Abstract
The functional and anatomical organization of the cingulate cortex across primate species is the subject of considerable and often confusing debate. The functions attributed to the midcingulate cortex (MCC) embrace, among others, feedback processing, pain, salience, action-reward association, premotor functions, and conflict monitoring. This multiplicity of functional concepts suggests either unresolved separation of functional contributions or integration and convergence. We here provide evidence from recent experiments in humans and from a meta-analysis of monkey data that MCC feedback-related activity is generated in the rostral cingulate premotor area by specific body maps directly related to the modality of feedback. As such, we argue for an embodied mechanism for adaptation and exploration in MCC. We propose arguments and precise tools to resolve the origins of performance monitoring signals in the medial frontal cortex, and to progress on issues regarding homology between human and nonhuman primate cingulate cortex.
Keywords: decision, learning, prefrontal, primate, reward
Introduction
Primates show a remarkable ability to adapt in the face of rapidly changing environments. Evaluation of decisions and of their outcomes, so-called performance monitoring, lies at the heart of such abilities. The search for computational and neurobiological principles of performance monitoring has been fruitful in the last 30 years, largely due to parallel research in rodents, monkeys, and humans (reviewed in Holroyd and Coles 2002; Montague et al. 2004; Rushworth et al. 2004; Shenhav et al. 2013).
Several studies have highlighted one subdivision of the cingulate cortex, the midcingulate cortex (MCC), as a central element of the performance monitoring network (Rushworth et al. 2007; Bush 2009; Shackman et al. 2011). Understanding the specific contribution of the MCC is an important challenge because of its putative key role in several aspects of human cognition, its association to a wide range of pathological conditions (Vogt 2009b) and, also, because physiological activity in parts of the cingulate cortical region might serve as markers of developmental and individual behavioral traits (Segalowitz and Dywan 2009).
In the search for MCC functions, discrepancies between human and monkey studies, and between functional and lesion data (Fellows and Farah 2005; di Pellegrino et al. 2007; Nachev 2011), have fueled debates on the exact contribution of this subdivision and, to some extent, on the validity of the nonhuman primate as a model of human cingulate functions (Cole et al. 2009; Schall and Emeric 2010). The debates have confronted multiple anatomical definitions of cingulate areas, as well as different functional interpretations of data obtained with multiple techniques. Important theoretical attempts have been made to integrate various pools of data (Botvinick 2007; Shenhav et al. 2013). However, we think that it is essential to clarify the fundamental issues in comparing empirical data obtained in humans and monkeys. These are the precision of anatomical descriptions and the experimental equivalence. In particular, the provision of juice reward and reward omission are central to the study of decision making in animals. The computational basis of adaptation relies on teaching signals that have been mostly studied using juice with animals. Juice reward and feedback must thus be taken into account as such when comparing human and monkey brain functions.
In the present contribution, we specifically address the issue of functional homology between human and monkey MCC, and its functional organization. To achieve this, we first deal with some difficulties in the anatomical and functional subdivisions of the cingulate region in the 2 species. Second, we show that the functional organization of the human MCC for juice feedback follows a systematic rule. The studies had 2 crucial constraints: behavioural protocols in human functional studies that are similar to those used in monkeys; and parsing the results on the basis of human interindividual morphological variability. Finally, we perform a meta-analysis of cingulate feedback-related unit activity in monkey to show a functional homology with human anterior MCC. This approach then allows us to discuss a possible functional organization principle in MCC, and to provide testable hypotheses.
Overview of Cingulate Cortical Organization in Primates
Part of the confusion in the functional definition of MCC arose from the multiplicity of labels naming subdivisions of the cingulate cortex (Laird et al. 2005; Vogt 2009b). It has become virtually impossible to understand what part of the medial frontal cortex is referred to when one uses the label anterior cingulate cortex (ACC). The label dorsal ACC (dACC) emerged in an attempt to reduce confusion, but it is based only on a rough estimate from brain imaging studies. Use of a common and consistent terminology is mandatory for further progress in this field. The regional model proposed by Vogt et al. is to date the clearest and most rigorous. It is based on multidimensional mappings in human and nonhuman species, including nonhuman primates (Vogt et al. 1995, 2005; Palomero-Gallagher et al. 2009; Vogt 2009c). The model describes 4 cingulate regions among which the most anterior is labeled ACC (See Fig. 1). The region just posterior, dorsal to the corpus callosum, is the MCC (mostly equivalent to dACC) with its most anterior part (aMCC) being the subject of the present study.
Figure 1.
