Abstract
Background
Clinical trials of implantable cardioverter defibrillators (ICDs) for primary prevention enrolled a limited number of women. We sought to examine clinical practice data to compare survival rates among women with heart failure (HF) with or without a primary prevention ICD.
Methods and Results
We linked data from 264 US hospitals included in the Get With The Guidelines for Heart Failure (GWTG-HF) registry with data from the Centers for Medicare and Medicaid Services (CMS). From these sources, we propensity score matched 430 women with HF who received a primary prevention ICD to 430 women who did not; we further adjusted using a Cox proportional hazards model. Median follow up was 3.4 and 3.0 years, respectively. For comparison, we matched 859 men receiving an ICD with 859 not; median follow-up was 3.9 vs 2.9 years. In the matched cohorts, an ICD was associated with similarly better survival in women (HR 0.78 95% CI 0.66-0.92 p=0.003) and men (HR 0.76 95% CI 0.67-0.87 p<0.001). There was no interaction between sex and presence of an ICD with respect to survival (p = 0.79).
Conclusions
Among patients with heart failure with reduced LVEF, a primary prevention ICD was associated with a significant survival advantage among women as well as among men. These findings support guideline-directed use of primary prevention ICDs in eligible patients.
Keywords: comparative effectiveness, heart failure, implantable cardioverter defibrillator, morbidity/mortality, women
Randomized clinical trials established a survival benefit of primary prevention implantable cardioverter defibrillators (ICDs) in patients with heart failure (HF) and reduced ejection fraction. 1-3 However, these trials generally enrolled a majority of men and were underpowered to assess benefits in the smaller subset of women which represented 10-30% of enrolled subjects. Some experts have questioned whether primary prevention ICDs provide benefit to women and have raised substantial concerns regarding underrepresentation of women in clinical trials for devices. 4 Nonetheless, the results of these trials were assumed in national guidelines to apply to otherwise eligible patients regardless of sex. 5 Despite sex neutral guideline recommendations, the actual use of primary prevention ICDs is lower in women versus men6, and one possible explanation for this may be concerns regarding the paucity of evidence supporting primary prevention ICDs in women.
Ethical challenges make it unlikely that there will ever be a trial of primary prevention ICDs in women. As such, two meta-analyses have been conducted to assess the impact of primary prevention ICDs on survival in women with benefit demonstrated in one 7, but not the other 8. Results in other post-hoc and observational analyses of primary prevention ICDs in women have been mixed. 9, 10 However, conclusions from these studies were fundamentally limited due to study design leaving unanswered questions about the benefit of primary prevention ICDs in women. We previously compared survival of women with an ICD from the National Cardiovascular Data Registry (NCDR) ICD Registry to matched women without an ICD from Get With The Guidelines for Heart Failure (GWTG – HF), a voluntary hospital based improvement program. 11 We found that the presence of an ICD was associated with improved survival, and there was no evidence of an interaction between sex and the presence of an ICD with respect to survival. However, patients could not be matched based on hospital characteristics, and this may have confounded the analysis.
In this analysis we sought to compare survival between women hospitalized for HF and implanted with a primary prevention ICD with eligible women from similar hospital settings without an ICD implanted. We then compared this with similar matched analyses among men.
Methods
Data Sources
Data for this investigation were acquired from the GWTG-HF registry and the Centers for Medicare & Medicaid Services (CMS). The GWTG-HF registry has been described previously.12 Briefly, it began in 2000 as a voluntary data collection and hospital-based quality improvement initiative. The HF module originated from the March 2005 Organized Program to Initiate Lifesaving Treatment of Patients Hospitalized with Heart Failure (OPTIMIZE-HF) study.13 All participating institutions are required to comply with local regulatory and privacy guidelines and, if required, to secure institutional review board approval. Because data were used primarily at the local site for quality improvement, sites were granted a waiver of informed consent under the common rule. Outcome, A Quintiles Company (Cambridge, MA), is the data collection and coordination center for the American Heart Association/American Stroke Association Get With The Guidelines programs, and the Duke Clinical Research Institute (DCRI) (Durham, NC) serves as the data analysis center. The DCRI has an agreement to analyze the aggregate de-identified data for research purposes. Hospital characteristics as well as patient demographic and clinical characteristics including comorbidities, previous therapies and interventions, contraindications to evidence-based therapies, and in-hospital outcomes are collected prospectively. Data related to ICD therapy for each hospitalization include whether an ICD was present at admission, implanted during the hospitalization, or planned after hospital discharge; contraindications to ICD therapy, and any reason documented by a physician for not implanting or prescribing an ICD.
Medicare data include Part A inpatient claims and the corresponding denominator files for 2005 through 2012. We linked the registry data to Medicare claims data using a validated method that uses combinations of indirect identifiers and identifies patients 91% of the time. 14
Study Population
Heart failure admissions in the GWTG-HF registry were merged with Medicare Part A inpatient claims, matching by admission and discharge dates, date of birth, sex, and hospital, using methodology previously described. 14 These linked data were available for admissions from January 1, 2005 through December 31, 2012.
For the present analysis, the initial group of interest included women in the GWTG-HF registry who were at least 65 years old, whose primary insurance was Medicare, and who were linked to CMS data as described above (n=58,742). We sequentially excluded from the analysis records of patients who died during hospital admission (n=2142); received comfort care only (n=2953); were not discharged to home (n=17,809); already had an ICD in place (n=1868); were missing an LVEF or medical history data (n=5398); had an LVEF >35% (n=20,821); or had a contraindication to ICD including recent onset of HF (i.e., HF diagnosis not predating the index admission), recent myocardial infarction (within 40 days) or coronary revascularization (percutaneous coronary intervention or CABG within 90 days), class IV HF symptoms, or no reasonable expectation of survival to one year (n=935); and those who received cardiac resynchronization therapy (CRT) (n=720) because , in these cases, the effect of CRT cannot be distinguished from that of the ICD. Records of subsequent hospitalizations were also excluded (n=716). After these exclusions, 3788 unique Medicare patients remained. Of these, 430 (11%) had an ICD implanted or prescribed during the index hospitalization, and this group made up the ICD population to which non-ICD patients were matched.
