Abstract
Objective:
To assess usability, bioavailability, and safety of subcutaneous self-administration of 0.3 mL of methotrexate 50 mg/mL solution via a prefilled autoinjector pen (methotrexate pen) in patients with rheumatoid arthritis.
Methods:
The study enrolled methotrexate-naïve and methotrexate-experienced patients aged ≥16 years. Visit 1 (Day 1) included methotrexate pen usage training with documentation, patient self-injection, and a patient-training questionnaire completed by the healthcare professional. Visit 2 (Days 8–10) included evaluation of patient self-injection through four scenarios: holding needle in place for 5 s, confirming methotrexate delivery, skin pinch, and pen disposal. At Visit 2, patient opinion and training retention (since Visit 1) were also assessed. Pharmacokinetic parameters were assessed in 25 patients, who were stratified by body weight and randomized to receive injections in the abdomen or the upper thigh.
Results:
At Visit 1, 12 of 106 patients had questions about the pen, and 4 required self-injection assistance. At Visit 2, the mean performance rating for all scenarios was ≥9.8 (scale: 1 (very difficult)−10 (very easy)). Successful completion rates were 96.2%–100%; 91.3%–100% of patients required no assistance. Impressions of the pen were favorable; 98.1% of patients passed the written examination. All methotrexate pens effectively delivered 0.3 mL methotrexate and were intact after use. Body weight >100 kg significantly decreased total and peak methotrexate exposure when administered abdominally. No adverse effects resulted in drug discontinuation.
Conclusion:
The methotrexate pen was used with a high degree of effectiveness, satisfaction, and safety, indicating that this delivery system may be a viable option for patients requiring subcutaneous methotrexate.
Keywords: Methotrexate, rheumatoid arthritis, injection, pharmacokinetics, questionnaire, effectiveness, delivery system
Methotrexate (MTX) is the most commonly used and recommended disease-modifying antirheumatic drug for the treatment of rheumatoid arthritis (RA).1,2 Oral MTX has variable absorption and bioavailability among patients3–9 and demonstrates saturation of the absorption mechanism with increasing doses.10 Subcutaneously administered MTX appears to circumvent these issues.8,10–13 For example, the bioavailability of oral MTX was found to plateau with a 15-mg dose, while subcutaneous (SC) delivery of MTX achieved higher bioavailability levels, and these levels increased with higher doses of MTX.14 In a previous trial of the autoinjector pen device used in this trial, SC administration of MTX by the pen resulted in a higher relative bioavailability than oral MTX without dosing saturation across several MTX concentrations commonly used in the treatment of RA.15
Importantly, in patients with RA who are intolerant or unresponsive to oral MTX treatment, parenteral SC MTX has been shown to improve disease control16–20 and to reduce the need for biologic therapy.17,18 SC MTX is increasingly being considered as an alternative to oral MTX for patients, but there are difficulties associated with SC delivery of MTX when a syringe is used. These challenges include the time necessary to train patients on proper syringe use (e.g. drawing and preparation of MTX, injection protocols, disposal), limitations in a patient’s ability to self-administer the drug due to physical disabilities (e.g. vision problem, poor manual dexterity), and adversity or fear of syringe use.
The development and implementation of medications delivered SC via autoinjector/pen devices and single-use syringes have become increasingly common for the treatment of chronic disorders such as migraine, diabetes, and multiple sclerosis.21–23 Newer injection systems have been designed with shorter and smaller gauge needles to minimize injection pain and decrease the risk of accidental intramuscular (IM) injection.23 Importantly, patients with RA and other chronic disorders have been found to prefer autoinjection pen devices versus more conventional treatments (e.g. vial and syringe, prefilled syringe) in clinical trials because of their ease of use and convenience,24–26 with some patients reporting improved quality of life.27,28
The purpose of this study was to assess the usability, label comprehension, robustness, safety, and bioavailability of SC self-administration of the prefilled MTX autoinjector pen (MTX pen) containing 0.3 mL of MTX 50 mg/mL solution (medac GmbH, Germany) in a typical population of patients with RA who require MTX treatment.
Patients and methods
Study design
This actual-use study was performed at five clinical research centers in the United States (Pinellas Park, FL; Duncansville, PA; South Miami, FL; Hot Springs, AR; and Houston, TX) over a 2-week period between October 2012 and January 2013. Each site was scheduled to enroll approximately 25–30 patients with a 10% over-enrollment to allow for dropouts to achieve a minimum of 100 participants who had successfully completed at least one self-injection. This number of participants is representative of a typical human factors evaluation for a device and was deemed necessary by the US Food and Drug Administration (FDA) for US-labeling of the autoinjector pen.
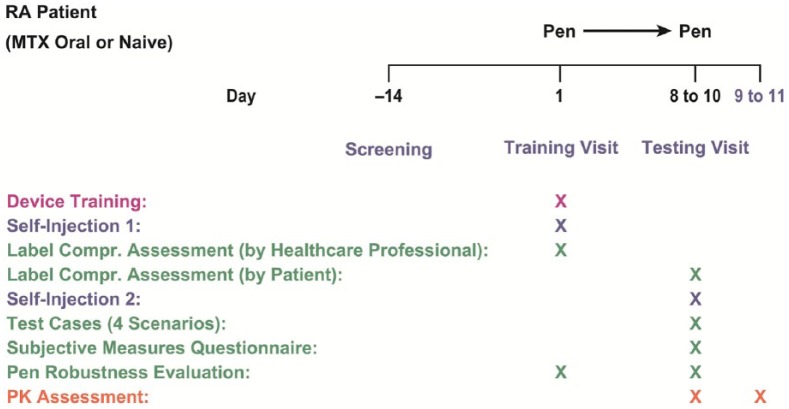
Design of the study is shown in Figure 1. Screening of patients took place at Day −14 (14 days prior to the start of the first study visit. Study Visit 1 (V1; Day 1) consisted of training on the use of the device followed by patient self-injection in the presence of a qualified healthcare professional. Afterwards, healthcare professionals completed a questionnaire of patient training (part 1 of the label comprehension assessment) and evaluated pen robustness. To achieve interrater reliability, the healthcare professionals of the different study sites were trained by the same individual on the use of the device. This assured a standardized level and quality of patient training at V1 between study sites. At Study Visit 2 (V2; Days 8–10), patients completed a written examination in order to evaluate how well they comprehended training information they received at V1 (part 2 of the label comprehension assessment). V2 also consisted of human factors/usability testing in which patients were evaluated under direct observation of an independent monitor for evaluation of conduct while performing a self-injection. A subjective-measures questionnaire was used to record patient impressions, any difficulties, and general comments on the device. Pen robustness was again evaluated by an independent monitor. Pharmacokinetic assessments were also performed beginning at Day 8 and extended until Day 11.
