Abstract
Objective
To describe demographic and behavioral characteristics of persons with acute HIV infection (AHI) over time.
Methods
We conducted a retrospective assessment of AHI identified through the STAT (Screening and Tracing Active Transmission) Program from 2003 through 2012 in North Carolina (NC). AHI was identified using pooled nucleic acid amplification for antibody negative samples and individual HIV-1 RNA for antibody indeterminate samples. The STAT program provides rapid notification and evaluation. We compared STAT collected demographic and risk characteristics to all persons requesting tests and all non-AHI diagnoses from the NC State Laboratory of Public Health.
Results
The STAT Program identified 236 AHI cases representing 3.4% (95% CI 3.0–3.9%) of all HIV diagnoses. AHI cases were similar to those diagnosed during established HIV. On pre-test risk-assessments, AHI cases were predominately Black (69.1%), male (80.1%), young (46.8% < 25 years), and men who have sex with men (MSM) (51.7%). Per post-diagnosis interviews, the median age decreased from 35 (IQR 25–42) to 27 (IQR 22–37) years, and the proportion <25 years increased from 23.8% to 45.2% (trend p=0.04) between 2003 and 2012. AHI men were more likely to report MSM risk post-diagnosis than on pre-test risk-assessments (64% to 82.9%; p<0.0001). Post-diagnosis report of MSM risk in men with AHI increased from 71.4% to 96.2%.
Conclusions
In NC, 3.4% of individuals diagnosed with HIV infection have AHI. AHI screening provides a real-time source of incidence trends, improves the diagnostic yield of HIV testing and offers an opportunity to limit onward transmission.
Keywords: Acute HIV infection, surveillance, incidence, HIV transmission
INTRODUCTION
Acute HIV Infection (AHI), the period from initial viral transmission to the presence of detectable HIV antibodies, is estimated to account for 25–50% of HIV-1 transmission. [1] Routine HIV screening algorithms utilizing only antibody testing may therefore miss a critical portion of individuals with AHI in whom very high levels of HIV virus are typical, and most closely associated with the risk of onward HIV transmission. [2, 3] Effective testing for AHI followed by linkage to care can facilitate early engagement in care and initiation of antiretroviral therapy (ART) leading to viral suppression. [4] Coupling early initiation of ART with behavior changes associated with awareness of HIV status, the ability to detect AHI may decrease infectiousness and reduce HIV transmission. [3, 5, 6] Accordingly, routine screening for AHI represents an important, yet not widely implemented component of treatment as prevention.
Prior to 2013, detecting HIV in the acute/early stages was challenging as HIV testing standards relied primarily on the presence of HIV antibodies. Therefore, HIV tests such as the antibody enzyme immunoassay (EIA) or the Western Blot (WB), missed AHI diagnoses. [7–9] Since AHI symptoms cannot reliable identify infection, [10–12] failure to order an HIV RNA or antigen test with an HIV EIA postponed or prevented disease detection. [13–16]
The Screening and Tracing Active Transmission (STAT) Program[13, 15] was implemented by the North Carolina (NC) Division of Public Health (DPH) in November 2002, making AHI screening part of routine statewide HIV testing. The NC State Laboratory of Public Health (SLPH) used pooled nucleic acid amplification (pNAAT) to identify AHI among persons testing EIA negative or indeterminate. [15, 17, 18] For over 10 years, NC successfully implemented AHI screening. The testing population is reflective of persons living in the southeastern region of the United States. [12, 19, 20] NC is notably the only state to conduct statewide AHI screening; larger metropolitan areas have community-based AHI screening programs focusing on high risk populations defined by venue or self-report. [16, 21–24] In contrast, NC screens a population that has a substantial rural component[25] and testing is universal for all people testing at publicly-funded sites across the state.
The STAT program provides a rich source of information about testing trends in acutely infected persons in NC. The current analysis includes data collected over 10 years, building on previously published results from the first years of the program. [10, 13, 26–30] We compared demographic and behavioral trends in both the AHI and established HIV infection (EHI) diagnosed at publicly-funded testing sites in NC between 2003 and 2012.
METHODS
Testing for AHI
The NCDPH initially integrated AHI screening into HIV testing practices as a 12-month observational study. The identification of 22 AHI cases led to the initiation of the STAT program, a collaborative research project with the University of North Carolina at Chapel Hill (UNC) from January 2003 until August 2005. [13, 15, 17] In 2005, the NCSLPH incorporated HIV-1 pNAAT testing into the state’s HIV testing program, making AHI screening a statewide standard for all serum samples from publicly-funded testing sites.
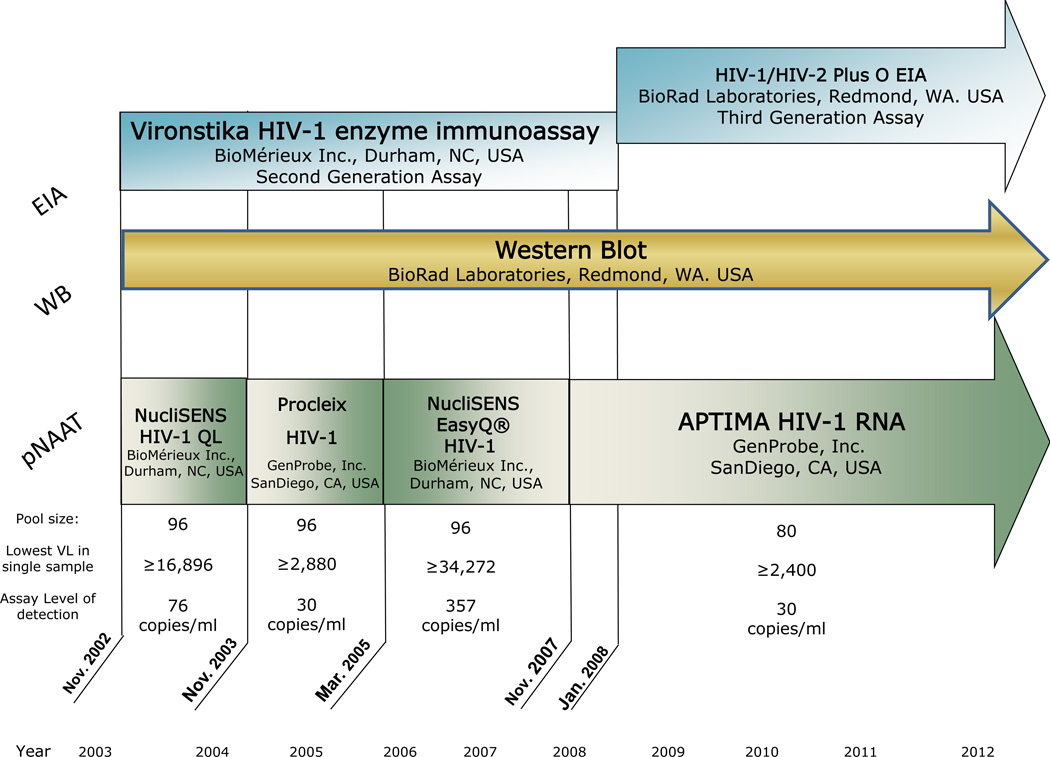
All samples for HIV testing underwent an individual antibody EIA. Antibody negative samples underwent a pooling strategy to test for HIV-1 RNA via pNAAT. The assays used to test for the HIV antibody and HIV RNA changed between 2003 and 2012 (Figure 1). HIV antibody positive samples with a negative or indeterminate WB tests were individually tested for the presence of HIV-1 RNA.
