Abstract
Importance
Depression and inadequate self-care are common and interrelated problems that increase the risks of hospitalization and mortality in patients with heart failure (HF).
Objective
To determine the efficacy of an integrative cognitive behavior therapy (CBT) intervention for depression and HF self-care.
Design, Setting, and Participants
Randomized clinical trial with single-blind outcome assessments. Eligible patients were enrolled at Washington University Medical Center in St. Louis between January 4, 2010 and June 28, 2013. The participants were 158 outpatients in New York Heart Association Class I, II, and III heart failure with comorbid major depression.
Interventions
Cognitive behavior therapy delivered by experienced therapists plus usual care (UC), or usual care alone. Usual care was enhanced in both groups with a structured HF education program delivered by a cardiac nurse.
Main Outcomes and Measures
The primary outcome was severity of depression at 6 months as measured by the Beck Depression Inventory. The Self-Care of Heart Failure Index Confidence and Maintenance subscales were co-primary outcomes. Secondary outcomes included measures of anxiety, depression, physical functioning, fatigue, social roles and activities, and quality of life. Hospitalizations and deaths were exploratory outcomes.
Results
One hundred fifty-eight patients were randomized to UC (n=79) or CBT (n=79). Within each arm, 26 (33%) of the patients were taking an antidepressant at baseline. One hundred thirty-two (84%) of the participants completed the 6-month posttreatment assessments; 60 (76%) of the UC and 58 (73%) of the CBT participants completed every follow-up assessment (P−.88). Six-month depression scores were lower in the CBT than the UC arm on the Beck Depression Inventory (BDI-II) (12.8 [10.6] vs 17.3 [10.7]; P−.008). Remission rates differed on the BDI-II (46% vs 19%; number needed to treat [NNT] = 3.76; 95% CI, 3.62-3.90; P<.001) and the Hamilton Depression Scale (51% vs 20%; NNT=3.29; 95% CI, 3.15-3.43; P<.001). The groups did not differ on the Self-Care Maintenance or Confidence subscales. The mean (SD) Beck Depression Inventory scores 6 months after randomization were lower in the CBT (12.8[10.6]) than the UC arm (17.3 [10.7]), P=.008. There were no statistically significant differences between the groups on the Self-Care Maintenance or Confidence subscales scores or on physical functioning measures. Anxiety and fatigue scores were lower and mental- and HF-related quality of life and social functioning scores were higher at 6 months in the CBT than the UC arm, and there were fewer hospitalizations in the intervention than the UC arm.
Conclusions and Relevance
A CBT intervention that targets both depression and heart failure self-care is efficacious for depression but not for HF self-care or physical functioning relative to enhanced usual care. Additional benefits include reduced anxiety and fatigue, improved social functioning, and better health-related quality of life.
Trial Registration clinicaltrials.gov Identifier: NCT01028625
Major depression is a common comorbidity in heart failure (HF).1-3 It is associated with poor quality of life4,5 and an increased risk for hospitalization6-10 and mortality.6,9,11,12 It is also difficult to treat. Sertraline Against Depression and Heart Disease in Chronic Heart Failure (SADHART-CHF) is the largest (n=469) randomized clinical trial (RCT) to date of an antidepressant for major depression in HF. There was no difference in post-treatment depression between the sertraline and placebo arms.13 More recently, the Mortality, Morbidity, and Mood in Depressed Heart Failure Patients (MOOD-HF) trial14 found no difference in post-treatment depression between the escitalopram and placebo arms in 372 patients with heart failure.15 There are well-established behavioral treatments for depression in psychiatric patients16, but little is known about their efficacy for comorbid major depression in HF.
Inadequate self-care is also common in HF.17 Self-care includes behaviors that maintain physical functioning and prevent acute exacerbations, such as following a low-sodium diet, exercising, taking prescribed medications, and monitoring edema. Heart failure self-care reduces the risk of hospitalization18 and improves HF-related quality of life.19 Like depression, however, inadequate HF self-care can be difficult to modify. Two of the largest HF self-care trials yielded modest20 or no21 differences between the intervention and comparison arms.
Depression is a barrier to HF self-care, and poor self-care is associated with depression.22-24 Thus, an intervention that targets both problems might achieve better outcomes than interventions for only one of them.17 The Depression and Self-Care of Heart Failure trial evaluated the efficacy of an integrative behavioral intervention for depression and HF self-care.
Methods
Participants
Patients with HF at Washington University Medical Center in St. Louis, Missouri were invited to participate in this study between January 4, 2010 and June 28, 2013. The primary statistical data analyses were conducted in February 2015. The inclusion criteria were 1) HF diagnosed 3 or fewer months prior to screening, 2) current major depressive episode25, and 3) a “depressed” score (≥14) on the Beck Depression Inventory (BDI-II).26 Heart failure self-care deficits were not required. The exclusion criteria were 1) inability to participate due to cognitive impairment, frailty, a communication deficit, or a logistical barrier; 2) poor 1-year prognosis due to a non-cardiac comorbidity; 3) hospitalization within the past month; 4) suicidality, psychosis, or substance abuse; or 5) initiation of an antidepressant within the past 8 weeks. Patients who had been on an antidepressant for more than 8 weeks were allowed to continue. Participants provided written informed consent and were compensated for completing the assessments. The study was approved by the Human Research Protection Office at Washington University Medical Center.
Randomization
The study was a single-blind, parallel groups, randomized controlled trial. After completing the baseline evaluation, participants were randomly assigned in a 1:1 ratio to cognitive behavior therapy (CBT) plus usual care or to usual care alone. Randomization with permuted blocks of 2, 4, or 6 pairs was stratified by antidepressant use at baseline. Allocations were concealed in sequentially numbered opaque envelopes (one set per stratum) and opened by the study coordinator after the baseline evaluation.
Enhanced Usual Care
Participants continued their usual medical care during the trial, with no restrictions on the continuation or initiation of non-study medications. All participants received educational materials on HF self-care from the Heart Failure Society of America27 and the American Heart Association.28 A cardiac nurse reviewed the materials with the participant during the baseline visit and on three 30-minute telephone calls over 3 to 4 weeks post-randomization.
Intervention
The treatment followed standard CBT manuals29,30 and a supplemental manual31 on CBT for cardiac patients. The initial clinical evaluation included a review of the Self-Care of Heart Failure Index32. If self-care deficits or other barriers to self-care were identified, standard CBT techniques were used to address them (eMethods 1 in Supplement 1).