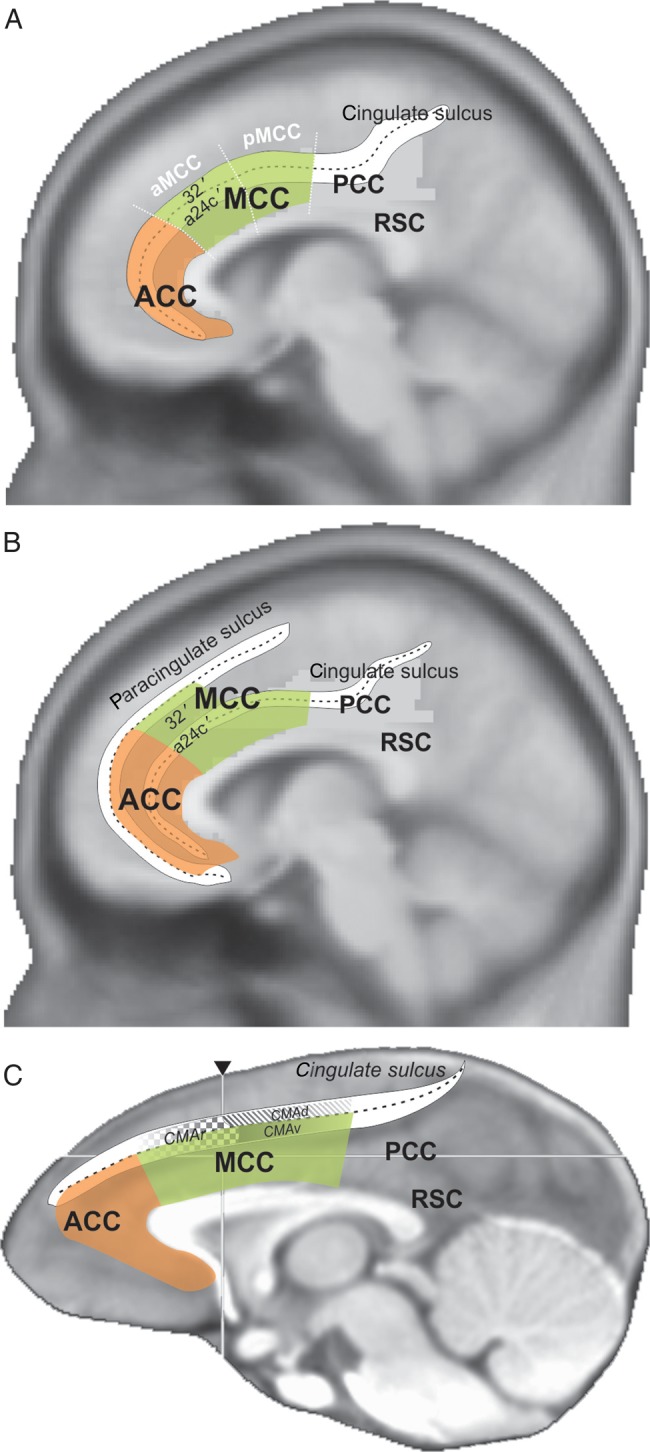
Schematic representations of the ACC and MCC region in the human (A,B) and macaque (C) brains according to Vogt et al. Overlap on brain anatomical scans average in MNI standard spaces for both species. The regions ACC, MCC, PCC, and RSC are based on the 4 regions subdivision by Vogt et al. (Vogt et al. 2005; Palomero-Gallagher et al. 2009; Vogt 2009b). The human representations schematize the organization of cingulate subdivisions in the case of absence (A) or presence (B) of the paracingulate sulcus. Area 32′ and a24c′ were defined by the same authors. In A, the schematic limits of anterior and posterior MCC (aMCC and pMCC) are shown. In C, the schematic position of cingulate motor areas (CMAr, CMAd, CMAv) are presented as in He et al. (1995).
It is important to note that the cytoarchitectonic limits of the MCC appear to relate to the morphology of sulci in primates and such relationships have been in fact another important source of confusion regarding the functional organization of MCC. Based on classical cytoarchitectonic studies and their own research, Vogt et al. propose that the human MCC comprises cytoarchitectonic areas 24a′, 24b′, 24c′, 24d, 33′, and 32′ (Vogt et al. 1995, see schema Fig. 1B; Palomero-Gallagher et al. 2008). According to these studies, area 32′ is always dorsal to area 24c′, but the relationship between these areas and the sulci on the medial wall is not trivial. Specifically, this is because of individual variations in morphology. Whereas all humans possess a cingulate sulcus in each hemisphere, a double cingulate sulcus known as the paracingulate sulcus is variably present (Petrides 2012). The paracingulate sulcus is observed in ∼70% of subjects at least in one hemisphere, and runs dorsal and parallel to the cingulate sulcus through the MCC (Vogt et al. 1995; Paus et al. 1996; Fornito et al. 2008). A paracingulate sulcus can be observed in both hemispheres in some brains, in only one hemisphere in most cases (see Supplementary Material), or in neither hemisphere. Morphological variability appears clearly in surface-based standardized analyses (Hill et al. 2010).
The question of interest here is the relationship between these sulci and the cytoarchitecture, and although this requires further study, current understanding is depicted in Figure 1. Areas 32′ and 24c′ cover the dorsal and ventral banks of the cingulate sulcus in the absence of paracingulate sulcus (Fig. 1A). In contrast, area 32′ was observed in the paracingulate gyrus above the cingulate sulcus and in the paracingulate sulcus when the latter is present, with area 24c′ covering both banks of the cingulate sulcus (Fig. 1B, Vogt et al. 1995). In the standard stereotaxic space (MNI), as used in brain imaging experiments, the cortex lying in the paracingulate and cingulate sulci have different coordinates. This suggests that the location of area 32′ in standard space is different for the 2 types of morphology. Crucially, this means that population averaging procedures should significantly decrease the reliability of activation measures in that region, unless individual morphology is rigorously taken into account (Shackman et al. 2011; Amiez et al. 2013).