Figure 1.
Study Population
All results from samples tested for HIV at the NCSLPH between January 1, 2003 and December 31, 2012 were included in this analysis. Informed consent for HIV testing was obtained according to NCDPH regulations. The UNC Institutional Review Board and the NCDPH approved this project.
Definitions
The testing population is defined as all HIV test results processed through the NCSLPH during the analysis period. Point-of-care (rapid) test results were not included since it was not available for the entire analysis period and AHI screening samples were not collected with rapid testing. Some duplication of persons exists in the testing population because a person testing negative may test multiple times and be counted more than once. The results from persons previously diagnosed with HIV by self-report or on record at the NCDPH, were excluded. AHI was defined as detectable HIV-1 RNA in an antibody negative or indeterminate sample. EHI was defined as both a positive EIA and WB test.
Data Collection
Time of Testing
The US Centers for Disease Control and Prevention (CDC) counseling, testing and referral (CTR) behavioral risk assessment forms are completed for all persons testing for HIV at publicly-funded sites in NC. Completed as pre-test risk-assessment, the standardized CTR form captures self-reported demographics, risk behaviors, reason for testing, testing site and county of residence and accompanies all samples sent to the NCSPLH for HIV testing. The information collected on the CTR form is reviewed and cleaned by NC Epidemiologists, and reported annually. [31] We reviewed information reported on the CTR behavioral risk assessment form for all HIV samples tested between 2003 and 2012 (Table 1).
Table 1.
Demographics Recorded on CTR forms at the Time of Testing for New HIV Diagnoses at Publicly-Funded Testing Sites in NC 2003–2012
| Number of HIV Tests Performed‡ |
Total HIV Cases Diagnosed | Established HIV Infection | Acute HIV Infection | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | Positivity Rate^ |
N | % | Positivity Rate^ |
N | % | Positivity Rate^ |
% Acute of All HIV Cases |
|
| Total | 1,799,864 | 100.0 | 6,869 | 100.0 | 0.38 | 6,633 | 100.0 | 0.37 | 236 | 100.0 | 0.01 | 3.4 |
| Age | ||||||||||||
| <25 | 847,385 | 47.2 | 1888 | 27.5 | 0.22 | 1778 | 26.9 | 0.21 | 110 | 46.8 | 0.01 | 5.8 |
| ≥25 | 947,009 | 52.8 | 4968 | 72.5 | 0.52 | 4843 | 73.2 | 0.51 | 125 | 53.2 | 0.01 | 2.5 |
| Sexual Risk Category | ||||||||||||
| Female | 1,232,070 | 69.0 | 1814 | 26.7 | 0.15 | 1771 | 27.0 | 0.14 | 43 | 18.5 | 0.00 | 2.4 |
| Heterosexual Male | 441,663 | 24.7 | 1870 | 27.5 | 0.42 | 1817 | 27.7 | 0.41 | 53 | 22.8 | 0.01 | 2.8 |
| MSM | 49,621 | 2.8 | 2359 | 34.7 | 4.75 | 2239 | 34.1 | 4.51 | 120 | 51.7 | 0.24 | 5.1 |
| Unknown Male | 63,385 | 3.6 | 760 | 11.2 | 1.20 | 744 | 11.3 | 1.17 | 16 | 6.9 | 0.03 | 2.1 |
| Race | ||||||||||||
| Black NH | 819,350 | 47.2 | 4787 | 73.2 | 0.58 | 4635 | 73.4 | 0.57 | 152 | 69.1 | 0.02 | 3.2 |
| White NH | 540,538 | 31.1 | 1110 | 17.0 | 0.21 | 1058 | 16.8 | 0.20 | 52 | 23.6 | 0.01 | 4.7 |
| Hispanic | 334,412 | 19.3 | 531 | 8.1 | 0.16 | 516 | 8.2 | 0.15 | 15 | 6.8 | 0.00 | 2.8 |
| Other/Multi Race | 42,270 | 2.4 | 110 | 1.7 | 0.26 | 109 | 1.7 | 0.26 | 1 | 0.5 | 0.00 | 0.9 |
| Reason for testing | ||||||||||||
| Symptoms | 4,763 | 0.3 | 67 | 1.0 | 1.41 | 64 | 1.0 | 1.34 | 3 | 1.3 | 0.06 | 4.5 |
| Test Setting | ||||||||||||
| STD Clinic | 702,002 | 40.8 | 2712 | 40.8 | 0.39 | 2586 | 40.3 | 0.37 | 126 | 55.5 | 0.02 | 4.7 |
| HIV Test Site | 86,040 | 5.0 | 1262 | 19.0 | 1.47 | 1220 | 19.0 | 1.42 | 42 | 18.5 | 0.05 | 3.3 |
| Family Planning/Prenatal/OB | 619,111 | 35.9 | 270 | 4.1 | 0.04 | 266 | 4.2 | 0.04 | 4 | 1.8 | 0.00 | 1.5 |
| TB Clinic | 15,215 | 0.9 | 31 | 0.5 | 0.20 | 30 | 0.5 | 0.20 | 1 | 0.4 | 0.01 | 3.2 |
| DIS Field Visit | 14,370 | 0.8 | 408 | 6.1 | 2.84 | 397 | 6.2 | 2.76 | 11 | 4.9 | 0.08 | 2.7 |
| Outreach | 78,499 | 4.6 | 540 | 8.1 | 0.69 | 516 | 8.0 | 0.66 | 24 | 10.6 | 0.03 | 4.4 |
| Prison/Jail | 81,801 | 4.8 | 525 | 7.9 | 0.64 | 517 | 8.1 | 0.63 | 8 | 3.5 | 0.01 | 1.5 |
| Drug Treatment | 20,485 | 1.2 | 88 | 1.3 | 0.43 | 86 | 1.3 | 0.42 | 2 | 0.9 | 0.01 | 2.3 |
| Comm. Health Center | 33,367 | 1.9 | 288 | 4.3 | 0.86 | 282 | 4.4 | 0.85 | 6 | 2.6 | 0.02 | 2.1 |
| Other | 71,945 | 4.2 | 519 | 7.8 | 0.72 | 516 | 8.0 | 0.72 | 3 | 1.3 | 0.00 | 0.6 |
Per 100
From NC SLPH CTR—number of HIV Tests performed, previous positives removed, acute follow-up tests removed. (Missing NOT included in Denominator)
CTR form = Counseling, Testing and Referral form
NC = North Carolina
SLPH = State Laboratory of Public Health
HIV = Human Immunodeficiency Virus
MSM = Men who have Sex with Men
NH = Non-Hispanic
STD = Sexually Transmitted Disease
OB = Obstetrical
TB = Tuberculous
DIS = Disease Intervention Specialist
Disease Intervention Specialist (DIS) Interview
NCDPH DIS conduct standardized partner notification with HIV. [32] NCDPH contracts with Whetstone Consultations to provide a required 2-day HIV CTR Training for all DIS. Routine partner services requires that DIS interview all new HIV positive diagnosis, however the NC STAT program prioritizes rapid notification and confirmatory testing within 72 hours of a positive HIV result for all individuals with suspected AHI.