The intensive phase of the intervention consisted of up to 6 months of weekly 1-hour sessions. Collaborative problem lists and treatment plans were developed to individualize the treatment. Progress toward treatment goals was monitored, and treatment plans were adjusted as needed. A treat-to-target strategy was followed so that the therapy schedule thinned when a set of depression, HF self-care, and CBT skill criteria were met. Sessions tapered to biweekly and then monthly between the end of intensive (weekly) treatment and 6 months post-randomization. Up to four 20 to 30 minute relapse prevention telephone contacts were provided as needed between 6 and 12 months post-randomization.
The cases were divided between 2 masters-level and 2 doctoral-level therapists, all of whom had prior training and experience with CBT for depression. Weekly clinical supervision meetings included reviews of case conceptualizations, treatment plans, and clinical progress.
Treatment Fidelity and Adherence
The therapists completed a CBT technique checklist after each session to document fidelity to the intervention protocol. They also completed ratings of homework, use of CBT techniques in daily life, and HF self-care to assess the participant’s adherence. Data on nonstudy medical and psychiatric care were collected to evaluate the potential for co-intervention bias.33,34
Measures
Baseline assessments were conducted between February 2010 and April 2013, and follow-up assessments were conducted between May 2010 and July 2014. The outcome assessors were blinded to group assignments. Baseline and 6-month assessments included the Depression Interview and Structured Hamilton35 to diagnose major depression and to rate the severity of depression on the Hamilton Rating Scale for Depression36, a 6-minute walk test of submaximal exercise capacity37-39, 1 week of actigraphy to assess physical activity40, and several of the National Institute of Health’s Patient-Reported Outcomes Measurement Information System (PROMIS) measures, including the Depression, Anxiety, Physical Functioning, Satisfaction with Discretionary Social Activities, and the Satisfaction with Social Roles scales.41 Questionnaires administered at baseline and at 3-, 6-, 9-, and 12-month assessments included the BDI-II26, Beck Anxiety Inventory 42, Self-Care of Heart Failure Index32, Kansas City Cardiomyopathy Questionnaire43, and Medical Outcomes Study 12-item Short Form.44
Outcomes
The primary outcome was the BDI-II depression score at 6 months, as specified in the trial protocol (Supplement 2), and the original co-primary outcomes were the total score on the Self-Care of Heart Failure Index and the Kansas City Cardiomyopathy Questionnaire. However, shortly before the start of the trial, the authors of the Self-Care of Heart Failure Index advised researchers to switch from using the total score to subscale scores instead.32 Consequently, the 6-month Self-Care Maintenance and Confidence subscale scores were defined as co-primary outcomes, and the Kansas City Cardiomyopathy Questionnaire was made a secondary outcome. The Self-Care Maintenance subscale assesses self-care behaviors such as daily weight checks and dietary compliance. The Confidence subscale assesses the patient’s confidence in his or her HF self-care skills. Inferences about the primary and co-primary outcomes were constrained by the following decision rules. 1) Whether the intervention is efficacious for comorbid depression in HF does not depend on whether it is also efficacious for HF self-care. 2) Both self-care confidence and maintenance behaviors must improve for the intervention to be considered efficacious for HF self-care. 3) The intervention is not efficacious if it improves self-care but not depression.
Remission of major depression was defined as a score of 9 or less on the BDI-II.45 Secondary outcomes at 6 months included scores on the Hamilton Rating Scale for Depression (with ≤7 as the criterion for remission), the Beck Anxiety Inventory, the Kansas City Cardiomyopathy Questionnaire, the SF-12 Mental and Physical component subscales, a set of NIH-PROMIS patient-reported outcome measures, 6-minute walk test distance, and average daily activity level on wrist actigraphy. Maintenance-phase questionnaire scores at 9 and 12 months, and hospitalizations and deaths over 12 months, were exploratory outcomes.
Power and Statistical Analyses
Based on previous trials, clinically significant effects on the primary and the original co-primary outcomes were initially defined as a between-group difference of 3 or more points on the BDI-II46,47, 20 or more points on the Self-Care total score48, and 11 or more points on the Kansas City Cardiomyopathy Questionnaire.49 The Bonferroni-corrected type 1 error rate for these outcomes was set at 0.016 (α=.05/3). Power was set at 0.80 or greater for all 3 outcomes, and expected attrition was set at 25% or less. Based on these assumptions as well as estimates of variability and intraclass correlation, a simulation-based power analysis yielded a target sample size of 240 (eMethods 2 in Supplement 1).
At baseline, χ2 and t tests were used to compare the groups at baseline. Multiple imputation was used to impute data that were plausibly missing at random, consistent with the intention-to-treat analysis plan (eMethods 3 in Supplement 1). Separate imputers’ models were developed for each outcome (eMethods 3 in Supplement 1). Each model included the terms that were to be used in the analysis of that outcome as well as variables that correlated with the presence or absence of the outcome data.50 Parameter estimates were aggregated over 20 imputed datasets for statistical inference.51
Linear mixed models with an autoregressive covariance structure were used to evaluate continuous outcomes. Each model included antidepressant use (the stratification factor), group, time, and the group by time interaction as fixed factors, and baseline intercept and patient as random factors. Pre-planned moderator analyses of the primary and co-primary outcomes were conducted to determine whether the effects of treatment were moderated by sex, race, or antidepressant use, and a pre-planned subgroup analysis was performed to determine whether the effects of treatment on HF self-care depended on the presence of self-care deficits at baseline. The number of hospitalizations over 12 months was regressed on treatment group and antidepressant use in a Poisson model, with the treatment effect defined as the incidence rate ratio. Cox regression was used to model the time to the first all-cause hospitalization or death with the same predictors as in the Poisson model. Standard diagnostics were performed to ensure that there were no violations of model assumptions. To account for multiplicity, a Bonferroni correction was applied to the primary and co-primary outcome analyses, whereby the corrected Type 1 error rate was set at 0.016 (α = 0.05 / 3). The Type 1 error rate for all other outcomes was set at 0.05 per comparison. All analyses were performed with SAS 9.3 software (SAS Institute, Inc).