In monkeys, the cingulate cortex presents important similarities in anatomical organization with the human cingulate region. Figure 1C represents the subdivision of the rostral cingulate region as proposed by Vogt et al. who studied a comparative anatomy in humans and monkeys. The different subdivisions of the cingulate cortex are organized around the single cingulate sulcus as there is no paracingulate sulcus in macaque monkeys. This cingulate sulcus contains several cytoarchitectonnic areas that have been mostly shown to be comparable with human cytoarchitectonic subdivisions. The exceptions are areas 32′ in MCC and 33′. Earlier cytoarchitectonic studies of the macaque monkey midcingulate region had not identified area 32′, and it was attributed to the human species only (Vogt 2009a). In macaque, the 4-region model uses the fundus of the cingulate sulcus as the dorsal limit of the MCC, excluding the dorsal bank of the sulcus (Vogt et al. 2005). However, neuroanatomical studies from several groups observed that the cortex in the dorsal bank of the cingulate sulcus includes cingulate or transition areas (Matelli et al. 1991; Petrides and Pandya 1994; Zilles et al. 1995; Geyer et al. 1998; Paxinos et al. 2009; for review Sallet et al. 2011). In the human brain, the cortex above the cingulate sulcus when there is a paracingulate sulcus, that is, on the paracingulate gyrus, is a transitional dysgranular area that separates agranular cingulate cortex (classical area 24) from medial dorsal frontal areas (see Petrides and Pandya 1994, 1999). The corresponding region in the macaque brain lies in the dorsal bank of the cingulate sulcus, above the anterior part of the corpus callosum (Petrides and Pandya 1994). Interestingly, the dorsal bank is where the most dorsal MCC lies in the human brain when there is only a single cingulate sulcus (Vogt et al. 1995; Palomero-Gallagher et al. 2008 and see Fig. 1B).
In conclusion, some architectonic studies suggest important primate species difference in the cingulate cortex, with a dorsal limit in monkey cingulate sulcus, supporting the theoretical argument on primate interspecies difference (Cole et al. 2009). However, based on cytoarchitectonic studies (e.g., Petrides and Pandya 1994), this dorsal limit can be challenged. Also, as we shall see, most of the physiological recordings in monkey cingulate cortex that are compared with human functional neuroimaging data have been performed in the dorsal bank and fundus of the cingulate sulcus, that is, outside of Vogt's definition of the MCC. In addition, the layout of cingulate motor areas (CMAs) also favors the extension of MCC onto the dorsal bank.
Cingulate Motor Areas
Crucially, the MCC region overlaps with or includes CMAs. The CMAs have been defined in monkeys using intracortical microstimulation, as well as by anatomical demonstration of connection to the premotor cortex, the primary motor cortex, and the spinal cord (Woolsey et al. 1952; Hutchins et al. 1988; Mitz and Godschalk 1989; Dum and Strick 1991, 1996; Godschalk et al. 1995; He et al. 1995; Hatanaka et al. 2001). Cortical labeling following tracer injections in the cervical or lumbar segment of the spinal cord showed that several representations of the arm and of the lower limb are present in the cortex of the dorsal and ventral banks of the cingulate sulcus, which contrasts with a cytoarchitectonic limit in the fundus of the sulcus. Three major subdivisions were defined by Strick et al.: CMAr, CMAd, and CMAv (for rostral, dorsal, and ventral CMAs) each containing somatomotor representations (Hutchins et al. 1988; Dum and Strick 1991). It is unclear how Vogt's borders relate to motor areas on the medial wall in nonhuman primates (Fig. 1C). For instance, because the posterior representation of the limbs (in CMAd) are found in the dorsal bank of the sulcus (He et al. 1995; Hatanaka et al. 2001), a rigorous application of the dorsal border in the cingulate sulcus results in double arm and leg representations in the primary motor cortex and supplementary area, respectively.
Although CMAr (the main subject of this paper) is often discussed in relation to its well-known arm and hand representations, a representation of the face/eye field has also been described using experimental anatomical tract tracing and microstimulation, just anterior to the arm representation (Mitz and Godschalk 1989; Morecraft et al. 1996, 2007; Tokuno et al. 1997). Further information on the rostral cingulate face representation is provided below.