Upon detection, each AHI case is assigned a unique STAT identification code. DIS attempt to interview, expedite HIV care referrals and initiate partner notification for all AHI cases. Prior to meeting and interviewing each case, the DIS review the information obtained during the pre-test risk-assessment. During the interview, DIS systematically collect details related to HIV acquisition, including symptoms of AHI, sexually transmitted co-infections, high risk sexual behaviors, drug use, and sexual orientation. This new information is documented on standardized STAT forms. The de-identified DIS interview data collected after diagnosis is maintained in a central, secure database at UNC specifically for AHI analysis. The DIS interview data is also maintained as part of NC surveillance data.
For our analysis, we looked at both the information collected at the time of testing (CTR form) and the data collected at the DIS interview (STAT form). We compiled the information collected at the time of testing, providing cumulative and comparative data for the entire testing population including those testing negative as well as those diagnosed with both EHI and AHI (Table 1). We then compared the difference between the data collected on the CTR form to the STAT form data.
Statistical Analysis
We calculated the percent positivity and descriptive statistics for both AHI and EHI, stratified by demographic and behavioral characteristics. Cochran-Armitage tests of trend were used to assess HIV prevalence over time. Wilcoxon rank-sum tests for continuous variables and a 2-sided Pearson chi-squared test for categorical variables were used to compare AHI and EHI diagnoses. We used the McNemar test to compare differences between the demographics and testing circumstances of AHI diagnoses at the time of testing and after DIS interviews. All analyses were performed using SAS v 9.2 (SAS Institute, Cary, NC).
RESULTS
Testing for AHI
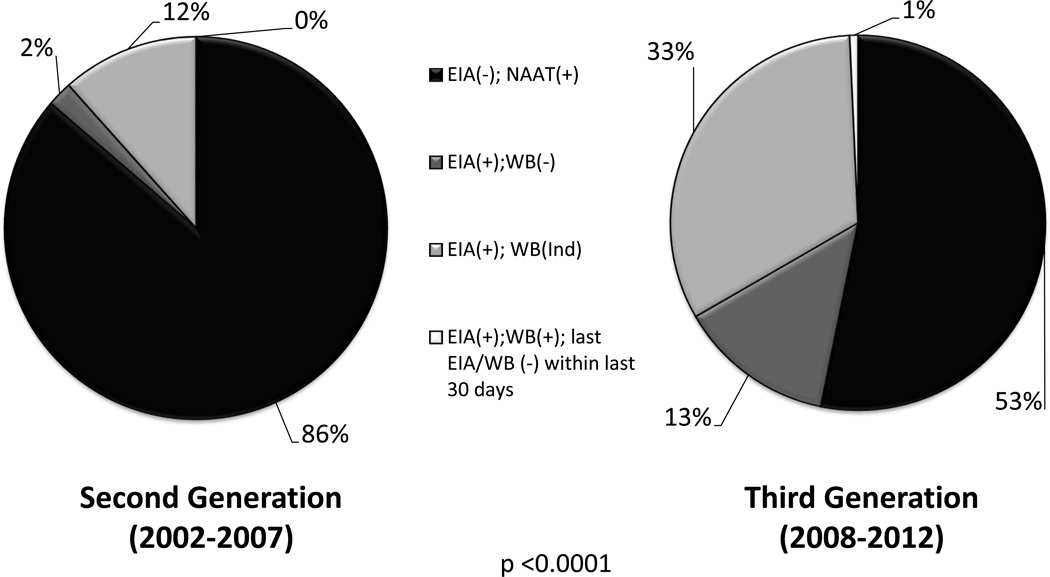
From 2003 to 2007, 2nd generation EIA tests were used to detect HIV antibodies. In 2008, the NCSLPH transitioned to a 3rd generation EIA, [8] and the increased sensitivity of this test reduced the window period that an HIV antibody test was non-reactive (Figure 1). Consequently, the observed frequency of AHI as diagnosed by a non-reactive EIA and a positive NAAT test result declined from 86.3% to 53.2%, while tests with an EIA-reactive/WB-negative or indeterminate result increased from 13.7% to 46.1% (p <0.0001) (Figure 2).
Figure 2.
Time of Testing
Between January 1, 2003 and December 31, 2012, 1,807,997 samples were tested for HIV at the NCSLPH (test/year range 112,712 – 258,719; trend p=0.001). We excluded 5,794 (0.1%) tests with missing results (insufficient sample, laboratory errors) and 2,339 (0.1%) positive test results for persons known to be HIV positive at the time of testing. The remaining 1,799,864 HIV test results are included in this analysis. Among the tested samples, 6633 (0.4%) were newly diagnosed with EHI. The remaining 1,793,231 tests were EIA negative or indeterminate for HIV and received additional (pooled or individual) HIV-1 RNA testing. Of these, 236 (0.01%) were HIV RNA positive and confirmed to have AHI. Those with AHI represented 3.4% (95% CI, 3.0 – 3.9%) of HIV-positive cases (Table 1). AHI represented a similar proportion of all new HIV diagnoses in the testing population for each year of analysis (range: 2.3– 4.9%).
The number of HIV cases detected varied substantially by HIV testing site (Table 1). HIV testing occurred most frequently at STD clinics (40.8% of tests) accounting for 40.8% of the new HIV cases (40.3% of EHI and 55.5% of AHI cases). In STD clinics, 4.7% of all new HIV diagnoses were diagnosed during AHI. Similar proportions of AHI among all new diagnoses were seen in outreach testing events (4.4%) and routine HIV testing sites (3.3%). However, AHI cases were diagnosed in all testing venue types.