Results
Recruitment, Retention, and Baseline Characteristics
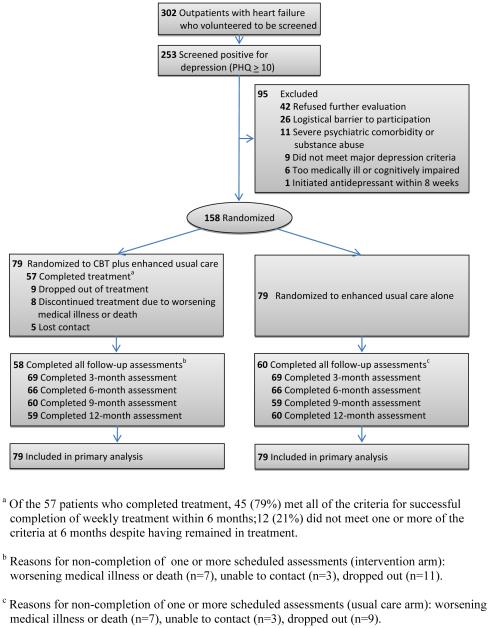
One hundred fifty-eight patients (52% of HF patients screened, 66% of the target sample size) were randomized to usual care (n=79) or CBT (n=79). Within each arm, 26 (33%) of the patients were taking an antidepressant at baseline. One hundred thirty-two (84%) of the participants completed the 6-month post-treatment assessments; 60 (76%) of the usual care and 58 (73%) of the CBT participants completed every follow-up assessment (p=0.88) (Figure 1).
Figure 1.
Consort Diagram of Screening, Enrollment, Randomization, and Follow-up
Table 1 presents the participants’ baseline characteristics. There were more minority patients in the usual care than the intervention arm (p=0.02), but no other differences at baseline.
Table 1.
Baseline Patient Characteristicsa
| Total Sample (n=158) |
Usual Care (n=79) |
CBT + Usual Care (n=79) |
p value |
|
|---|---|---|---|---|
| Demographic variables | ||||
| Age | 55.8 ± 11.2 | 55.5 ± 10.9 | 56.2 ± 11.5 | 0.69 |
| Female | 73 (46.2) | 33 (41.8) | 40 (50.6) | 0.26 |
| Caucasian | 100 (63.3) | 57 (72.2) | 43 (54.4) | 0.02 |
| Married or partnered | 74 (46.8) | 40 (50.6) | 34 (43.0) | 0.33 |
| Education ≥ 12 years | 135 (85.4) | 68 (86.1) | 67 (84.8) | 0.82 |
| Employed (full- or part-time) | 34 (21.5) | 17 (21.5) | 17 (21.5) | 0.99 |
| Annual income ≥ $30,000 | 66 (48.9) | 32 (48.5) | 34 (49.3) | 0.93 |
| Medical variables | ||||
| NYHA class at enrollment | 0.87 | |||
| I-II | 91 (57.6) | 45 (57.0) | 46 (58.2) | |
| III | 67 (42.4) | 34 (43.0) | 33 (41.8) | |
| LVEF | ||||
| Interval-scaled | 38.9 ± 15.5 | 37.6 ± 15.5 | 40.3 ± 15.5 | 0.27 |
| <45% | 84 (53.9) | 46 (59.0) | 38 (48.7) | 0.20 |
| Prior hospitalization for heart failure | 132 (84.6) | 67 (85.9) | 65 (83.3) | 0.66 |
| Body mass index (kg/m2) | 33.6 ± 7.9 | 32.6 ± 7.8 | 34.7 ± 7.9 | 0.11 |
| Current smoker | 18 (11.3) | 11 (13.9) | 7 (8.9) | 0.32 |
| History of MI | 49 (31.6) | 24 (31.6) | 25 (31.7) | 0.99 |
| Revascularization | 43 (27.2) | 20 (25.3) | 23 (29.1) | 0.59 |
| History of atrial fibrillation | 56 (35.4) | 30 (38.0) | 26 (32.9) | 0.51 |
| Diabetes | 60 (38.0) | 33 (41.8) | 27 (34.2) | 0.33 |
| COPD | 29 (18.4) | 18 (22.8) | 11 (13.9) | 0.15 |
| Hypertension | 113 (71.5) | 59 (74.7) | 54 (68.4) | 0.38 |
| Sodium | 139.1 + 2.7 | 138.8 + 2.8 | 139.3 ± 2.7 | 0.23 |
| Creatinine | 1.32 + 1.40 | 1.23 ± 0.98 | 1.40 ± 1.7 | 0.46 |
| BUN | 23.2 + 12.5 | 23.3 ± 14.9 | 23.1 ± 9.7 | 0.94 |
| BNP | 225 + 340 | 230 ± 338 | 220 ± 344 | 0.86 |
| Medications | ||||
| Antidepressant (stratification factor) | 52 (32.9) | 26 (32.9) | 26 (32.9) | n/a |
| Diuretic | 124 (78.5) | 59 (74.7) | 65 (82.3) | 0.25 |
| Beta blocker | 145 (91.8) | 73 (92.4) | 72 (91.1) | 0.77 |
| ACE inhibitor or ARB | 128 (81.0) | 63 (79.8) | 65 (82.3) | 0.69 |
| Aldosterone antagonists | 62 (39.2) | 32 (40.5) | 30 (38.0) | 0.74 |
| Devices | ||||
| Automatic implantable cardioverter defibrillator | 88 (55.7) | 50 (63.3) | 38 (48.1) | .06 |
| Left ventricular assist device | 6 (3.8) | 2 (2.5) | 4 (5.1) | .41 |
Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association.
Continuous variables are reported as mean ± (SD); categorical variables are reported as number of patients (%).
Treatment Fidelity and Adherence
Participation in HF education ranged from 95% at the first session to 85% at the third, with no between-group differences. Seventeen (21%) of the intervention participants identified HF self-care as one of the high-priority items on their initial CBT problem list, and the therapists targeted self-care deficits or barriers in 38 (55%) of the CBT cases. Participants in the intervention arm were exposed to mean (SD) of 8.1 (2.2) CBT techniques out of the 11 that were tracked. The CBT participants completed 10.8 (5.8) treatment sessions, with mean duration of 59 (21) minutes and with 96% (9%) of CBT homework assignments at least partially completed. Therapist ratings (range, 1 [worst] to 6 [best]) of the participants’ CBT homework compliance, use of CBT techniques in daily life, and adherence to HF self-care goals averaged 4.1 (0.9), 3.4 (1.1), and 4.1 (0.8), respectively, consistent with satisfactory adherence.