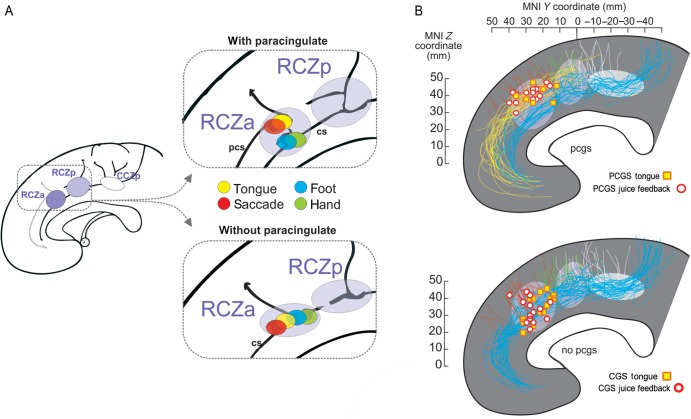
Until recently, the definition of human equivalents of the monkey CMAs relied mainly on the comprehensive meta-analysis by Picard and Strick (1996), who provisionally identified based on positron emission tomography studies 3 subdivisions labeled anterior and posterior rostral cingulate zones (RCZa and RCZp) and a caudal cingulate zone (CCZ) that might be equivalent to the respective CMAr, CMAv, and CMAd identified in the macaque monkey. These investigators predicted the presence of face (related to studies on eye movements and speech) and arm representations in the 2 RCZ and an arm representation in the CCZ. However, no single study had ever tested such somatomotor mappings in individual human subjects paying particular attention to the sulcal morphological variability. Amiez and Petrides (2014) have recently performed this experiment by mapping, with functional magnetic resonance imaging (fMRI) activations in the cingulate regions for eye, tongue, arm, and foot movements (Amiez and Petrides 2014). As in the monkey, they uncovered 3 cingulate motor regions, each including a focus for arm and foot movements and with the 2 anterior regions including in addition foci for movements of the eye and of the tongue. (Note that it was not possible to find a significant spatial dissociation between eye and tongue-related activations, unpublished observation.) This suggests that limb representations are present in all cingulate zones but that only the most anterior ones (RCZa and RCZp) appear to have representations of the face, including eye-related fields, although a separation between face and eye remains to be investigated.
Importantly, using a single-subject approach, it was shown that the face activations are located in the paracingulate sulcus when it is present, but in the cingulate sulcus in the absence of the paracingulate sulcus. In either case, the eye/face focus is always located at the junction of the cingulate or paracingulate sulci with a small perpendicular sulcus (Fig. 2A) (Amiez and Petrides 2014). Arm and foot activations were always in the cingulate sulcus. Together with the observations of displacement in the presence of a paracingulate sulcus (Fig. 1), these functional data suggest that the rostral eye/face representation in aMCC is displaced in a similar way to the displacement of area 32′ described by Vogt et al. (1995). A clear morphological landmark might thus be used to track the location of RCZ face area in humans and allow precise functional mappings.
Figure 2.
Human cingulate motor areas, feedback activity, and sulcal morphology. (A) Schematic illustration of the 3 human cingulate motor areas (RCZa, RCZp, and CCZ) as described by Amiez and Petrides (2014). Colored disks represent the average location of activation peaks in response to simple voluntary movements for hemispheres with (top) and without (bottom) a paracingulate sulcus. cs: cingulate sulcus, pcs: paracingulate sulcus. (B) Overlap of tongue movement-related activation peaks (individual peak locations are represented by squares) and peaks for feedback-related activation (circles) during exploration for hemispheres with and without a paracingulate sulcus. Each individual sulcus path has been retraced, and all paracingulate (blue) and cingulate (yellow) sulci, as well as vertical branches (red, green, and white), have been overlapped for the populations of subjects. Data taken from Amiez et al. (2013) and Amiez and Petrides (2014). Note, the activation data come from 2 separate experiments. The approximate location of RCZa, RCZp, and CCZ is indicated by ellipses (rostrocaudal extent estimated from Amiez and Petrides 2014).
Feedback Evaluation and the MCC
Functional data have accumulated on the role of MCC in outcome-based decisions and adaptation, in both humans and monkeys. Single-unit and local field potential recordings in the anterior section of the dorsal bank of the cingulate sulcus in monkeys have revealed particularly prominent activity related to outcome or feedback detection and evaluation (e.g., Amiez et al. 2005; Matsumoto et al. 2007; Seo and Lee 2007; Kennerley and Wallis 2009; Luk and Wallis 2009). During an explore/repeat task (searching for a rewarded response and then repeat), single-unit activity has been shown to be related to the coding and discrimination of various forms of feedback relevant for adaptation (negative feedback: no reward, positive feedback: juice delivery, etc.), in particular during exploration (Quilodran et al. 2008). How does this relate to human MCC? Learning and decision behavioral protocols in monkeys use juice reward as feedback and incentive. Delivery or omission of reward provides the relevant information to guide behavior. Thus, a proper comparison of human and macaque studies requires the use of reward in similar ways in both species. For a direct comparison with monkey studies, we recently adapted the task used in monkey experiments, including exploration (trial and error) and repetition periods and using fruit juice as outcome or feedback, in a human fMRI protocol (Amiez et al. 2013). As predicted from monkey electrophysiological results, very reliable aMCC activation was observed at feedback during exploration, but not at feedback during repetition. Most importantly, the location of feedback-related activation could be related to the local sulcal morphology in the cingulate region as was the case for the location of the rostral CMA. The feedback-related activation was located in the paracingulate sulcus when present, or in the cingulate sulcus in the absence of the secondary paracingulate sulcus.
Taken together, our recent functional neuroimaging experiments in human subjects reveal organizational principles in MCC that stimulate a reconsideration of data in monkeys (Amiez et al. 2013; Amiez and Petrides 2014). The data suggest that the juice feedback activations and the CMAs are related, and that this relationship could be similar in human and nonhuman primates. To address this homology issue, we proceeded in 2 ways: 1) we combined single human subject fMRI data to test the relationships between feedback-related activations and cingulate motor areas and 2) we performed a meta-analysis of monkey outcome-related and CMA-related data. The aim was to provide a comparative assessment of the relationship between juice feedback-related activity and CMAs in the 2 species.