The median age for HIV-negative tests over the 10-year period was younger at 25 years (IQR 21–33) as compared to newly-diagnosed persons (32 years, [IQR 24–42]; p<0.0001). Almost half (46.8%) of persons diagnosed with AHI were <25 years of age, compared with 26.9% of those diagnosed with EHI (p<0.001). Nearly half (47.2%) of HIV tests were obtained from persons who reported their race and ethnicity as Black. This group represented 73.2% of persons diagnosed with HIV infection (73.4% EHI and 69.1% AHI diagnoses). Newly-diagnosed, black persons were less likely to be diagnosed with AHI (3.2%), as compared to white non-Hispanics (4.7%) (p=0.01), (Table 1).
Women provided 69.0% of the total number of samples tested for HIV, but represented only 26.7% of new HIV diagnoses (27.0% of EHI and 18.5% of AHI diagnoses), (Table 1). The proportion of HIV-infected women diagnosed during AHI was 2.4%, significantly lower than the proportion of HIV-infected men diagnosed during AHI (3.8%) (p=0.006).
Only 2.8% of all tested samples were obtained from persons who self-identified as men who have sex with men (MSM). However, MSM as assessed by the CTR represented 34.7% of all newly-diagnosed cases (Table 1); 34.1% of persons diagnosed during EHI reported MSM behavior compared to 51.7% of AHI diagnosed (p<0.0001). When including only men with HIV, 47.3% reported MSM behavior at the time of HIV testing; (46.6% EHI versus 63.5% AHI [p=<0.0001]).
CTR collected data showed that young (< 25 years) Black MSM (YBMSM) were disproportionately represented among AHI cases. Although only comprising 1.9% of all men tested, 25.1% of males diagnosed during AHI were Black and <25 years of age. The median age of MSM with AHI was younger for Black MSM (22, IQR 20–25) compared to all non-Black MSM (28, IQR 24–37) (p<0.0001). The proportion of tests attributed to YBMSM was consistent each year of the analysis period. However, the absolute number of YBMSM tested increased each year (data not shown).
DIS Interview
DIS found and interviewed 228 (96.6%) persons diagnosed with AHI. Similar to other HIV testing programs utilizing rapid partner service [33, 34], a total of 220 (96.5%) of the 228 cases had confirmatory testing. The median time from the date of the initial HIV test to the date results were available from the lab was 12 days (IQR 9–14 days). DIS took a median of 1 additional day (IQR 0–3 days) to interview the AHI case. More than 75% of confirmatory testing occurred during the DIS interview (range 0–29 days).
Data collected on the CTR form agreed with the DIS interview data on all variables considered except reason for testing and sexual risk status. While only 3 (1.3%) AHI cases reported symptoms as the reason for HIV testing at the time of testing, 43 (18.9%) of AHI cases interviewed reported this as the reason for testing during DIS interview (p<0.0001). An additional 132 (57.9%) AHI cases reported AHI symptoms within the 8 weeks prior to HIV diagnosis when interviewed, despite not providing it as a reason for testing. Women with AHI were more likely to be asymptomatic when compared to men (38.1% vs.19.9%; p= 0.01).
The proportion of men with AHI reporting sex with men increased from 62.9% (117/186) at time of testing to 82.8% (154/186) at the time of the DIS interview (p=<0.0001). Similarly, among young black men, the proportion of cases reporting sex with men increased from 61.2% (52/85) at testing to 92.9% (79/85) during DIS interviews (p=<0.0001). Among persons who changed risk category during the post-test interview, two initially self-identified as female, 20 identified as heterosexual and 9 indicated unknown risk; all subsequently identified as MSM. The majority of these (26/31) were YBMSM.
Trends
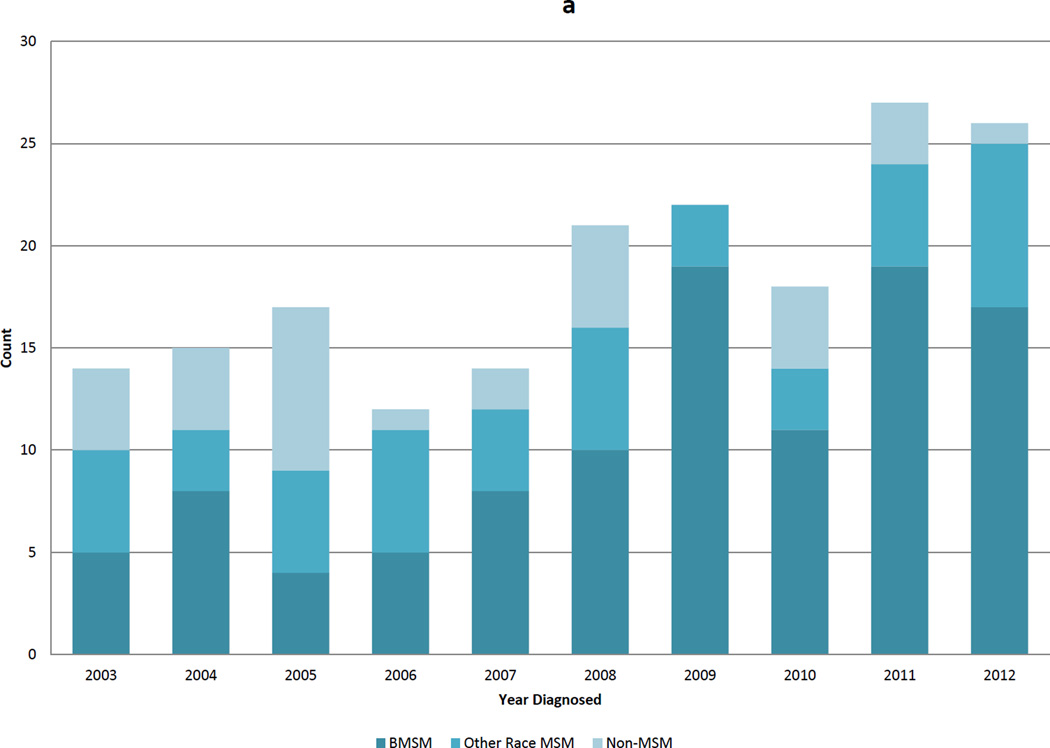
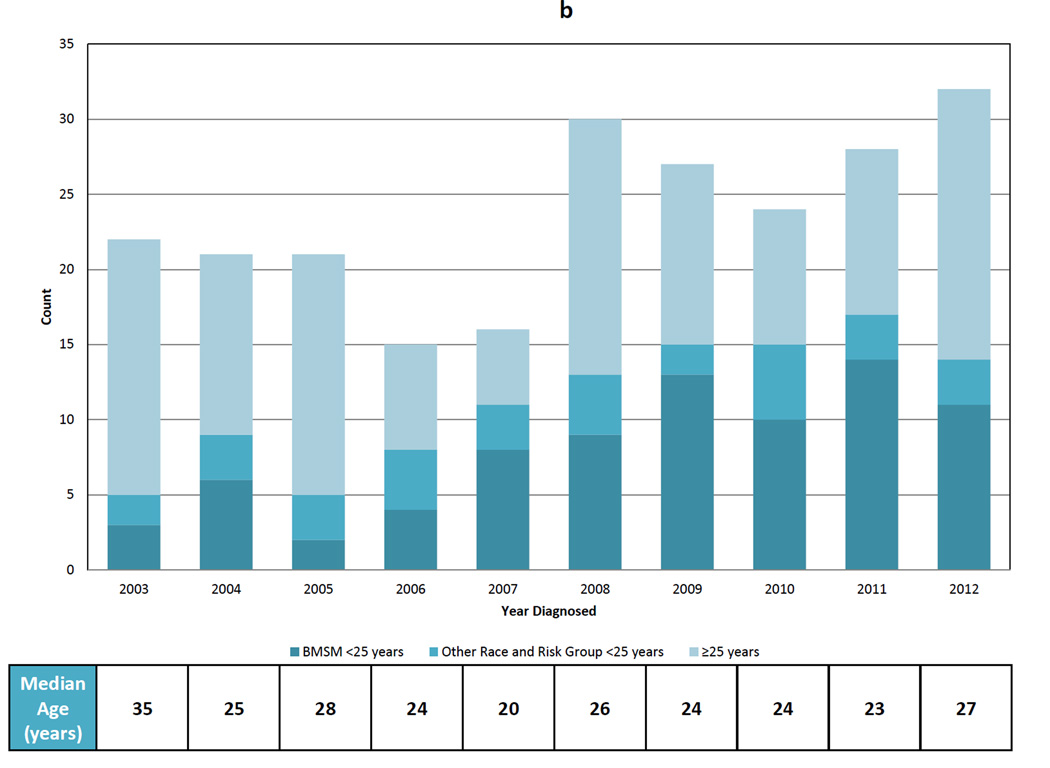
The DIS interview data revealed several trends over the 10-year period. The proportion of MSM among men with AHI increased from 71.4% in 2003 to 96.2% in 2012 (p=0.002) (Figure 3a). The median age decreased from 35 years (IQR 25–42) to 27 years (IQR 22–37), with the lowest median age (20; IQR 19–27) observed in 2007 (Figure 3b). The proportion of AHI cases <25 years of age at diagnosis increased from 23.8% to 45.2% (trend p=0.03) (Figure 3b). This decrease in median age was driven by an increase in the proportion of YBMSM diagnosed from 14.3% to 35.5%. YBMSM represented 50.0% of AHI cases in 2007, 2009, and 2011 (Figure 3b).
Figure 3.
DISCUSSION