Figure 1.
Study design—Label comprehension assessment (by healthcare professional): questionnaire for healthcare professionals regarding patient training and ability to self-inject using the device following training at V1. Label comprehension assessment (by patient): examination of patients’ retention and comprehension of training received at V1. Screening: 14 days prior to V1; V1: Visit 1 (Day 1); V2: Visit 2 (Days 8–10 or Days 9–11 for the pharmacokinetic study population).
A total of 25 patients enrolled in the human factors/usability study were selected for the pharmacokinetic study. To ensure that the pharmacokinetic study enrolled a sufficient number of patients across a broad range of body weights, patients in the pharmacokinetic study were stratified into three classifications of body weight groups as follows: <60 kg, 60–100 kg, or >100 kg. The sample size of six completers in each body weight group was estimated to ensure a statistical power of 90% for the confidence intervals (CIs) to be within 70% and 143% of the mean values. Using a 20% dropout rate, eight patients in each body weight category (a total of 24 patients) were to be enrolled in the pharmacokinetic study for each group. These patients were centrally and randomly assigned in a 1:1 ratio to receive injections in the abdomen or upper thigh in each of the three body weight categories. Within each category, the sites of injection were the abdomen in four patients and the upper thigh in four patients.
The study was conducted according to the International Conference on Harmonisation (ICH) harmonized tripartite guideline E6(R1). This affirms that the study followed the requirements of the Declaration of Helsinki. Approval was given by a central Institutional Review Board (Quorum; registration number: 27446) in the United States. This trial is registered at ClinicalTrials.gov under identifier NCT01871961.
Study medication
Study medication consisted solely of prefilled pens containing 0.3 mL of MTX (50 mg/mL) solution.
Patients
This study included male and female patients aged 16 years or older, who had moderate to severe RA, and were suitable candidates for MTX treatment, including newly diagnosed and MTX-naïve patients and those switching from an oral route of administration or those already receiving MTX via an SC route of administration. Key exclusion criteria included the following: females who were pregnant or nursing, past or current inflammatory arthritis or rheumatic autoimmune disease other than RA, history of hypersensitivity to MTX, and/or positive for hepatitis B surface antigen or hepatitis C antibody. Patients were excluded from the pharmacokinetic study population (PKP) if they were under 21 years of age. Written informed consent was obtained from each patient before entering the study.
Patient populations
The population sets included in this study were the all-enrolled population, the safety population (SP), the intention-to-treat population, and the PKP. The all-enrolled population included all patients who were enrolled in the study regardless of whether they had self-injected using the prefilled pen. In this study, the all-enrolled population equaled the SP, which included all patients who received at least one dose of MTX using the MTX pen and was used for all safety analyses. The intention-to-treat population included all patients who attempted or completed injection of one dose of MTX using the MTX pen at V1 and attempted or completed injection of one dose of MTX at V2; this population was used for all human factors analyses. The PKP included all patients who participated in the pharmacokinetic study and had any plasma MTX concentration data available.
Patient training and first self-injection using the prefilled pen
During V1, patients participated in a group training session or were trained individually on the proper technique for using the prefilled pen. Patients were also provided a copy of the Patient Instructions For Use (IFU; Medication Guide) for reference to be used any time during and after V1. Training was provided to the patients in the physician’s office or clinic by a healthcare professional (i.e. nurse, physician, or pharmacist) to ensure proper understanding of the Patient IFU and confirm that the device could be properly used. Following training, the patients, assisted by the healthcare professionals as needed, used the pen to perform a self-injection.
Label comprehension assessment
Label comprehension, defined as the extent to which patients understand the information on the drug product labeling and how well patients apply this information,29 was assessed in patients in a two-part process described below.
Label comprehension assessment, part 1: questionnaire of patient training completed by healthcare professionals
Following a patient’s training and self-injection, the healthcare professional completed a questionnaire about the patient training that included the following queries: (1) Was the patient trained on the use of the MTX pen? (2) Did the patient review the MTX pen IFU? (3) Did the patient understand the MTX pen IFU? (4) Did the patient have questions related to the use of the MTX pen? (5) Were all patient questions related to the MTX pen able to be answered? (6) Did the patient perform a self-injection using the MTX pen? (7) Did the patient need assistance to perform the injection? (8) Did the patient have any difficulties using the MTX pen? and (9) Did the patient experience any adverse events (AEs) at this visit? The questionnaire responses were summarized by the number and percentage of patients for each response, “Yes” or “No.”
Label comprehension assessment, part 2: written examination completed by patients
A written examination, which tested patients’ retention and comprehension of training received at V1, was completed by patients at V2. A score of at least 80% on written examination was considered a passing score.
Human factors testing
Test-case scenarios
At V2, patients performed a self-injection under the direct observation of the healthcare professional and were evaluated on four task scenarios: (1) holding needle in place for 5 s, (2) checking window of MTX pen to confirm delivery of MTX, (3) appropriately performing skin pinch for injection, and (4) properly disposing of MTX pen after injection. Patients were monitored for evidence of usability difficulties or use errors under direct observation of an independent monitor. Time on task for Scenario 1 was observed and documented. Additionally, data were collected regarding patient ease of performance, successful completion, and assistance required for success (requests, prompts, incorrect step, and self-corrected step) at each test-case scenario.
Subjective-measures questionnaire
At V2, patients also completed a subjective-measures questionnaire that included patients’ ratings of the ease or difficulty of using the prefilled pen, open-ended questions about their impressions of the device, and what changes, if any, they might make to the design of the prefilled pen or the IFU of the medication.
Pen robustness
After the patients completed self-injection at V1 and V2, the pens were collected and evaluated for evidence of failure (robustness). Pen robustness was evaluated by an independent monitor using the following criteria: (1) Was the pen still intact with all pieces remaining as one unit? (2) Was there any fluid left in the transparent control zone? (3) Was the needle intact? Did it bend or break? and (4) Did the protective needle shield move back into place to cover the needle?
