and considerations in treating depression for YLWH such as drug-drug interactions and side effects. The algorithm specifies the order and doses of antidepressant medications at each stage taking into consideration prior psychotropic medication history and measurement of current symptoms (see Table 1). After four to six weeks, doses could be increased as shown, or antidepressants augmented with lithium, bupropion, or an atypical antipsychotic. [A general overview of the treatment stages, doses for augmentation, and common medications to treat associated symptoms are found in the Figure and Tables in the Supplemental Digital Content.] The MMA was implemented by site prescribing clinicians who received a one-day training and participated in twice monthly monitoring and supervision calls.
Table 1.
| a. Medication Algorithm | |
|---|---|
| Stage | Treatment |
| Stage 0 | No medication |
| Stage 1 | Monotherapy with SSRI |
| Stage 2 | Monotherapy with 2nd SSRI |
| Stage 3 | Monotherapy with non-SSRI |
| Stage 4 | Combination Treatment |
| b. Antidepressant Doses for Depression | ||||
|---|---|---|---|---|
| Antidepressant | Initial dose (mg/day) |
Target dose to achieve by week 4 (mg/day) |
Dose titration for partial or non- responders (mg/day) |
Usual dose schedule |
| SSRIs | ||||
| Fluoxetine | 10–20 | 20 | 30–60 | q AM |
| Sertraline | 25–50 | 100 | 150–200 | q AM |
| Citalopram | 10–20 | 20 | 30–60 | q AM |
| Escitalopram | 5–10 | 10 | 20–30 | q AM |
| Paroxetine | 10–20 | 20 | 30–50 | q AM |
| Non-SSRIs | ||||
| Bupropion, Bupropion SR | 75–100 | 75–300 | 450 | bid-tid (≤ 150 mg/dose) |
| Bupropion XL | 150 | 300–450 | 450 | q AM |
| Mirtazapine | 15–30 | 30 | 45 | q hs |
| Venlafaxine-XR | 37.5–75 | 75–150 | 225 | q d |
| Duloxetine | 20 | 40–60 | 60 | 20 mg bid 60 mg q d |
Health and Wellness Cognitive Behavioral Therapy (herein referred to as CBT) decreases negative mood and unhealthy cognitions, while simultaneously enhancing strengths, positive experiences, and healthy cognitions [29]. CBT was adapted for YLWH to address factors such as medical symptoms, poverty, stigma, and alienation from families. It also integrated motivational interviewing (MI) skills in order to engage patients in treatment [30,31]. The development and feasibility of the manual for YLWH was reported previously [32]. The study CBT is comprised of three stages of treatment. Stage 1: Psychoeducation and Motivation for Treatment addresses the numerous psychosocial stresses of HIV infection, as well as treatment of current depressive symptoms. Participants may be uninterested in treatment for depression because of the seriousness of their medical condition or may be avoidant of all medical care. The therapy is non-confrontational and includes MI. Stage 2: Reducing Depressive Symptoms has six CBT sessions that teach core skills of mood monitoring, behavioral activation, reducing negative thinking, and problem-solving. Symptom reports guide the use of additional modules. Stage 3: Achieving and Maintaining Wellness identifies areas of strength and techniques to promote continued wellness because YLWH can become entrenched in the “patient” mode. All site therapists possessed either a clinical Master’s or Doctoral degree. COMB therapists received a two-day CBT training (e.g. didactic instruction, role plays, and vignettes), 4 hours of training and 3 “booster” training from MI network trainer, and participated in weekly supervision calls. A random sample of 40% of CBT sessions were rated by the CBT supervisor and 95% were of acceptable quality using the Cognitive Therapy Rating Scale [33].
MEASURES
Assessments
All participants provided self-report assessments via an audio computer-assisted self-interview [at baseline and weeks 6, 12, 24 (end of treatment), 36, and 48 (end of follow-up)]. The baseline assessment included demographic information. Health data were abstracted from medical records. Also, a mental health clinician trained in the Quick Inventory of Depressive Symptomology-Clinician Rating (QIDS-C) [34] assessed baseline and end of treatment (week 24) symptoms at all sites. Also, at the COMB sites, the QIDS-C was used to guide treatment decisions 6 and 12 week medication visits.
Primary outcome measures
Participants completed the Quick Inventory of Depressive Symptomatology-Self-Report (QIDS-SR), a 16-item scale assessing 9 depressive symptoms. QIDS-SR is a reliable and valid measure of depression in adults and adolescents [34,35]. Total scores range from 0–42, whereby scores of 6–10 reflect mild symptoms, 11–15 moderate, and ≥16 severe. Score range and symptom severity categories are the same for the QIDS-C. QIDS-SR scores over time were used in two binary outcomes. Response to treatment was defined as a ≥50% decrease from baseline, and remission from depression was defined as a QIDS-SR score <5.
Secondary outcome measures
Adherence to antiretroviral medications was assessed using items from the Adherence Module developed by the ATN Adherence Workgroup [31]. Hopelessness was measured with the Beck Hopelessness Scale (BHS) [36]. This scale has been shown to predict dropout from psychosocial treatment and poorer treatment response [37] and has been used with adolescent psychiatric outpatients [38]. Life satisfaction was measured with the Multidimensional Student's Life Satisfaction Scale (MSLSS) [39], which examines five domains (Friends, Family, School, Self, and Living Environment) and general (total) life satisfaction. It has acceptable psychometric properties [40]. The Child and Adolescent Services Assessment (CASA) [41], a semi-structured face-to-face interview, obtained information about service use for mental health problems. Adequate reliability has been demonstrated (ICCs=.74 –.76). Each participant’s biomarker values (CD4 and VL) within the past three months were abstracted from the medical chart.