In NC, persons diagnosed with AHI consistently represented 3.4% of all HIV diagnoses from 2003 to 2012. The proportion of persons with newly diagnosed HIV who were acute varied slightly by age, race and sex, with the highest proportion observed in MSM and persons <25 years. AHI was detected at all testing sites, including testing sites with very low HIV prevalence, such as family planning clinics. The consistent AHI detection, regardless of demographics, suggests that AHI screening is important and should be incorporated into all HIV testing algorithms. [35, 36] Failure to test for AHI increases the risk of onward transmission and misses opportunities for intervention and early ART initiation. [1, 4, 37, 38]
The in-person DIS interviews after diagnosis revealed a substantial increase in the number and proportion of AHI persons reporting sex with men, particularly in YBMSM. The high rate of AHI among YBMSM is consistent with national trends in overall HIV diagnosis. [20, 39–41] The continued increase in the proportion of AHI cases among YBMSM in NC indicates that this group remains a focal point of HIV incidence. [42] If demographic and behavioral trends seen in AHI diagnoses are predictive of overall trends in HIV incidence, then targeted prevention and testing is imperative. Any shifts in these parameters should be considered a forecaster for trends in new EHI diagnoses and prevention efforts should be re-evaluated and adjusted accordingly.
Persons diagnosed during AHI were consistently younger than those diagnosed during EHI; however, the age distribution of the general testing population mirrored those with AHI. A plausible explanation for the observed age difference may reflect an increase in the number of younger persons, particularly MSM, undergoing routine testing. Increased testing frequency increases the likelihood of AHI diagnosis. [43] Because our dataset allows for the same person to test more than once, it is possible that high risk persons, who may be younger, are testing more frequently, increasing AHI detection in a younger population, or the shift in demographics may represent a true increase in incidence among young MSM.
NC women are infrequently diagnosed during AHI despite representing the majority of HIV testing events. Identification of at-risk women needs to be prioritized in order to test and diagnose women effectively. Similar to previous reports, women reported fewer symptoms than men during the acute phase [12, 44], suggesting that women may overlook or be less likely to experience AHI symptoms, potentially delaying HIV testing and care-seeking behaviors. Additionally, women may be seen as lower risk and HIV testing may not be offered even when there are classic symptoms and risk behaviors.
Notably, we found that reported pre-test risk behaviors were frequently inaccurate, particularly among YBMSM. Those diagnosed with AHI under-reported MSM risk; 26 (33.8%) of YBMSM reporting sex with men during the DIS interview did not report this risk on the CTR form at the time of testing and is consistent with previously published pre-test behaviors. [45–47] Furthermore, AHI symptoms are often not elicited, reported or considered as a reason for testing by individuals or providers. [11, 12] However, after diagnosis, three-quarters of AHI cases in our study reported symptoms consistent with AHI, suggesting persons may not accurately report risk or associate symptoms with HIV infection until specifically asked or made aware of their diagnosis. We show that current standardized assessment forms used to collect HIV risk and symptoms have limited utility in assessing risk for AHI or EHI given substantial MSM underreporting, [45–47] making precise prevention counseling or targeted screening for AHI challenging. [29] Many factors and perceptions influence disclosure at testing, and though varied, these are primarily associated with comfort with one’s sexual identity and perceived acceptance. [48, 49] Incorporating computer based risk assessment tools might allow more accurate disclosure by removing fear of judgment from the process. [47]. Obtaining more accurate pre-test behavioral and clinical information will elicit better assessment of true risk and facilitate effective testing of high risk groups. Routine screening of all samples for AHI is a strength of the STAT program, allowing effective identification of AHI despite under-reported risk at the time of testing.
In our analysis, nearly three-quarters of all new HIV diagnoses were Black, while less than one-quarter of the total NC population is Black. [31] Because our analysis excludes testing done in private settings, our results are limited to the subset of those diagnosed through publicly-funded sites, which comprise approximately one third of all HIV positive diagnoses in NC each year. [31] Accordingly, people testing at publicly-funded testing sites may differ from those testing at private sites in terms of socio-demographic, HIV risk and geographic factors.