Materials and Methods
Brain Imaging
Individual peaks of statistically identified clusters reported in Amiez and Petrides (2014) and Amiez et al. (2013) were plotted in the MNI standard stereotaxic space (Fig. 2B). The course of the cingulate sulcus, paracingulate sulcus, and 3 major branching vertical sulci were drawn from single-subject T1 sagittal views. The most posterior of the 3 vertical sulci is the paracentral sulcus (pacs), followed by the preparacentral sulcus (prpacs), and then the vertical paracingulate sulcus (vpcgs), which is the most anterior (see Amiez et al. 2013).
Principles of Monkey Meta-analysis
We evaluated, from the literature, the location of reported outcome or feedback-related single-unit activity in relation to CMAr representations. To do this, we performed a meta-analysis of published neurophysiological and neuroanatomical data obtained in monkeys.
Our aim was to co-register, using the same anatomical reference framework, data from unit recordings, microstimulation mappings, and neuroanatomical tract tracing, and to investigate whether outcome-related activity was likely to come from recordings in CMAr/face region. Data from 26 articles were used (see Supplementary Material). Reconstructions of recording sites were based on the data available in those published articles.
For unit recordings, the selection of articles was based on whether the investigators reported outcome-, feedback-, or more generally juice-related changes in single-unit activity and also on whether there was sufficient information to reconstruct the recording zone. The recording zone retained for analysis corresponds, for each article, to the entire extent of recordings that included outcome- or feedback-related activity.
Published articles reporting anatomical data were selected for this review when they presented cortical map reconstructions or sufficient comprehensive data to reconstruct the rostrocaudal extent of the face/eye or arm representation identified by retrograde tracing or microstimulation mapping.
Co-registration
There is unfortunately no accepted standard method to report the location of data in monkeys, although an effort is made to provide an MNI standard monkey stereotaxic space (Frey et al. 2011). The investigators report either the extent of recordings relative to morphological landmarks (genu of the arcuate sulcus, genu of the corpus callosum, anterior commissure), relative to stereotaxic binaural zero, or both, or none of the above. The most comprehensive approach is to report all that information on a reconstructed cortical surface map.
In order to co-register the rostrocaudal coordinates reported in all articles considered, we have taken the level of the genu of the arcuate sulcus (ArcGen) as a reference. This landmark is indeed the most reported landmark. When the position of recordings relative to ArcGen was available, we aligned data to the ArcGen position. When stereotaxic coordinates were provided but the location of ArcGen was absent we realigned data on the average ArcGen location obtained from a database of 11 monkey MRIs. This average was AP + 24 (SD 2.6). The average location for the genu of corpus callosum (32.69 mm) was also used in some cases (on average 8.67 mm between ArcGen and Ccgen).
Results
Human fMRI
In Amiez et al. (2013), a single-subject analysis revealed that the feedback-related activation was systematically (15/15 subjects) located in the paracingulate sulcus when present, or in the cingulate sulcus in the absence of the paracingulate sulcus. The activation was also always observed at the junction with a specific short perpendicular sulcus, the vertical paracingulate sulcus. A less consistent (6 of 15 subjects) posterior peak was systematically located at the intersection between the cingulate sulcus (if there was no paracingulate sulcus) or the paracingulate sulcus (if present) and the preparacentral sulcus. It important to note here that we observed 2 distinct peaks and not a single peak that spread out.
Based on the description of the cingulate motor zones described above, such properties suggest that the juice feedback-related activation in the aMCC overlaps with an orofacial representation of RCZa. To evaluate this overlap, we compared the activation coordinates obtained for tongue movements in Amiez and Petrides (2014) and for juice feedback (Amiez et al. 2013) provided in Figure 2B. We chose to represent only the anterior activation peak because of its consistency in 100% of subjects bilaterally. In the explore/exploit task, activation of RCZp was obtained only in 50% of subjects and is not considered further. The single-subject data reported on individual morphology for hemispheres with and without a paracingulate sulcus, and in relation to the extent of the 3 cingulate motor zones as described by Amiez and Petrides (2014) reveal that both activations for juice feedback in exploration and for tongue movements are located in RCZa and are associated with the paracingulate sulcus when this sulcus is present. Experiments are currently being performed to further test this overlap.
Monkey Meta-analysis
If the orofacial representation in human aMCC processes feedback provided by juice, then can we find the same correspondence in monkeys? If so this would converge towards a clear anatomical and functional homology between human and monkey performance monitoring systems, in particular regarding the aMCC/RCZa subregion. Most unit recording experiments in monkeys reported data acquired close to or just anterior to CMAr in the dorsal bank and fundus of the cingulate sulcus, a region often referred to as the dACC. Because an eye/face representation exists anterior to the hand representation of CMAr, we re-evaluated from the literature the location of outcome or feedback-related activity relative to CMAr representations. We performed a meta-analysis of published data acquired in macaques (see Materials and Methods). This approach is quite rare in the monkey literature, and is in fact quite difficult, mostly because of a lack of a convention in the reporting of the location of recordings or of anatomical data. Nevertheless, this approach allowed us to synthesize and map available functional data in the cingulate sulcus.