DATA ANALYSIS
Chi-square or Fisher’s exact tests were used to examine the visit-specific differences in proportions between study groups (COMB vs. TAU) for categorical outcomes. The two-sample t-test or non-parametric test was used to assess the visit-specific mean differences between study groups.
The univariate and multivariable mixed effects models with repeated measurements for continuous outcomes (QIDS-SR, QIDS-C, BHS, MSLSS, log10 VL, and CD4 absolute counts) and the generalized linear models with generalized equation estimation (GEE) approach for categorical outcomes (remission, response to treatment, and antidepressant medication) were used to examine the intervention effect over time while adjusting for site as a cluster effect. Additional analytic covariates considered included study visit, interaction of intervention and visit, study site, value of outcome measure at baseline, birth gender, and mode of HIV transmission. The relationship of HIV health status outcomes (CD4 counts and log10 VL) and depression status (QIDS-SR, response and remission status) also were examined. Covariates with p-value < 0.2 were entered in the initial multivariable full model for model selection. Backward elimination and stepwise model selection were used to choose the best final model.
The SAS MIXED procedure was used to adjust for site effects; this method also accommodates missing data [42] by using the likelihood-based approach. Generalized linear models can estimate the working correlations from the data containing missing values by using the “all available pairs” method, in which all non-missing pairs of data are used in estimating the working correlation parameters defined previously. These procedures have the capability of using all available data even for subjects who have missing observations.
Results
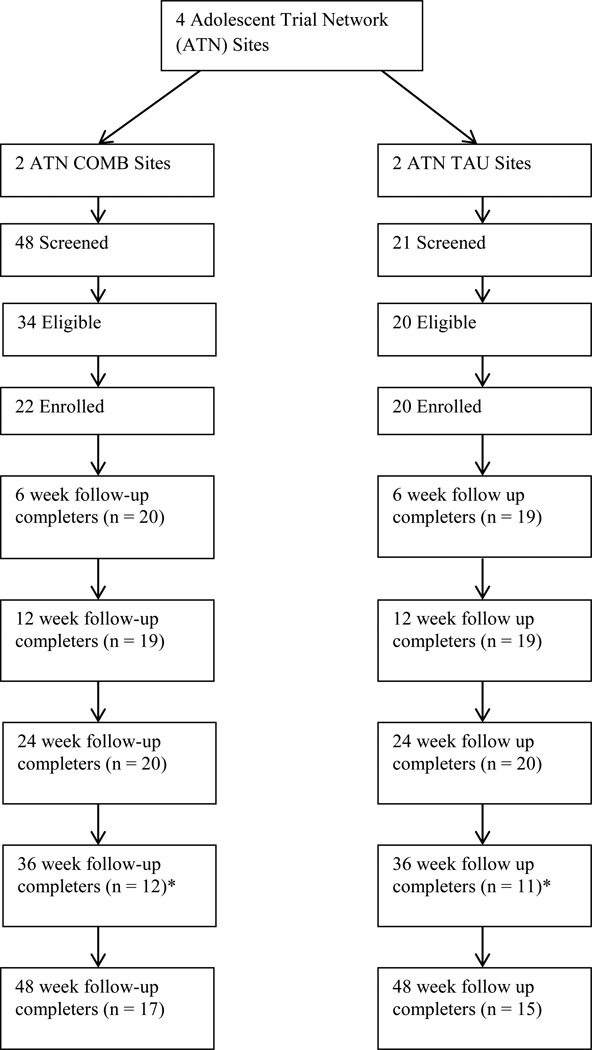
The 44 participants were mostly male (69%); Black/African-American (83%), and non-Hispanic (79%), with a mean age of 21.5 years. Table 2 shows significant differences between the COMB and TAU groups at baseline based on gender and transmission category. The COMB group was comprised of a significantly greater proportion of males (96% vs. 40%, p<0.001) and those who had acquired HIV via behavioral contact (95% vs. 85%, p<0.001). There were no differences at baseline between groups based on age, Hispanic ethnicity, CD4, VL, or drug and alcohol use. The CONSORT is shown in Figure 1 with 42 participants allocated to COMB (n=22) or TAU (n=20) based on random assignment of the four sites and 95% of the sample was assessed at week 24 (end of treatment) and 76% at week 48. Over the study period, five subjects prematurely discontinued (COMB, n=3; and TAU, n=2) because of a move out of state (2), loss to follow-up (2) or incarceration (1). Baseline QIDS-SR scores were compared between subjects with and without missing scores at follow-up, and there were no significant differences except Week 48 where missing subjects reported more depressive symptoms at baseline (18.5 (4.4) vs. 14.4 (4.0), t=2.74, p=0.009). Proportions of missing QIDS-SR scores by treatment condition at each study visit were not significantly different.
Table 2.