Our 10-year study period illustrates the evolution of HIV testing assays and its impact on AHI diagnosis. New diagnostic algorithms continue to evolve, decreasing time from testing to the reporting of results. The new combination p24 antigen and HIV antibody EIA assay further shortens the window between HIV acquisition and diagnosis, making AHI detection more feasible in the context of routine HIV testing. [35, 50] Approximately 10–20% AHI diagnosis are detected only due to a positive HIV RNA [2], and AHI cases previously detected by a positive EIA antibody and a negative or indeterminate WB may no longer be identified as acute by 4th generation assay (46% of AHI in our study from 2008-12), but instead as established infection. For AHI screening programs like ours, which facilitates rapid tracing of persons with AHI (and their contacts) and thus early ART initiation, the switch to 4th generation testing will miss very early and later stages of acute infection, potentially diminishing the impact on transmission. STAT, and programs endorsing rapid notification, successfully locate 96–100% new positives. [33, 34] Accordingly, in high risk populations, HIV RNA screening and alternative assays to detect recent HIV infection [9] should be considered as supplements to fourth generation testing for fully optimal AHI detection.
For more than 10 years, NC successfully sustained a statewide screening program for AHI. Despite a relatively low risk testing population, 3.4% of all persons diagnosed with HIV had AHI. AHI testing projects (Seattle, WA, San Francisco, San Diego and Los Angeles, CA, Lilongwe, Malawi and Cape Town, South Africa) reported similar increase in AHI detection when using pooled NAAT screenings. [16, 18, 24, 51–54] Notably, most of these programs are clinic-based within cities, and the STAT program remains the only statewide screening program to our knowledge. Data from the STAT program provides predictive value for assessing emerging trends in HIV incidence [55–57], observes an increase in AHI in YBMSM corresponding to national incidence estimates, [20, 39] and facilitates real time evaluation of individual AHI cases. [2, 4] Thorough DIS interviews lead to the identification of individuals at risk, other undiagnosed HIV infections (some of which are acute), and high risk sexual networks. [42] The STAT program allows for immediate intervention and testing of persons participating in these high risk sexual networks, potentially impacting transmission. [26, 55–57]
The benefits of the STAT program are substantial and offer a unique opportunity to identify AHI cases and their at-risk and infected partners. [1, 2, 4, 58] This provides persons with AHI an opportunity for behavior change and, if linked to care, the opportunity to initiate ART early, thereby improving health outcomes and decreasing infectiousness. By identifying high-risk HIV negative partners of AHI cases, the STAT program provides an avenue for identifying and potentially initiating pre-exposure prophylaxis (PrEP) to a population most likely to gain benefit. [59, 60] Thus, the STAT Program allows for a prioritized focus on prevention, education, HIV testing, rapid linkage to care and immediate initiation of ART, all of which are critical and will impact the HIV epidemic.
Acknowledgements
We thank the Disease Intervention Specialist of the Communicable Disease Branch of the NC Department of Health and Human Services for contacting and interviewing all newly diagnosed AHI persons and their partners. Without their hard work and dedication, the STAT program would not have been successfully implemented. We thank the staff of the Laboratory of Public Health Serology/Virology who did the antibody testing and pooling. Myron Cohen, MD, University of North Carolina at Chapel Hill, for his work on the initial establishment of the STAT program and Christopher Pilcher, MD, University of California at San Francisco, for his role in the establishment and implementation of the program. Sandy McCoy, PhD, MPH, University of California, Berkley, Sabrina Zadrozny, MS., Gillings School of Public Health, UNC and Ashley Mayo, MSPH, FHI 360, Research Triangle, NC for their important role in STAT data collection and analysis over the 10 year period. The NC Division of Public Health has been instrumental to the success of the STAT Program, we would like to thank Jacquelyn Clymore, MS, Todd VanHoy and Wallace Lambert, BA for their continued assistance and support.
Role of the Sponsors
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Source of Funding
The STAT program was funded in part during the first 5 years (2002 - 2007) by National institute of Allergy and Infectious Disease, grants, R01 MH068686 and K23 AI001781.
JE is supported by the UNC Center of AIDS Research (P30 AI050410).
CG was supported by a grant from the Center for Disease Control (5U01PS001559-04) outside the submitted work,
JE reports grants from NIH, grants from the North Carolina Department of Health and Human Services, during the conduct of the study; grants from Abbvie and ViiV Healthcare and personal fees from Merck, ViiV Healthcare, and Abbvie, Bristol Meyers Squibb and personal fees from Gilead Sciences, and Janssen, outside the submitted work.
DM reports grant from NIH, NIAID, during the conduct of the study; personal fees from Merck and other from Gilead, Abbvie and Abbott, outside the submitted work.
CG reports grants from Argos Therapeutics, Abbott Laboratories, Janssen, Gilead, amFAR and BMS, and personal fees from UpToDate, outside submitted work.
PL reports personal fees from Abbott Diagnostics outside the submitted work.
WM reports grants from NIH during the conduct of the study; grants from NIH outside the submitted work
Footnotes
Previous Presentations
This work was presented in part at the 19th Conference on Retroviruses and Opportunistic Infections, Seattle, WA, March 2012, abstract 566 and at the 5th Conference on HIV Pathogenesis, Treatment and Prevention, Cape Town, South Africa, July 2009, abstract MOPEB006.
Conflicts of Interest
All other authors, Drs. Cope and Sampson, Mr. Barnhart and Mss. Foust, Brinson, Ashby and Kuruc report no conflict of interest.