The same process was employed to obtain a study sample of men (n=48,478) which resulted in 5,273 unique Medicare patients; 863 of these had an ICD implanted or prescribed during the hospitalization.
Outcomes
All-cause mortality was the primary outcome of this analysis, determined from the Medicare denominator file through 12/31/2012. Patients with no record of death in the denominator file were considered alive as of 12/31/2012 or the date at which the patient was no longer enrolled in Part A & Part B fee-for-service Medicare, whichever came first.
Statistical Analysis
We compared the baseline characteristics of women with and without an ICD using the Pearson chi-square test for categorical variables and the Wilcoxon rank-sum test for continuous variables. Summary statistics are reported as percentages for categorical variables and as medians and 25th and 75th percentiles for continuous variables. The standardized difference between groups for each variable was defined as the absolute value of the difference in group means or proportions, divided by the average standard deviation and expressed as a percentage.
Significant differences between ICD and non-ICD patients were expected in this non-randomized sample, and a preliminary examination of the data confirmed this. We used the methods of Rosenbaum and Rubin to develop matched groups. 15 First, for continuous variables, we excluded non-ICD patients whose value was below the minimum or above the maximum for ICD patients. Second, missing data were imputed. Missing rates were generally quite low, but up to 15% of data on medications were missing. When a contraindication to the medication was noted, the value for that medication was set to 0; otherwise missing data were imputed by using a single Markov chain Monte Carlo (MCMC) imputation. Third, a propensity model was built using logistic regression in which the dependent (outcome) variable was an indicator of whether each patient belonged to the group with an ICD or without an ICD, and the independent (predictor) variables were baseline characteristics including age; race (white versus other); LVEF; systolic blood pressure (SBP); medical history including ischemic heart disease, prior atrial arrhythmia (including atrial fibrillation and/or atrial flutter), diabetes, hypertension, chronic renal insufficiency, depression, chronic obstructive pulmonary disease (COPD) or asthma, anemia, or prior CVA/TIA; medications at discharge including angiotensin converting enzyme inhibitor (ACE-I) or angiotensin receptor blocker (ARB), beta blocker, calcium channel blocker, digoxin, diuretic, and statin; relevant laboratory values including hemoglobin, sodium, BNP, and creatinine from admission when available and otherwise from discharge; and hospital characteristics including geographic region, teaching hospital, number of beds, and whether the hospital performs advanced cardiac procedures. From the logistic regression model, an estimated propensity score (the probability – p—of being an ICD patient) and a corresponding logit for the propensity score (loge[p/(1-p)]) were calculated for each patient.
Fourth, for the matching process, a caliper width of 0.25*(standard deviation of the logit) was used. For a given patient with an ICD, all patients without an ICD were considered whose logit differed from the ICD patient's logit by less than the caliper width. Among these patients, the patient without an ICD with the shortest Mahalanobis distance from the ICD patient was selected as a match. Variables used in calculating the Mahalanobis distance were all significant predictors from the propensity model. Each patient without an ICD was matched no more than once; there were no patients with an ICD left unmatched. These procedures were repeated to develop a subgroup of men.
A Cox proportional hazards model was used to evaluate the association of the presence of an ICD with the risk of all-cause mortality among the matched patients. The model included all women and men, a term for sex, and a term for the interaction between sex and presence of an ICD. Because the patient cohorts were matched, the unadjusted results are considered the primary results. A robust sandwich variance estimator was used to account for correlation among patients at the same hospital. The proportional hazards assumption for the ICD term was assessed and determined to have been met. As a sensitivity analysis, to determine whether residual confounding affected the estimates, the model was repeated adjusting for all variables in the propensity model, and stratified by quartile of propensity score. Missing values of covariates were imputed using multiple imputation. Risk relationships are expressed as hazard ratios (HR) with 95% confidence intervals (CI) within the subgroups of women and men derived from each Cox model. Mortality rates at 1 and 3 years are presented as Kaplan-Meier estimates in the primary results and as predicted (adjusted) rates in the sensitivity results.
Differences were declared to be statistically significant at p < .05, and all statistical tests were 2-sided. For all analyses, SAS version 9.2 (SAS Institute, Cary NC) was used.
Preliminary examination of the primary outcome data demonstrated very early separation in survival curves between the groups of patients with and without an ICD. We explored whether excluding patients who died in the first 30 days after hospitalization would reduce this effect. A landmark model was performed beginning 30 days after discharge which resulted in omission of 29 ICD patients (2%) and all similar patients without an ICD (n=343, 4%). The samples were re-matched and the Cox model was recreated.
