Abstract
Maternal education differences in children’s academic skills have been strongly linked to parental investment behaviors. This study extended this line of research to investigate whether these same maternal education patterns in parenting are observed among a set of parenting behaviors that are linked to young children’s health. Drawing on data from the Early Childhood Longitudinal Study, Birth Cohort (n = 5,000) and longitudinal models incorporating random effects, the authors found that higher levels of maternal education were associated with more advantageous health investment behaviors at each phase of early development (9 months, 2 years, 4 years, 5 years). Moreover, these disparities were typically largest at the developmental stage when it was potentially most sensitive for children’s long-term health and development. These findings provide further evidence of a developmental gradient associated with mothers’ education and new insight into the salience of mothers’ education for the short- and long-term health and well-being of their children.
Keywords: child health, early childhood, family processes, health disparities, parent education, parenting
The classic status attainment model argues that education begets various benefits that help parents promote the academic success, earning potential, and social class position of their children. As such, parents’ socioeconomic status is reproduced in their children (Blau & Duncan, 1967; Sewell, Haller, & Portes, 1969). More contemporary studies have expanded on the basic status attainment model to yield additional insights into how parents’ status is reproduced in their children. First, this intergenerational phenomenon is set in motion when children are young. For example, parents’ education has been linked to their children’s wages in adulthood via their early academic skills (Heckman, 2006). Second, parental education differences in children’s early academic skills are connected to parents’ investment behaviors, perhaps more so than the economic factors that correlate with education (Augustine, 2014; Mayer, 1997). Third, parental education not only begets higher levels of parental investment but also helps parents better adapt to meet the changing developmental needs of their children (Kalil, Ryan, & Corey, 2012). Finally, the connection between parents’ and children’s socioeconomic status that is formed during early childhood extends beyond children’s academic skills to their health (Palloni, 2006). For example, child health has forecast adult labor market participation and mortality (Case, Fertig, & Paxson, 2005; Haas, Glymour, & Berkman, 2011).
In this study we weaved together these four ideas to examine whether mothers’ education is positively associated with investments in children’s health during early childhood and whether education differences in these investments will be most pronounced at child ages when a specific health need is more intensive or developmentally important. Our specific focus on health-related parenting aimed to advance our understanding of the reproduction of inequality by connecting maternal education to several health investment behaviors previously associated with children’s health outcomes that have yet to be explicitly and consistently linked to maternal education throughout early childhood. Our investigation on variation in the levels of such practices by mothers’ education at different “sensitive” periods of early childhood speaks to a widening socioeconomic gap in children’s opportunity for mobility observed by various scholars and often termed diverging destinies (McLanahan, 2004).
To preview our approach, one hypothesis we tested is that maternal education disparities would consistently be associated with greater frequency of meeting the recommended number of and timing of child well-child visits when children are young (birth through kindergarten) but that this difference would be widest during infancy, when the number of recommended pediatric visits is much greater (around four vs. one) and thus the difficulty in meeting such recommendations is too. In addition to preventative health, we tested hypotheses related to children’s nutrition, shared family dinners, physical activity, television watching, safety, and smoke exposure—all concepts previously linked to child health outcomes. Our sample was a large, nationally representative cohort of children born in 2001 whose families participated in the Early Childhood Longitudinal Study, Birth Cohort (ECLS-B; see http://nces.ed.gov/ecls/birth.asp). The results of this study provide a fresh understanding of the salience of maternal education to parenting behaviors connected to children’s health and further insight into the origins of the reproduction of inequality.
Background
Maternal Education and Health-Related Parenting
At present, scholars have amassed substantial evidence linking mothers’ education with various child physical health outcomes, such as general health and asthma (Chen, Martin, & Matthews, 2006; Spencer, 2005). The main explanation for the connection between maternal education and child health (net of income or other economic resources) is that education provides women with more knowledge of and commitment to appropriate health practices (e.g., nutrition information, safety behaviors; Black, Morris, Smith, Townsend, & Whitehead, 1988; Gage, Fang, O’Neill, & Dirienzo, 2013). Thus, it stands to reason that we would observe maternal education differences in the parenting behaviors that promote children’s health. Yet few studies have examined this association. Studies of maternal education have focused on various prenatal health behaviors, such as drinking or health care utilization (Mangrio, Hansen, Lindström, Köhler, & Rosvall, 2011; U.S. Department of Health and Human Services, 2013). The research on health-related parenting behaviors beyond this prenatal period, however, have typically focused on other socioeconomic indicators as predictors, such as race and ethnic background or income (Hesketh, Ball, Crawford, Campbell, & Salmon, 2007; Tandon et al., 2012).
Thus, the first aim of this study was to document that maternal education is associated with higher levels of investment in children’s health, net of other socioeconomic factors, across various domains (e.g., nutrition, physical activity) and child ages (9 months, 2 years, 4 years, 5 years). In doing so, we created a link to the literatures noted above as well as to the research linking various health-related parenting behaviors to a variety of general (obesity) and specific child health disorders (asthma). Among these behaviors, child nutrition, preventative health, physical activity, television watching, maternal smoking, and safety are considered the key (or least most observable) factors that fall within parents’ control (e.g., Case & Paxson, 2002; Østbye et al., 2013; Philips, Sioen, Michels, Sleddens, & De Henauw, 2014). Consistent with economic models of health production (see Currie, 2009), we conceptualized such behaviors as health investments because they require time, energy, and a strategy to effectively and efficiently implement. They also pave the way for good long-term health (Aizer & Currie, 2014).
This investigation of the link between maternal education and health-related parenting during early childhood also has other theoretical roots: literatures exploring maternal education differences in the types of parenting activities that promote the academic skills needed to prepare their children to succeed at school (e.g., Bodovski & Farkas, 2008; Cheadle, 2008; Crosnoe & Huston, 2007). This research suggests that education yields a range of social and psychological resources, above and beyond the financial rewards associated with the jobs often occupied by highly educated parents (Augustine, Cavanagh, & Crosnoe, 2009; Mirowsky & Ross, 2003). Such skills include greater locus of control and persistence, expanded personal networks, and better health self-efficacy (Kingston, Hubbard, Lapp, Schroeder, & Wilson, 2003). Parents activate these skills and resources to glean advantages for their children at school (Lareau, 2002), to overcome practical barriers to parenting (Augustine, 2014), and to develop a network of parents that sets norms and standards for their children’s achievement (Carbonaro, 1998).
We argue that these skills can be applied to parental health practices that affect their children’s physical health. For example, education improves parents’ ability to seek access to specialized information on children’s health needs via the web (Radey & Randolf, 2009) or other sources. Attending college and subsequently working in white-collar industries exposes parents to a network of highly educated individuals, who may exhibit healthier behavior and be direct (e.g., medical professionals) or indirect sources of medical advice regarding their children (Greenberger, O’Neil, & Nagel, 1994; Grzywacz & Butler, 2005). The practice of intensive parenting (Lareau, 2002), thought to support children’s cognitive development, could spill over to children’s health. Indeed, the “overscheduled” child’s participation in organized sports likely results in increased physical activity and less television time and, potentially, learned exercise and a reduced risk of obesity (Robinson, 1999). Finally, there is evidence that education begets skills that help adults manage their own health (Mirowsky & Ross, 2003), which would likely translate to the management of their children’s health as well.
Parenting Health Investments and the Early Developmental Gradient
The review above highlights why parental education may be associated with parenting behaviors known to promote young children’s health, but in a static way. Developmental theory, however, suggests that parenting behaviors are likely—and should be—dynamic by pointing to the diverse and changing needs of children. This idea is further developed in a study by Kalil and colleagues (2012) in which they found that mothers with more education not only spent more time with their children but also were more likely to spend a greater proportion of that time on more “developmentally appropriate” academic-related activities. What constituted developmentally appropriate tasks changed according to the child’s developmental needs; a phenomenon the authors called, and a term we borrow, the developmental gradient. The explanation for this maternal education gradient was based on an extension of the theoretical framework outlined above. Maternal education begets skills that not only help mothers invest in their children’s well-being but also to do so in an efficient way that maximizes their life chances.
Borrowing this idea, we believe a developmental gradient could be observed among parenting behaviors and activities that reflect the changing health needs of children. Both health recommendations, such as those issued by the American Academy of Pediatrics (AAP) and the National Institutes of Health (NIH), and extant research point to the changing nature of children’s health needs as well as how and when parents’ health practices should change. We drew across such studies and health recommendations to hone in on several parenting behaviors that have been shown to matter to children’s health, conceptualized more formally when the sensitive stages of each parenting behavior are likely to be, and developed several hypotheses about when we would see the largest maternal education differences in health-related parenting.
First, preventative health practices, such as well-child visits, are much more intensive during infancy, with more visits recommended (four to five) compared to later years, when only annual visits are necessary (NIH, n.d.). It also represents a time when these visits are most important in terms of receiving vaccinations and identifying health issues that could be more problematic if left undiagnosed (e.g., hearing loss, for language development; Committee on Children with Disabilities, 1994; Yoshinaga-Itano, Sedey, Coulter, & Mehl, 1998). Thus, infancy (measured at 9 months in the ECLS-B) may be a sensitive period in the area of preventative health.