Acute Treatment Phase
Primary Outcomes
Six-month depression scores were lower in the CBT than the usual care arm on the BDI-II (12.8 / 10.6 vs. 17.3 / 10.7; P=0.008) (Table 2). Remission rates differed on the BDI-II (46% vs. 19%; number needed to treat [NNT]=3.76; 95% C.I., 3.62-3.90; P=0.001) and the Hamilton Depression Scale (51% vs. 20%; NNT=3.29; 95% C.I., 3.15-3.43; P<0.0001). The groups did not differ on the Self-Care Maintenance or Confidence subscales.
Table 2.
Primary and Secondary Outcomesa
| Outcome Measure | Usual Care (n=79) |
CBT + Usual Care (n=79) |
Differencec
(95% CI) |
P Value |
|---|---|---|---|---|
| Beck Depression Inventory (BDI-II) (p=0.002)b | ||||
| Baseline | 29.6 ± 10.2 | 30.7 ± 10.2 | 1.05 (−2.09, 4.19) |
0.51 |
| 3 months | 18.2 ± 10.5 | 16.0 ± 10.7 | −2.24 (−5.56, 1.09) |
0.19 |
| 6 months | 17.3 ± 10.7 | 12.8 ± 10.6 | −4.43 (−7.68, −1.18) |
0.008 |
| 9 months | 16.9 ± 11.2 | 12.4 ± 11.3 | −4.53 (−8.03, −1.02) |
0.01 |
| 12 months | 16.0 ± 10.8 | 11.2 ± 10.7 | −4.82 (−8.14, −1.49) |
0.005 |
| Hamilton Rating Scale for Depression (p=0.001)b |
||||
| Baseline | 25.1 ± 5.7 | 25.1 ± 5.7 | −0.01 (−1.76, 1.74) |
0.99 |
| 6 months | 12.1 ± 6.0 | 8.2 ± 5.9 | −3.95 (−5.81, −2.08) |
<.0001 |
| Self-Care of Heart Failure Index Maintenance (p=0.42)b |
||||
| Baseline | 62.1 ± 15.5 | 61.9 ± 15.5 | −0.17 (−4.92, 4.59) |
0.94 |
| 3 months | 67.3 ± 16.0 | 68.6 ± 16.0 | 1.32 (−3.57, 6.20) |
0.59 |
| 6 months | 67.7 ± 16.1 | 69.4 ± 16.1 | 1.70 (−3.16, 6.56) |
0.49 |
| 9 months | 67.2 ± 16.3 | 69.9 ± 16.6 | 2.70 (−2.39, 7.79) |
0.30 |
| 12 months | 68.1 ± 17.3 | 68.7 ± 16.3 | 0.58 (−4.48, 5.64) |
0.82 |
| Self-Care of Heart Failure Index Confidence (p=0.10)b |
||||
| Baseline | 55.9 ± 21.1 | 55.4 ± 21.1 | −0.49 (−6.99, 6.01) |
0.88 |
| 3 months | 60.1 ± 22.1 | 64.9 ± 21.8 | 4.78 (−2.03, 11.59) |
0.17 |
| 6 months | 63.1 ± 21.9 | 69.0 ± 21.5 | 5.94 (−0.83, 12.71) |
0.08 |
| 9 months | 64.3 ± 23.7 | 64.0 ± 23.6 | −0.25 (−7.41, 6.92) |
0.95 |
| 12 months | 62.8 ± 23.7 | 67.1 ± 22.4 | 4.23 (−2.75, 11.22) |
0.23 |
| Anxiety | ||||
| Beck Anxiety Inventory (p=0.03)b | ||||
| Baseline | 19.6 ± 10.3 | 19.4 ± 10.3 | −0.24 (−3.39, 2.91) |
0.88 |
| 3 months | 15.7 ± 10.6 | 12.9 ± 10.5 | −2.74 (−5.99, 0.50) |
0.10 |
| 6 months | 15.6 ± 10.5 | 11.2 ± 10.6 | −4.47 (−7.70, −1.25) |
0.007 |
| 9 months | 13.9 ± 11.1 | 10.4 ± 10.7 | −3.52 (−6.95, −0.10) |
0.04 |
| 12 months | 14.8 ± 11.4 | 11.1 ± 11.2 | −3.71 (−7.23, −0.19) |
0.04 |
| Health-Related Quality of Life | ||||
| Kansas City Cardiomyopathy Questionnaire (p=0.01)b |
||||
| Baseline | 46.1 ± 23.3 | 45.2 ± 23.3 | −0.96 (−8.11, 6.19) |
0.79 |
| 3 months | 55.7 ± 23.8 | 59.7 ± 24.0 | 3.99 (−3.37, 11.35) |
0.29 |
| 6 months | 54.5 ± 23.8 | 63.1 ± 24.1 | 8.51 (1.15, 15.88) |
0.02 |
| 9 months | 55.8 ± 26.0 | 63.2 ± 25.6 | 7.36 (−0.62, 15.35) |
0.07 |
| 12 months | 56.0 ± 24.0 | 64.2 ± 25.2 | 8.14 (0.63, 15.66) |
0.03 |
| SF-12 Mental Component Score (p<.0001)b | ||||
| Baseline | 34.7 ± 11.4 | 31.7 ± 11.4 | −3.07 (−6.59, 0.45) |
0.09 |
| 3 months | 41.8 ± 12.2 | 43.3 ± 12.2 | 1.50 (−2.30, 5.30) |
0.44 |
| 6 months | 42.7 ± 12.0 | 46.9 ± 12.0 | 4.18 (0.43, 7.93) |
0.03 |
| 9 months | 43.0 ± 13.0 | 45.5 ± 12.5 | 2.52 (−1.53, 6.56) |
0.22 |
| 12 months | 39.8 ± 13.2 | 48.1 ± 12.3 | 8.29 (4.20, 12.38) |
0.0001 |
| SF-12 Physical Component Score (p=0.06)b | ||||
| Baseline | 31.9 ± 11.1 | 34.5 ± 11.1 | 2.60 (−0.82, 6.01) |
0.14 |
| 3 months | 34.0 ± 11.4 | 34.3 ± 11.4 | 0.35 (−3.22, 3.92) |
0.85 |
| 6 months | 32.0 ± 11.6 | 35.1 ± 11.8 | 3.10 (−0.53, 6.73) |
0.09 |
| 9 months | 32.7 ± 11.9 | 34.4 ± 11.5 | 1.71 (−1.83, 5.26) |
0.34 |
| 12 months | 33.1 ± 12.7 | 33.5 ± 12.5 | 0.38 (−3.36, 4.12) |
0.84 |
| Physical Measures | ||||
| Actigraphy 7-day average activity counts (p=0.71)b |
||||
| Baseline | 98.0 ± 55.3 | 92.7 ± 55.1 | −5.34 (−22.20, 11.52) |
0.53 |