Demographic and Baseline Characteristics by Study Group
| Total N=42 n (%) |
COMB* N=22 n (%) |
TAU** N=20 n (%) |
p-value | |
|---|---|---|---|---|
| Age (years) | ||||
| Mean (SD) [range 18 – 24] | 21.5 (1.6) | 21.5 (1.6) | 21.5 (1.6) | 0.98 |
| Gender | ||||
| Male | 29 (69.0) | 21 (95.5) | 8 (40.0) | < 0.001 |
| Ethnicity | ||||
| Hispanic or Latino | 9 (21.4) | 3 (13.6) | 6 (30.0) | 0.27 |
| Race | ||||
| Black or African American | 35 (83.3) | 19 (86.4) | 16 (80.0) | 0.64 |
| Mode of HIV transmission | ||||
| Perinatal transmission | 4 (9.5) | 1 (4.5) | 3 (15.0) | < 0.001 |
| High-risk heterosexual contact | 5 (11.9) | 1 (4.5) | 4 (20.0) | |
| Male-to-male sexual contact | 28 (66.7) | 20 (90.9) | 8 (40.0) | |
| Presumed heterosexual contact | 5 (11.9) | 0 (0.0) | 5 (25.0) | |
| Baseline CD4 count - categorical | ||||
| < 200 | 6 (14.3) | 4 (18.2) | 2 (10.0) | 0.93 |
| 200 – 350 | 6 (14.3) | 3 (13.6) | 3 (15.0) | |
| 350 – 500 | 10 (23.8) | 5 (22.7) | 5 (25.0) | |
| ≥ 500 | 20 (47.6) | 10 (45.5) | 10 (50.0) | |
| Log 10 Viral Load | ||||
| Mean (SD) | 2.9 (1.4) | 2.7 (1.3) | 3.1 (1.5) | 0.33 |
| Baseline Viral load - categorical | ||||
| < 400 | 21 (50.0) | 12 (54.5) | 9 (45.0) | 0.93 |
| 400 – 9,999 | 1 (2.4) | 1 (4.5) | 0 (0.0) | |
| 1,000 – 9,999 | 9 (21.4) | 4 (18.2) | 5 (25.0) | |
| 10,000 – 99,999 | 8 (19.0) | 4 (18.2) | 4 (20.0) | |
| ≥ 100,000 | 3 (7.1) | 1 (4.5) | 2 (10.0) | |
| Did you drink alcohol (beer, wine or wine coolers, etc.) in the past 3 months? | ||||
| Yes | 31 (86.1) | 16 (84.2) | 15 (88.2) | 1.00 |
| Have you smoked marijuana, other than just trying a few puffs, in the past 3 months? | ||||
| Yes | 20 (55.6) | 11 (57.9) | 9 (52.9) | 1.00 |
| Have you used any other king of drug in the past 3 months? | ||||
| Yes | 3 (8.3) | 2 (10.5) | 1 (5.9) | 1.00 |
COMB = combined manualized CBT and medication management algorithm
TAU = treatment as usual
Figure 1.
Participant consent, randomization, and retention (CONSORT Flowchart)
*Delay in site approval for 36 week follow-up reduced eligible participant
DEPRESSION SEVERITY
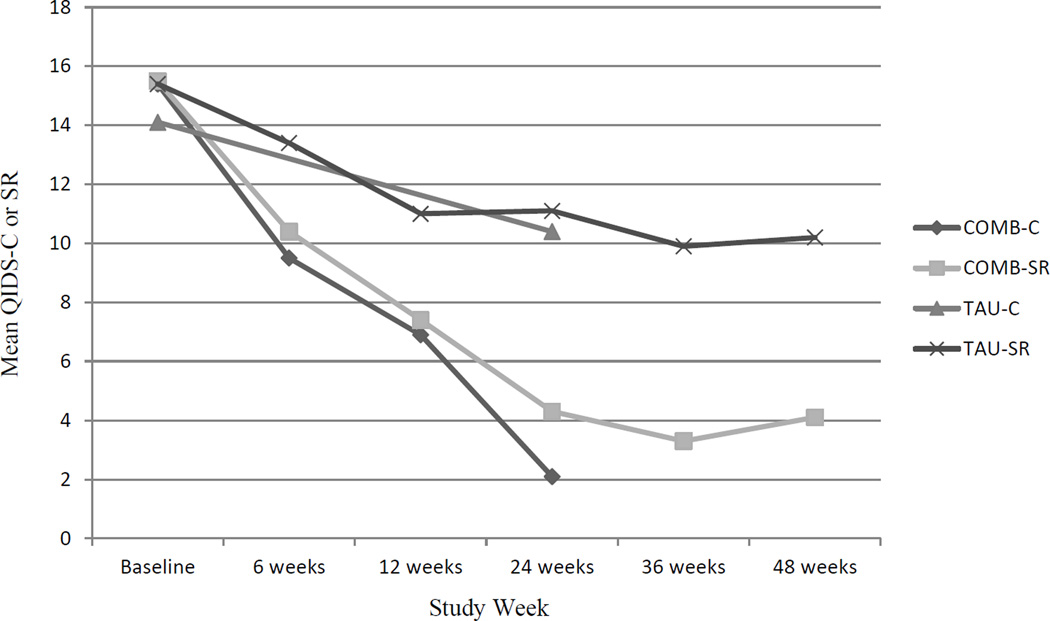
The unadjusted mean QIDS-SR and QIDS-C for both groups over time are shown in Figure 2 and Table 3 (significance tests over time adjusted for site and for covariates with p<0.2). At baseline, the groups did not differ and were moderately to severely depressed as reflected by QIDS-SR mean scores of 15. By the end of treatment (week 24), COMB participants, compared to TAU, reported significantly fewer depressive symptoms (4.3 vs. 11.1, p<0.001) and this effect was maintained at week 48 (4.1 vs. 10.2, p<0.001). Clinician symptom ratings (QIDS-C) reflected a similar pattern of a greater reduction in depressive symptoms for COMB at week 24. The relationship between QIDS-SR scores and condition (COMB or TAU) for each assessment time-point is shown in Table 3 and demonstrates that the COMB group reported significantly fewer symptoms at all time-points after baseline.
Figure 2.
Quick Inventory of Depression Symptomatology (QIDS) over Time* and by Condition
*Note: QIDS-C not administered at 36 and 48 weeks
Table 3.