Our aim was to co-register, using the same anatomical reference framework, data from unit recordings, microstimulation mappings, and neuroanatomical tract tracing, and to investigate whether outcome-related activity was likely to come from recordings in the CMAr/face region. As pointed out above, 26 articles formed the basis of this meta-analysis (see Supplementary Material regarding selection criteria and methods).
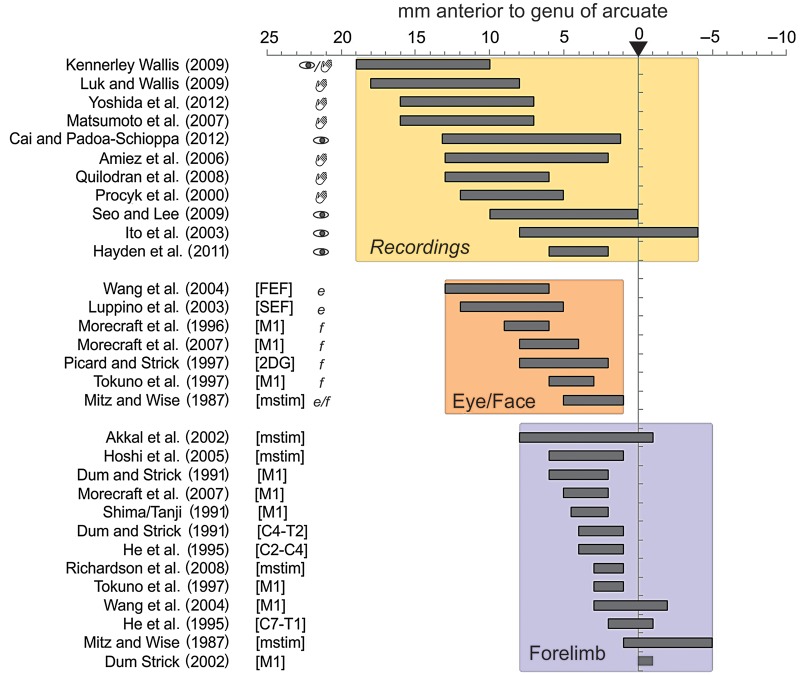
Figures 3 and 4 present the major findings. The rostrocaudal extent of regions of interest collected from the 26 articles are grouped according to whether they reported data on outcome-/feedback-related unit activity, data on the location of a face or eye-related area (Face: tracing studies or microstimulations) and, data on the hand region of CMAr (Forelimb) (Fig. 3). Note that the figure reports several specific points regarding each study, including the effectors used to respond in single-unit recording studies (see also Supplementary Material). This information is provided because the effector might be a key factor in determining the functional organization of CMAr. The raw data show that the eye/face representation clearly overlaps with the recordings reporting outcome-related activity. These 2 regions are somewhat anterior to the forelimb representation in CMAr. Most recordings were performed in the dorsal bank of the cingulate sulcus, and most neuroanatomical data regarding CMAr were in the dorsal bank with some extensions in the ventral bank (see Supplementary Material).
Figure 3.
Database for meta-analysis in monkeys. Rostrocaudal extent of (top) recording sites in studies reporting feedback/outcome-related activity, (middle) regions with face-related effects of microstimulations and regions with anatomical connections with face-related areas and nuclei, and (bottom) regions with arm-related effects of microstimulations and regions with connections with arm-related areas and spinal levels. On the left of recording sites extent, symbols of an eye and of a hand indicate the effector used by animals to respond. On the left of Eye/Face studies, “e” and “f” relate to studies focusing on eye-related data (e.g., connections to FEE) or to face-related data (e.g., connection to M1 face), respectively. The specificity of anatomical studies is indicated in brackets (FEF, SEF, M1, C4-T2, C2-C4, C7-T1: injections of tracer in the respective cortical or spinal regions; mstim: microstimulation study; 2DG: study using 2-deoxyglucose). All data are aligned to the level of the genu of the arcuate sulcus (anterior 0, ArcGen). See Supplementary Information for details.
Figure 4.
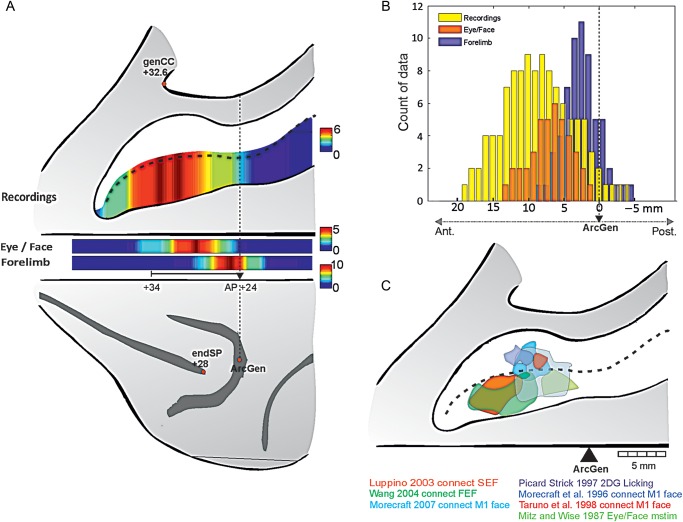
Meta-analysis of functional and anatomical data in monkeys. (A) Number of studies covering the rostrocaudal regions of the dorsal bank of the cingulate sulcus (data from Fig. 3). Single-unit recording studies are represented in the opened sulcus. Eye/face data and forelimb-related data are shown just below. Red in the color scale indicates a greater number of studies. AP coordinates for genu of the arcuate (genArc), caudal end of principalis (endSP), and genu of the Corpus Callosum (genCC) are averages taken from a population of 11 rhesus monkeys (from MRI images). (B) Histogram of data reported along the cingulate sulcus for recordings related to outcome/feedback (yellow), and for anatomical maps for Eye/Face representation (orange) and Forelimb (purple). Comparing distributions reveals that data for Eye/Face and outcome/feedback are different in terms of antero-posterior coverage at P < 0.01, but that both differ from the distribution related to Forelimb at P < 10−8. The rostrocaudal extent is aligned on ArcGen. (C) Schematic overlap of eye/face-related data reconstructed from 7 studies. Maps are aligned on the rostrocaudal level of the genu of the arcuate sulcus (ArcGen).