| 6 months | 98.8 ± 58.5 | 90.6 ± 59.0 | −8.20 (−26.46, 10.07) |
0.38 |
| 6-minute walk test distance (feet) (p=0.47)b | ||||
| Baseline | 1025.6 ± 410.6 | 981.1 ± 409.6 | −44.5 (−169.7, 80.8) |
0.48 |
| 6 months | 1026.7 ± 431.2 | 1017.0 ± 430.4 | −9.64 (−142.8, 123.5) |
0.89 |
| NIH PROMIS Measures | ||||
| Anxiety (p<.0001)b | ||||
| Baseline | 62.2 ± 7.5 | 62.8 ± 7.5 | 0.56 (−1.74, 2.87) |
0.63 |
| 6 months | 57.8 ± 8.4 | 51.4 ± 8.1 | −6.40 (−8.95, −3.86) |
<.0001 |
| Depression (p<.0001)b | ||||
| Baseline | 62.6 ± 7.5 | 63.8 ± 7.5 | 1.19 (−1.12, 3.50) |
0.31 |
| 6 months | 58.1 ± 8.3 | 51.6 ± 8.2 | −6.49 (−9.14, −3.84) |
<.0001 |
| Fatigue (p=0.04)b | ||||
| Baseline | 63.4 ± 8.2 | 62.3 ± 8.1 | −1.07 (−3.57, 1.43) |
0.40 |
| 6 months | 60.5 ± 9.1 | 56.5 ± 8.8 | −4.08 (−6.87, −1.29) |
0.005 |
| Physical Functioning (p=0.22)b | ||||
| Baseline | 35.1 ± 6.6 | 35.8 ± 6.6 | 0.65 (−1.37, 2.66) |
0.53 |
| 6 months | 36.0 ± 7.1 | 37.7 ± 7.1 | 1.71 (−0.51, 3.94) |
0.13 |
| Satisfaction with Discretionary Social Activities (p=0.005)b |
||||
| Baseline | 40.2 ± 7.0 | 40.4 ± 7.0 | 0.19 (−1.97, 2.35) |
0.86 |
| 6 months | 44.1 ± 7.8 | 48.4 ± 7.6 | 4.27 (1.85, 6.69) |
0.001 |
| Satisfaction with Social Roles (p=0.001)b | ||||
| Baseline | 38.7 ± 7.5 | 37.7 ± 7.5 | −1.00 (−3.30, 1.29) |
0.39 |
| 6 months | 41.5 ± 8.0 | 44.9 ± 8.3 | 3.34 (0.78, 5.89) |
0.01 |
Scores are reported as covariate-adjusted least-square mean ± standard deviation.
These p values are for the group X time interactions from the mixed models. Statistically significant values indicate that the group’s trajectories differed over time.
Difference scores were calculated by subtracting the scores in the usual care arm from the scores in the CBT arm, i.e., Difference = CBT – UC.
Secondary Outcomes
Six-month outcomes were superior in the CBT relative to the usual care arm on secondary measures of depression (Hamilton Depression, P<0.0001; PROMIS Depression, P<0.0001), anxiety (Beck Anxiety Inventory, P=0.007; PROMIS Anxiety, P<0.0001), HF-related quality of life (Kansas City Cardiomyopathy Questionnaire, P=0.02), mental health-related quality of life (SF-12 Mental, P=0.03), fatigue (PROMIS Fatigue, P=0.01), and social functioning (PROMIS Discretionary Social Activities, P=0.001; PROMIS Social Roles, P=0.01). The groups did not differ on any of the physical functioning measures (SF-12 Physical, PROMIS Physical Functioning, 6-minute walk test distance, average daily activity level on actigraphy).
Follow-up Phase
The BDI-II criteria for remission of depression were met by 23 (29.1%) of the usual care and 42 (53.2%) of the CBT patients at 12 months (NNT, 4.16; 95% C.I., 4.01-4.31; P=0.002). Statistically significant group by time interactions were found on the BDI-II (P=0.002), Beck Anxiety Inventory (P=0.03), Kansas City Cardiomyopathy Questionnaire (P=0.01), and the SF-12 Mental score (P<0.001), indicating better maintenance of gains over 1 year in depression, anxiety, HF-related quality of life, and mental health quality of life in the CBT than the usual care arm. There were no group by time interactions on the Self-Care of Heart Failure Index or the SF-12 Physical score. Thirty-five patients in the usual care arm were hospitalized at least once within 12 months of enrollment, compared with 32 in the CBT arm (P = .63). After controlling for antidepressant use, adjusting for the overdispersed Poisson model, and counting multiple readmissions, patients in the CBT arm had a lower rate of hospitalizations compared to those in the usual care arm (incidence rate ratio, 0.47; 95% C.I., 0.30-0.76; P = .002). There was no statistically significant difference in the time to the first all-cause hospitalization or death between the usual care and CBT groups (37 [47%] vs 34 [43%], respectively; hazard ratio (HR), 1.17; 95% C.I., 0.73-1.86; P = .52). There were no study-related serious adverse events.