Blood collection/plasma preparation and pharmacokinetic assessments
Patients in the PKP were stratified by three body weight categories (under 60 kg, 60–100 kg, and over 100 kg) and were randomly assigned to inject MTX in either the abdomen or in the upper thigh. Single blood samples were collected by the study physician or study staff from these patients at the clinical research centers from 2 h to 30 min before dosing at Visit 2 (Day 8–10) and at the following time points after dosing: 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4 h (±2 min), and 6, 8, 12, 16, and 24 h (±5 min). To accommodate blood sampling over this time-course, patients remained in the clinic overnight. A validated liquid chromatography–tandem mass spectrometry method with a lower limit of quantitation of 1.00 ng/mL was used for the bioanalysis of MTX in plasma samples. The fluorescence polarization immunoassay typically used for monitoring MTX concentrations has been reported to be non-specific following administration of certain other agents.30,31 Conversely, liquid chromatography–tandem mass spectrometry has been previously validated as rapid, highly accurate, and sensitive for use in evaluating MTX drug exposure in patients, even when MTX is used in combination with other pharmacotherapies.32 Pharmacokinetic assessments of MTX in plasma included evaluation of the following parameters: area under the plasma concentration versus time curve from time zero to the last measurable concentration (AUC0-t), area under the plasma concentration versus time curve from time zero extrapolated to infinity (AUC0-inf), maximum observed MTX plasma concentration directly from plasma concentration–time curve (Cmax), and time to reach the maximum (peak) observed plasma concentration directly from plasma concentration–time curve (Tmax).
Safety assessments
Safety assessments included monitoring and recording all AEs, serious adverse events (SAEs), adverse device effects (ADEs), and unanticipated adverse device effects (UADEs). An ADE was related to the use of the investigational device and included ADEs resulting from insufficient or inadequate IFU, deployment, implantation, installation, or operation/malfunction of the investigational device. ADEs included any event resulting from use error or from intentional misuse of the investigational device. A UADE was any serious adverse effect on health or safety or any life-threatening problem or death caused by or associated with the investigational device, if that effect, problem, or death was not previously identified in nature, severity, or degree of incidence in the investigational plan or any other unanticipated serious problem associated with a device that relates to the rights, safety, or welfare of patients.
Results
Patient disposition and characteristics
Of the 106 patients enrolled, 104 patients (98.1%) qualified for the intention-to-treat population and completed the study, whereas 2 patients (1.9%) discontinued. Discontinuations after V1 were due to voluntary withdrawal and physician decision. The patient demographic and baseline characteristics for the all-enrolled population are summarized in Table 1.
Table 1.
Summary of patient demographic and baseline characteristics.
| Characteristics | All enrolled-population |
|---|---|
| Patient group, n | 106 |
| Age, yearsa | |
| Mean (SD) | 56.8 (12.75) |
| Minimum, maximum | 27, 82 |
| Age categories, years, n (%) | |
| Under 21 | 0 |
| 21–40 | 12 (11.3) |
| 41–60 | 53 (50.0) |
| Over 60 | 41 (38.7) |
| Gender, n (%) | |
| Male | 28 (26.4) |
| Female | 78 (73.6) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 17 (16.0) |
| Not Hispanic or Latino | 89 (84.0) |
| Race, n (%) | |
| White | 93 (87.7) |
| Black | 8 (7.5) |
| Asian | 1 (0.9) |
| Other | 4 (3.8) |
| Baseline height, cm | |
| Mean (SD) | 165.67 (9.50) |
| Minimum, maximum | 142.8, 195.6 |
| Weight categories, kg, n (%) | |
| Under 60 | 17 (16.0) |
| 60–100 | 72 (67.9) |
| Over 100 | 17 (16.0) |
| BMI, kg/m2 | |
| Mean (SD) | 29.57 (7.61) |
| Minimum, maximum | 18.2, 59.9 |
| Duration of being diagnosed with RA, months | |
| Mean (SD) | 116.6 (113.62) |
| Minimum, maximum | 1, 576 |
| Previous MTX treatment | |
| Yes | 98 (92.5) |
| No | 8 (7.5) |
| Previous route of MTX treatment | |
| Intramuscular | 2 (1.9) |
| Oral | 89 (84.0) |
| Subcutaneous | 7 (6.6) |
| NA | 8 (7.5) |
| Previous use of autoinjector pen | |
| Yes | 55 (51.9) |
| No | 51 (48.1) |
BMI: body mass index; MTX: methotrexate; RA: rheumatoid arthritis; SD: standard deviation.
Age was calculated as date of informed consent minus the date of birth plus 1 divided by 365.25.
Human factors/usability testing
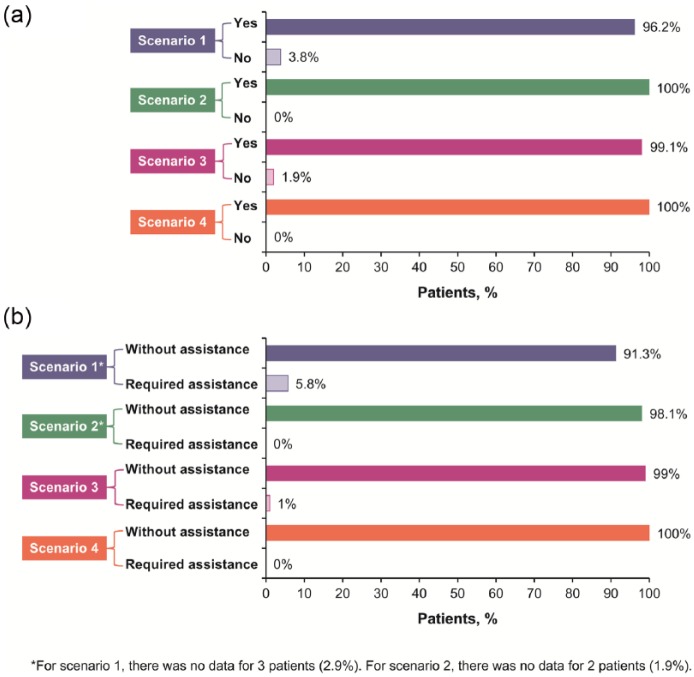
Test-case scenarios (all scenarios were rated on a scale of 1 (very difficult)–10 (very easy)) (Figure 2(a)–(d) and Figure 3(a) and (b)). The mean time on task for Scenario 1 (holding the needle in place) was 6.7 s (range, 3–23). This scenario received a mean rating of 9.8 (range, 7–10); a total of 91 patients (89.2%) assigned this task a performance rating of 10 (“very easy”; Figure 2(a) Scenario 2 (patient checking the MTX pen window to confirm delivery of MTX) received a mean rating of 10 (range, 9–10); a total of 102 patients (99.0%) assigned this task a performance rating of 10 (Figure 2(b)). Scenario 3 (patient performing the skin pinch) received a mean rating of 9.9 (range, 1–10); a total of 102 patients (98.1%) assigned this task a performance rating of 10 (Figure 2(c)). Scenario 4 (properly disposing of MTX pen after use) received a mean rating of 10 (no range); all patients rated the ease of disposal as “very easy” (Figure 2(d)).