Next, although nutritional feeding practices across early childhood have been shown to be important predictors of children’s health, including appropriate physical growth and a bolstering of children’s resistance to short-term illness (Kaplan, Liverman, & Kraak, 2004), the earliest years may be a sensitive period for child nutrition given how breastfeeding is a documented predictor of later childhood obesity—which research suggests may actually begin in infancy—and other types of disease (Gillman, 2008). Research also suggests that breastfeeding may be a critical predictor of children’s diversity in food acceptance and learned response to internal cues of hunger and satiety related to energy intake (Birch & Fisher, 1998). In contrast, as children age and the nutritional quality of their diets tend to become poorer (Kudlová & Schneidrová, 2012), the effect of those dietary changes for long-term health becomes more variable too.
Parenting practices that encourage physical activities and discourage sedentary ones such as television watching have also been linked to children’s short- and long-term health in general via an association with body composition, fitness, metabolic syndrome and cardiovascular disease risk factors (Tremblay et al., 2011), and risk of obesity in particular (Lindsay, Sussner, Kim, & Gortmaker, 2006). Research suggests that the critical period for physical activity may be when all children have entered a structured school setting (kindergarten), where they spend more hours of the day seated, with less time for play (Janz, Burns, & Levy, 2005). Moreover, as children grow older, peer networks begin to exert an influence on children’s food and activity choices (Salvy, de la Haye, Bowker, & Hermans, 2012) and adiposity “rebounds” (Han, Lawlor, & Kimm, 2010), and thus parental efforts to promote children’s physical activity may be most important. For similar reasons, the critical period for television watching—which has been linked to increased food consumption (Matheson, Killen, Wang, Varady, & Robinson, 2004)—may be similar. This is also a time when unhealthy food advertising during children’s programming begins to have an impact on children’s food preferences and choices (Andreyeva, Rashad, & Harris, 2011; Goris, Petersen, Stamatakis, & Veerman, 2010).
Finally, parenting practices that support healthy eating routines, such as eating family meals together, are important to children’s physical health because they provide an opportunity to perform certain types of healthy role modeling (Benton, 2004). For example, studies have shown that family meals reduce children’s resistance to low-energy-dense (and healthier) foods during early childhood, when children tend to avoid new and novel foods (Salvy et al., 2012). Thus, we view age 5 as a sensitive period for family meals as well, because it is a time when—as noted above—peer networks begin to exert more influence over children’s eating preferences and thus family dinners become an important opportunity for the role-modeling of eating behaviors and exposure to diverse foods (Salvy et al., 2012). Family dinners around age 5 also take on additional significance compared to earlier years because they represent a key routine during a major transitory period: the transition to formal schooling. Stability in family routines during this sensitive period have been correlated with other health outcomes, such as decreased disruptive sleep patterns and lower psychological stress and cortisol levels (Wildenger, McIntyre, Fiese, & Eckert, 2008).
Two behaviors, however, we expect not to change: (a) car seat use, which is associated with other forms of childhood safety (Case & Paxson, 2002; Hendricks & Reichert, 1996), and (b) smoke exposure. The AAP recommends that children ride in a rear-facing car seat or belt-positioning bolster seat in the back of the car until they are at least 4 feet, 9 inches tall (about 8–12 years old; Durbin, 2011). The policy of the AAP is that there is “no safe level or duration of exposure to secondhand tobacco smoke” for children (Best, 2009). Thus, if the association between these “unchanging” health investments and maternal education remains stable, this pattern would further validate our argument that education helps mothers tailor their parenting to the developing needs of their children, in cases where their health needs change.
Hypotheses
We hypothesized that, in line with prior research on educational differences in parenting, mothers with more education would be more likely to practice behaviors that are advantageous to children’s present and future health. We did not go on to link these parenting behaviors with children’s health but instead focused on behaviors that have proven significance for children’s health in either the short term or long term. We then tested the hypothesis that educational disparities in parental investments in children’s health would be largest during periods when children’s needs are most complex and/or most sensitive to their long-term health. Although the current literature points to the periods when different health-related parenting behaviors may be most critical for producing later inequalities in children’s health, no study to our knowledge has provided explicit evidence of these sensitive periods. Thus, should our findings support our hypotheses, this study will both reveal a new source of children’s diverging destinies and provide a more concrete structure for future studies to extend this initial examination to specific child health outcomes.
Method
Data and Sample
Data came from the ECLS-B, a nationally representative sample of children born in the United States in 2001. Data were sponsored by the National Center for Education Statistics, with the aims of capturing the home and education experiences of U.S. children from birth (Snow et al., 2007). The original cohort included 10,600 children identified though a clustered list frame sample of births registered in the National Center for Health Statistics Vital Statistics system, and their parents. Data were collected when children were 9 months, 2, 4 (preschool), and 5 years old (kindergarten) from in-home interviews with the primary caregiver (generally, the mother) on topics such as parenting practices, household characteristics (e.g., income), and characteristics of the child (e.g., behaviors, health).
The analytic sample began with the full sample of mothers participating in the ECLS-B. We then excluded 400 who did not continuously coreside with their children; 50 who were less than age 16 at their time of birth (to whom the ECLS-B data cannot be generalized); and around 1,500 mothers whose children participated in the later 2007 kindergarten data collection, for whom many of the detailed questions asked in the main 2006 kindergarten data collection were not asked. About 3,650 mothers who did not have valid longitudinal weights because of attrition and/or not being interviewed at each wave were also excluded, leaving us with a final sample of around 5,000 mothers. Longitudinal sampling weights (described soon) adjusted for differential patterns of attrition and nonresponse, but there were some differences between those in the final analytic sample and those excluded or lost to attrition. Excluded mothers had less education and characteristics that, in regression models, were correlated with decreased likelihood of practicing health investments (e.g., racial/ethnic minority, low income). Such patterns suggest that estimates produced from our analytical sample are, if anything, likely to be more conservative than if we had not lost mothers due to attrition or had full data for the 2007 kindergarten cohort sample.
Measures
Dependent variables
We conceptualized seven types of maternal parenting investments in children’s health. Preventative health, nutrition, and secondhand smoke (SHS) exposure were measured at each wave (at the 9-month and 2-, 4-, and 5-year interviews). Dinner together, physical activity, car seat safety, and television watching were assessed at the 2-, 4-, and 5-year interviews but not at the 9-month wave, either because the relevant questions were not included in the survey (i.e., dinner together, car seat use, television watching) or were not appropriate (e.g., about physical activity). The operationalization of these concepts, which we explain next, was limited by the available data, although each has been used widely in studies based on the ECLS-B data and other sources (e.g., Anderson & Whitaker, 2010; Tandon, Zhou, Lozano, & Christakis, 2011).
First, preventative health was indicated by a binary variable for whether, at each wave, the child had attended the minimum appropriate number of well-child pediatrician visits between interviews. At age 9 months, this meant at least four pediatrician visits between birth and the 9- month interview. At ages 2 and 4, this meant at least two visits, and at age 5 it meant at least one visit to the pediatrician (NIH, n.d.).
Second, nutrition was captured by a standardized z score indicating a degree of deviation from the sample’s mean for an age-specific measure of better child nutrition practices. We used z scores because the indicators of better child nutrition practices changed across waves, and we needed a standardized approach. The 9-month measure was based on a scale indicating the number of months the mother had breastfed her child (0 = never/less than a month, 6 = 6 months or more). The 2-year scale counted whether the mother reported giving her child sugary drinks or soda (0 = both, 1 = one or the other, 2 = neither). The 4-year and 5-year nutrition variables were assessed with scales indicating the frequency during the past week mothers reported their child had eaten fast food, drank soda, and had sweets (e.g., candy, cookies). The scale was averaged across the three food areas and ranged from 0 (4 or more times per day) through 7 (did not eat/drink any of these things in the past week). Again, these measures reflect the data that were available at each wave, because waves did not contain the same questions or degree of information (particularly at age 2).
Third, dinner together—a successful predictor of healthy weight in childhood and adolescence (Hammons & Fiese, 2011)—was measured on a scale that ranged from 0 (never) through 7 (every day) of the number of evenings the mother reported that they typically ate dinner together as a family. This question was asked at the 2-, 4-, and 5-year interviews.
Fourth, physical activity was a binary outcome indicating whether the child played outside daily (at the 2-year interview), played outside daily and/or participated in organized sports or dance lessons (at the 4-year interview), and whether the child participated in organized sports or dance lessons (at the 5-year interview). Again, these coding decisions reflect the fact that some questions were asked at one wave and not the others and that this was the only information on children’s physical activity that was collected.
Fifth, television watching was a standardized z score indicating deviation from the sample mean hours of television mothers reported their children watched daily at the 2-, 4-, and 5-year interviews. Sixth, car seat safety was a binary measure, assessed at the 2-, 4-, and 5-year interviews, for whether the mother reported that her child was always in a car seat when n the car. Last, SHS exposure was a binary measure that captured mother reports of whether she smoked or someone smoked inside the home. Smoke exposure was assessed at each wave.
Focal independent variables
The focal independent variables were maternal education and child’s age. Maternal education was captured by four dummy variables indicating mothers’ highest educational attainment reported at the 9-month interview: (a) no high school diploma or GED (Certificate of High School Equivalency for students who did not earn a high school diploma), (b) high school diploma/GED, (c) some college experience or associate’s degree, or (d) bachelor’s degree. Child’s age corresponded to the interview wave: 9 months, 2 years, 4 years, or 5 years. In our multivariate models—which, to preview, were estimated in long format in which each time interval corresponds to the interview wave—a continuous measure for months accounted for deviations in the child’s age from the interview wave. For example, a child 25 months old at the 2-year interview received a value of 1 (25 − 24).