We calculated the overall rostrocaudal distributions of reported regions of interest, and display them in Figure 4A on a flat map reporting the main anatomical landmarks on a macaque brain. Statistical comparisons of the antero-posterior distributions (Fig. 4B) show a small difference between feedback/outcome recordings and face regions but a highly significant difference between those locations and the distribution for reports related to the forelimb CMAr representation (Distributions were compared by Wilcoxon rank-sum tests. Recordings versus Eye/Face: P = 0.011, ns; Recordings versus Forelimb: P < 10–9, zval: −7.65 ; Eye/Face versus Forelimb: P < 10−8, zval: 6.14. Two-sample Kolmogorov–Smirnov tests led to the exact same conclusions with P = 0.019 for Recordings versus Eye/Face and all P < 10−8 for tests against Forelimb). Indeed, some authors have specifically noted the drop of prevalence of outcome encoding when recording in posterior parts of the cingulate sulcus (see the Conclusion in Luk and Wallis 2009). Selected articles for which clear (anatomical) maps were provided reveal an eye/face-related area mostly in the dorsal bank and fundus of the cingulate sulcus and for some study in the ventral bank, overlapping with the licking activity obtained with 2-deoxyglucose by Picard and Strick (1997) (Fig. 4C). Note that discriminating between putative eye and face fields remains difficult with the analyzed data.
In conclusion, the meta-analysis strongly suggests that most recordings of feedback-related activity (juice in all cases but in Seo and Lee 2009) were most likely overlapping with the eye/face representation of CMAr, a functional overlap comparable with the one found in humans.
Discussion
We have provided evidence for the functional organization of the midcingulate cortical region in humans (Amiez et al. 2013; Amiez and Petrides 2014) and for a functional homology between the human and the monkey MCC by using comparable behavioral protocols in both humans and monkeys, and by taking into account the interindividual sulcal variability in humans. Based on this research, we propose that, in both species, the anterior MCC processes feedback provided by juice in a specialized somatomotor orofacial field. We further argue that feedback processing in general is embodied in the rostral cingulate motor area (CMAr) which is a specialized area of the MCC that may have evolved for higher control of motor action and decision making in both species.
Monkey Cingulate Maps
The data suggest that juice feedback is processed by homologous areas in both human and nonhuman primates, namely in the rostral cingulate premotor field. In contrast to previous suggestions (Cole et al. 2009), the present data support a functional homology between the aMCC in humans and a part of the dorsal bank of the cingulate sulcus in macaque monkeys and suggest an extension of MCC in the dorsal bank of the cingulate sulcus in the monkey brain. This finding is consistent with cytoarchitectonic studies showing that the upper bank of the cingulate sulcus in macaque monkeys is distinct from the dorsomedial frontal gyrus above and the cingulate gyrus below (Petrides and Pandya 1994) as is area 32′ defined by Vogt et al. (1995) in the MCC of the human brain. It also agrees with the coherent scheme of premotor field organizations observed in both monkeys and humans within the cingulate sulcus (He et al. 1995; Amiez and Petrides 2014). To clarify this point further, future studies will have to combine neural recordings during reward-based decision tasks and control sensorimotor tasks, in both the arm and face representations of CMAs. Ideally such experiments would include microstimulation and neuroanatomical tracing as performed in the study by Shima and Tanji (1998) on voluntary arm movement selection. Interestingly, these authors mentioned anatomical and microstimulation data supporting a location of recordings in the forelimb representation of CMAr. Their report of cingulate activity selectively modulated by changing arm movement after a reward decrease suggests that the arm representation would be involved when specific movement selection is required. In any case, the data reviewed above weakens previous arguments on species differences regarding MCC functions.
MCC Integrative Function and Embodied Feedback Processing
The value of a single individual approach and of using comparable protocols between human and monkeys have already been emphasized (Bush et al. 2002; Amiez et al. 2006; Bush 2009; Shackman et al. 2011). The present synthesis highlights their relevance to a better understanding of anatomo-functional relationships. The MCC is considered as an integration zone between cognition, motivation, and action (Paus 2001; Shackman et al. 2011). Orofacial fields in CMAs might contribute to the control of facial expressions (Morecraft et al. 2004). We propose that those fields process face-related information in the context of information-seeking during exploration or learning. In monkey experiments, juice reward provides important feedback information to resolve behavioral tasks. The orofacial representation in the most CMAr would participate in harvesting the information relevant for adaptive behavior. Although there is no evidence regarding a specific role of an orofacial versus eye representation, we propose that juice feedback engaged the former.