Results
Baseline Characteristics
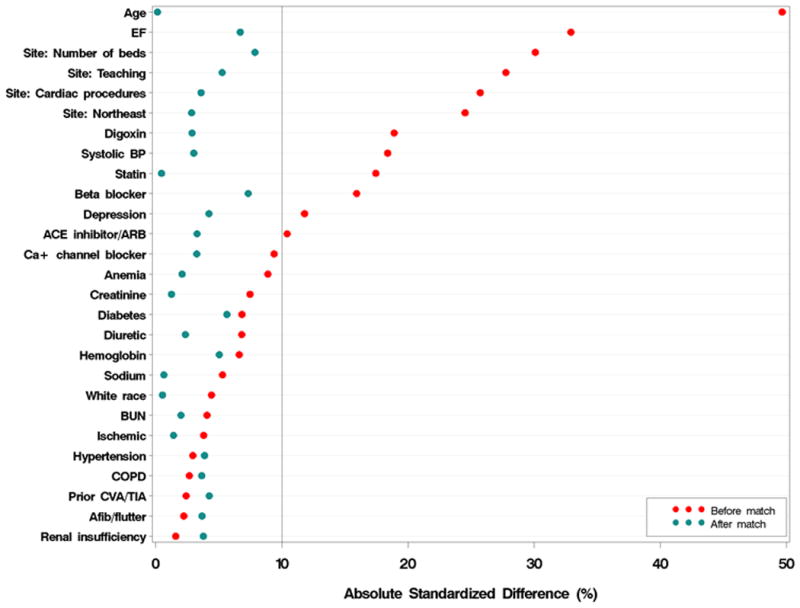
The unmatched baseline characteristics of women from GWTG-HF with and without an ICD are shown in Table 1. A similar table for men is included in Appendix I. Compared with women with HF and no ICD, those with an ICD were younger and were more likely to have been admitted to a larger teaching hospital. The rates of comorbid conditions including ischemic heart disease, diabetes, hypertension, renal failure, depression, and history of stroke or TIA were similar between the 2 groups. After propensity score matching, the differences between groups were smaller (Table 2 and Figure 1) with an absolute standardized difference on all variables less than 10%. In this group, 64% of patients received an ICD during the index admission with the remaining patients (36%) being prescribed an ICD on discharge. Matching in the subgroup of men achieved absolute standardized differences on all variables no greater than 10% (Figure 1).
Table 1. Baseline characteristics for women with or without an ICD.
| Baseline characteristic | ICD N=430 |
No ICD N=3358 |
|---|---|---|
| Age, years | 76 (71, 81) | 80 (73, 86) |
| White race | 76% (317) | 77% (2534) |
| Presentation | ||
| Systolic BP | 133 (117, 149) | 139 (121, 157) |
| Heart rate | 80 (70, 94) | 86 (74, 101) |
| LVEF (%) | 25 (20, 30) | 28 (20, 32) |
| BMI | 26.3 (22.0, 30.9) | 25.0 (21.3, 29.6) |
| QRS duration, ms | 113 (94, 140)(n=124) | 114 (94, 145)(n=1126) |
| Medical history | ||
| Anemia | 12% (53) | 15% (517) |
| Ischemic heart disease | 58% (251) | 57% (1897) |
| Prior atrial arrhythmia | 28% (119) | 29% (963) |
| Diabetes | 41% (177) | 38% (1270) |
| Hypertension | 76% (328) | 75% (2519) |
| Smoking in past 12 months | 13% (55) | 10% (323) |
| Chronic renal insufficiency | 16% (67) | 15% (504) |
| Dialysis | 2% (7) | 3% (94) |
| COPD or asthma | 27% (114) | 25% (851) |
| Prior CVA or TIA | 13% (56) | 14% (465) |
| PAD | 9% (39) | 11% (354) |
| Depression | 6% (24) | 9% (289) |
| Medications | ||
| ACE-inhibitor or ARB | 91% (331) | 89% (2432) |
| Anticoagulant therapy | 32% (119) | 32% (894) |
| Beta blocker | 95% (387) | 92% (2845) |
| Calcium channel blocker | 13% (49) | 18% (504) |
| Digoxin | 37% (146) | 29% (824) |
| Diuretic | 79% (308) | 83% (2488) |
| Statin | 49% (193) | 39% (1198) |
| Labs | ||
| BNP (pg/mL) | 1119 (453, 2106) | 1290 (664, 2343) |
| Sodium (mEq/L) | 138 (136, 140) | 138 (135, 141) |
| Hemoglobin (g/dL) | 12.3 (11.0, 13.3) | 12.0 (10.8, 13.2) |
| Creatinine (mg/dl) | 1.2 (0.9, 1.5) | 1.2 (0.9, 1.6) |
| BUN (mg/dl) | 23 (17, 33) | 24 (17, 35) |
| Hospital characteristics | ||
| Geographic region | ||
| Northeast | 41% (175) | 29% (977) |
| Midwest | 19% (83) | 22% (751) |
| South | 32% (139) | 36% (1205) |
| West | 8% (33) | 13% (425) |
| Teaching hospital | 75% (321) | 62% (2077) |
| Rural site | 2% (10) | 7% (224) |
| Number of beds | 438 (339, 593) | 372 (236, 536) |
| Performs PCI for acute MI | 92% (378) | 85% (2679) |
| Performs cardiac surgery | 90% (377) | 73% (2303) |
| Performs heart transplants | 18% (75) | 8% (268) |
Continuous variables are shown as median (25th, 75th percentiles) and are compared with Wilcoxon rank sum tests. Categorical variables are shown as percent (number) and are compared with Pearson chi-square tests. Only non-imputed values are used.
QRS duration has been collected in the GWTG-HF registry since February 2008, and has been required since Oct 2011.
Medications are from discharge where available, otherwise from admission.
Labs are from admission where available, otherwise from discharge.