Figure 2.
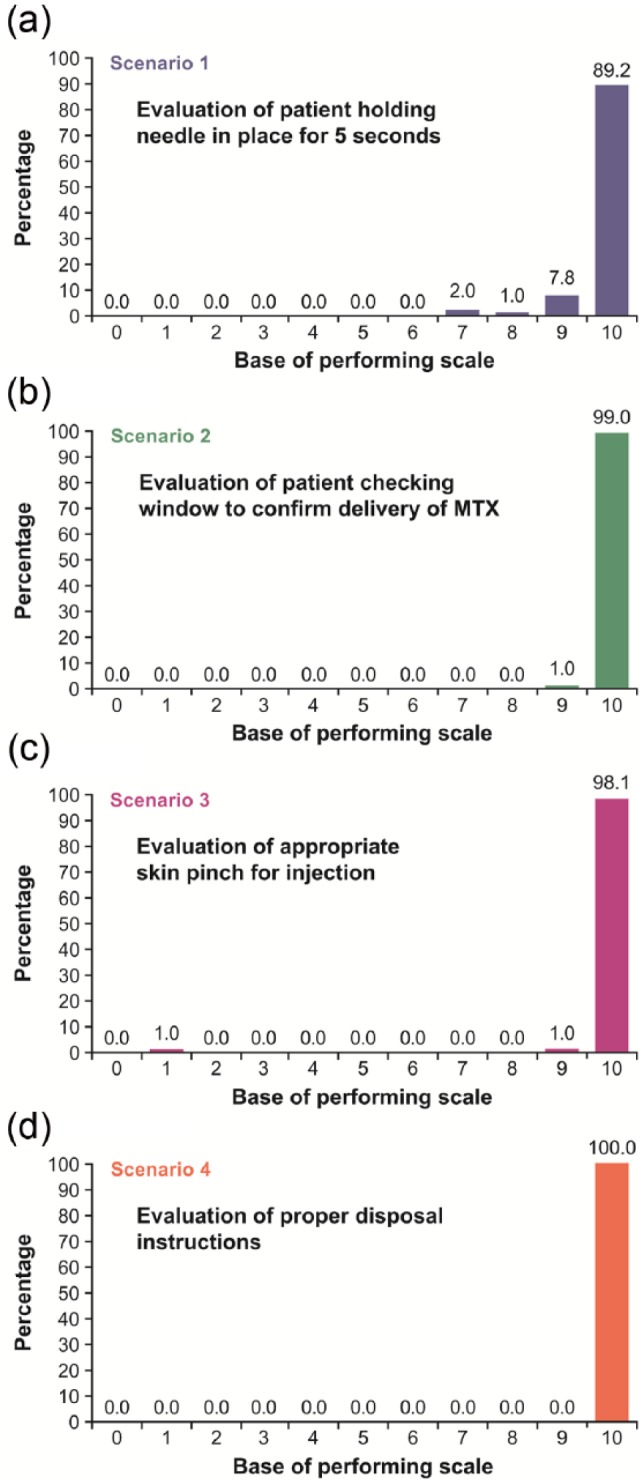
Percentage of patients assigning a performance rating of 10 (“very easy”) for the following: (a)Scenario 1, evaluation of the patient holding the needle in place for 5 s; (b)Scenario 2, evaluation of the patient checking the window of the MTX pen to confirm delivery of MTX; (c)Scenario 3, evaluation of the patient appropriately performing the skin pinch for injection; and (d)Scenario 4, evaluation of the patient properly disposing of the MTX pen after injection.
Figure 3.
(a) Rate of successful completion of each test-case scenario and (b) percentage of patients requiring assistance for success at each test-case scenario.
For each of the four test-case scenarios:
Scenario 1, evaluation of the patient holding the needle in place for 5 s.
Scenario 2, evaluation of the patient checking the window of the MTX pen to confirm delivery of MTX.
Scenario 3, evaluation of the patient appropriately performing the skin pinch for injection.
Scenario 4, evaluation of the patient properly disposing of the MTX pen after injection.
The rate of successful completion of each test-case scenario is shown in Figure 3(a). For all scenarios, over 96% of patients were able to complete the tasks successfully; all patients completed Tasks 2 (checking window to confirm delivery of MTX) and 4 (proper disposal) successfully. The percentage of patients requiring assistance for success at each test-case scenario is shown in Figure 3(b). Again, the vast majority (>91%) were able to complete all tasks without assistance. The subjective-measures questionnaire indicated a high level of patient satisfaction with the pen, with 95.2% of patients indicating they had no difficulty in using the pen and 98% of patients stating they had a positive overall impression of the pen. The ease of use for the pen was rated at a mean score of 10 on a 10-point scale, with 10 being classified as very easy to use. Favorable aspects of the pen included that it was painless, easy to use, drug delivery could be visualized, and that the pen eliminated the need for using a syringe. Some patients reported difficulty in removing the cap and expressed a desire for the pen to be refillable. Overall, patients preferred the pen to oral MTX, and it was considered easy to use by patients with RA that affected their hands.
Label comprehension assessment, part 1
Data from the questionnaire of patient training filled by healthcare professionals at V1 showed that a total of 12 patients (11.3%) had questions related to the use of the MTX pen at V1. These questions were successfully answered and primarily concerned proper use of the autoinjector pen. Only one patient (0.9%) was unsuccessful at self-injection; this was due to lifting the pen from the injection site before the needle was engaged. A total of four patients (3.8%) required assistance to perform the self-injection, and seven patients (6.6%) were recorded as having difficulty using the MTX pen.
Label comprehension assessment, part 2
The written examination taken by patients at V2 assessed patient retention of training provided at V1. A summary of the patient scores on the written examination by question is shown in Table 2. Overall, the written examination median score was 100% (range 60%–100%). A total of 102 patients (98.1%) scored 80% or better, and 1 patient (1.0%) retested.
Table 2.