Covariates
We accounted for several factors that could be related to selection into different education groups or be endogenous to maternal education in a way that, without controlling for, remained a plausible alternative to the education “effect” we aimed to reveal. In the former case, we included time-invariant measures for maternal race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, other), maternal nativity (foreign born or not), language spoken in the home (English or not), and mothers’ age at the child’s birth (years). This information came from the baseline interview or the child’s birth certificate. In the latter case, we included time-varying measures assessed at each data collection point (9 months, 2 years, 4 years, and 5 years) for maternal employment status (full time, part time, or not working), family structure (married biological parents, cohabiting biological parents, married/cohabiting with a stepfather, or single mother), annual household income (on a continuous scale ranging from 1 = $5,000 or less through 13 = $200,000 or more), mother’s self-rated health (a scale ranging from 1 = excellent through 5 = poor), a continuous measure for the number of other children in the home, regional residence (Northeast, Midwest, South, and West), and maternal depression based on a modified version of the Center for Epidemiologic Studies–Depression scale (Radloff, 1977; 1 = rarely or never through 4 = most of all days). Because this scale was not administered at the 2-year interview, for this wave we averaged the scores from the 9-month and 4-year waves. To account for assortative mating patterns and the parenting role of fathers, we controlled for paternal education, assessed at baseline and coded the same way as maternal education.
In addition to these mother-level covariates we included a number of child-level controls to account for any confounding between mother’s education and her response to her child’s health, recorded before the very first parenting measure was collected. We did not include such measures collected after the first parenting measure was recorded because they could, in fact, be caused by mothers’ health behaviors. Available measures were a binary indicator for whether the mother had a risk factor during pregnancy (e.g., eclampsia, alcohol use during pregnancy), whether the child was born at a clinically low birth weight, and the child’s sex (female = 1). We also included a time-varying indicator for whether the child was without health insurance at the time of the interview or experienced a lapse in health insurance since the last interview.
Analytical Plan
We began by examining bivariate associations among our four categories of maternal education and the seven parenting health investment concepts. We estimated this association across all waves by creating an average score for each parenting concept and at each wave by examining the association between the categories of maternal education and the wave-specific value of each of the seven parenting measures. We used t tests and chi-square tests to assess whether mean values across maternal education groups were statistically significant. To adjust for attrition over time, nonresponse at baseline, and the sampling design in which children from subgroups (e.g., Asian, twins, low birth weight) were oversampled, we used the longitudinal sampling weight (W4R0) provided by National Center for Education Statistics (Snow et al., 2007).
Next, we examined whether patterns observed in the bivariate framework persisted in a multivariate one. Here, we turned to a longitudinal modeling approach in which we pooled the data across waves (three waves predicting physical activity, car seat use, dinners, and television watching, and four waves for preventive health, nutrition, and SHS exposure). To account for correlated errors in the repeated measures, we used random effects, a class of hierarchical models that can adjust for autocorrelation (or nonindependence) in the repeated measures of the dependent variable by correlating the errors within subgroups (mothers) while allowing the error term to vary across them (Laird & Ware, 1982). Thus, random effects models also help account for unmeasured factors that are stable within individuals yet likely to vary across them (i.e., the random effect), although to a lesser degree than within person models such as fixed effects (Allison, 2009).
We estimated seven models in which each parenting behavior was the dependent variable, conditional on maternal education, the child’s age (wave), and the full set of controls. These models revealed whether there was an association between maternal education and investments in her child’s health, averaged across time. For each model, our metric of time corresponded to the child’s general age at the interview wave. We used logistic regression to predict binary outcomes and ordinary least squares regression to predict continuous outcomes. We then added interactions between maternal education and child age to assess whether this association varied along different points of the developmental gradient. To help interpret statistically significant interactions and better observe any education and age-related patterns, we graphed the point estimates. For all multivariate analyses, we used the survey weight but also adjusted for the complex survey design in which the sample of children was stratified along various factors (e.g., income) and clustered within primary sampling units.
All models were estimated in Stata Version 11. To address the problem of item-level data missing on the covariates (there were none missing on the measures of maternal education or child age), we used multiple imputation procedures using mice to produce 100 fully imputed data sets and the mi estimate suite of commands to analyze the multiply imputed data in the multivariate analyses. Assuming data are missing at random, this approach has been shown to provide an unbiased strategy for dealing with missing data (Allison, 2001).
Results
A description of the sample of mothers (with some information on their children included), by maternal education group, is presented in Table 1. For factors that vary across time (e.g., income, employment), we present mothers’ information taken from the 9-month interview. As expected, mothers with a college education were more socioeconomically advantaged compared to mothers with less education on a range of indicators. They had higher family incomes, were older at the time of their child’s birth, had consistent health insurance coverage for their child, and most were married to the child’s father; differences that highlight the need to account for such factors in the multivariate models. At the same time, we did not observe maternal education differences in the measures of/proxies for her child’s early health, such as child’s birth weight and pregnancy complications, which we anticipated might confound mothers’ subsequent health behaviors.
Table 1.
Descriptive Statistics of the Sample at the 9-Month Interview
| Variable | % or M | ||||
|---|---|---|---|---|---|
| Total (N = 5,000) |
No HS/GED (n = 900) |
HS/GED (n = 1,450) |
Some college (n = 1,200) |
College (n = 1,350) |
|
| Maternal age at child’s birth (years) | 27.5 | 24.0a,b,c | 26.0b,c,d | 28.4a,c,d | 31.9a,b,d |
| (6.2) | (6.1) | (5.9) | (5.3) | (4.4) | |
| Maternal race/ethnicity | |||||
| Non-Hispanic White | 55.4 | 24.5a,b,c | 54.0b,c,d | 62.9a,c,d | 79.a,b,d |
| Non-Hispanic Black | 13.7 | 17.1c,d | 18.1c,d | 13.5a,c,d | 4.7a,b,d |
| Hispanic White | 24.2 | 53.2a,a,b,c | 22.6b,c,d | 16.6a,c,d | 6.4a,b,d |
| Other | 6.7 | 5.2c,b | 5.4c,d | 6.9a,c,d | 9.2a,b,d |
| Mother foreign born | 21.5 | 43.9a,b,c | 15.9b,c,d | 16.2a,c,d | 12.8a,b,d |
| Family structure | |||||
| Married biological | 65.7 | 41.4a,b,c | 56.1b,c,d | 74.2a,c,d | 93.6a,b,d |
| Cohabiting biological | 13.6 | 26.2a,b,c | 14.4b,c,d | 11.3a,c,d | 2.6a,b,d |
| Stepparents (cohabiting or married) | 0.9 | 1.3c | 1.2d | 1.0c | 0.1a,b,d |
| Single mother | 19.9 | 31.2a,b,c | 28.5b,c,d | 13.5a,c,d | 3.8a,b,d |
| Maternal employment status | |||||
| Not working | 46.5 | 64.6a,b,c | 47.9b,c,d | 39.8a,c,d | 35.1a,b,d |
| Part time | 19.5 | 12.2a,b,c | 18.2a,d | 20.4d | 27.6a,d |
| Full time | 34.1 | 23.2a,b,c | 33.9b,c,d | 39.8a,d | 37.3a,d |
| Paternal education | |||||
| No HS/GED | 22.7 | 56.8a,b,c | 24.6b,c,d | 12.0a,c,d | 1.0a,b,d |
| HS diploma/GED | 26.3 | 30.8a,b,c | 39.6b,c,d | 24.7a,c,d | 6.6a,b,d |
| Some college/associate’s degree | 26.0 | 11.4a,b,c | 27.3b,c,d | 40.9a,c,d | 21.8a,b,d |
| College or higher | 25.0 | 1.0a,b,c | 8.5b,c,d | 22.4a,c,d | 70.6a,b,d |
| Household income (average in $1,000s) |
$30–$35 | $20–$25a,b,c | $25–$30b,c,d | $35–$40a,c,d | $50–$75a,b,d |
| (3.4) | (4.6) | (3.1) | (2.9) | (1.8) | |
| Lapse in or no child health insurance | 10.7 | 17.1a,b,c | 11.0c,d | 10.8a,c | 3.8a,b,d |
| Maternal self-reported excellent/good health |
68.7 | 55.9a,b,c | 63.4b,c,d | 71.8a,c,d | 84.7a,b,d |
| At least one pregnancy risk factor | 29.6 | 28.1 | 29.0 | 31.8 | 29.6 |
| Maternal depression (0–4) | 1.43 | 1.51b,c | 1.48b,c | 1.41a,c,d | 1.30a,b,d |
| (0.47) | (0.56) | (0.48) | (0.43) | (0.35) | |
| Child born at low birth weight | 7.7 | 8.7 | 8.8 | 6.9 | 6.0 |
| Child female | 49.7 | 49.2 | 48.4 | 49.5 | 51.7 |
| English is child’s home language | 19.7 | 45.1a,b,c | 15.5c,d | 12.4c,d | 9.1a,b,d |
| Number of siblings in the home (average) |
0.98 | 1.12c | 0.98c | 1.01c | 0.83a,b,d |
| (1.10) | (1.32) | (1.04) | (1.08) | (0.94) | |
| Region | |||||
| Northeast | 19.2 | 13.4a,b,c | 18.5c,d | 18.3c,d | 25.8a,b,d |
| Midwest | 19.6 | 13.9b,c | 18.5b,c | 23.8a,d | 21.a,d |
| South | 35.0 | 40.5b,c | 37.0b,c | 31.1a,d | 32.1a,d |
| West | 26.3 | 32.2c | 26.0 | 26.8 | 20.3d |
Note. Table data are weighted %s/Ms (t tests and χ2s) and unweighted Ns. Ns are rounded to nearest 50th per National Center for Education Statistics guidelines. Numbers in parentheses are standard deviations.