Depression Status and Treatment Response by Study Group and Visit
| Baseline | Week 6 | Week 12 | Week 24 | Week 36 | Week 48 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COMB | TAU | COMB | TAU | COMB | TAU | COMB | TAU | COMB | TAU | COMB | TAU | p-value1 | |
| Response to treatment1 | |||||||||||||
| n | 22 | 20 | 20 | 19 | 19 | 19 | 20 | 20 | 12 | 11 | 17 | 15 | |
| Number Yes | -- | -- | 5 | 0 | 10 | 4a | 17 | 4** | 11 | 4** | 15 | 5** | < 0.001 |
| Proportion | -- | -- | 25.0 | 0.0 | 52.6 | 21.1 | 85.0 | 20.0 | 91.7 | 36.4 | 88.2 | 33.3 | |
| Remission from depression2 | |||||||||||||
| n | 22 | 20 | 20 | 19 | 19 | 19 | 20 | 20 | 12 | 11 | 17 | 15 | |
| Number Yes | -- | -- | 4 | 0 | 7 | 2 | 13 | 2 | 9 | 1 | 12 | 1 | < 0.001 |
| Proportion | -- | -- | 20.0 | 0.0 | 36.8 | 10.5 | 65.0 | 10.0 | 75.0 | 9.1 | 70.6 | 6.7 | |
| Antidepressant medication use | |||||||||||||
| n | 22 | 20 | 20 | 19 | 19 | 19 | 20 | 20 | 12 | 11 | 17 | 15 | |
| Number Yes | 5 | 10 | 7 | 8 | 9 | 9 | 7 | 9 | 5 | 4 | 3 | 6 | |
| Proportion | 22.7 | 50 | 35 | 42.1 | 47.4 | 47.4 | 35 | 45 | 41.7 | 36.4 | 17.6 | 40 | 0.368 |
| Continuous Scale Outcomes | |||||||||||||
| Quick Inventory of Depressive Symptoms-Self Report (QIDS-SR)3 | |||||||||||||
| n | 22 | 20 | 20 | 19 | 19 | 19 | 20 | 20 | 12 | 11 | 17 | 15 | |
| mean | 15.5 | 15.4 | 10.4 | 13.4a | 7.4 | 11.0a | 4.3 | 11.1** | 3.3 | 9.9* | 4.1 | 10.2** | 0.001 |
| standard deviation | 4.9 | 4.0 | 5.0 | 4.2 | 4.4 | 5.6 | 2.9 | 5.6 | 1.9 | 4.6 | 3.5 | 5.5 | |
| Quick Inventory of Depressive Symptoms-Clinician (QIDS-C) 4 | |||||||||||||
| n | 22 | 20 | 20 | 19 | 19 | 19 | 20 | 20 | 12 | 11 | 17 | 15 | |
| mean | 15.4 | 14.1 | 9.5 | -- | 6.9 | -- | 2.1 | 10.4** | -- | -- | -- | -- | ≤ 0.001 |
| standard deviation | 4.4 | 4.1 | 3.4 | -- | 4.3 | -- | 2.6 | 5.5 | -- | -- | -- | -- | |
| Beck Hopelessness Scale (BHS) 5 | |||||||||||||
| n | 22 | 20 | 20 | 19 | 19 | 19 | 20 | 20 | 12 | 11 | 17 | 15 | |
| mean | 10.3 | 8.3 | 7.0 | 7.9* | 5.9 | 7.5* | 4.8 | 6.1* | 4.2 | 6.2a | 3.6 | 6.0* | 0.351 |
| standard deviation | 4.1 | 3.8 | 4.2 | 5.3 | 4.5 | 5.7 | 3.6 | 3.3 | 2.4 | 4.0 | 1.8 | 3.7 | |
| Multidimensional Student’s Life Satisfaction Scale (MSLSS)6 | |||||||||||||
| n | 22 | 20 | 20 | 19 | 19 | 19 | 20 | 20 | 12 | 11 | 17 | 15 | |
| mean | 4.0 | 4.1 | 4.2 | 4.1 | 4.3 | 4.3 | 4.7 | 4.5 | 4.7 | 4.4 | 4.8 | 4.4 | 0.547 |
| standard deviation | 0.6 | 0.7 | 0.8 | 0.9 | 0.7 | 0.9 | 0.7 | 0.7 | 0.5 | 1.0 | 0.6 | 0.7 | |
| CD4 count | |||||||||||||
| n | 22 | 20 | 7 | 6 | 12 | 13 | 16 | 17 | 10 | 11 | 16 | 13 | 0.736 |
| mean | 511.4 | 507.2 | 548.4 | 438.8 | 475.6 | 487.6 | 513.3 | 577.2 | 383.9 | 536.7 | 575.6 | 509.6 | |
| standard deviation | 337.0 | 297.5 | 304.0 | 142.3 | 314.3 | 354.0 | 317.4 | 409.6 | 240.6 | 352.1 | 331.1 | 316.2 | |
| Log 10 Viral Load | 0.104 | ||||||||||||
| n | 22 | 20 | 7 | 6 | 12 | 14 | 16 | 17 | 10 | 11 | 16 | 13 | |
| mean | 2.6 | 3.0 | 2.3 | 2.6 | 2.1 | 2.6 | 2.2 | 2.9 | 1.3 | 2.5 | 1.7 | 2.7 | |
| standard deviation | 1.4 | 1.6 | 1.4 | 1.0 | 1.3 | 1.6 | 1.2 | 1.7 | 0.2 | 1.4 | .0.8 | 1.3 | |
p-value is of the test of intervention effect of each variable of interest over time.
Quick Inventory of Depressive Symptoms – Self Report ↓ ≥ 50% from baseline.
Quick Inventory of Depressive Symptoms-Self Report score < 5.
QIDS-SR: Higher score indicates a greater depression, range 0–27.