REFERENCES
- 1.Smith MK, Rutstein SE, Powers KA, Fidler S, Miller WC, Eron JJ, Jr, et al. The detection and management of early HIV infection: a clinical and public health emergency. Journal of acquired immune deficiency syndromes (1999) 2013;63(Suppl 2):S187–S199. doi: 10.1097/QAI.0b013e31829871e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gay C, Dibben O, Anderson JA, Stacey A, Mayo AJ, Norris PJ, et al. Cross-sectional detection of acute HIV infection: timing of transmission, inflammation and antiretroviral therapy. PloS one. 2011;6:e19617. doi: 10.1371/journal.pone.0019617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powers KA, Ghani AC, Miller WC, Hoffman IF, Pettifor AE, Kamanga G, et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet. 2011;378:256–268. doi: 10.1016/S0140-6736(11)60842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gay CL, Mayo AJ, Mfalila CK, Chu H, Barry AC, Kuruc JD, et al. Efficacy of NNRTI-based antiretroviral therapy initiated during acute HIV infection. AIDS. 2011;25:941–949. doi: 10.1097/QAD.0b013e3283463c07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller WC, Rosenberg NE, Rutstein SE, Powers KA. Role of acute and early HIV infection in the sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5:277–282. doi: 10.1097/COH.0b013e32833a0d3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. Journal of acquired immune deficiency syndromes (1999) 2005;39:446–453. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 7.Branson BM, Stekler JD. Detection of acute HIV infection: we can't close the window. The Journal of infectious diseases. 2012;205:521–524. doi: 10.1093/infdis/jir793. [DOI] [PubMed] [Google Scholar]
- 8.Branson BM. The future of HIV testing. Journal of acquired immune deficiency syndromes (1999) 2010;55(Suppl 2):S102–S105. doi: 10.1097/QAI.0b013e3181fbca44. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg NE, Pilcher CD, Busch MP, Cohen MS. How can we better identify early HIV infections? Current opinion in HIV and AIDS. 2015;10:61–68. doi: 10.1097/COH.0000000000000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hightow-Weidman LB, Golin CE, Green K, Shaw EN, MacDonald PD, Leone PA. Identifying people with acute HIV infection: demographic features, risk factors, and use of health care among individuals with AHI in North Carolina. AIDS and behavior. 2009;13:1075–1083. doi: 10.1007/s10461-008-9519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weintrob AC, Giner J, Menezes P, Patrick E, Benjamin DK, Jr, Lennox J, et al. Infrequent diagnosis of primary human immunodeficiency virus infection: missed opportunities in acute care settings. Arch Intern Med. 2003;163:2097–2100. doi: 10.1001/archinte.163.17.2097. [DOI] [PubMed] [Google Scholar]
- 12.McKellar MS, Cope AB, Gay CL, McGee KS, Kuruc JD, Kerkau MG, et al. Acute HIV-1 Infection in the Southeastern United States: A Cohort Study. AIDS Res Hum Retroviruses. 2012 doi: 10.1089/aid.2012.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilcher CD, Fiscus SA, Nguyen TQ, Foust E, Wolf L, Williams D, et al. Detection of acute infections during HIV testing in North Carolina. N Engl J Med. 2005;352:1873–1883. doi: 10.1056/NEJMoa042291. [DOI] [PubMed] [Google Scholar]
- 14.Zetola NM, Pilcher CD. Diagnosis and management of acute HIV infection. Infect Dis Clin North Am. 2007;21:19–48. doi: 10.1016/j.idc.2007.01.008. vii. [DOI] [PubMed] [Google Scholar]
- 15.Pilcher CD, McPherson JT, Leone PA, Smurzynski M, Owen-O'Dowd J, Peace-Brewer AL, et al. Real-time, universal screening for acute HIV infection in a routine HIV counseling and testing population. JAMA. 2002;288:216–221. doi: 10.1001/jama.288.2.216. [DOI] [PubMed] [Google Scholar]
- 16.Priddy FH, Pilcher CD, Moore RH, Tambe P, Park MN, Fiscus SA, et al. Detection of acute HIV infections in an urban HIV counseling and testing population in the United States. J Acquir Immune Defic Syndr. 2007;44:196–202. doi: 10.1097/01.qai.0000254323.86897.36. [DOI] [PubMed] [Google Scholar]
- 17.Voelker R. Detecting acute HIV infections feasible, North Carolina program demonstrates. JAMA. 2003;289:2633–2634. doi: 10.1001/jama.289.20.2633. [DOI] [PubMed] [Google Scholar]
- 18.Fiscus SA, Pilcher CD, Miller WC, Powers KA, Hoffman IF, Price M, et al. Rapid, real-time detection of acute HIV infection in patients in Africa. The Journal of infectious diseases. 2007;195:416–424. doi: 10.1086/510755. [DOI] [PubMed] [Google Scholar]
- 19.Prejean J, Tang T, Hall HI. HIV diagnoses and prevalence in the southern region of the United States, 2007–2010. J Community Health. 2013;38:414–426. doi: 10.1007/s10900-012-9633-1. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan PS, Rosenberg ES, Sanchez TH, Kelley CF, Luisi N, Cooper HL, et al. Explaining racial disparities in HIV incidence in black and white men who have sex with men in Atlanta, GA: a prospective observational cohort study. Ann Epidemiol. 2015;25:445–454. doi: 10.1016/j.annepidem.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Facente SN, Pilcher CD, Hartogensis WE, Klausner JD, Philip SS, Louie B, et al. Performance of risk-based criteria for targeting acute HIV screening in San Francisco. PLoS One. 2011;6:e21813. doi: 10.1371/journal.pone.0021813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinberg M, Cook DA, Gilbert M, Krajden M, Haag D, Tsang P, et al. Towards targeted screening for acute HIV infections in British Columbia. J Int AIDS Soc. 2011;14:39. doi: 10.1186/1758-2652-14-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease C, Prevention. Acute HIV infection - New York City, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:1296–1299. [PubMed] [Google Scholar]
- 24.Patel P, Klausner JD, Bacon OM, Liska S, Taylor M, Gonzalez A, et al. Detection of acute HIV infections in high-risk patients in California. Journal of acquired immune deficiency syndromes (1999) 2006;42:75–79. doi: 10.1097/01.qai.0000218363.21088.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.2010 Census Urban and Rural Classification and Urban Area Criteria. United States Census Bureau; [Google Scholar]
- 26.Patterson KB, Leone PA, Fiscus SA, Kuruc J, McCoy SI, Wolf L, et al. Frequent detection of acute HIV infection in pregnant women. AIDS. 2007;21:2303–2308. doi: 10.1097/QAD.0b013e3282f155da. [DOI] [PubMed] [Google Scholar]
- 27.Westreich DJ, Hudgens MG, Fiscus SA, Pilcher CD. Optimizing screening for acute human immunodeficiency virus infection with pooled nucleic acid amplification tests. J Clin Microbiol. 2008;46:1785–1792. doi: 10.1128/JCM.00787-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCoy SI, Eron JJ, Kuruc JD, Strauss RP, Macdonald PD, Fiscus SA, et al. Sexually transmitted infections among patients with acute HIV in North Carolina. Sex Transm Dis. 2009;36:372–374. doi: 10.1097/OLQ.0b013e3181997252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller WC, Leone PA, McCoy S, Nguyen TQ, Williams DE, Pilcher CD. Targeted testing for acute HIV infection in North Carolina. AIDS. 2009;23:835–843. doi: 10.1097/QAD.0b013e328326f55e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore ZS, McCoy S, Kuruc J, Hilton M, Leone P. Number of named partners and number of partners newly diagnosed with HIV infection identified by persons with acute versus established HIV infection. J Acquir Immune Defic Syndr. 2009;52:509–513. doi: 10.1097/QAI.0b013e3181ac12bf. [DOI] [PubMed] [Google Scholar]