Statistically different from college.
Statistically different from HS/GED.
Statistically different from some college.
Statistically different from no HS (high school)/GED at p < .05.
Bivariate Results
Our first set of analyses (see Table 2) examined bivariate associations between maternal education and seven health investment outcomes, overall and at each wave. With all interview waves combined (see top panel of table), there were significant differences across education groups in almost all of the health investment outcomes, with mothers without high school diplomas least likely to practice more advantageous health investment behaviors and college-educated mothers most likely to practice them. Mothers with a high school diploma and some college fell in between. As one example of this pattern, 41% of children of mothers without a high school diploma or high school degree were exposed to SHS, compared to one quarter of children of mothers with some college experience and less than 10% of the children of mothers with a college degree.
Table 2.
Weighted Means and Percentages of Outcomes by Maternal Education Group
| Variable | % or M | ||||
|---|---|---|---|---|---|
| Total (person N = 5,000) |
No HS (person n = 900) |
HS/GED (person n = 1,450) |
Some college (person n = 1,200) |
College (person n = 1,350) |
|
| All interviews | |||||
| Well-child checkups | 89.8 | 87.4a,b | 89.2a,b | 90.1b,c,d | 92.7a,c,d |
| Nutrition (z score) | −0.03 | −0.31a,b,d | −0.15a,b,c | 0.05b,c,d | 0.31a,c,d |
| Dinner together (0–7) | 5.66 | 5.56a,a,b,c | 5.57a,b | 5.77c,d | 5.76c,d |
| Physical activity | 56.2 | 45.6a,b,d | 51.3a,b,c | 57.9b,c,d | 71.1a,c,d |
| Television watching (z score) | 0.01 | 0.28a,b,d | 0.08a,b,c | −0.06b,c,d | −0.26a,c,d |
| Car seat use | 84.2 | 78.7a,b,d | 82.0a,b,c | 84.5b,c,d | 91.5a,c,d |
| SHS exposure | 30.0 | 41.3a,b,d | 41.0a,b,c | 25.1b,c,d | 9.8a,c,d |
| 9 months | |||||
| Well-child checkups | 87.9 | 80.9a,b | 86.8b | 90.1b,d | 93.5a,c,d |
| Nutrition (z score) | 0.03 | −0.21a,b | −0.21a,b | 0.07b,c,d | 0.55a,c,d |
| Dinner together (0–7) | |||||
| Physical activity | |||||
| Television watching (z score) | |||||
| Car seat use | |||||
| SHS exposure | 30.9 | 43.2a,b,d | 40.5a,b,c | 26.7b,c,d | 11.2a,c,d |
| 2 years | |||||
| Well-child checkups | 89.1 | 90.3 | 89.3 | 89.0 | 88.4 |
| Nutrition (z score) | −0.04 | −0.34a,b,d | −0.15a,b,c | 0.06b,c,d | 0.29a,c,d |
| Dinner together (0–7) | 5.97 | 5.93 | 5.96 | 6.09 | 5.9 |
| Physical activity | 53.4 | 57.8a,b,d | 52.0c | 51.4c | 53.5c |
| Television watching (z score) | −0.00 | 0.19a,b | 0.11a,b | −0.06b,c,d | −0.29a,c,d |
| Car seat use | 96.7 | 92.2a,b,d | 97.0a,b,c | 97.6b,c,d | 99.3a,c,d |
| SHS exposure | 30.1 | 39.9a,b | 41.6a,b | 25.2b,c,d | 10.5a,c,d |
| 4 years | |||||
| Well-child checkups | 86.0 | 82.2d | 86.2b | 84.6d | 90.7a,c,d |
| Nutrition (z score) | −0.05 | −0.35a,b,d | −0.09a,b,c | 0.02b,c,d | 0.20a,c,d |
| Dinner together (0–7) | 5.44 | 5.35a,b | 5.31a,b | 5.56a,b | 5.62c,d |
| Physical activity | 64.4 | 56.4a,b | 60.1a,b | 64.5b,c,d | 77.5a,c,d |
| Television watching (z score) | 0.03 | 0.37a,b,d | 0.07a,b,c | −0.04b,c,d | −0.28a,c,d |
| Car seat use | 82.4 | 76.2a,b | 80.0a,b | 82.4b,c,d | 90.9a,c,d |
| SHS exposure | 29.5 | 40.3a,b | 41.2a,b | 24.7b,c,d | 8.8a,c,d |
| 5 years | |||||
| Well-child checkups | 96.2 | 96.1a | 94.7b | 96.8d | 98.2a,c,d |
| Nutrition (z score) | −0.05 | −0.33a,b,d | −0.14a,b,c | 0.05b,c,d | 0.20a,c,d |
| Dinner together (0–7) | 5.56 | 5.41b | 5.44a,b | 5.66b,d | 5.75a,c,d |
| Physical activity | 50.7 | 22.6a,c,d | 41.6a,b,c | 57.7b,c,d | 82.1a,c,d |
| Television watching (z score) | 0.01 | 0.28a,c,d | 0.07a,b,c | −0.08b,c,d | −0.23a,c,d |
| Car seat use | 73.3 | 67.6a,c,d | 68.7a,b,c | 73.5b,c,d | 84.2a,c,d |
| SHS exposure | 29.4 | 42.0a,d | 40.7a,b | 23.8b,c,d | 8.7a,c,d |
Note. Table data are weighted %s/Ms (t tests and χ2s) and unweighted Ns. Ns are rounded to the nearest 50th per National Center for Education Statistics guidelines. Standard errors for continuous measures (nutrition and television z scores) not shown. SHS = secondhand smoke.
Statistically different from some college.
Statistically different from college.
Statistically different from no HS/GED at p < .05.
Statistically different from HS (high school)/GED.
When we examined these trends across different interview waves we observed similar patterns for nearly all health behaviors (television watching, SHS exposure, car seat use, nutrition), although in some cases there were nonsignificant differences between neighboring groups, in particular between those with less than high school and high school. We did detect some more nuanced patterns when looking at preventative health and physical activity. As one example, at the 9-month interview college-educated mothers were most likely to report they had attended the appropriate number of well-child visits (94%), compared to 81% of mothers without a high school diploma, 87% of mothers with a high school diploma, and 90% of the mothers with some college experience. At the 2-year interview there were no statistical differences in having attended well-child visits across the different education groups, but at the 4- and 5-year interviews the education-related pattern emerged once again. We observed a nearly identical pattern for physical activity.
Overall, the findings from the bivariate analyses suggested that differences exist across education groups in terms of mothers’ health investment behaviors, but there may be variation in how these trends play out over the early developmental gradient. Next, we tested these patterns in a more robust modeling framework, accounting for key confounds, unmeasured heterogeneity across children and within child over time, and nonindependence in the parenting measures.
Multivariate Analyses
Turning to a multivariate framework. in Table 3 we present the results of the ordinary least squares and logistic random effects models in which the seven maternal health investment behaviors were predicted by maternal education, net of the covariates (full model results are available on request). In Model 1, we noted that, across all maternal health investment behaviors, mothers with a college degree were more likely to practice advantageous health behaviors compared to all other education groups, except for eating dinner together. They were more likely to make the appropriate number of well-child visits, to promote physical activity and advantageous nutrition, and to use a car seat; and they were less likely to expose their children to SHS; and their children watched fewer hours of television, on average. Moreover, these educational differences appeared to follow an education gradient whereby mothers with college degrees had the highest levels of health investment and mothers without a high school diploma the least.
Table 3.