QIDS-C: Higher score indicates a greater depression, range 0–27.
BHS: Higher score reflects a higher level of hopelessness, range 0–20.
MSLSS: average of items, range 1–6.
p <.05 for difference between TAU and COMB in model test adjusting for baseline value and significant covariates retained in final model.
p <.01 for difference between TAU and COMB in model test adjusting for baseline value and significant covariates retained in final model.
p <.001 for difference between TAU and COMB in model test adjusting for baseline value and significant covariates retained in final model.
RESPONSE RATES (Table 3)
Those in COMB were significantly more likely to respond (QIDS-SR decrease ≥50% from baseline) at week 24 (end of treatment), compared to those in TAU (85% vs. 20%, p<0.001) and the pattern was maintained at week 48 (88% vs. 33%, p<0.001).
REMISSION RATES (Table 3)
Those in COMB, compared to those in TAU were significantly more likely to be in remission (QIDS-SR <5) over the course of the study, p<0.001. Week-specific rate differences were not calculated due to the few TAU cases in remission but the pattern was evident from week 24, (65% vs. 10%) to week 48 (71% vs. 7%).
SECONDARY OUTCOMES (Table 3)
The COMB group, relative to TAU reported significantly less hopelessness (BHS) at all visits after baseline. There was no change in life satisfaction reports over the course of the study. Similarly, CD4 counts and ARV adherence (approximately 80% at all visits (data not shown)) did not differ between groups during the study. HIV log10 VL decreased for the entire sample (main effect for time at weeks 36 and 38, p<0.01), but groups did not differ in the rate of change. A post hoc analysis of variance, accounting for baseline viral load, found an association between reduction in QIDS-SR scores and the final log10 viral loads (F=4.37, p<.05) among the entire sample.
TREATMENT CHARACTERISTICS
Participants in COMB were more likely to receive psychotherapy than in TAU (95% vs. 45%, X2=12.44, p<0.001) and attended more sessions over the 24 weeks treatment period (12.6 (SD=3.1) vs. 5.0 (SD=2.2), t=9.01, p<0.001). There was no difference in the rate of antidepressant use between the COMB and TAU groups over the course of the study (see Table 3) and the complex details of medication usage in each condition will be reported subsequently. No significant adverse effects attributable to treatment medications were reported in either group.
Discussion
Combined cognitive behavioral therapy and medication management algorithm (COMB) delivered in HIV medical care sites by existing staff out-performed mental health treatment as usual (TAU) and resulted in a large and sustained reduction in depressive symptoms in youth living with HIV. The clinical improvement was reported by the participants and the change was confirmed by structured assessments by mental health clinicians. In addition to COMB significantly reducing the number of reported depressive symptoms, 70% of youth in the combined treatment achieved a period of symptom free functioning at the end of one year, compared to less than ten percent of the group receiving standard care who also had access at their sites to mental health treatment and psychopharmacological management. Similar, clinically meaningful reductions in depressive symptoms have been associated with improvement in other measures of health and wellness over time [43,44]. This study demonstrates that combining manualized CBT with MI skills and medication algorithms guided by symptom measurement is a highly effective intervention for depression in YLWH. Existing staff can deliver these interventions successfully after training and with continued off-site supervision by an experienced interventionist.
Remission rates in this study are somewhat higher (71%) than reported in other clinical trials of combined CBT and medication management with non-medically ill youth, where remission rates were between 37–45% at week 24 [45,46,47,48]. Several factors could account for the excellent rates observed in this study. Collaboration between the mental health therapist and medication prescriber has been shown to be a particularly effective approach in treating depression among adolescents in medical treatment settings and the COMB treatment may have prompted increased collaboration [18]. Also, the CBT and MMA were tailored for the unique issues of those YLWH, perhaps increasing the appeal, engagement, and efficacy of the treatments over and above TAU.
COMB was more effective than TAU in engaging youth in psychotherapy (95% vs. 45%) and delivering more sessions (12 vs. 5). COMB asked youth to attend 14 CBT treatment sessions. This increased therapeutic contact could have resulted in a greater impact, irrespective of its content. MI was emphasized in COMB training and supervision to help engage and retain youth, particularly those who were withdrawn, reluctant, or skeptical of therapy and/or psychotropic medication. It is possible that YLWH who suffer from depression respond uniquely well to a time-specified, collaborative, non-confrontational treatment approach because of their experienced stigma, economic stress, somatic symptoms, and cognitive distortions. Although we do not know which aspects of the psychotherapy sessions account for its impact, the data suggests that COMB was able to address the complex psychosocial issues inherent for depressed YLWH, if they are retained in mental health care and COMB appears to have engaged and retained youth. It is important to note, many in COMB did not receive psychotropic medications and most showed improvement, despite being moderately to severely depressed at baseline. This finding suggests that the greater impact of COMB, relative to TAU, was not simply due a greater use of psychotropic medication. COMB also resulted in decreased hopelessness, consistent with the reduction in depressive symptoms, which was expected given the strong relationship between the two constructs and their responsiveness to CBT and medication.
Reduction in VL were observed over time, but was unrelated to treatment condition. Rather, reduced VL was significantly associated with reduced depression in the entire sample, confirming the general relationship between improvement in depression and better health. The lack of COMB impact on health indices could be due to the relatively small sample and associated lack of statistical power. Further, participants were receiving care in HIV-specialty clinics whereby many participants were taking antiretroviral medications at baseline with relatively good adherence reported at the outset; as a result, there may have been less room for improvement.