Summary of patient label comprehension/written examination scores by question.
| Written examination question | Patients, n (%) (overall n = 104) |
|---|---|
| Question 1: Identify all sites of the body where it is suitable to subcutaneously self-inject the prefilled pen of MTX. (Select all that apply) | |
| (a) arm | 4 (3.8) |
| (b) abdomena | 104 (100.0) |
| (c) upper thigha | 103 (99.0) |
| (d) chest | 0 |
| (e) hand | 0 |
| Question 2: How can it be determined that the medication was properly delivered from the prefilled pen? (Select only one) | |
| (a) listen for clicks | 10 (9.6) |
| (b) view the content of syringe through windowa | 92 (88.5) |
| (c) delivered automatically when button pushed | 8 (7.7) |
| (d) no way to determine delivery | 0 |
| (e) call physician | 0 |
| Question 3: About how long should you hold the prefilled pen in place after pushing the button for the MTX to be injected? (Select only one) | |
| (a) 1 s | 0 |
| (b) 2 s | 0 |
| (c) 5 sa | 103 (99.0) |
| (d) 8 s | 0 |
| (e) 10 s | 1 (1.0) |
| Question 4: When does the prefilled pen start the injection? (Select only one) | |
| (a) when pressed firmly against the skin | 8 (7.7) |
| (b) when placed in a perpendicular position | 0 |
| (c) after it is removed from the skin | 0 |
| (d) when the injection button is pushed and a click is hearda | 95 (91.3) |
| (e) when there is no liquid left | 1 (1.0) |
| Question 5: To avoid injury, never do the following: (Select only one) | |
| (a) insert fingers in the opening of the protective tubea | 103 (99.0) |
| (b) look at the plastic window to see liquid contents | 1 (1.0) |
| (c) press the injection button on the prefilled pen | 0 |
| (d) hold the needle against pinched skin until all of the medication is injected (5 s) | 1 (1.0) |
| (e) press the prefilled pen firmly against the skin | 0 |
| Question 6: Never do the following: (Select only one) | |
| (a) call your physician with a question | 0 |
| (b) wash your hands before using the prefilled pen | 0 |
| (c) use alcohol swab to clean the injection site | 0 |
| (d) use medication with expired datea | 104 (100.0) |
| (e) place prefilled pen on a flat surface | 0 |
| Question 7: Do not use the prefilled pen if the following is observed: (Select all that apply) | |
| (a) it appears to be damageda | 101 (97.1) |
| (b) the package has an expired datea | 102 (98.1) |
| (c) the device is ready for use | 7 (6.7) |
| (d) you did not receive a dose last week | 1 (1.0) |
| (e) your caregiver is unavailable | 1 (1.0) |
| Question 8: When is it acceptable to pinch the skin for subcutaneous injection? (Select only one) | |
| (a) it is always acceptable and helps to assure proper subcutaneous injectiona | 102 (98.1) |
| (b) it is never acceptable to pinch the skin for injection because it hurts | 0 |
| (c) only if there is no discomfort when the skin is pinched | 2 (1.9) |
| (d) it helps to remove the needle perpendicular to the skin | 0 |
| (e) only if this is the second attempt to use the prefilled pen today | 0 |
| Question 9: Each prefilled pen is designed to be used only once. (Select only one) | |
| (a) false, it is used with a new refillable syringe each time | 0 |
| (b) false, it can be used multiple times with the same syringe | 0 |
| (c) true, the prefilled pen is designed for single use onlya | 104 (100.0) |
| (d) true, this means it can only be used once per day | 0 |
| (e) false, the prefilled pen is recycled for reissue | 0 |
| Question 10: Who should I contact in case of an emergency? (Select only one) | |
| (a) my physician, pharmacist, or healthcare providera | 104 (100.0) |
| (b) the US FDA to report all emergencies | 0 |
| (c) the manufacturer, they accept toll calls | 0 |
| (d) my caregiver at home if he or she is not with me | 0 |
| (e) my insurance company if it is a question about medicine | 0 |
MTX: methotrexate; FDA: Food and Drug Administration.
Indicates correct or acceptable answers.
Pen robustness
All prefilled pens used in the study were examined, and all were found to be intact with all pieces remaining as one unit. There was one pen with evidence of fluid within the transparent control zone (it was noted that this was a “failed injection and the MTX deposited on the wall of the shield”). One pen was reported to have a bent needle, although the degree of bending was not noted. The protective shield at the end of all the prefilled pens moved into the correct position immediately after the MTX pen was lifted from the injection site.
Pharmacokinetic findings
Of the 25 patients included in the PKP, 7 patients weighed under 60 kg, 10 patients weighed 60–100 kg, and 8 patients weighed over 100 kg. Patients in the PKP were randomly assigned to either inject MTX in the abdomen or in the upper thigh; a total of 13 patients injected MTX in the abdomen and 12 patients injected MTX in the upper thigh. There were no remarkable differences in the demographics between the summarization types.
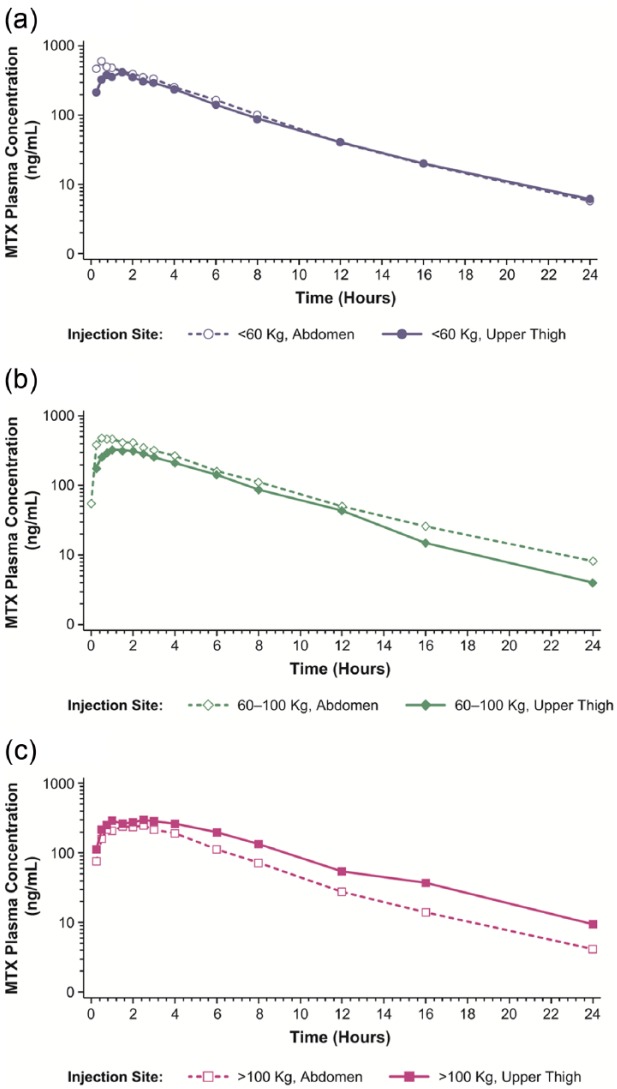
Mean MTX plasma concentration–time curves are displayed in Figure 4 and results of the pharmacokinetic analyses are summarized in Table 3. Mean AUC0-t, AUC0-inf, and Cmax values in patients weighing under 60 kg and 60–100 kg were higher in patients who self-administered in the abdomen compared with patients in those weight groups that self-administered in the upper thigh. Conversely, mean AUC0-t, AUC0-inf, and Cmax values in patients weighing over 100 kg were lower in those who received SC MTX administration in the abdomen compared with patients who received SC administration in the upper thigh.