Logit and Ordinary Least Squares (OLS) Regression Random Effects Models Predicting Maternal Health Behaviors: Unstandardized B Coefficients (With Standard Errors in Parentheses)
| Predictor | Well-child checkups | Nutrition | Dinner together | Physical activity | TV watching | Car seat use | Smoke exposure | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1a | Model 2a | Model 1a | Model 2a | Model 1b | Model 2b | Model 1b | Model 2b | Model 1b | Model 2b | Model 1b | Model 2b | Model 1a | Model 2a | |
| Maternal education (ref.: college or more) |
||||||||||||||
| No HS/GED | −0.38** | −0.80*** | −0.38*** | −0.52*** | −0.04 | 0.21* | −0.34*** | 0.95*** | 0.25*** | 0.14** | −0.45* | −1.11** | 2.82*** | 2.90*** |
| (0.13) | (0.18) | (0.04) | (0.05) | (0.08) | (0.10) | (0.09) | (0.12) | (0.04) | (0.05) | (0.18) | (0.37) | (0.27) | (0.31) | |
| HS/GED | −0.40*** | −0.67*** | −0.24*** | −0.49*** | −0.08 | 0.14† | −0.35*** | 0.46*** | 0.20*** | 0.19*** | −0.44** | −0.38 | 2.30*** | 2.10*** |
| (0.10) | (0.15) | (0.03) | (0.04) | (0.07) | (0.08) | (0.07) | (0.10) | (0.03) | (0.04) | (0.15) | (0.36) | (0.23) | (0.27) | |
| Some college | −0.26** | −0.46** | −0.12*** | −0.28*** | 0.03 | 0.14† | −0.20** | 0.30** | 0.12*** | 0.12** | −0.29* | −0.13 | 1.48*** | 1.47*** |
| (0.09) | (0.15) | (0.03) | (0.04) | (0.06) | (0.08) | (0.07) | (0.10) | (0.03) | (0.04) | (0.14) | (0.39) | (0.21) | (0.26) | |
| Child’s age (ref.: 9 months) |
||||||||||||||
| 2 years | 0.39*** | −0.15 | 0.01 | −0.19*** | −0.19* | −0.03 | ||||||||
| (0.07) | (0.14) | (0.02) | (0.04) | (0.08) | (0.19) | |||||||||
| 4 years | −0.48*** | −0.71*** | 0.01 | −0.17*** | −0.54*** | −0.34*** | 0.46*** | 1.01*** | 0.03 | −0.03 | −2.25*** | −2.48*** | −0.01 | −0.23 |
| (0.08) | (0.15) | (0.02) | (0.04) | (0.04) | (0.07) | (0.06) | (0.10) | (0.02) | (0.04) | (0.13) | (0.32) | (0.10) | (0.21) | |
| 5 years | 1.02*** | 1.14*** | 0.01 | −0.18*** | −0.45*** | −0.25*** | −0.23*** | 1.04*** | 0.04† | 0.04 | −3.00*** | −3.14*** | −0.04 | −0.23 |
| (0.10) | (0.23) | (0.02) | (0.04) | (0.05) | (0.07) | (0.06) | (0.10) | (0.03) | (0.04) | (0.14) | (0.32) | (0.10) | (0.21) | |
| Maternal education × age interactions (ref.: college or more, 9 months) |
||||||||||||||
| No HS/GED × 2 years | 0.84*** | 0.20*** | −0.37 | |||||||||||
| (0.20) | (0.06) | (0.25) | ||||||||||||
| HS/GED × 2 years | 0.77*** | 0.36*** | −0.04 | |||||||||||
| (0.19) | (0.05) | (0.23) | ||||||||||||
| Some college × 2 years | 0.51** | 0.25*** | −0.24 | |||||||||||
| (0.20) | (0.05) | (0.24) | ||||||||||||
| No HS/GED × 4 years | 0.50* | 0.16*** | −0.41*** | −1.13*** | 0.20*** | 0.90† | −0.02 | |||||||
| (0.20) | (0.05) | (0.09) | (0.14) | (0.05) | (0.36) | (0.25) | ||||||||
| HS/GED × 4 years | 0.22 | 0.33*** | −0.35*** | −0.76*** | 0.06 | −0.05 | 0.45† | |||||||
| (0.18) | (0.05) | (0.08) | (0.12) | (0.05) | (0.36) | (0.24) | ||||||||
| Some college × 4 years | 0.19 | 0.19*** | −0.10 | −0.55*** | 0.05 | −0.10 | 0.23 | |||||||
| (0.19) | (0.05) | (0.09) | (0.13) | (0.05) | (0.39) | (0.25) | ||||||||
| No HS/GED × 5 years | 0.13 | 0.22*** | −0.34*** | −2.92*** | 0.13† | 0.67† | 0.13 | |||||||
| (0.30) | (0.05) | (0.10) | (0.15) | (0.05) | (0.36) | (0.25) | ||||||||
| HS/GED × 5 years | −0.28 | 0.32*** | −0.30*** | −1.75*** | −0.02 | −0.08 | 0.43† | |||||||
| (0.27) | (0.05) | (0.08) | (0.12) | (0.05) | (0.36) | (0.24) | ||||||||
| Some college × 5 years | −0.16 | 0.22*** | −0.23** | −1.02*** | −0.04 | −0.24 | 0.06 | |||||||
| (0.29) | (0.05) | (0.09) | (0.13) | (0.05) | (0.39) | (0.25) | ||||||||
| R2 (OLS)/−2 LL (Logit) | 12,174 | 12,143 | 0.094 | 0.097 | 0.049 | 0.051 | 19,449 | 18,938 | 0.070 | 0.071 | 10,865 | 10,181 | 14,816 | 15,001 |
Note. The regression includes the full set of controls. Ns are rounded to nearest 50th per National Council on Educational Statistics guidelines. Unweighted Ns. Person N = 5,000 for each model. ref. = reference category; HS = high school; LL = log likelihood.
n = 19,900.
n = 14,950.
p < .10.
p < .05.
p < .01.
p < .001.
Next, Model 2 in Table 3 displays the main and interaction effects of maternal education and child age on maternal health investment behaviors. Recall that we hypothesized that there are health investment behaviors that should not change over the early childhood developmental gradient (i.e., SHS exposure and car seat use) and investment behaviors that should change and adapt over early childhood (i.e., well-child visits, nutrition, dinner together, physical activity, and television watching). As expected, when we examined behaviors for which we did not expect to see any change across, there was no moderation effect of mothers’ education. In other words, the maternal education and child age interaction terms were not statistically significant when predicting SHS exposure or car seat use, suggesting that although women with more education were consistently less likely to smoke (or expose their children to smoke), and more likely to use a car seat, this difference did not narrow or widen over early childhood.
Turning to behaviors that we did expect to change at different developmental stages, we found significant moderation effects (i.e., significant interactions) for four of the five outcomes, with the strength and size of these interaction effects varying across child age. To help interpret these interactions, we graphed the predicted probabilities (for dichotomous outcomes) or average values (for continuous ones) based on the point estimates for each maternal education group and at each child age. These graphs appear in Figures 1–4.
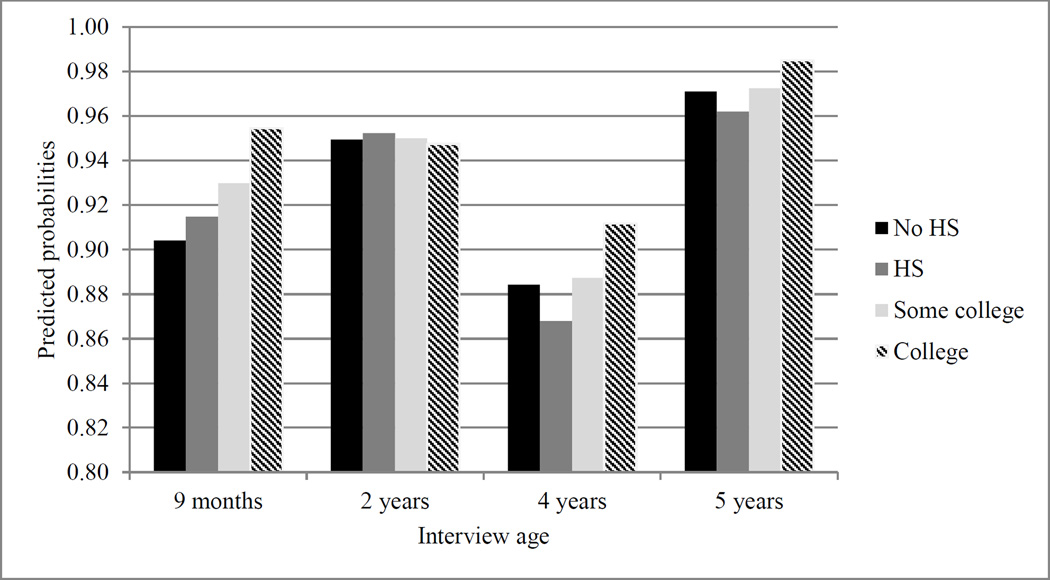
Figure 1.
Point Estimates Predicting Appropriate Number of Well-Child Checkups.
Note. HS = high school.
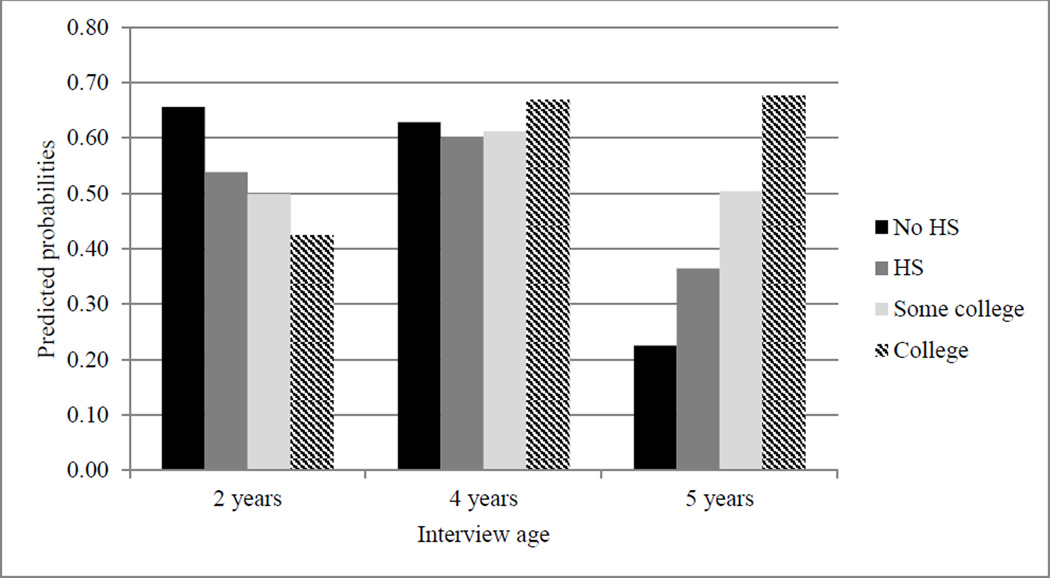
Figure 4.