Table 2. Variables used in the propensity model for women and men with standardized differences after matching.
| Baseline characteristic | Women with ICDN=430 | Women after 1:1 matching | Men with ICDN=859 | Men after 1:1 matching | ||
|---|---|---|---|---|---|---|
| No ICDN=430 | % standardized difference* | No ICDN= 859 | % standardized difference* | |||
| Age, years | 76 (71, 81) | 76 (71, 80) | 0 | 75 (70, 80) | 75 (71, 80) | 4 |
| White race | 76% (325) | 75% (324) | 1 | 84% (720) | 85% (731) | 4 |
| LVEF (%), mean (SD) | 24.4 (6.93) | 24.9 (6.7) | 7 | 24.8 (6.9) | 24.9 (6.4) | 1 |
| Systolic BP | 133 (116, 150) | 135 (120, 151) | 3 | 130 (112, 148) | 130 (114, 146) | 2 |
| Ischemic heart disease | 58% (251) | 59% (254) | 1 | 74% (633) | 77% (661) | 8 |
| Prior atrial arrhythmia | 28% (119) | 26% (112) | 4 | 32% (274) | 32% (273) | 0 |
| Diabetes | 41% (177) | 44% (189) | 6 | 36% (310) | 37% (315) | 1 |
| Hypertension | 76% (328) | 78% (335) | 4 | 67% (577) | 70% (598) | 5 |
| Chronic renal insufficiency | 16% (67) | 17% (73) | 4 | 16% (138) | 16% (138) | 0 |
| Depression | 6% (24) | 5% (20) | 4 | 5% (47) | 5% (46) | 1 |
| COPD/asthma | 27% (114) | 28% (121) | 4 | 23% (201) | 21% (184) | 5 |
| Anemia | 12% (53) | 13% (56) | 2 | 8% (70) | 8% (71) | 0 |
| Prior CVA or TIA | 13% (56) | 12% (50) | 4 | 12% (102) | 11% (95) | 3 |
| ACE-inhibitor or ARB | 77% (331) | 76% (325) | 3 | 77% (661) | 78% (672) | 3 |
| Beta blocker | 90% (387) | 92% (396) | 7 | 90% (769) | 90% (772) | 1 |
| Calcium channel blocker | 13% (55) | 16% (67) | 3 | 13% (114) | 13% (109) | 2 |
| Digoxin | 37% (158) | 37% (158) | 3 | 35% (297) | 31% (270) | 7 |
| Diuretic | 79% (339) | 81% (349) | 2 | 78% (666) | 82% (701) | 10 |
| Statin | 47% (201) | 47% (203) | 0 | 47% (400) | 47% (408) | 2 |
| Sodium | 138 (136, 140) | 138 (136, 140) | 1 | 138 (136, 141) | 138 (136, 141) | 2 |
| BUN | 24 (17, 35) | 25 (18, 35) | 2 | 26 (19, 37) | 27 (20, 38) | 5 |
| Creatinine | 1.2 (0.9, 1.7) | 1.2 (0.9, 1.7) | 1 | 1.4 (1.1, 1.9) | 1.4 (1.1, 1.9) | 2 |
| Hemoglobin | 12.3 (11.0, 13.4) | 12.0 (10.9, 13.3) | 5 | 13.0 (11.8, 14.3) | 12.8 (11.7, 14.0) | 10 |
| Site: Northeast | 41% (175) | 39% (169) | 3 | 138 (136, 141) | 138 (136, 141) | 2 |
| Site: Teaching hospital | 75% (321) | 72% (311) | 5 | 26 (19, 37) | 27 (20, 38) | 5 |
| Site: Number of beds | 438 (339, 593) | 435 (311, 559) | 8 | 1.4 (1.1, 1.9) | 1.4 (1.1, 1.9) | 2 |
| Site: Advanced cardiac procedures performed† | 92% (397) | 93% (398) | 1 | 13.0 (11.8, 14.3) | 12.8 (11.7, 14.0) | 10 |
Dataset used in matching is shown here, i.e., with a single imputation for missing data, which affects race, systolic BP, medications, labs, and site performance of cardiac procedures (no other variables had missing data); therefore slight differences may be noted in these variables between this table and Table 1. For medications, patients with noted contraindications are counted as “no” in Table 2 but as missing in Table 1.
The standardized difference is the absolute difference in means (or proportions) divided by the average standard deviation.
Hospital performs PCI, cardiac surgery, or heart transplants. Continuous variables are shown as median (25th, 75th percentiles), except where noted, and categorical variables as percent (number).
Figure 1. Standardized differences in baseline characteristics in women before and after matching.
Mortality
The median follow up was 3.4 and 3.0 years respectively for the propensity matched groups of women with and without an ICD. The overall risk of mortality was significantly lower in women with an ICD compared with those without an ICD (HR 0.78 95% CI 0.66-0.92, p=0.003). This mortality difference appeared early and persisted throughout follow up with mortality at 3 years of 40.2% in the group with an ICD and 48.7% in the group without an ICD (Table 3, Figure 2A). A similar survival benefit was seen in the propensity matched group of men with an ICD compared to those without an ICD (HR 0.76 95% CI 0.067-0.087, p<0.001) (Table 3, Figure 2B). A test for interaction demonstrated that improved survival associated with implantation of an ICD did not depend on sex (p=0.79).
Table 3. Results of mortality analysis in women and men. (A) Primary analysis propensity matched (B) Propensity matched and adjusted model (C) Propensity matched 30-day landmark analysis.