Moderator, Subgroup, and Bias Analyses
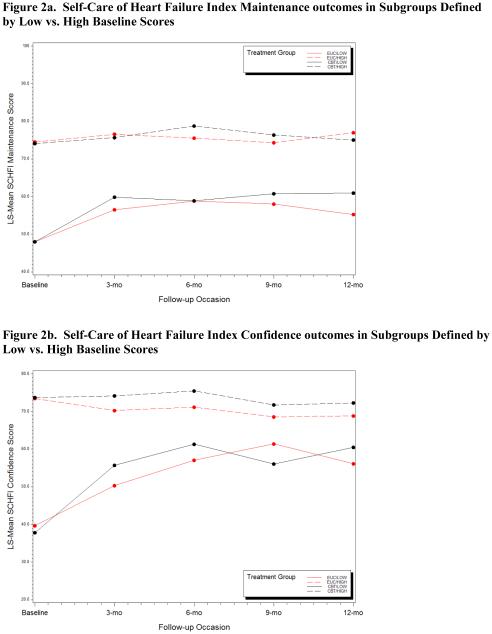
None of the moderator tests were statistically significant, suggesting that efficacy of CBT did not depend on sex, race, or use of nonstudy antidepressants. When the sample was stratified by the median baseline Self-Care Maintenance score, there was no treatment effect on the Maintenance subscale in the low-score stratum but a marginal effect (P=0.07) in the high-score stratum. When stratified by the median baseline Self-Care Confidence score, there was a marginal treatment effect in the low-score stratum (P=0.05) but not in the high-score stratum (Figure 2).
Figure 2.
Figure 2a. Self-Care of Heart Failure Index Maintenance outcomes in Subgroups Defined by Low vs. High Baseline Scores. Figure 2b. Self-Care of Heart Failure Index Confidence outcomes in Subgroups Defined by Low vs. High Baseline Scores
Because inadequate blinding can bias trial results, the outcome assessors were instructed to try to guess the participant’s group assignment after each contact. The resulting weighted kappa statistics were 0.20, 0.16, 0.17, and 0.16 at 3, 6, 9, and 12 months respectively. These figures are only slightly better than chance, suggesting that the assessments were adequately blinded.
Co-intervention bias is also a potential threat to the validity of single-blinded RCTs. It was evaluated in 3 ways. First, research staff and interventionists who had direct patient contact recorded all instances in which patients were advised to seek nonstudy medical or psychiatric care or referred to a nonstudy health care professional. Recommendations or referrals were received by 19 (24%) of the usual care and 42 (53%) of the CBT participants (P=0.003). However, controlling for this difference had no effect on any outcome. Second, changes during the trial use of antidepressants and HF guideline-recommended medications were tracked (eTable in Supplement 1). There were few medication changes after randomization and no statistically significant differences between the groups. Finally, in an exploratory analysis, the between-group difference in the hospitalization rate did not account for any of the other outcomes. Thus, no evidence of co-intervention bias was found.
Discussion
This trial tested the efficacy of a behavioral intervention that targeted major depression and inadequate HF self-care. Cognitive behavior therapy was superior to usual care at 6 months on the primary (BDI-II) measure of depression. The depression remission rate was higher in the CBT than the usual care arm, and most participants maintained their gains for at least 12 months after initiating CBT. The results suggest that CBT is superior to usual care for depression in patients with HF. This is an especially encouraging result in light of the negative findings of the SADHART-CHF13 and MOOD-HF15 antidepressant trials. The effects of the intervention on several secondary outcomes, including anxiety, fatigue, HF-related quality of life, mental health-related quality of life, and satisfaction with social roles and activities, suggest that CBT also offers other benefits for patients with HF and depression. The preintervention/postintervention change in the CBT arm on the Kansas City Cardiomyopathy Questionnaire is consistent with a large improvement in HF-related quality of life.49 The intervention might also help to reduce rehospitalization rates among clinically depressed HF patients. However, hospitalization was an exploratory outcome, and the groups did not differ on the composite endpoint of hospitalization or death, so this finding should be interpreted with caution and evaluated more definitively in future trials.
The intervention was not efficacious for HF self-care maintenance or confidence, the co-primary outcomes. This was the first attempt to modify HF self-care in a clinically depressed patient population. Nevertheless, it is the latest of a growing list of studies to find weak or null effects for HF self-care interventions.20,21 Thus, HF self-care behaviors are proving to be difficult to modify. In this trial, self-care deficits were not required for eligibility and both groups received intensive HF education. However, a secondary analysis of self-care outcomes revealed a surprising pattern: The intervention tended to improve self-care behaviors in patients who were already engaging in relatively good self-care practices, but it had no effect on the patients with relatively deficient self-care behaviors. Conversely, self-care confidence improved in patients with low confidence at baseline but did not increase in those who were already relatively confident at baseline. More importantly, as shown in Figure 2, the baseline differences between these subgroups were much larger than the intervention effects within the subgroups. Furthermore, the scores within the relatively deficient subgroup never converged with those of the relatively proficient subgroup. More research is needed to identify the characteristics and needs of patients with persistent self-care deficits. This could lead to interventions that would either directly target or compensate for durable barriers to HF self-care in depressed patients.
The aim of the behavioral activation component of CBT is to reduce depression by increasing engagement in pleasurable and productive activities. Although the intervention did not include physical exercise, we had hoped that behavioral activation would have detectable effects on physical functioning. However, there were no effects on the 6-minute walk test or the SF-12 and PROMIS Physical scores. The absence of a treatment effect on actigraphy suggests that even if the patients were engaging in more pleasurable and/or productive activities, they were doing so in ways that did not depend on increasing physical activity. This suggests that physical activity is unlikely to increase unless it is made an explicit target of intervention. The HF-ACTION trial showed that exercise can help to reduce depression in HF.52 A combination of CBT and exercise might produce better depression outcomes than either intervention alone.53
The primary purpose of this study was to determine whether the intervention, when added to usual medical care and HF education, is superior to usual care and education alone. The trial was not designed to control for exposure to clinical attention or determine whether other interventions (including simpler, briefer, or less expensive ones) might have comparable effects. However, the trial design was appropriate for evaluating the efficacy of the intervention.33,54
This study has several limitations. First, despite vigorous efforts to reach the study’s enrollment target, recruitment fell short of the goal. However, this neither obscured the effect of the intervention on depression nor accounted for the absence of a clinically significant effect on HF self-care. However, the moderator and subgroup analyses were underpowered and should be interpreted with caution. Second, like most behavioral trials, it was necessarily single- rather than double-blinded. However, we found no evidence of co-intervention bias. Third, treatment fidelity was assessed by the therapists themselves and closely monitored by the clinical supervisor but not evaluated by independent raters. Fourth, the multiplicity of secondary outcomes increases the risk of Type 1 errors. Finally, the participants were enrolled at a single academic medical center and were treated by experienced therapists who received intensive clinical supervision. Independent replications are needed to clarify the extent to which these findings are generalizable to patients treated in other settings.