Point Estimates Predicting Advantageous Physical Activity Practices.
Note. HS = high school.
Figure 1 displays the predicted probabilities of attending the appropriate number of well-child visits, revealing that the difference between college-educated mothers and mothers with less education was largest at the 9-month interview. This is noteworthy because the period between birth and 9 months requires many more visits to the pediatrician than at any other time during childhood and are sensitive for detecting longer term health problems. In this way, the disparity in health investment behaviors across education groups appeared largest when the child’s needs are potentially most complex and cumbersome and amenable to early intervention.
Second, Figure 2 displays the predicted average nutrition z score, indicating deviation from the sample mean on better nutritional practices at each interview wave. Again, this figure reveals that differences between mothers with a college degree and mothers with less education was largest at the 9-month interview. This effect was driven by differences in breastfeeding practices, which is a considered one of the most significant predictors of childhood obesity and other childhood and adult health, including chronic illnesses (Gillman, 2008).
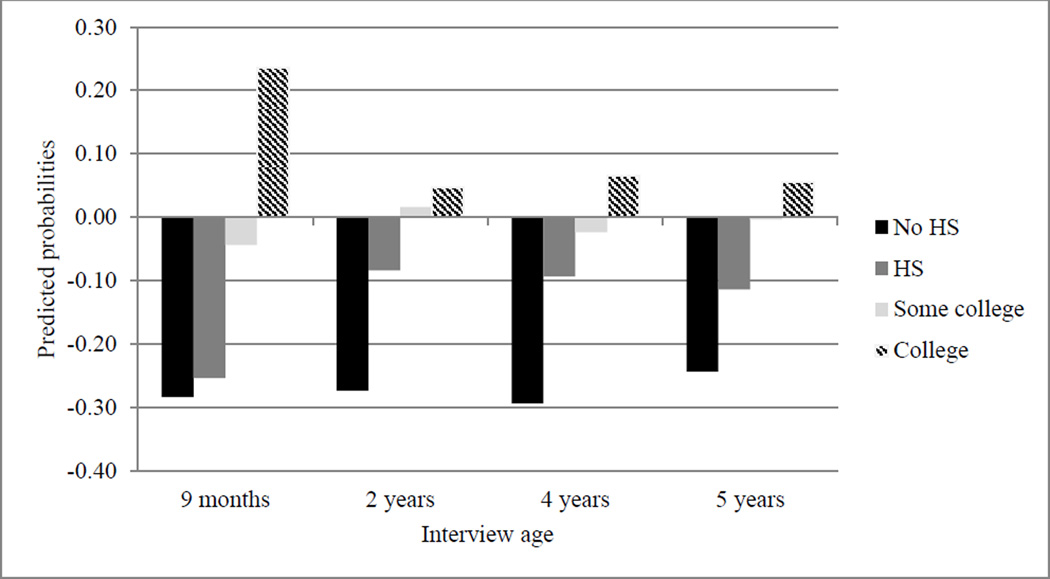
Figure 2.
Point Estimates Predicting Advantageous Nutrition Practices.
Note. HS = high school.
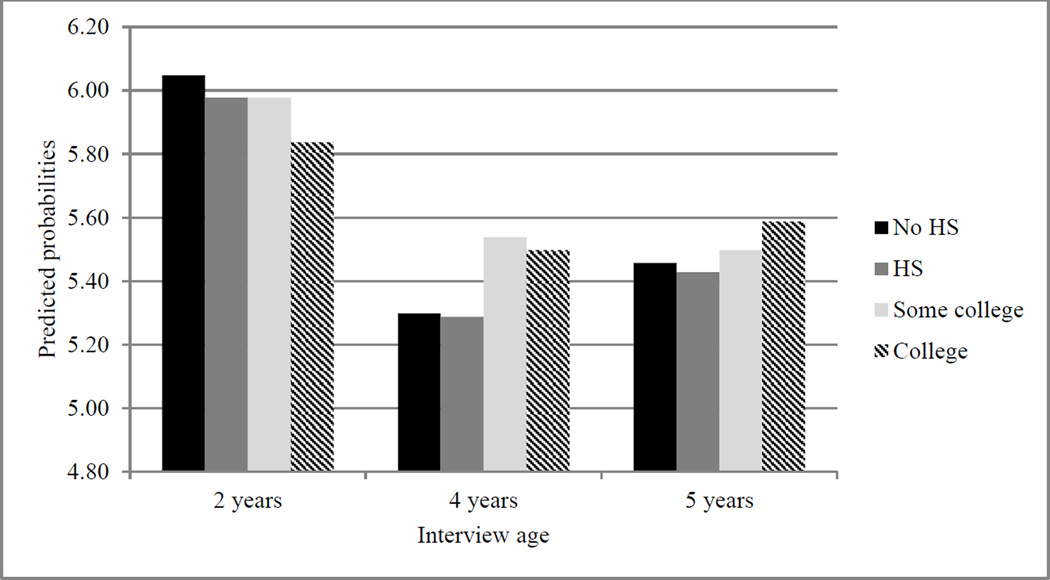
Third, Figure 3 plots the predicted number of times the family ate dinner together during a typical week. The difference between mothers with a college degree (and mothers with some college experience) and mothers with less education appears larger as children aged. Again, these later ages may be sensitive in terms of nutritional intake as children gain more autonomy over food consumption and are more susceptible to outside influences, such as peer groups and television advertising (Andreyeva et al., 2011).
Figure 3.
Point Estimates Predicting Frequency of Eating Dinner Together.
Note. HS = high school.
Finally, Figure 4 shows the predicted probabilities of mothers’ reports of their child’s physical activity. Mothers with no high school diploma were most likely to report that their child played outside daily at the 2-year interview, but this trend reversed, with the gap largest across education groups at the 5-year interview, when college-educated mothers were most likely to report their child was participating in some physical activity. This disparity appeared mostly driven by participation in organized sports/dance and occurred at a time when play becomes a smaller part of children’s days as they enter more formal schooling. In addition, the ages of 5 to 7 years have been identified as another sensitive period for the development of persistent obesity (Han et al., 2010), making the start of school an important time for physical activity.
In regards to television watching, we expected there to be a moderation effect between maternal education and child’s age, with the disparity in the number of hours children watch television largest at earlier ages, when suggested guidelines around screen time are most stringent. For the most part, there was no obvious moderation effect, except for a slight but notable growth in the gap between college-educated mothers and mothers without a high school at the 4-year interview (vs. their child’s screen time at the 2-year interview). A potential explanation is the higher rates of preschool participation among children of college-educated mothers (Augustine et al., 2009), meaning less time at home and thus less potential for television watching. This gap narrowed to the point of nonsignificance once children began school.
Discussion
A robust literature highlights how maternal education differences in parenting practices when children are young are key predictors of inequalities in children’s academic development, which persist across the early life course and eventually translate to disparities in other indicators of adult well-being (e.g., wages, labor force participation; Haveman & Wolf, 1995; McLanahan, 2004; Palloni, 2006). Yet within this framework the role of parenting in explaining maternal education differences in the development of children’s health has often been overlooked. It is important to note that the extant research underscores the connection between early and later health, on the one hand, and maternal education differences in child health on the other. Such research laid the foundation for the current study, in which we examined maternal education differences in mothers’ health investment behaviors across early childhood (birth through age 5) and, going a step further, whether these differences are greatest during the time when children’s needs are the most demanding, sensitive to parental inputs, or foundational to long-term health.
In the first step, we found that mothers with a college degree were most likely to practice more advantageous health investment behaviors in terms of preventative care, nutrition, SHS exposure, car seat use, physical activity, and television watching throughout early childhood. These results echoed those found in the large literature linking maternal education to parenting behaviors related to children’s academic skills (e.g., Bodovski & Farkas, 2008; Cheadle, 2008). Yet we also examined how the association between maternal education and health investment behaviors changed over early childhood. We compared behaviors that we expected to change in response to their child’s changing needs at different ages to those that we would expect to be stable across early childhood, and our results for the most part matched our specific hypotheses.