| Women | Men | |||
|---|---|---|---|---|
|
| ||||
| ICD | No ICD | ICD | No ICD | |
|
| ||||
| N | 430 | 430 | 859 | 859 |
|
| ||||
| Follow-up duration among survivors (years) | ||||
| Median | 3.4 | 3.0 | 3.9 | 2.9 |
| 25th, 75th percentiles | 1.9, 5.3 | 1.7, 4.7 | 2.1, 5.2 | 1.6, 4.7 |
| Min, max | 0.03, 7.8 | 0.01, 7.9 | 0.04, 7.7 | 0.01, 8.0 |
|
| ||||
| (A) Propensity matched (primary results) | ||||
|
| ||||
| Mortality rate (KM) at 1 year (95% CI) | 17.0% (13.7, 21.0) | 24.5% (20.7, 29.0) | 19.8% (17.3, 22.7) | 27.5% (24.6, 30.7) |
|
| ||||
| Mortality rate (KM) at 3 years (95% CI) | 40.2% (35.4, 45.4) | 48.7% (43.7, 53.9) | 42.9% (39.5, 46.5) | 52.9% (49.3, 56.6) |
|
| ||||
| Unadjusted HR (95% CI) for ICD vs. no ICD | 0.78 (0.66, 0.92), p=0.003 | 0.76 (0.67, 0.87), p<0.001 | ||
|
| ||||
| P-value for interaction of sex with ICD | 0.79 | |||
|
| ||||
| (B) Propensity matched and adjusted | ||||
|
| ||||
| Adjusted mortality rate at 1 year (95% CI) | 18.3% (17.6,19.0) | 23.1% (22.3,23.9) | 21.3% (20.7,21.8) | 26.7% (26.0,27.3) |
|
| ||||
| Adjusted mortality rate at 3 years (95% CI) | 39.1% (38.0,40.3) | 47.1% (45.9,48.3) | 44.2% (43.3,45.0) | 52.5% (51.6,53.4) |
|
| ||||
| Adjusted HR (95% CI) for ICD vs. no ICD | 0.75 (0.63, 0.90), p=0.002 | 0.76 (0.67, 0.86), p<0.001 | ||
|
| ||||
| p-value for interaction of sex with ICD | 0.97 | |||
| (C) Propensity matched 30-day landmark analysis | ||||
|
| ||||
| N | 422 | 422 | 839 | 839 |
|
| ||||
| Mortality rate at 1 year (95% CI) | 17.3% (13.9, 21.3) | 23.6% (19.8, 28.1) | 19.4% (16.8, 22.3) | 25.0% (22.2, 28.2) |
|
| ||||
| Mortality rate at 3 years (95% CI) | 40.1% (35.3, 45.3) | 48.6% (43.6, 54.0) | 43.3% (39.9, 47.0) | 50.9% (47.3, 54.7) |
|
| ||||
| Unadjusted HR (95% CI) for ICD vs no ICD | 0.80 (0.68, 0.94), p=0.007 | 0.81 (0.71, 0.92), p=0.002 | ||
|
| ||||
| p-value for interaction of sex with ICD | 0.86 | |||
Figure 2. Unadjusted Kaplan-Meier estimates of mortality with and without an ICD placed during or after a HF hospitalization (A) Women (B) Men.
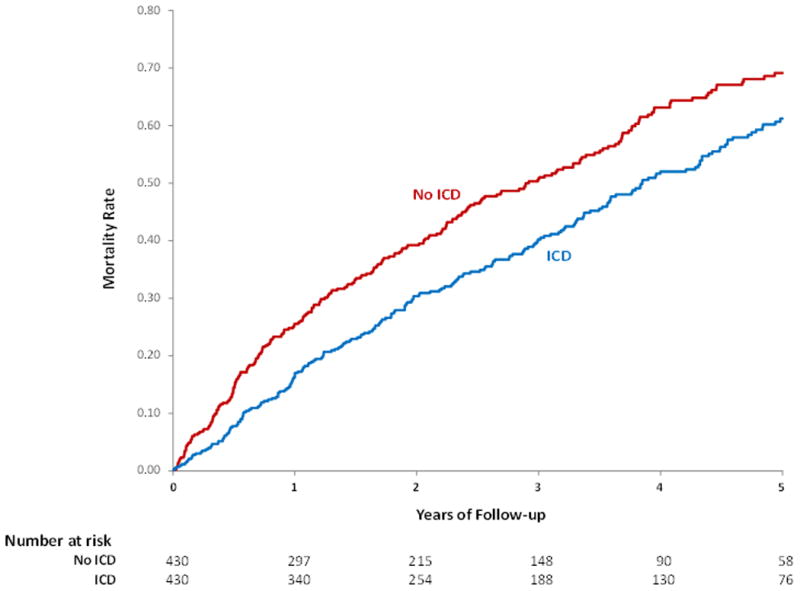
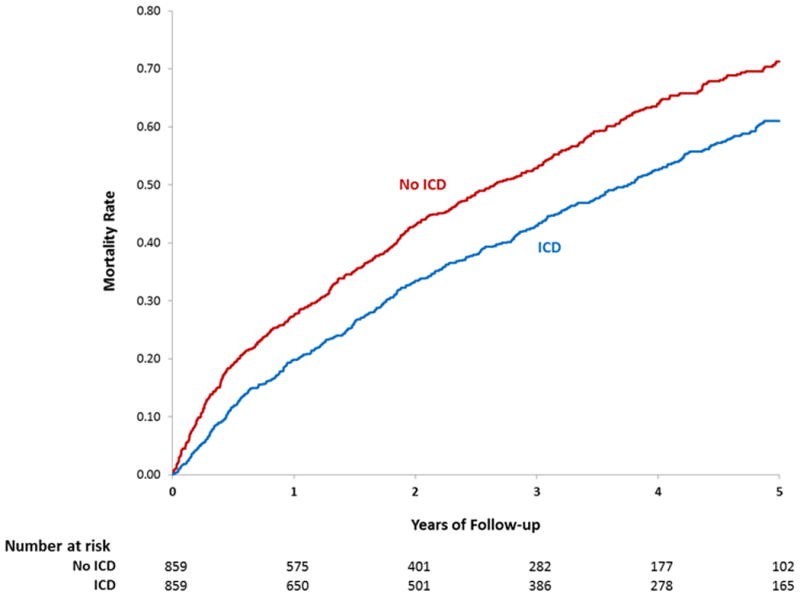
To further adjust for small remaining imbalances between groups, the primary propensity matched results were adjusted for the covariates listed in Table 2. In this propensity matched and adjusted model, the risk of mortality in women with an ICD compared with those without an ICD was nearly identical to the primary propensity matched results (HR 0.75 95% CI 0.63-0.90, p=0.002) (Table 3). This was also true in men (HR 0.76 95% CI 0.67- 0.86, p<0.001) (Table 3).