Conclusions
Cognitive behavior therapy was effective relative to usual care for major depression in patients with heart failure. It did not improve HF self-care or physical functioning, but it did improve anxiety, fatigue, social functioning, and quality of life, and an exploratory analysis suggested that the intervention might help to decrease the hospitalization rate in clinically depressed patients. Comorbid major depression in heart failure may respond to CBT even if antidepressant therapy is unsuccessful. Further research is needed on interventions to improve depression, self-care, physical functioning, and quality of life in patients with heart failure and comorbid major depression.
Supplementary Material
Acknowledgements
Funding/Support: This study was conducted with support from the National Heart, Lung, and Blood Institute, grant R01HL091918 (Dr. Freedland).
Footnotes
Author Contributions: Dr. Freedland had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Freedland, Carney, Rich, Rubin.
Acquisition, analysis, or interpretation of data: Freedland, Carney, Steinmeyer, Rubin.
Analysis and interpretation of data: Freedland, Carney, Rich, Rubin.
Drafting of the manuscript: Freedland, Steinmeyer.
Critical revision of the manuscript for important intellectual content: Freedland, Carney, Rich, Rubin.
Statistical analysis: Freedland, Steinmeyer.
Obtained funding: Freedland, Carney.
Administrative, technical, or material support: Freedland, Carney.
Study supervision: Freedland, Rubin.
Conflict of Interest Disclosures: None reported.
Role of the Sponsor: The National Heart, Lung, and Blood Institute had no role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
REFERENCES
- 1.Freedland KE, Rich MW, Skala JA, et al. Prevalence of depression in hospitalized patients with congestive heart failure. Psychosom Med. 2003;65(1):119–128. doi: 10.1097/01.psy.0000038938.67401.85. [DOI] [PubMed] [Google Scholar]
- 2.Haworth JE, Moniz-Cook E, Clark AL, et al. Prevalence and predictors of anxiety and depression in a sample of chronic heart failure patients with left ventricular systolic dysfunction. Eur J Heart Fail. 2005;7(5):803–808. doi: 10.1016/j.ejheart.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Koenig HG. Depression in hospitalized older patients with congestive heart failure. Gen Hosp Psychiatry. 1998;20(1):29–43. doi: 10.1016/s0163-8343(98)80001-7. [DOI] [PubMed] [Google Scholar]
- 4.Schowalter M, Gelbrich G, Stork S, et al. Generic and disease-specific health-related quality of life in patients with chronic systolic heart failure: impact of depression. Clin Res Cardiol. 2013;102(4):269–278. doi: 10.1007/s00392-012-0531-4. [DOI] [PubMed] [Google Scholar]
- 5.Muller-Tasch T, Peters-Klimm F, Schellberg D, et al. Depression is a major determinant of quality of life in patients with chronic systolic heart failure in general practice. J Card Fail. 2007;13(10):818–824. doi: 10.1016/j.cardfail.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Albert NM, Fonarow GC, Abraham WT, et al. Depression and clinical outcomes in heart failure: an OPTIMIZE-HF analysis. Am J Med. 2009;122(4):366–373. doi: 10.1016/j.amjmed.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 7.Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42(7):1226–1233. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 8.Lesman-Leegte I, van Veldhuisen DJ, Hillege HL, et al. Depressive symptoms and outcomes in patients with heart failure: data from the COACH study. Eur J Heart Fail. 2009;11(12):1202–1207. doi: 10.1093/eurjhf/hfp155. [DOI] [PubMed] [Google Scholar]
- 9.Moraska AR, Chamberlain AM, Shah ND, et al. Depression, healthcare utilization, and death in heart failure: a community study. Circ Heart Fail. 2013;6(3):387–394. doi: 10.1161/CIRCHEARTFAILURE.112.000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherwood A, Blumenthal JA, Trivedi R, et al. Relationship of depression to death or hospitalization in patients with heart failure. Arch Intern Med. 2007;167(4):367–373. doi: 10.1001/archinte.167.4.367. [DOI] [PubMed] [Google Scholar]
- 11.Jiang W, Kuchibhatla M, Cuffe MS, et al. Prognostic value of anxiety and depression in patients with chronic heart failure. Circulation. 2004;110(22):3452–3456. doi: 10.1161/01.CIR.0000148138.25157.F9. [DOI] [PubMed] [Google Scholar]
- 12.O'Connor CM, Jiang W, Kuchibhatla M, et al. Antidepressant use, depression, and survival in patients with heart failure. Arch Intern Med. 2008;168(20):2232–2237. doi: 10.1001/archinte.168.20.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Connor CM, Jiang W, Kuchibhatla M, et al. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56(9):692–699. doi: 10.1016/j.jacc.2010.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angermann CE, Gelbrich G, Stork S, et al. Rationale and design of a randomised, controlled, multicenter trial investigating the effects of selective serotonin re-uptake inhibition on morbidity, mortality and mood in depressed heart failure patients (MOOD-HF) Eur J Heart Fail. 2007;9(12):1212–1222. doi: 10.1016/j.ejheart.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Angermann CE. Annual Scientific Sessions of the American College of Cardiology. San Diego, California: Effects of selective serotonin re-uptake inhibition on mortality, morbidity, and mood in depressed heart failure patients: MOOD-HF clinical trial. 3-16-2015. [Google Scholar]
- 16.Cuijpers P, Karyotaki E, Weitz E, et al. The effects of psychotherapies for major depression in adults on remission, recovery and improvement: a meta-analysis. J Affect Disord. 2014:159118–126. doi: 10.1016/j.jad.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 17.Riegel B, Moser DK, Anker SD, et al. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120(12):1141–1163. doi: 10.1161/CIRCULATIONAHA.109.192628. [DOI] [PubMed] [Google Scholar]
- 18.McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol. 2004;44(4):810–819. doi: 10.1016/j.jacc.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 19.Grady KL. Self-care and quality of life outcomes in heart failure patients. J Cardiovasc Nurs. 2008;23(3):285–292. doi: 10.1097/01.JCN.0000305092.42882.ad. [DOI] [PubMed] [Google Scholar]
- 20.Dracup K, Moser DK, Pelter MM, et al. Randomized, controlled trial to improve self-care in patients with heart failure living in rural areas. Circulation. 2014;130(3):256–264. doi: 10.1161/CIRCULATIONAHA.113.003542. [DOI] [PubMed] [Google Scholar]