Figure 4.
Mean MTX plasma concentration versus time following SC injection of MTX (0.3 mL of 50 mg/mL solution) to the abdomen or upper thigh. Results are presented on a semi-logarithmic scale: (a) body weight <60 kg, (b) body weight 60–100 kg, and (c) body weight >100 kg.
Table 3.
Summary of pharmacokinetic data and statistical analyses of interactions of body weight and injection site following SC MTX administration via MTX pen.
| Parameter | Body weight category | Injection site | n | Geometric LS mean | Comparison | Ratio (%) of geometric LS mean | 90% CI of the ratio (%) |
|---|---|---|---|---|---|---|---|
| AUC0-t (h•ng/mL) | <60 kg | Abdomen | 4 | 2670.54 | <60 kg/60–100 kg | 97.18 | 72.90–129.55 |
| 60–100 kg | 5 | 2748.07 | |||||
| >100 kg | 4 | 1580.31 | >100 kg/60–100 kg | 57.51 | 43.14–76.66 | ||
| <60 kg | Upper thigh | 3 | 2351.47 | <60 kg/60–100 kg | 113.39 | 82.92–155.06 | |
| 60–100 kg | 5 | 2073.76 | |||||
| >100 kg | 4 | 2528.83 | >100 kg/60–100 kg | 121.94 | 91.47–162.56 | ||
| AUC0-inf (h•ng/mL) | <60 kg | Abdomen | 4 | 2697.90 | <60 kg/60–100 kg | 96.22 | 71.75–129.03 |
| 60–100 kg | 5 | 2803.99 | |||||
| >100 kg | 4 | 1599.23 | >100 kg/60–100 kg | 57.03 | 42.53–76.49 | ||
| <60 kg | Upper thigh | 3 | 2391.13 | <60 kg/60–100 kg | 114.27 | 83.02–157.28 | |
| 60–100 kg | 5 | 2092.53 | |||||
| >100 kg | 4 | 2584.80 | >100 kg/60–100 kg | 123.53 | 92.11–165.66 | ||
| Cmax (ng/mL) | <60 kg | Abdomen | 4 | 619.14 | <60 kg/60–100 kg | 120.88 | 91.77–159.24 |
| 60–100 kg | 5 | 512.19 | |||||
| >100 kg | 4 | 256.87 | >100 kg/60–100 kg | 50.15 | 38.07–66.07 | ||
| <60 kg | Upper thigh | 3 | 444.00 | <60 kg/60–100 kg | 125.18 | 92.74–168.98 | |
| 60–100 kg | 5 | 354.68 | |||||
| >100 kg | 4 | 319.89 | >100 kg/60–100 kg | 90.19 | 68.47–118.81 |
AUC0-t: area under the plasma concentration versus time curve from time zero to the last measurable concentration; AUC0-inf: area under the plasma concentration versus time curve from time zero extrapolated to infinity; CI: confidence interval; Cmax: maximum observed plasma concentration of drug; LS: least squares; MTX: methotrexate; SC: subcutaneous.
When the pharmacokinetics of MTX administered subcutaneously to the abdomen of patients weighing under 60 kg were compared with that of patients weighing 60–100 kg, the 90% CIs of the ratios of geometric least squares (LS) means for AUC0-t, AUC0-inf, and Cmax were not contained within the predefined no-difference range of 80%–125% (Table 3). Thus, the results were not sufficient to statistically support a claim of equivalence in exposure for these weight groups when MTX was administered subcutaneously to the abdomen. When administration of SC MTX to the abdomen of patients weighing over 100 kg was compared with patients weighing 60–100 kg, the 90% CIs of the ratios of geometric LS means for AUC0-t, AUC0-inf, and Cmax were outside the predefined no-difference range, demonstrating that excessive body weight (>100 kg) significantly decreased both AUC and Cmax of MTX when administered SC to the abdomen.
When MTX administered subcutaneously to the upper thigh of patients weighing less than 60 kg was compared with that of the patients weighing 60–100 kg, the 90% CIs of the ratios of geometric LS means for AUC0-t, AUC0-inf, and Cmax were not contained within the predefined no-difference range of 80%–125% (Table 3). Similar results were found when MTX administered subcutaneously to the upper thigh of patients weighing over 100 kg was compared with patients weighing 60–100 kg. Thus, results of both of these sets of comparisons showed that the data were not sufficient to statistically support a claim of equivalence in exposure for these weight groups when MTX was administered subcutaneously to the upper thigh.
Differences were also noted between weight groups in Tmax values (data not shown). Specifically, the median Tmax of SC MTX was similar in patients weighing up to 100 kg (ranging from 0.75 to 1.50 h); however, median Tmax was slightly delayed for patients weighing over 100 kg (ranging from 1.78 to 2.25 h).
Safety outcomes
The mean duration of exposure was 8.8 days and a total of 102 patients (96.2%) received two injections. Overall, three AEs were reported by three patients (one event of diarrhea, one event of toothache, and one event of upper respiratory tract infection). All of the AEs were mild in severity and only one of the AEs (diarrhea) was deemed to be related to MTX treatment. There were no reports of burning while receiving the injection, and no patients experienced an AE as a result of the self-administration at V1. No ADEs, UADEs, deaths, SAEs, or AEs leading to study drug discontinuation were reported.
Discussion
The purpose of this study was to assess the usability, label comprehension, robustness, bioavailability, and safety of SC self-administration of MTX using the MTX pen in patients with RA who required MTX treatment. This study was designed to assess user/MTX pen interaction issues that could pose a safety risk for patients. The vast majority of patients were able to successfully and comfortably use the MTX pen during their first attempt, and no AEs were associated with use of the pen. Overall, the subjective-measures questionnaire indicated that patients felt the MTX pen was easy to use and their overall impression was favorable. Other studies of autoinjector pens in patients with chronic disorders (including RA) requiring long-term injection treatments have also reported favorable patient opinions,24–26 and that autoinjector pens were preferred by patients over syringes mostly because of their ease of use and convenience. In this study, some patients remarked that they felt the MTX pen was even more convenient than taking oral MTX.