In a 30-day landmark analysis we removed early deaths. Even after removing those with early mortality the propensity matched mortality HR was nearly identical to that observed in the primary analysis (0.80 for women and 0.81 for men (Table 3).
Finally, we conducted a survival analysis in women and men in which all patients with an ICD – including those with an ICD at the time of HF hospitalization – were included in the ICD group. While the survival benefits were attenuated modestly in both men and women, no interaction of ICD and sex was seen (Appendix II).
Discussion
Our study found that in both older women and men with HF and reduced LVEF, implantation or prescription of a primary prevention ICD on discharge was associated with improved survival. Relative to those not receiving an ICD, those receiving (or prescribed) an ICD had similarly improved survival in both women and men, with no significant sex-based interactions. These hazard ratios are similar to those seen overall in the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) (HR for mortality in ICD versus placebo groups = 0.77) which, among the landmark randomized clinical trials of primary prevention ICDs, most closely resembles the population studied here. 1
Despite the survival benefits of primary prevention ICDs in HF patients demonstrated in randomized clinical trials, benefit in the subgroup of women from these trials has not been definitively proved. 7-9 This uncertainty regarding survival benefit may be one of several contributing factors to the lower rates of ICD referral and implantation in eligible women. 6, 16, 17 Indeed, in this cohort, only 11% of eligible women and 16% of eligible men received an ICD or a prescription for one at the time of HF hospitalization. These low rates are consistent with other investigations which found underutilization of ICDs. 18, 19 In the absence of an adequately powered analysis from a randomized clinical trial, we previously compared survival of women with an ICD from the National Cardiovascular Data Registry (NCDR) ICD Registry to matched women without an ICD from GWTG–HF. 11 The survival benefit of a primary prevention ICD for women was similar to that seen in this analysis, but we were unable to match for certain patient and hospital characteristics resulting in possible confounding Moreover, the cohort size of women with a primary prevention ICD studied in this analysis (and our previous investigation) is greater than any examined in a randomized clinical trial.
In this analysis we matched women hospitalized for HF who were eligible for a primary prevention ICD and either received one (or were prescribed one on discharge) or did not receive one. Given the observational nature of these data, we used propensity score matching to create groups that were as similar as possible using a model that included variables representing demographic and clinical patient characteristics as well as characteristics of the hospital in which patients were treated for HF. Notably, hospital characteristics were very similar after matching (Table 2 and Figure 1) differing by no more than 2% on geographic region, teaching versus non-teaching, size, and availability of advanced cardiac procedures. This indicates that each woman with an ICD was generally compared with a woman without an ICD from a similar hospital; therefore, hospital site does not explain differences in survival.
Importantly, the survival curves in this analysis separated early (Figure 2). In part, this may be due to the high event rates observed in this population based on relatively older age and more comorbidities compared to clinical trial patients.20 In addition, patients in this analysis were necessarily identified based on a HF hospitalization which has been associated with worse outcomes in Medicare patients21, 22. Indeed, when the benefits of a primary prevention ICD have been examined in the sickest subgroup of patients in clinical trials, a similar finding of early curve separation has been seen as in the case of New York Heart Association (NYHA) class III patients in SCD-HeFT and Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) trials. 1, 2 However, to fully investigate early curves separation in this context, we performed a 30-day landmark analysis to examine the effect of early deaths on the survival curves (Table 3). Exclusion of patients who died early simply delayed the early separation of curves by 30 days. This suggests that the differences in survival were not explained by a lead time bias.
Investigation of the mortality benefit of a primary prevention ICD in women has been ongoing since the landmark clinical trials which did not answer this question definitively. Various retrospective, post hoc, registry-based, and/or meta-analytic studies have sought to answer this question and have arrived at varying results. 7-10 In light of this controversy, providers, professional societies, guideline committees, regulators, and others have assumed that, on average, the potential benefit of a primary prevention ICD in women outweighs the associated risks. Therefore, there is insufficient equipoise to justify a randomized controlled clinical trial, and in the absence of such a trial, analyses of non-randomized clinical cohorts such as this are important to inform clinical decision making.
While this analysis and others demonstrate a mortality benefit for women from a primary prevention ICD, this benefit must be weighed against potential risks. This is particularly important for women because complication rates associated with primary prevention ICD implantation tend to be higher compared with men. 23 Future research is needed to identify ways to reduce complication rates in order to maximize the net benefit from primary prevention ICDs in women.
Limitations
The primary limitation of this analysis is that treatment assignment was not assigned randomly, and despite propensity matching and additional risk adjustment there remains the potential for residual measured and unmeasured confounding by variables not captured in the GWTG-HF registry, and a provider's assessment of a patient's overall fitness for ICD implantation includes consideration of many of these factors together. Those patients without an ICD implanted or prescribed may have been too sick to undergo the procedure such that mortality differences may be a reflection of underlying comorbidities. For example, NYHA class was not available for this analysis, nor were characteristics describing quality of life and patient and provider preferences, and these variables may have contributed to decisions surrounding ICD implantation as well as survival differences. This analysis was concerned with outcomes in patients who had an ICD implanted or prescribed during a HF hospitalization, but planned implantations cannot be confirmed. Most patients undergoing ICD implantation in the US do so during a hospital stay that is less than 24 hours24, so data related to these implants are not available in the Medicare Part A claims to which we had access. We relied on a propensity score matching process to develop groups for comparison which necessarily excludes patients who are too dissimilar to match (e.g., those with a high burden of disease). Lastly, we limited our analysis to Medicare patients hospitalized at a hospital participating in GWTG-HF which is a voluntary quality improvement program. While this group has previously been demonstrated to be similar to the Medicare population as a whole 25-27, our results may not generalize to younger, healthier patients or those in alternative clinical settings.