We found no widening or narrowing of the education disparity over early childhood for child’s SHS exposure and car seat use—for which the recommended pediatrician guidelines do not change (i.e., children’s exposure to SHS should be limited; they should always travel in a car seat; Best, 2009; Durbin, 2011). When we examined behaviors that we expected to change over early childhood, however, we found that, in four of the five outcomes we examined, the gap in the practice of advantageous health investment behaviors between college-educated mothers and mothers with less education was widest at ages when their children’s health needs were most complex or sensitive for health and well-being. For example, education disparities in meeting the appropriate number of well-child visits were largest during infancy, when the appointments are more numerous and identifying health problems potentially most important for intervention (Committee on Children with Disabilities, 1994; NIH, n.d.). As another example, the education disparities in children’s physical activity were widest when children were age 5—a period highly predictive of later childhood obesity (Han et al., 2010), when children transition to more hours spent in school and the potential to become more sedentary increases.
Although we expected there to be a developmental gradient in children’s television watching (whereby education gaps in television watching may be larger at younger ages), we did not find one. One potential explanation for this could be related to the much younger ages (compared to the AAP’s recommendation of at least age 2 years) of television watching initiation (Vandewater et al., 2007). It remains possible, however, that if questions had been asked on this topic at 9 months we may have seen a gradient at this earlier wave that narrowed by the 2-year interview, when television watching is a more accepted activity for children and rules and routines around television watching are more firmly established.
Taken together, we view these two sets of results (comparing changing and unchanging health needs) as new evidence for how maternal education is linked to children’s short- and long-term health—above and beyond correlated sources of income or demographic factors, which we controlled for. We also view these results as evidence of an unrecognized pathway in the intergenerational transmission of inequality and a source of disadvantage for children raised by less educated mothers. Not only are their mothers less likely to exhibit the health investment behaviors linked to more advantageous health outcomes, but also they are even less likely to exhibit those parenting behaviors when those behaviors are potentially most vital to their child’s long-term well-being.
Despite this contribution to research and theory, our study also has limitations. Perhaps the greatest is the difference in measurement of certain variables over time that may account for some compounded disparities. Differences in measurement could capture more variability at certain times (nutrition was measured on a scale of 0–6 at 9 months, 0–2 at 2 years, and a 1–7 at the 4- and 5-year interviews), qualitatively different dimensions of the concept we wanted to measure, or differences in a just one specific behavior (e.g., in breastfeeding behavior but not other nutrition behaviors). Given the study’s goals, we needed to develop a measurement system that captured the same concept across time. We understand our approach was vulnerable to the problems mentioned above, but it was also a limitation for only some measures. For example, we found a developmental gradient for eating dinners together, which was in fact consistently measured across waves. In addition, despite the use of different measures across time, we also consistently found the widest maternal education differences at the child age we expected to.
An additional measurement limitation is that the health behaviors were self-reported. Mothers with more education may have more knowledge of the socially desirable responses (regardless of their actual behaviors) or fear of stigma if they report less desirable responses, which would exaggerate the differences between education groups. This is a limitation, however, that is shared by most studies on parental investment or time use that must rely on parent reports. There is also some indication that social desirability bias in reporting health-related parenting behaviors, though present, does not differ by mothers’ education (Fisher et al., 2008).
We also note that there may be other confounds that are associated with both maternal education and parenting behaviors that remain unmeasured and unaccounted for in our study. We included a rich array of controls and used statistical models to try and negate this limitation, but we cannot say with certainty whether there is a causal association. Nevertheless, should we have failed to account for some selection factors, our results would still point to a socioeconomic-related pattern that reflects a dimension of inequality that was not previously recognized.
Next, we did not take the additional step of connecting these parenting health behaviors to children’s actual physical health, although we did select concepts shown to matter in other studies for children’s health. Yet had we done so, we also acknowledge the possibility that the parent health behaviors we explored may produce little variability in children’s health, either because young children are generally healthy and larger variations in health are often not seen until late childhood or early adulthood (Newacheck & Halfon, 1998), or the operationalization of our concepts (i.e., nutrition), though based on widely used measures that appropriately proxy for the concept, may have limited power to predict child health. Still, we argue that the behaviors we studied lay the foundation for good health in various ways across the life course. Thus, identifying early disparities in parenting prior to the development of the actual health outcomes themselves is important to early intervention efforts.
In sum, we found that not only are there pronounced maternal education differences in health investment behaviors but also that these differences are generally widest during the periods in a child’s life when they are more complex or demanding to practice or are more important developmentally. These empirical findings, taken more broadly, shed new light on how mothers’ education is connected to the diverging destinies of children today. Given how policy efforts targeting disparities in young children’s health tend to focus on the children themselves, our study shows how investments in the formal education of women may also benefit child health and help reduce social inequalities across generations. A more modest recommendation would be to target the public health knowledge and health behaviors of less educated mothers at sensitive periods, although given the multifaceted returns to education for which our study provides evidence, such approaches would likely have a lesser impact.
Acknowledgments
We acknowledge Population Research Center (5 R24 HD042849) and Training Program in Population Studies (5 T32 HD007081) grants awarded to the Population Research Center at The University of Texas at Austin by the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Contributor Information
Kate C. Prickett, Email: kate.prickett@utexas.edu, The Population Research Center, University of Texas at Austin, 305 E. 23rd St., G1800, Austin, TX 78712.
Jennifer M. Augustine, Department of Sociology, Sloan College, University of South Carolina, 911 Pickens St., Columbia, SC 29208
References
- Aizer A, Currie J. The intergenerational transmission of inequality: Maternal disadvantage and health at birth. Science. 2014 May 23;344:856–861. doi: 10.1126/science.1251872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison PD. Missing data. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- Allison PD. Fixed effects regression models. Thousand Oaks, CA: Sage; 2009. [Google Scholar]
- Anderson SE, Whitaker RC. Household routines and obesity in US preschool-aged children. Pediatrics. 2010;125:420–428. doi: 10.1542/peds.2009-0417. [DOI] [PubMed] [Google Scholar]
- Andreyeva T, Rashad I, Harris JL. Exposure to food advertising on television: Associations with children’s fast food and soft drink consumption and obesity. Economics & Human Biology. 2011;9:221–233. doi: 10.1016/j.ehb.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Augustine JM. Maternal education and the unequal significance of family structure for children’s early achievement. Social Forces. 2014;93:687–718. [Google Scholar]
- Augustine JM, Cavanagh S, Crosnoe R. Maternal education, child care, and the reproduction of advantage. Social Forces. 2009;88:1–30. doi: 10.1353/sof.0.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton D. The role of parents in the determination of the food preferences of children and the development of obesity. International Journal of Obesity. 2004;28:858–869. doi: 10.1038/sj.ijo.0802532. [DOI] [PubMed] [Google Scholar]
- Best D. Tobacco use: A pediatric disease. Pediatrics. 2009;124:1474–1487. doi: 10.1542/peds.2009-2114. [DOI] [PubMed] [Google Scholar]
- Birch LL, Fisher JO. Development of eating behaviors among children and adolescents. Pediatrics. 1998;101:539–549. [PubMed] [Google Scholar]
- Black D, Morris JN, Smith C, Townsend P, Whitehead M. Inequalities in health: The Black Report. The health divide. London: Penguin; 1988. [Google Scholar]
- Blau PM, Duncan OD. The American occupational structure. New York: Wiley; 1967. [Google Scholar]
- Bodovski K, Farkas G. “Concerted cultivation” and unequal achievement in elementary school. Social Science Research. 2008;37:903–919. [Google Scholar]
- Carbonaro W. A little help from my friends’ parents: Intergenerational closure and educational outcomes. American Journal of Sociology. 1998;94:S95–S120. [Google Scholar]
- Case A, Fertig A, Paxson C. The lasting impact of childhood health and circumstance. Journal of Health Economics. 2005;24:365–389. doi: 10.1016/j.jhealeco.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Case A, Paxson C. Parental behavior and child health. Health Affairs. 2002;21:164–178. doi: 10.1377/hlthaff.21.2.164. [DOI] [PubMed] [Google Scholar]
- Cheadle JE. Educational investment, family context, and children’s math and reading growth from kindergarten through the third grade. Sociology of Education. 2008;81:1–31. [Google Scholar]