Several patients in this study indicated that one of the things they liked best about the injection was that it was relatively pain free. This is not surprising, given that automatic needle insertion has been found to be significantly less painful than manual needle insertion.33 In a study of patients with RA assessing preference and tolerability of adalimumab via a prefilled syringe or an autoinjector pen,24 approximately 77% of patients reported that the pen was less painful to use than the syringe. Additionally, a study in healthy patients comparing SC self-injection using an autoinjector pen versus injections administered by a nurse using a syringe found that pain associated with the autoinjector pen was significantly lower than that from the syringe.34 Berteau et al.34 also reported that all patients preferred self-administration using the autoinjector pen for the final study injection and for potential future treatment of a chronic disorder. Patients in this study also commented that they liked the ability to view the injection fluid in a window to confirm that they had indeed received the medication.
In this study, patient comprehension and retention of the usage training and IFU of the MTX pen were validated by the written examination administered at V2. The delay between training at V1 and examination at V2 allowed for a period of training decay. Despite this delay, 98.1% of patients passed their written examination at V2, indicating that almost all patients had learned how to properly use the MTX pen and retained the training information.
Data from the pharmacokinetic analyses demonstrated that the MTX pen reliably delivered drug across a wide range of body weights; however, we found that excessive body weight (over 100 kg) significantly decreased both total and peak exposure of MTX when administered subcutaneously to the abdomen. Therefore, consideration should be given to using the SC MTX pen in the upper thigh rather than the abdomen for patients weighing over 100 kg. For all other AUC and Cmax comparisons with the 60–100 kg weight group, statistical analyses were inconclusive.
Overall, data from the SP showed that the MTX pen was well tolerated, and no patients discontinued the study due to AEs, ADEs, UADEs, deaths, or SAEs. A study evaluating tolerability of beta-1α treatment in patients with multiple sclerosis has shown that autoinjectors are associated with a significantly reduced incidence of injection-site reactions (pain, bruising, and/or transient erythema; inflammation with or without induration; necrosis at injection site and necrosis necessitating plastic surgery) compared with manual injections.22 None of the few AEs reported in this study were attributed to local reactions at the injection site.
One of the benefits of SC MTX is that it demonstrates exceptional efficacy in treating RA12,13 and potentially decreases the need for patients to progress to costly biologic therapy.17,18 SC administration of MTX offers more predictable drug bioavailability resulting in less AEs and better disease control. However, in patients weighing over 100 kg, both total and peak MTX levels are decreased with abdominal MTX injection, and it should be recommended that these patients inject SC MTX in the upper thigh. Additionally, it has been speculated that optimized drug-delivery systems, such as autoinjector pens, may potentially increase patient adherence to treatment for chronic disorders, likely improving disease outcomes in those patients.25,35 Current options for SC MTX administration are hampered by requirements of a hand not limited by disability or disease, dedicated office staff, and a concern as to whether patients are actually getting the dose prescribed. Our data showed that the MTX pen used in this study was consistently and reliably used by a heterogeneous population of patients with moderate to severe RA with a high degree of effectiveness, patient satisfaction, and safety. The MTX pen was rated highly by newly diagnosed/MTX naïve patients, patients switching from oral MTX, and current SC MTX users, potentially providing physicians with a new treatment option for patients with RA requiring SC MTX.
Acknowledgments
This trial is registered at ClinicalTrials.gov under identifier NCT01871961.
Footnotes
Declaration of conflicting interests: J.A. Pachon received research grant from medac GmbH and speaker honoraria from Pfizer, Takeda, and UCB; A.J. Kivitz received research grant from medac GmbH and Antares Pharma; K-U. Heuer is a full-time employee of medac GmbH; and U. Pichlmeier is a full-time employee of medac GmbH.
Funding: medac GmbH has supported this research and also funded the technical assistance (aid in preparation of draft versions of the article, copyediting, and styling of the article for submission) provided by Oxford PharmaGenesis Inc., Philadelphia, PA. The authors were fully responsible for all content and editorial decisions and received no financial support or other form of compensation related to the development of this article.
References
- 1. Pavy S, Constantin A, Pham T, et al. Methotrexate therapy for rheumatoid arthritis: clinical practice guidelines based on published evidence and expert opinion. Joint Bone Spine 2006; 73: 388–395. [DOI] [PubMed] [Google Scholar]
- 2. Swierkot J, Szechinski J. Methotrexate in rheumatoid arthritis. Pharmacol Rep 2006; 58: 473–492. [PubMed] [Google Scholar]
- 3. Herman RA, Veng-Pedersen P, Hoffman J, et al. Pharmacokinetics of low-dose methotrexate in rheumatoid arthritis patients. J Pharm Sci 1989; 78: 165–171. [DOI] [PubMed] [Google Scholar]
- 4. Oguey D, Kolliker F, Gerber NJ, et al. Effect of food on the bioavailability of low-dose methotrexate in patients with rheumatoid arthritis. Arthritis Rheum 1992; 35: 611–614. [DOI] [PubMed] [Google Scholar]
- 5. Lebbe C, Beyeler C, Gerber NJ, et al. Intraindividual variability of the bioavailability of low dose methotrexate after oral administration in rheumatoid arthritis. Ann Rheum Dis 1994; 53: 475–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hamilton RA, Kremer JM. Why intramuscular methotrexate may be more efficacious than oral dosing in patients with rheumatoid arthritis. Br J Rheumatol 1997; 36: 86–90. [DOI] [PubMed] [Google Scholar]
- 7. Kurnik D, Loebstein R, Fishbein E, et al. Bioavailability of oral vs. subcutaneous low-dose methotrexate in patients with Crohn’s disease. Aliment Pharmacol Ther 2003; 18: 57–63. [DOI] [PubMed] [Google Scholar]
- 8. Hoekstra M, Haagsma C, Neef C, et al. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J Rheumatol 2004; 31: 645–648. [PubMed] [Google Scholar]