Despite the strengths of this study, there are a number of limitations. The sample was recruited from HIV clinical care sites and youth with substance abuse disorders were excluded, so results may not be generalizable to all YLWH. Also, the results may not be generalizable to sites outside of the U.S. or those without trained mental health professionals. Depressive symptoms were not measured by a “blind” rater but the reports of clinicians and participants were similar. Only four clinical sites were selected for randomization, therefore site-specific characteristics (participants and staff) may have contributed to the outcomes. COMB sites enrolled a smaller proportion of eligible participants, possibly introducing group differences. In fact, COMB and TAU groups differed by gender and transmission category, although these factors were not found to affect study outcomes. Previous research has found that gender does not predict acute response, although it may impact relapse [49,50]. Although youth with perinatal transmission may differ from those with other modes in their extent of medical care and neuropathology, which could impact psychiatric treatment, the small number of perinatal youth (four) precludes examination of this important issue. COMB sites implemented structured treatment with supervision to assure treatment fidelity but treatment at TAU sites was not monitored. Inherent differences in skill or impact of providers cannot be ruled out. Nonetheless, once enrolled, sites in both conditions did equally well in retaining youth for study assessments, so, by that measure, the relationship between the sites and participants appears comparable. Data on the mental utilization at sites was not obtained after the end of treatment, limiting our understanding of the reason for the sustained remission rate in COMB.
This preliminary trial found that combined cognitive behavioral therapy with MI skills and stepped-care medication management algorithm guided by symptom measures resulted in a significant reduction in depressive symptoms for YLWH. The combined intervention was delivered in the medical care setting by existing staff, suggesting that COMB, tailored for YLWH is feasible in other U.S. medical care sites. The improvement in depression was maintained for an additional 24 weeks beyond the 24 week intervention period, suggesting that COMB could have a lasting impact. It is left to future research to confirm these findings in other sites, examine the moderating impact of factors such as gender, age, or route of transmission, and any sustained changes in treatment delivery by sites. The extension of efficacious interventions for depression to centers outside of the U.S., where the burden of HIV is greater, is also imperative.
Supplementary Material
Acknowledgements
Supported by the Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) from the National Institutes of Health (U01 HD 040533 and U01 HD 040474) through the Eunice Kennedy Shriver National Institute of Child Health and Human Development (B. Kapogiannis, C. Worrell) with supplemental funding from the National Institutes on Drug Abuse (S. Kahana, K. Davenny) and Mental Health (S. Allison). The following ATN sites participated in the study from which the manuscript evolved: University of South Florida, Tampa (Emmanuel, Lujan-Zilbermann, Julian), Children’s Hospital of Philadelphia (Douglas, Tanney, DiBenedetto), Montefiore Medical Center (Futterman, Enriquez-Bruce, Campos, Fernandez), Children’s Diagnostic & Treatment Center (Puga, Leonard, Inman), St. Jude Children’s Research Hospital (Flynn, Dillard, Wilkins). The study was scientifically reviewed by the ATN’s Behavioral Leadership Group. Thanks to the ATN Coordinating Center for Network for scientific and logistical support (C. Wilson and C. Partlow); the ATN Data and Operations Center at Westat (J. Korelitz and B. Driver); the ATN Community Advisory Board and the youth who participated in the study. Support also provided by the Lifespan Tufts Brown Center for AIDS Research, a NIH-funded program (P30 AI 042853, PI: C. Carpenter).
Source of Funding:
Geetha A. Subramaniam is an employee of the National Institute on Drug Abuse (NIDA) at the National Institutes of Health, which co-funded this research study. She served as scientific collaborator on the U award but had no role with respect to the P30 grant. The content of this manuscript does not in any way reflect the official position of NIDA.
Footnotes
Conflicts of Interest:
For the remaining authors, none were declared.
Data presented at Adolescent AIDS Trials Network for HIV/AIDS Interventions Meeting on October 6, 2014 in Bethesda, MD
REFERENCES
- 1.Gaughan DM, Hughes MD, Oleske JM, Malee K, Gore CA, Nachman S, et al. Psychiatric hospitalizations among children and youths with human immunodeficiency virus infection. Pediatrics. 2004;113:e544–e551. doi: 10.1542/peds.113.6.e544. [DOI] [PubMed] [Google Scholar]
- 2.Brown LK, Whiteley L, Harpe GW, Nichols S, Nieves A. The ATN 086 Protocol Team for The Adolescent Medicine Trials Network for HIV/AIDS Interventions. Psychological symptoms among 2032 youth living with HIV: A multisite study. AIDS Patient Care STDs. doi: 10.1089/apc.2014.0113. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez J, Hosek SG, Carleton RA. Screening and Assessing Violence and Mental Health Disorders in a Cohort of Inner City HIV-Positive Youth between 1998–2006. AIDS Patient Care STDs. 2009;23(6):469–475. doi: 10.1089/apc.2008.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pao M, Lyon M, D'Angelo LJ, Schuman WB, Tipnis T, Mrazek DA. Psychiatric diagnoses in adolescents seropositive for the human immunodeficiency virus. Arch Pediatr Adolesc Med. 2000;154:240–244. doi: 10.1001/archpedi.154.3.240. [DOI] [PubMed] [Google Scholar]