Conclusion
In a propensity score matched analysis of Medicare patients with HF and reduced LVEF, we found both women and men implanted with a primary prevention ICD during or following a heart failure hospitalization had significantly longer survival compared with their counterparts who did not receive an ICD, and there were no significant sex-based interactions for the survival benefits associated with ICD placement. The associated survival benefit appeared early post hospitalization but was not sensitive to the exclusion of patients who died within a month of discharge, and this benefit was present throughout available follow up. These data support current guideline recommendations for the implantation of a primary prevention ICD in eligible women as well as men with heart failure and reduced LVEF.
Supplementary Material
Clinical Perspective.
Clinical trials of implantable cardioverter defibrillators (ICDs) for primary prevention enrolled a limited number of women and were underpowered to assess benefits in this important subgroup. However, in light of the overall results of these landmark trials, the benefits of primary prevention ICDs have been assumed in national guidelines to apply to all eligible heart failure (HF) patients regardless of sex. Ethical limitations make it unlikely that there will ever be a randomized trial of primary prevention ICDs in women. As such, various post hoc, retrospective, and meta-analytic evaluations of the effect of ICDs on mortality in women have generated varying results. Therefore, in this analysis from the Get With The Guidelines for Heart Failure (GWTG-HF) Registry, women hospitalized for heart failure who had an ICD implanted or prescribed were matched to similar women without an implanted or prescribed ICD using a propensity score model. When survival was compared between these two groups, those women who had a primary prevention ICD implanted or prescribed had a significant survival advantage over women without an ICD. A parallel analysis of men from GWTG-HF demonstrated similar results. These findings support guideline-directed use of primary prevention ICDs in eligible patients regardless of sex.
Acknowledgments
Sources of Funding: This project was supported in part by grant number U19HS021092 from the Agency for Healthcare Research and Quality (AHRQ). The content is solely the responsibility of the authors and does not necessarily represent the official views of the AHRQ or the AHA.
Footnotes
Disclosures: Dr. Zeitler was funded by National Institutes of Health (NIH) T-32 training grant #2 T32 HL 69749-11 A1. However, no relationships exist related to the analysis presented. Ms Hellkamp and Drs Al-Khatib, Hernandez, Peterson, Sanders, Schulte, and Yancy report no relevant disclosures. Dr. Fonarow reports consulting for Medtronic.
References
- 1.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH, Sudden Cardiac Death in Heart Failure Trial I Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 2.Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, Calkins H, Hoch D, Goldberger J, Shalaby A, Sanders WE, Schaechter A, Levine JH, Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation I Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–8. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–40. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 4.Redberg RF. Disparities in use of implantable cardioverter-defibrillators: moving beyond process measures to outcomes data. JAMA. 2007;298:1564–6. doi: 10.1001/jama.298.13.1564. [DOI] [PubMed] [Google Scholar]
- 5.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Tracy CM, Epstein AE, Darbar D, DiMarco JP, Dunbar SB, Estes NA, 3rd, Ferguson TB, Jr, Hammill SC, Karasik PE, Link MS, Marine JE, Schoenfeld MH, Shanker AJ, Silka MJ, Stevenson LW, Stevenson WG, Varosy PD, American College of Cardiology F, American Heart Association Task Force on Practice G and Heart Rhythm S 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2013;61:e6–75. doi: 10.1016/j.jacc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Al-Khatib SM, Hellkamp AS, Hernandez AF, Fonarow GC, Thomas KL, Al-Khalidi HR, Heidenreich PA, Hammill S, Yancy C, Peterson ED, Get With the Guidelines Steering C and Hospitals Trends in use of implantable cardioverter-defibrillator therapy among patients hospitalized for heart failure: have the previously observed sex and racial disparities changed over time? Circulation. 2012;125:1094–101. doi: 10.1161/CIRCULATIONAHA.111.066605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santangeli P, Pelargonio G, Dello Russo A, Casella M, Bisceglia C, Bartoletti S, Santarelli P, Di Biase L, Natale A. Gender differences in clinical outcome and primary prevention defibrillator benefit in patients with severe left ventricular dysfunction: a systematic review and meta-analysis. Heart Rhythm. 2010;7:876–82. doi: 10.1016/j.hrthm.2010.03.042. [DOI] [PubMed] [Google Scholar]
- 8.Ghanbari H, Dalloul G, Hasan R, Daccarett M, Saba S, David S, Machado C. Effectiveness of implantable cardioverter-defibrillators for the primary prevention of sudden cardiac death in women with advanced heart failure: a meta-analysis of randomized controlled trials. Archives of internal medicine. 2009;169:1500–6. doi: 10.1001/archinternmed.2009.255. [DOI] [PubMed] [Google Scholar]