- 9. Rau R. Bioavailability of higher dose MTX comparing oral and subcutaneous administration in patients with RA. J Rheumatol 2005; 32: 1412–1413. [PubMed] [Google Scholar]
- 10. Balis FM, Mirro J, Jr, Reaman GH, et al. Pharmacokinetics of subcutaneous methotrexate. J Clin Oncol 1988; 6: 1882–1886. [DOI] [PubMed] [Google Scholar]
- 11. Jundt JW, Browne BA, Fiocco GP, et al. A comparison of low dose methotrexate bioavailability: oral solution, oral tablet, subcutaneous and intramuscular dosing. J Rheumatol 1993; 20: 1845–1849. [PubMed] [Google Scholar]
- 12. Braun J, Kastner P, Flaxenberg P, et al. Comparison of the clinical efficacy and safety of subcutaneous versus oral administration of methotrexate in patients with active rheumatoid arthritis: results of a six-month, multicenter, randomized, double-blind, controlled, phase IV trial. Arthritis Rheum 2008; 58: 73–81. [DOI] [PubMed] [Google Scholar]
- 13. Islam MS, Haq SA, Islam MN, et al. Comparative efficacy of subcutaneous versus oral methotrexate in active rheumatoid arthritis. Mymensingh Med J 2013; 22: 483–488. [PubMed] [Google Scholar]
- 14. Schiff MH, Simon LS, Freundlich B, et al. Drug exposure limitations of oral methotrexate (MTX) at doses >15 mgs may be overcome by using a subcutaneous MTX auto-injector in patients with rheumatoid arthritis (RA). Arthritis Rheum 2013; 65(Suppl. 10): S337–S338; Abstract 796. [Google Scholar]
- 15. Pichlmeier U, Heuer KU. Subcutaneous administration of methotrexate with a prefilled autoinjector pen results in a higher relative bioavailability compared with oral administration of methotrexate. Clin Exp Rheumatol 2014; 32: 563–571. [PubMed] [Google Scholar]
- 16. Osman A, Mulherin D. Is parenteral methotrexate worth trying? Ann Rheum Dis 2001; 60: 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bharadwaj A, Agrawal S, Batley M, et al. Use of parenteral methotrexate significantly reduces the need for biological therapy. Rheumatology 2008; 47: 222. [DOI] [PubMed] [Google Scholar]
- 18. Thornton C, Ong V, Ward J, et al. Comment on: use of parenteral methotrexate significantly reduces the need for biological therapy. Rheumatology 2008; 47: 1438. [DOI] [PubMed] [Google Scholar]
- 19. Bakker MF, Jacobs JW, Welsing PM, et al. Are switches from oral to subcutaneous methotrexate or addition of ciclosporin to methotrexate useful steps in a tight control treatment strategy for rheumatoid arthritis? A post hoc analysis of the CAMERA study. Ann Rheum Dis 2010; 69: 1849–1852. [DOI] [PubMed] [Google Scholar]
- 20. Stamp LK, Barclay ML, O’Donnell JL, et al. Effects of changing from oral to subcutaneous methotrexate on red blood cell methotrexate polyglutamate concentrations and disease activity in patients with rheumatoid arthritis. J Rheumatol 2011; 38: 2540–2547. [DOI] [PubMed] [Google Scholar]
- 21. Gobel H, Baar H, Beikufner HD, et al. Practicability and acceptance of subcutaneous self-administration of the selective serotonin agonist sumatriptan. Headache 1998; 38: 267–269. [DOI] [PubMed] [Google Scholar]
- 22. Mikol D, Lopez-Bresnahan M, Taraskiewicz S, et al. A randomized, multicentre, open-label, parallel-group trial of the tolerability of interferon beta-1a (Rebif) administered by autoinjection or manual injection in relapsing-remitting multiple sclerosis. Mult Scler 2005; 11: 585–591. [DOI] [PubMed] [Google Scholar]
- 23. Ludescher B, Rommel M, Willmer T, et al. Subcutaneous adipose tissue thickness in adults—correlation with BMI and recommendations for pen needle lengths for subcutaneous self-injection. Clin Endocrinol 2011; 75: 786–790. [DOI] [PubMed] [Google Scholar]
- 24. Kivitz A, Cohen S, Dowd JE, et al. Clinical assessment of pain, tolerability, and preference of an autoinjection pen versus a prefilled syringe for patient self-administration of the fully human, monoclonal antibody adalimumab: the TOUCH trial. Clin Ther 2006; 28: 1619–1629. [DOI] [PubMed] [Google Scholar]
- 25. Lugaresi A, Durastanti V, Gasperini C, et al. Safety and tolerability in relapsing-remitting multiple sclerosis patients treated with high-dose subcutaneous interferon-beta by Rebiject autoinjection over a 1-year period: the CoSa study. Clin Neuropharmacol 2008; 31: 167–172. [DOI] [PubMed] [Google Scholar]
- 26. Lim WH, Chan D, Boudville N, et al. Patients’ perceptions of subcutaneous delivery of darbepoetin alfa by autoinjector prefilled pen versus prefilled syringe: a randomized, crossover study. Clin Ther 2012; 34: 1948–1953. [DOI] [PubMed] [Google Scholar]
- 27. Hornquist JO, Wikby A, Andersson PO, et al. Insulin-pen treatment, quality of life and metabolic control: retrospective intra-group evaluations. Diabetes Res Clin Pract 1990; 10: 221–230. [DOI] [PubMed] [Google Scholar]
- 28. Rubin RR, Peyrot M. Quality of life, treatment satisfaction, and treatment preference associated with use of a pen device delivering a premixed 70/30 insulin aspart suspension (aspart protamine suspension/soluble aspart) versus alternative treatment strategies. Diabetes Care 2004; 27: 2495–2497. [DOI] [PubMed] [Google Scholar]
- 29. United States Food and Drug Administration Label Comprehension, http://www.accessdata.fda.gov/scripts/cder/training/OTC/topic3/topic3/da_01_03_0170.htm (2014, accessed 23 August 2014).
- 30. Nair H, Lawrence L, Hoofnagle AN. Liquid chromatography-tandem mass spectrometry work flow for parallel quantification of methotrexate and other immunosuppressants. Clin Chem 2012; 58: 943–945. [DOI] [PubMed] [Google Scholar]
- 31. Kumar VS, Law T, Kellogg M. Liquid chromatography-tandem mass spectrometry (LC-MS-MS) method for monitoring methotrexate in the setting of carboxypeptidase-G2 therapy. Methods Mol Biol 2010; 603: 359–363. [DOI] [PubMed] [Google Scholar]
- 32. Laverdiere I, Caron P, Couture F, et al. A liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for monitoring drug exposure in hematopoietic stem cell transplant recipients. J Chromatogr B Analyt Technol Biomed Life Sci 2012; 885–886: 131–137. [DOI] [PubMed] [Google Scholar]
- 33. Main KM, Jorgensen JT, Hertel NT, et al. Automatic needle insertion diminishes pain during growth hormone injection. Acta Paediatr 1995; 84: 331–334. [DOI] [PubMed] [Google Scholar]
- 34. Berteau C, Schwarzenbach F, Donazzolo Y, et al. Evaluation of performance, safety, subject acceptance, and compliance of a disposable autoinjector for subcutaneous injections in healthy volunteers. Patient Prefer Adherence 2010; 4: 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lugaresi A. Addressing the need for increased adherence to multiple sclerosis therapy: can delivery technology enhance patient motivation? Expert Opin Drug Deliv 2009; 6: 995–1002. [DOI] [PubMed] [Google Scholar]