- Chen E, Martin A, Matthews KA. Socioeconomic status and health: Do gradients differ within childhood and adolescence? Social Science & Medicine. 2006;62:2161–2170. doi: 10.1016/j.socscimed.2005.08.054. [DOI] [PubMed] [Google Scholar]
- Committee on Children with Disabilities. Screening infants and young children for developmental disabilities. Pediatrics. 1994;93:863–865. [PubMed] [Google Scholar]
- Crosnoe R, Huston AC. Socioeconomic status, schooling, and the developmental trajectories of adolescents. Developmental Psychology. 2007;43:1097–1110. doi: 10.1037/0012-1649.43.5.1097. [DOI] [PubMed] [Google Scholar]
- Currie J. Healthy, wealthy, and wise: Socioeconomic status, poor health in childhood, and human capital development. Journal of Economic Literature. 2009;47:87–122. [Google Scholar]
- Durbin DR. Child passenger safety. Pediatrics. 2011;127:e1050–e1066. doi: 10.1542/peds.2011-0215. [DOI] [PubMed] [Google Scholar]
- Fisher JO, Butte NF, Mendoza PM, Wilson TA, Hodges EA, Reidy KC, Deming D. Overestimation of infant and toddler energy intake by 24-h recall compared with weighed food records. American Journal of Clinical Nutrition. 2008;88:407–415. doi: 10.1093/ajcn/88.2.407. [DOI] [PubMed] [Google Scholar]
- Gage TB, Fang F, O’Neill E, Dirienzo G. Maternal education, birth weight, and infant mortality in the United States. Demography. 2013;50:615–635. doi: 10.1007/s13524-012-0148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman MW. The first months of life: A critical period for the development of obesity. American Journal of Clinical Nutrition. 2008;87:1587–1589. doi: 10.1093/ajcn/87.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goris JM, Petersen S, Stamatakis E, Veerman JLT. Television food advertising and the prevalence of childhood overweight and obesity: A multicountry comparison. Public Health Nutrition. 2010;13:1003–1012. doi: 10.1017/S1368980009992850. [DOI] [PubMed] [Google Scholar]
- Greenberger E, O’Neil R, Nagel SK. Linking workplace and homeplace: Relations between the nature of adults’ work and their parenting behaviors. Developmental Psychology. 1994;30:990–1002. [Google Scholar]
- Grzywacz JG, Butler AB. The impact of job characteristics on work-to-family facilitation: Testing a theory and distinguishing a construct. Journal of Occupational Health Psychology. 2005;10:97–109. doi: 10.1037/1076-8998.10.2.97. [DOI] [PubMed] [Google Scholar]
- Haas SA, Glymour MM, Berkman LF. Childhood health and labor market inequality over the life course. Journal of Health and Social Behaviors. 2011;52:298–313. doi: 10.1177/0022146511410431. [DOI] [PubMed] [Google Scholar]
- Hammons AJ, Fiese BH. Is frequency of shared family meals related to the nutritional health of children and adolescents? Pediatrics. 2011;127:e1565–e1574. doi: 10.1542/peds.2010-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Lawlor D, Kimm SYS. Childhood obesity. The Lancet. 2010;375:1737–1748. doi: 10.1016/S0140-6736(10)60171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haveman R, Wolfe B. The determinants of children’s attainments: A review of methods and findings. Journal of Economic Literature. 1995;33:1829–1878. [Google Scholar]
- Heckman J. Skill formation and the economics of investing in disadvantaged children. Science. 2006 Jun 30;312:1900–1902. doi: 10.1126/science.1128898. [DOI] [PubMed] [Google Scholar]
- Hendricks CM, Reichert A. Parents’ self-reported behaviors related to health and safety of very young children. Journal of School Health. 1996;66:247–251. doi: 10.1111/j.1746-1561.1996.tb06279.x. [DOI] [PubMed] [Google Scholar]
- Hesketh K, Ball K, Crawford D, Campbell K, Salmon J. Mediators of the relationship between maternal education and children’s TV viewing. American Journal of Preventive Medicine. 2007;33:41–47. doi: 10.1016/j.amepre.2007.02.039. [DOI] [PubMed] [Google Scholar]
- Janz KF, Burns TL, Levy SM. Tracking activity and sedentary behaviors in childhood: The Iowa Bone Development Study. American Journal of Preventative Medicine. 2005;29:171–178. doi: 10.1016/j.amepre.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Kalil A, Ryan R, Corey M. Diverging destinies: Maternal education and the developmental gradient in time with children. Demography. 2012;49:1361–1383. doi: 10.1007/s13524-012-0129-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JP, Liverman CT, Kraak VI. Preventing childhood obesity: Health in the balance. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- Kingston PW, Hubbard R, Lapp B, Schroeder P, Wilson J. Why education matters. Sociology of Education. 2003;76:53–71. [Google Scholar]
- Kudlová E, Schneidrová D. Dietary patterns and their changes in early childhood. Central European Journal of Public Health. 2012;20:126–134. doi: 10.21101/cejph.a3703. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Lareau A. Invisible inequality: Social class and childrearing in Black families and White families. American Sociological Review. 2002;67:747–776. [Google Scholar]
- Lindsay AC, Sussner KM, Kim J, Gortmaker SL. The role of parents in preventing childhood obesity. The Future of Children. 2006;16:169–186. doi: 10.1353/foc.2006.0006. [DOI] [PubMed] [Google Scholar]
- Mangrio E, Hansen K, Lindström M, Köhler M, Rosvall M. Maternal educational level, parental preventive behavior, risk behavior, social support, and medical care in 8-month-old children in Malmör, Sweden. BMC Public Health. 2011;11:891–899. doi: 10.1186/1471-2458-11-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson DM, Killen JD, Wang Y, Varady A, Robinson TN. Children’s food consumption during television viewing. American Journal of Clinical Nutrition. 2004;79:1088–1094. doi: 10.1093/ajcn/79.6.1088. [DOI] [PubMed] [Google Scholar]
- Mayer SE. What money can’t buy: Family income and children’s life chances. Cambridge, MA: Harvard University Press; 1997. [Google Scholar]
- McLanahan SS. Diverging destinies: How children are faring under the Second Demographic Transition. Demography. 2004;41:607–627. doi: 10.1353/dem.2004.0033. [DOI] [PubMed] [Google Scholar]
- Mirowsky J, Ross CE. Education, social status, and health. New York: Transaction; 2003. [Google Scholar]
- National Institutes of Health. Well-child visits. (n.d.). Retrieved from www.nlm.nih.gov/medlineplus/ency/article/001928.htm.
- Newacheck PW, Halfon N. Prevalence and impact of disability chronic conditions in childhood. American Journal of Public Health. 1998;88:610–617. doi: 10.2105/ajph.88.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østbye T, Malhotra R, Stroo M, Lovelady C, Brouwer R, Zucker N, Fuemmeler B. The effect of home environment on physical activity and dietary intake in preschool children. International Journal of Obesity. 2013;37:1287–1412. doi: 10.1038/ijo.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palloni A. Reproducing inequalities: Luck, wallets, and the enduring effects of childhood health. Demography. 2006;43:587–615. doi: 10.1353/dem.2006.0036. [DOI] [PubMed] [Google Scholar]
- Philips N, Sioen I, Michels N, Sleddens E, De Henauw S. The influence of parenting style on health related behavior of children: Findings from the ChiBS study. International Journal of Behavioral Nutrition and Physical Activity. 2014;11:95–108. doi: 10.1186/s12966-014-0095-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radey M, Randolf KA. Parenting sources: How do parents differ in their efforts to learn about parenting? Family Relations. 2009;58:536–548. [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Robinson T. Reducing children’s television viewing to prevent obesity: A randomized controlled trial. Journal of the American Medical Association. 1999;282:1561–1567. doi: 10.1001/jama.282.16.1561. [DOI] [PubMed] [Google Scholar]
- Salvy S, de la Haye K, Bowker JC, Hermans RCJ. Influence of peers and friends on children’s and adolescents’ eating and activity behaviors. Physiology & Behavior. 2012;106:369–378. doi: 10.1016/j.physbeh.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell WH, Haller AO, Portes A. The educational and early occupational attainment process. American Sociological Review. 1969;34:82–92. [Google Scholar]
- Snow K, Thalji L, Derecho A, Wheeless S, Lennon J, Kinsey S, Park J. Early Childhood Longitudinal Study, Birth Cohort (ECLS-B), Preschool year 2005 and 2006 data file user’s manual Publication No. NCES 2008-024. Washington, DC: National Center for Education Statistics; 2007. [Google Scholar]
- Spencer N. Maternal education, lone parenthood, material hardship, maternal smoking, and longstanding respiratory problems in childhood: Testing a hierarchical conceptual framework. Journal of Epidemiology & Community Health. 2005;59:842–846. doi: 10.1136/jech.2005.036301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon PS, Zhou C, Lozano P, Christakis DA. Preschoolers’ total daily screen time at home and by type of child care. The Journal of Pediatrics. 2011;158:297–399. doi: 10.1016/j.jpeds.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Tandon PS, Zhou C, Sallis JF, Cain KL, Frank LD, Saelens BE. Home environment relationships with children’s physical activity, sedentary time, and screen time by socioeconomic status. International Journal of Behavioral Nutrition and Physical Activity. 2012;9:88. doi: 10.1186/1479-5868-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay MS, LeBlanc AG, Kho ME, Saunders TJ, Larouche R, Colley RC, Grober SC. Systematic review of sedentary behaviour and health indicators in school-aged children and youth. International Journal of Behavioral Nutrition and Physical Activity. 2011;8:98–119. doi: 10.1186/1479-5868-8-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Child Health USA 2013. Rockville, MD: Health Resources and Services Administration, Maternal and Child Health Bureau, U.S. Department of Health and Human Services; 2013. [Google Scholar]
- Vandewater EA, Rideout VJ, Wartella EA, Huang X, Lee JH, Shim M. Digital childhood: Electronic media and technology use among infants, toddlers, and preschoolers. Pediatrics. 2007;119:e1006–e1015. doi: 10.1542/peds.2006-1804. [DOI] [PubMed] [Google Scholar]
- Wildenger LK, McIntyre LL, Fiese BH, Eckert TL. Children’s daily routines during kindergarten transition. Early Childhood Education Journal. 2008;36:69–74. [Google Scholar]
- Yoshinaga-Itano C, Sedey AL, Coulter DK, Mehl AL. Language of early- and later-identified children with hearing loss. Pediatrics. 1998;102:1161–1171. doi: 10.1542/peds.102.5.1161. [DOI] [PubMed] [Google Scholar]