- 5.Reisner SL, Mimiaga MJ, Skeer M, Perkovich B, Johnson CV, Safren SA. A review of HIV antiretroviral adherence and intervention studies among HIV-infected youth. Top HIV Med. 2009;17(1):14–25. [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy DA, Belzer M, Durako SJ, Sarr M, Wilson CM, Muenz LR. Adolescent Medicine HIV/AIDS Research Network. Longitudinal antiretroviral adherence among adolescents infected with human immunodeficiency virus. Arch Pediatr Adolesc Med. 2005;159:764–770. doi: 10.1001/archpedi.159.8.764. [DOI] [PubMed] [Google Scholar]
- 7.Caballero J, Nahata MC. Use of selective serotonin-reuptake inhibitors in the treatment of depression in adults with HIV. Ann Pharmacother. 2005;39:141–145. doi: 10.1345/aph.1E248. [DOI] [PubMed] [Google Scholar]
- 8.Elliott AJ, Russo J, Roy-Byrne PP. The effect of changes in depression on health related quality of life (HRQoL) in HIV infection. Gen Hosp Psychiatry. 2002;24(1):43–47. doi: 10.1016/s0163-8343(01)00174-8. [DOI] [PubMed] [Google Scholar]
- 9.Horberg MA, Silverberg SJ, Hurley LB, Towner WJ, Klein DB, Bersoff-Matcha S, et al. Effects of depression and selective serotonin reuptake inhibitor use on adherence to antiretroviral therapy and on clinical outcomes in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;47:384–390. doi: 10.1097/QAI.0b013e318160d53e. [DOI] [PubMed] [Google Scholar]
- 10.Parsons JT, Golub SA, Rosof E, Holder C. Motivational interviewing and cognitive-behavioral intervention to improve HIV medication adherence among hazardous drinkers: a randomized controlled trial. J Acquir Immune Defic Syndr. 2007;46:443–450. doi: 10.1097/qai.0b013e318158a461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosello J, Bernal G. The efficacy of cognitive-behavioral and interpersonal treatments for depression in Puerto Rican adolescents. J Consult Clin Psychol. 1999;67:734–745. doi: 10.1037//0022-006x.67.5.734. [DOI] [PubMed] [Google Scholar]
- 12.Sweeney M, Robins M, Ruberu M, Jones J. African-American and Latino families in TADS: Recruitment and treatment considerations. Cogn Behav Pract. 2005;12:221–229. [Google Scholar]
- 13.Castro-Couch M. Review of cognitive-behavioral therapies with lesbian, gay, bisexual clients. Arch Sex Behav. 2007;36:626–627. [Google Scholar]
- 14.Safren SA, Rogers T. Cognitive-behavioral therapy with gay, lesbian, and bisexual clients. J Clin Psychol. 2007;57:629–643. doi: 10.1002/jclp.1033. [DOI] [PubMed] [Google Scholar]
- 15.Birmaher B, Brent D, AACAP Work Group on Quality Issues. Bernet W, Bukstein O, Walter H, et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1503–1526. doi: 10.1097/chi.0b013e318145ae1c. [DOI] [PubMed] [Google Scholar]
- 16.Cheung AH, Zuckerbrot RA, Jensen PS, Ghalib K, Laraque D, Stein RE. Guidelines for Adolescent Depression in Primary Care (GLAD-PC): II. Treatment and Ongoing Management. Pediatrics. 2007;120(5):E1313–E1326. doi: 10.1542/peds.2006-1395. [DOI] [PubMed] [Google Scholar]
- 17.The Treatment for Adolescents with Depression Study (TADS) Long-term Effectiveness and Safety Outcomes. Arch Gen Psychiatry. 2007;64(10):1132–1143. doi: 10.1001/archpsyc.64.10.1132. [DOI] [PubMed] [Google Scholar]
- 18.Richardson L, Ludman E, McCauley E, Lindenbaum J, Larison C, Zhou C, et al. Collaborative Care for Adolescents in Primary Care. J Am Med Assoc. 2014;312:809–816. doi: 10.1001/jama.2014.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown LK, Whiteley L, Harper GW, Nichols S, Nieves A. Adolescent Medicine Trials Network for HIV/AIDS Interventions. Psychological Symptoms among 2032 youth living with HIV: A multisite study. AIDS Patient Care STDs. 2015;29(4):212–219. doi: 10.1089/apc.2014.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudy BR, Murphy DA, Harris R, Muenz L, Ellen J. Adolescent Medicine Trials Network for HIV/AIDS Interventions. Patient-related risks for nonadherence to antiretroviral therapy among HIV-infected youth in the United States: A study of prevalence and interactions. AIDS Patient Care STDs. 2009;23(3):185–193. doi: 10.1089/apc.2008.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henkel V, Bussfeld P, Mӧller H, Hegerl U. Cognitive-behavioural theories of helplessness/hopelessness: Valid models of depression? Eur Arch Psychiatry Clin Neurosci. 2002;252(5):240–249. doi: 10.1007/s00406-002-0389-y. [DOI] [PubMed] [Google Scholar]
- 22.Rissanen T, Viinamäki H, Honkalampi K, Lehto SM, Hintikka J, Saharinen T, et al. Long term life dissatisfaction and subsequent major depressive disorder and poor mental health. BMC Psychiatry. 2011;11(1):140. doi: 10.1186/1471-244X-11-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Psychiatric Association (APA) Practice Guideline for the Treatment of Patients with Major Depressive Disorder Third Edition. 2010 [Google Scholar]
- 24.American Psychiatric Association (APA) Practice Guideline for the Treatment of Patients with HIV/AIDS. 2000 [Google Scholar]
- 25.Bridge JA, Iyengar S, Salary CB, Barbe RP, Birmaher B, Pincus H, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. J Am Med Assoc. 2007;297:1683–1696. doi: 10.1001/jama.297.15.1683. [DOI] [PubMed] [Google Scholar]