- 9.Henyan NN, White CM, Gillespie EL, Smith K, Coleman CI, Kluger J. The impact of gender on survival amongst patients with implantable cardioverter defibrillators for primary prevention against sudden cardiac death. Journal of internal medicine. 2006;260:467–73. doi: 10.1111/j.1365-2796.2006.01713.x. [DOI] [PubMed] [Google Scholar]
- 10.Russo AM, Poole JE, Mark DB, Anderson J, Hellkamp AS, Lee KL, Johnson GW, Domanski M, Bardy GH. Primary prevention with defibrillator therapy in women: results from the Sudden Cardiac Death in Heart Failure Trial. Journal of cardiovascular electrophysiology. 2008;19:720–4. doi: 10.1111/j.1540-8167.2008.01129.x. [DOI] [PubMed] [Google Scholar]
- 11.Zeitler EP, Hellkamp AS, Fonarow GC, Hammill SC, Curtis LH, Hernandez AF, Al-Khalidi HR, Curtis JP, Heidenreich PA, Anstrom KJ, Peterson ED, Mark DB, Hammill BG, Sanders GD, Al-Khatib SM. Primary prevention implantable cardioverter-defibrillators and survival in older women. JACC Heart failure. 2015;3:159–67. doi: 10.1016/j.jchf.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Pieper K, Sun JL, Yancy C, Young JB, Investigators O-H and Hospitals Association between performance measures and clinical outcomes for patients hospitalized with heart failure. JAMA. 2007;297:61–70. doi: 10.1001/jama.297.1.61. [DOI] [PubMed] [Google Scholar]
- 13.Fonarow GC, Abraham WT, Albert NM, Gattis WA, Gheorghiade M, Greenberg B, O'Connor CM, Yancy CW, Young J. Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF): rationale and design. Am Heart J. 2004;148:43–51. doi: 10.1016/j.ahj.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. doi: 10.1016/j.ahj.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. The American Statistician. 1985;39:33–38. [Google Scholar]
- 16.Chae SH, Koelling TM. Patient and physician determinants of implantable cardioverter defibrillator use in the heart failure population. Congestive heart failure. 2010;16:141–6. doi: 10.1111/j.1751-7133.2009.00139.x. [DOI] [PubMed] [Google Scholar]
- 17.Sherazi S, Zareba W, Daubert JP, McNitt S, Shah AH, Aktas MK, Block RC. Physicians' knowledge and attitudes regarding implantable cardioverter-defibrillators. Cardiology journal. 2010;17:267–73. [PMC free article] [PubMed] [Google Scholar]
- 18.Narayanan K, Reinier K, Uy-Evanado A, Teodorescu C, Chugh H, Marijon E, Gunson K, Jui J, Chugh SS. Frequency and determinants of implantable cardioverter defibrillator deployment among primary prevention candidates with subsequent sudden cardiac arrest in the community. Circulation. 2013;128:1733–8. doi: 10.1161/CIRCULATIONAHA.113.002539. [DOI] [PubMed] [Google Scholar]
- 19.Pokorney SD, Miller AL, Chen AY, Thomas L, Fonarow GC, de Lemos JA, Al-Khatib SM, Peterson ED, Wang TY. Implantable Cardioverter-Defibrillator Use Among Medicare Patients With Low Ejection Fraction After Acute Myocardial Infarction. JAMA. 2015;313:2433–40. doi: 10.1001/jama.2015.6409. [DOI] [PubMed] [Google Scholar]
- 20.Hess PL, Laird A, Edwards R, Bardy GH, Bigger JT, Buxton AE, Moss AJ, Lee KL, Hall WJ, Steinman R, Dorian P, Hallstrom A, Cappato R, Kadish AH, Kudenchuk PJ, Mark DB, Al-Khatib SM, Piccini JP, Inoue LY, Sanders GD. Survival benefit of primary prevention implantable cardioverter-defibrillator therapy after myocardial infarction: Does time to implant matter? A meta-analysis using patient-level data from 4 clinical trials. Heart Rhythm. 2013;10:828–35. doi: 10.1016/j.hrthm.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998-2008. JAMA. 2011;306:1669–78. doi: 10.1001/jama.2011.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dharmarajan K, Hsieh AF, Kulkarni VT, Lin Z, Ross JS, Horwitz LI, Kim N, Suter LG, Lin H, Normand SL, Krumholz HM. Trajectories of risk after hospitalization for heart failure, acute myocardial infarction, or pneumonia: retrospective cohort study. Bmj. 2015;350:h411. doi: 10.1136/bmj.h411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russo AM, Daugherty SL, Masoudi F, Wang Y, Curtis J, Lampert R. Gender and outcomes following primary prevention ICD implantation: Findings from the NCDR(R) Am Heart J. 2015;169 doi: 10.1016/j.ahj.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hess PL, Greiner MA, Al-Khatib SM, Masoudi FA, Varosy PD, Fogel RI, Curtis LH, Hernandez AF. Same-day discharge and risks of mortality and readmission after elective ICD placement for primary prevention. J Am Coll Cardiol. 2015;65:955–7. doi: 10.1016/j.jacc.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 25.Curtis LH, Greiner MA, Hammill BG, DiMartino LD, Shea AM, Hernandez AF, Fonarow GC. Representativeness of a national heart failure quality-of-care registry: comparison of OPTIMIZE-HF and non-OPTIMIZE-HF Medicare patients. Circulation Cardiovascular quality and outcomes. 2009;2:377–84. doi: 10.1161/CIRCOUTCOMES.108.822692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heidenreich PA, Fonarow GC. Are registry hospitals different? A comparison of patients admitted to hospitals of a commercial heart failure registry with those from national and community cohorts. Am Heart J. 2006;152:935–9. doi: 10.1016/j.ahj.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 27.Heidenreich PA, Hernandez AF, Yancy CW, Liang L, Peterson ED, Fonarow GC. Get With The Guidelines program participation, process of care, and outcome for Medicare patients hospitalized with heart failure. Circulation Cardiovascular quality and outcomes. 2012;5:37–43. doi: 10.1161/CIRCOUTCOMES.110.959122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