- 31.Raleigh, NC 27699-1902: Division of Public Health, Epidemiology Section. Communicable Disease Branch; 2012. Dec, North Carolina Epidemiologic Profile for HIV/STD Prevention and Care Planning. [Google Scholar]
- 32.Centers for Disease C, Prevention. Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection. MMWR Recomm Rep. 2008;57:1–83. quiz CE81–84. [PubMed] [Google Scholar]
- 33.Lin X, Dietz PM, Rodriguez V, Lester D, Hernandez P, Moreno-Walton L, et al. Routine HIV screening in two health-care settings--New York City and New Orleans, 2011–2013. MMWR. Morbidity and mortality weekly report. 2014;63:537–541. [PMC free article] [PubMed] [Google Scholar]
- 34.Wolpaw BJ, Mathews C, Chopra M, Hardie D, Lurie MN, Jennings K. Diagnosis and counselling of patients with acute HIV infection in South Africa. Sex Transm Infect. 2011;87:71–72. doi: 10.1136/sti.2009.041475. [DOI] [PubMed] [Google Scholar]
- 35.Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm - United States, 2011–2013. MMWR. Morbidity and mortality weekly report. 2013;62:489–494. [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease C, Prevention. Vital Signs: HIV Infection, Testing, and Risk Behaviors Among Youths - United States. MMWR. Morbidity and mortality weekly report. 2012;61:971–976. [PubMed] [Google Scholar]
- 37.Le T, Wright EJ, Smith DM, He W, Catano G, Okulicz JF, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. The New England journal of medicine. 2013;368:218–230. doi: 10.1056/NEJMoa1110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ananworanich J, Schuetz A, Vandergeeten C, Sereti I, de Souza M, Rerknimitr R, et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PloS one. 2012;7:e33948. doi: 10.1371/journal.pone.0033948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prejean J, Song R, Hernandez A, Ziebell R, Green T, Walker F, et al. Estimated HIV incidence in the United States, 2006–2009. PLoS One. 2011;6:e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balaji AB, Bowles KE, Le BC, Paz-Bailey G, Oster AM. High HIV incidence and prevalence and associated factors among young MSM, 2008. AIDS. 2013;27:269–278. doi: 10.1097/QAD.0b013e32835ad489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Purcell DW, Johnson CH, Lansky A, Prejean J, Stein R, Denning P, et al. Estimating the population size of men who have sex with men in the United States to obtain HIV and syphilis rates. The open AIDS journal. 2012;6:98–107. doi: 10.2174/1874613601206010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peters PJ, Gay C, Beagle S, Shankar A, Switzer WM, Hightow-Weidman LB. HIV infection among partners of HIV-infected black men who have sex with Men - North Carolina, 2011–2013. MMWR. Morbidity and mortality weekly report. 2014;63:90–94. [PMC free article] [PubMed] [Google Scholar]
- 43.Long EF. HIV screening via fourth-generation immunoassay or nucleic acid amplification test in the United States: a cost-effectiveness analysis. PloS one. 2011;6:e27625. doi: 10.1371/journal.pone.0027625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meditz AL, MaWhinney S, Allshouse A, Feser W, Markowitz M, Little S, et al. Sex, race, and geographic region influence clinical outcomes following primary HIV-1 infection. The Journal of infectious diseases. 2011;203:442–451. doi: 10.1093/infdis/jiq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu H. Conference on Retroviruses and Opportunistic Infections. Seattle, WA: 2015. Unreported Sexual Risk Behavior Among MSM Newly Diagnosed with HIV Infection. [Google Scholar]
- 46.Torrone EA, Thomas JC, Kaufman JS, Pettifor AE, Leone PA, Hightow-Weidman LB. Glen or Glenda: reported gender of sex partners in two statewide HIV databases. Am J Public Health. 2010;100:525–530. doi: 10.2105/AJPH.2009.162552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torrone EA, Thomas JC, Maman S, Pettifor AE, Kaufman JS, Sena AC, et al. Risk behavior disclosure during HIV test counseling. AIDS Patient Care STDS. 2010;24:551–561. doi: 10.1089/apc.2010.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hue S, Brown AE, Ragonnet-Cronin M, Lycett SJ, Dunn DT, Fearnhill E, et al. Phylogenetic analyses reveal HIV-1 infections between men misclassified as heterosexual transmissions. AIDS. 2014;28:1967–1975. doi: 10.1097/QAD.0000000000000383. [DOI] [PubMed] [Google Scholar]
- 49.Stekler J, Collier AC, Holmes KK, Golden MR. Primary HIV infection education: knowledge and attitudes of HIV-negative men who have sex with men attending a public health sexually transmitted disease clinic. J Acquir Immune Defic Syndr. 2006;42:123–126. doi: 10.1097/01.qai.0000218357.82970.67. [DOI] [PubMed] [Google Scholar]
- 50.Nasrullah M, Wesolowski LG, Meyer WA, 3rd, Owen SM, Masciotra S, Vorwald C, et al. Performance of a fourth-generation HIV screening assay and an alternative HIV diagnostic testing algorithm. AIDS. 2013;27:731–737. doi: 10.1097/QAD.0b013e32835bc535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stekler J, Maenza J, Stevens CE, Swenson PD, Coombs RW, Wood RW, et al. Screening for acute HIV infection: lessons learned. Clin Infect Dis. 2007;44:459–461. doi: 10.1086/510747. [DOI] [PubMed] [Google Scholar]
- 52.Pilcher CD, Price MA, Hoffman IF, Galvin S, Martinson FE, Kazembe PN, et al. Frequent detection of acute primary HIV infection in men in Malawi. AIDS. 2004;18:517–524. doi: 10.1097/00002030-200402200-00019. [DOI] [PubMed] [Google Scholar]
- 53.Pilcher CD, Louie B, Facente S, Keating S, Hackett J, Jr, Vallari A, et al. Performance of Rapid Point-of-Care and Laboratory Tests for Acute and Established HIV Infection in San Francisco. PloS one. 2013;8:e80629. doi: 10.1371/journal.pone.0080629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cohen MS, Gay CL, Busch MP, Hecht FM. The detection of acute HIV infection. J Infect Dis. 2010;202(Suppl 2):S270–S277. doi: 10.1086/655651. [DOI] [PubMed] [Google Scholar]
- 55.Hurt CB, Beagle S, Leone PA, Sugarbaker A, Pike E, Kuruc J, et al. Investigating a sexual network of black men who have sex with men: implications for transmission and prevention of HIV infection in the United States. Journal of acquired immune deficiency syndromes (1999) 2012;61:515–521. doi: 10.1097/QAI.0b013e31827076a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dennis AM, Hue S, Hurt CB, Napravnik S, Sebastian J, Pillay D, et al. Phylogenetic insights into HIV transmission in North Carolina. AIDS. 2012 doi: 10.1097/QAD.0b013e3283573244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hurt CB, Matthews DD, Calabria MS, Green KA, Adimora AA, Golin CE, et al. Sex with older partners is associated with primary HIV infection among men who have sex with men in North Carolina. J Acquir Immune Defic Syndr. 2010;54:185–190. doi: 10.1097/QAI.0b013e3181c99114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gunthard HF, Aberg JA, Eron JJ, Hoy JF, Telenti A, Benson CA, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA. 2014;312:410–425. doi: 10.1001/jama.2014.8722. [DOI] [PubMed] [Google Scholar]
- 59.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. The New England journal of medicine. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14:820–829. doi: 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]