- 21.Powell LH, Calvin JE, Jr., Richardson D, et al. Self-management counseling in patients with heart failure: the heart failure adherence and retention randomized behavioral trial. JAMA. 2010;304(12):1331–1338. doi: 10.1001/jama.2010.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cameron J, Worrall-Carter L, Page K, et al. Does cognitive impairment predict poor self-care in patients with heart failure? Eur J Heart Fail. 2010;12(5):508–515. doi: 10.1093/eurjhf/hfq042. [DOI] [PubMed] [Google Scholar]
- 23.Morgan AL, Masoudi FA, Havranek EP, et al. Difficulty taking medications, depression, and health status in heart failure patients. J Card Fail. 2006;12(1):54–60. doi: 10.1016/j.cardfail.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Riegel B, Vaughan D,V, Goldberg LR, Deatrick JA. Factors associated with the development of expertise in heart failure self-care. Nurs Res. 2007;56(4):235–243. doi: 10.1097/01.NNR.0000280615.75447.f7. [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association, American Psychiatric Association, Task Force on DSM-IV . Diagnostic and statistical manual of mental disorders (DSM-IV-TR) 4th. American Psychiatric Association; Washington, DC: 2000. text revision. [Google Scholar]
- 26.Beck AT, Steer RA, Brown GK. Beck Depression Inventory (BDI-II) Manual. Pearson Education, Inc.; San Antonio, TX: 1996. [Google Scholar]
- 27.Heart Failure Society Of America Education Modules. 2015 [Google Scholar]
- 28.American Heart Association Heart Failure Patient Education. 2015 [Google Scholar]
- 29.Beck AT. Cognitive therapy of depression. Guilford Press; New York: 1979. [Google Scholar]
- 30.Beck JS. Cognitive behavior therapy: basics and beyond. 2nd Guilford Press; New York: 2011. [Google Scholar]
- 31.Skala JA, Freedland KE, Carney RM. Heart disease. Hogrefe & Huber; Toronto: 2005. [Google Scholar]
- 32.Riegel B, Lee CS, Dickson VV, Carlson B. An update on the self-care of heart failure index. J Cardiovasc Nurs. 2009;24(6):485–497. doi: 10.1097/JCN.0b013e3181b4baa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freedland KE, Mohr DC, Davidson KW, Schwartz JE. Usual and unusual care: existing practice control groups in randomized controlled trials of behavioral interventions. Psychosom Med. 2011;73(4):323–335. doi: 10.1097/PSY.0b013e318218e1fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hrobjartsson A, Emanuelsson F, Skou Thomsen AS, Hilden J, Brorson S. Bias due to lack of patient blinding in clinical trials. A systematic review of trials randomizing patients to blind and nonblind sub-studies. Int J Epidemiol. 2014;43(4):1272–1283. doi: 10.1093/ije/dyu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freedland KE, Skala JA, Carney RM, et al. The Depression Interview and Structured Hamilton (DISH): rationale, development, characteristics, and clinical validity. Psychosom Med. 2002;64(6):897–905. doi: 10.1097/01.psy.0000028826.64279.29. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960:2356–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.American Thoracic Society ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 38.Balke B. A simple field test for the assessment of physical fitness. Rep Civ Aeromed Res Inst US. 1963;63(6):1–8. [PubMed] [Google Scholar]
- 39.Bittner V, Weiner DH, Yusuf S, et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. SOLVD Investigators. JAMA. 1993;270(14):1702–1707. [PubMed] [Google Scholar]
- 40.Burton C, McKinstry B, Szentagotai TA, et al. Activity monitoring in patients with depression: a systematic review. J Affect Disord. 2013;145(1):21–28. doi: 10.1016/j.jad.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Rose M, Bjorner JB, Becker J, Fries JF, Ware JE. Evaluation of a preliminary physical function item bank supported the expected advantages of the Patient-Reported Outcomes Measurement Information System (PROMIS) J Clin Epidemiol. 2008;61(1):17–33. doi: 10.1016/j.jclinepi.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 42.Beck AT, Steer RA. Beck Anxiety Inventory manual. Psychological Corporation - Harcourt Assessment, Inc.; San Antonio, Texas: 1990. [Google Scholar]
- 43.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 44.Hays RD, Sherbourne CD, Mazel R. User's manual for the medical outcomes study (MOS) core measures of health-related quality of life. RAND; Santa Monica, CA: 1995. [Google Scholar]
- 45.Frank E, Prien RF, Jarrett RB, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48(9):851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- 46.Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA. 2003;289(23):3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 47.Freedland KE, Skala JA, Carney RM, et al. Treatment of depression after coronary artery bypass surgery: a randomized controlled trial. Arch Gen Psychiatry. 2009;66(4):387–396. doi: 10.1001/archgenpsychiatry.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riegel B, Carlson B, Moser DK, et al. Psychometric testing of the self-care of heart failure index. J Card Fail. 2004;10(4):350–360. doi: 10.1016/j.cardfail.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Spertus J, Peterson E, Conard MW, et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150(4):707–715. doi: 10.1016/j.ahj.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 50.Graham JW. Missing data analysis: making it work in the real world. Annu Rev Psychol. 2009:60549–576. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- 51.Rubin DB. Multiple imputation for nonresponse in surveys. Wiley-Interscience; Hoboken, N.J: 2004. [Google Scholar]
- 52.Blumenthal JA, Babyak MA, O'Connor C, et al. Effects of exercise training on depressive symptoms in patients with chronic heart failure: the HF-ACTION randomized trial. JAMA. 2012;308(5):465–474. doi: 10.1001/jama.2012.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gary RA, Dunbar SB, Higgins MK, Musselman DL, Smith AL. Combined exercise and cognitive behavioral therapy improves outcomes in patients with heart failure. J Psychosom Res. 2010;69(2):119–131. doi: 10.1016/j.jpsychores.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freedland KE. Demanding attention: reconsidering the role of attention control groups in behavioral intervention research. Psychosom Med. 2013;75(2):100–102. doi: 10.1097/PSY.0b013e3182851b75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.