- 26.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 27.Emslie GJ, Mayes T, Porta G, Vitiello B, Clarke G, Wagner KD, et al. Treatment of Resistant Depression in Adolescents (TORDIA): Week 24 outcomes. Am J Psychiatry. 2010;167(7):782–791. doi: 10.1176/appi.ajp.2010.09040552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes C, Emslie GJ, Crismon M, Posner K, Birmaher B, Ryan N, et al. Texas children’s medication algorithm project: update for Texas consensus conference panel on medication treatment of childhood major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:667–686. doi: 10.1097/chi.0b013e31804a859b. [DOI] [PubMed] [Google Scholar]
- 29.Kennard B, Hayley C, Hughes J, Jones J, Hines T. CBT Treatment Manual for ATN 080. 2010 [Google Scholar]
- 30.Naar-King S, Suarez M. Motivational interviewing with adolescents and young adults. New York: Guilford Press; 2011. [Google Scholar]
- 31.Naar-King S, Parsons JT, Murphy DA, Chen XG, Harris DR, Belzer ME. Improving health outcomes for youth living with the human immunodeficiency virus a multisite randomized trial of motivational intervention targeting multiple risk behaviors. Arch Pediatr Adolesc Med. 2009;163(12):1092–1098. doi: 10.1001/archpediatrics.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kennard BD, Brown LK, Hawkins L, Risi A, Radcliffe J, Emslie G, et al. Development and implementation of health and wellness CBT for individuals with depression and HIV. Cogn Behav Pract. 2014;21(2):237–246. doi: 10.1016/j.cbpra.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young J, Beck A. Cognitive therapy scale: rating manual. 1980 Unpublished manuscript. [Google Scholar]
- 34.Bernstein IH, Rush AJ, Trivedi MH, Hughes CW, Macleod L, Witte BP, et al. Psychometric Properties of the Quick Inventory of Depressive Symptomatology in Adolescents. Int J of Methods Psychiatr Res. 2010;19(4):185–194. doi: 10.1002/mpr.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore HK, Hughes CW, Mundt JC, Rush AJ, Macleod L, Emslie GJ, et al. A pilot study of an electronic, adolescent version of the quick inventory of depressive symptomatology. J Clin Psychiatry. 2007;68:1436–1440. doi: 10.4088/jcp.v68n0917. [DOI] [PubMed] [Google Scholar]
- 36.Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: The Hopelessness Scale. J Consult Clin Psychol. 1974;4:861–865. doi: 10.1037/h0037562. [DOI] [PubMed] [Google Scholar]
- 37.Brent DA, Kolko D, Birmaher B, Baugher M, Bridge J, Roth C. A clinical trial for adolescent depression: Predictors of additional treatment in the acute and follow-up phases of the trial. J Am Acad Child Adolesc Psychiatry. 1999;38:263–270. doi: 10.1097/00004583-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Brent DA, Holder D, Kolko D, Birmaher B, Baugher M, Roth C, et al. A clinical psychotherapy trial for adolescent depression comparing cognitive, family, and supportive therapy. Arch Gen Psychiatry. 1997;54:877–885. doi: 10.1001/archpsyc.1997.01830210125017. [DOI] [PubMed] [Google Scholar]
- 39.Huebner ES. Preliminary development and validation of a multidimensional life satisfaction scale for children. Psychol Assess. 1994;6:149–158. [Google Scholar]
- 40.Gilman R, Huebner EW, Laughlin JE. A first study of the Multidimensional Student Life Satisfaction Scale with adolescents. Soc Indic Res. 2000;52:135–160. [Google Scholar]
- 41.Burns B, Angold A, Magruder-Habib K, Costello E, Patrick M. Child and Adolescent Services Assessment (version 4.2) Durham, NC: Duke University; 1997. [Google Scholar]
- 42.Rubin DB. Inference and missing data. Biometrika. 1976;63:581–592. [Google Scholar]
- 43.Katon W, Lin EHB, Von Korf M, Ciechanowski P, Ludman E, Young B, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363:2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Neil A, Sanderson K, Oldenburg B, Taylor B. Impact of depression treatment on mental and physical health-related quality of life of cardiac patients. J Cardiopulm Rehabil Prev. 2011;31:146–156. doi: 10.1097/HCR.0b013e3181fc0985. [DOI] [PubMed] [Google Scholar]
- 45.The Treatment for Adolescents with Depression Study (TADS) long-term effectiveness and safety outcomes. Arch Gen Psychiatry. 2007;64:1132–1144. doi: 10.1001/archpsyc.64.10.1132. [DOI] [PubMed] [Google Scholar]
- 46.Goodyear I, Dubicka B, Wilkinson P, Kelvin R, Roberts C, Byford S, et al. Selective serotonin reuptake inhibitors (SSRIs) and routine specialist care with and without cognitive behaviour therapy in adolescents with major depression: randomized controlled trial. Br Med J. 2007;335:142. doi: 10.1136/bmj.39224.494340.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brent D, Emslie G, Clarke G, Wagner K, Asarnow J, Keller M, et al. The treatment of adolescents with SSRI-resistant depression (TORDIA): a comparison of switch to venlafaxine or to another SSRI, with or without additional cognitive behavioral therapy. J Am Med Assoc. 2008;299:901–913. doi: 10.1001/jama.299.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warden D, Rush AJ, Trivedi MH, Fava M, Wisniewski SR. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9(6):449–459. doi: 10.1007/s11920-007-0061-3. [DOI] [PubMed] [Google Scholar]
- 49.Curry J, Silva S, Rohde P, Ginsburg G, Kratochvil C, Simons A, et al. Recovery and recurrence following treatment for adolescent major depression. Arch Gen Psychiatry. 2011;68(3):263–269. doi: 10.1001/archgenpsychiatry.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Emslie GJ, Kennard BD, Mayes TL, Nightingale-Teresi J, Carmody T, Hughes CW, et al. Fluoxetine vs. Placebo to Prevent Relapse of MDD in Children and Adolescents. Am J Psychiatry. 2008;165(4):459–467. doi: 10.1176/appi.ajp.2007.07091453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.