Further, a general principle can be proposed, namely that behaviorally relevant information is attended to and processed by MCC somatomotor maps, as an embodied mechanism that serves the search for information relevant for modifying behavior. This principle might be extended to other types or modalities of feedback. For instance, tactile feedback on the hand or feedback related to arm movement itself might be expected to involve the forelimb representation of CMAr/RCZa. Because of the sulcal morphology to functional relationships in humans, hand feedback-related activation should appear near the cingulate sulcus even if a paracingulate sulcus is present. Current experiments in our laboratories are evaluating these hypotheses.
Relationship to ‘Other’ MCC Functions
The embodied mechanism clarifies the often disregarded presence of premotor fields (CMAs) in a region often associated with higher cognitive functions. Yet, meta-analyses have shown notably that verbal and manual Stroop tasks often recruit the anterior and posterior parts of RCZa, respectively, which fits with the scheme, presented in Figure 2, of a dissociated face and arm representation (Laird et al. 2005).
Key questions can be tested using the proposed framework. The first concerns the role of eye-related fields in CMAr. There is currently no clear information regarding whether an eye field is segregated from a face field in the CMAr, and the data collected in Figure 4 are indeed unclear in this regard. Because our approach focused specifically on juice reward, we parsimoniously hypothesized that our data reflect activations of the orofacial subdivision. Yet more experiments are required to directly test this segregation. Using the structure-to-function relationship in humans, one can test for instance whether eye movement control in the context of active information-seeking activates a specific representation in aMCC. Indeed, eye movements are major tools for information-seeking in primates (Gottlieb et al. 2013). A further extension to be tested is that the rostral cingulate face representation processes others’ facial expressions as feedback for specific adaptation. Recent experiments suggest that CMAs or more anterior parts of the cingulate cortex might be involved in face processing (Mies et al. 2011; Morita et al. 2014). Also, the mapping of body-specific behaviorally relevant information, such as pain, could be processed by specific subdivisions of CMA maps. This is suggested by recent experiments (Misra and Coombes 2015), and by an anatomical overlap between CMAs fields and the spinothalamic pain-related inputs in monkeys (Dum et al. 2009). Similarly, motor error-related activity observed in MCC in most decision tasks might be processed in the cingulate representation of the corresponding effector.
However, the proposed mapping does not resolve certain aspects of MCC functions. If primary, physical feedback, is processed in cingulate somatomotor areas, then what about visual or abstract secondary feedback? Money, power or other types of feedback often used in human studies could be processed by generalized CMA processes or in regions specific to processing more abstract information. Only precise single-subject analyses using voluntary movement tasks to produce specific localizers can answer these questions. Moreover, most theoretical approaches of MCC have emphasized its role in producing teaching signals but also in updating value functions to drive positive and negative feedback-based adaptations (Botvinick 2007; Alexander and Brown 2011; Shackman et al. 2011; Khamassi et al. 2015). For instance, MCC is proposed to monitor control-relevant information to estimate values necessary for optimal control selection (Shenhav et al. 2013). Other investigators suggest that MCC promotes searching or exploring the environment based on value signals estimated from the environment (Rushworth et al. 2012). Searching for the relationship between these valuation functions, the cingulate motor maps, and the varying sulcal patterns and their relationships to cytoarchitectonic areas in the human brain will certainly contribute to major improvement of our comprehension of MCC function. This will also be one key route for a clear resolution of the homology between human and nonhuman primate cingulate cortex. Toward that goal, and as mentioned above, methodological issues and differences between the 2 species will have to be taken seriously. In addition to proper protocol designs, using fMRI in monkeys combined with traditional neurophysiological approach will provide major information.
Finally, just as interindividual variability is important for precise investigations of the anatomo-functional organization in the human brain, it should be useful also to clinical approaches such as deep brain stimulation or relatively localized lesions as currently performed in patients with behavioral or mood disorders (Richter et al. 2004). The precise functional mapping of aMCC will be crucial to the planning of targeted and efficient interventions and to the understanding of their differential clinical effects.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by Agence National de la Recherche, project ANR-11-BSV4-0006 LU2, and by the labex CORTEX ANR-11-LABX-0042, Canadian Institutes of Health Research (CIHR) grant FRN 37753 (M.P.), and Fondation Neurodis (C.A.). CREW is funded by a Marie Curie Intra-European Fellowship (PIEF-GA-2010-273790). F.M.S. is funded by Fondation pour la Recherche Médicale and ANR DECCA project ANR-10-SVSE4-1441. M.C.M.F. is funded by Ministère de l'Education Nationale, de l'Enseignement Supérieur et de la Recherche. E.P. and C.A. are employed by the Centre National de la Recherche Scientifique.
Supplementary Material
Notes
The authors thank the reviewers for their important comments and suggestions. Conflict of Interest: None declared.
References
- Alexander WH, Brown JW. 2011. Medial prefrontal cortex as an action-outcome predictor. Nat Neurosci. 14:1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiez C, Joseph JP, Procyk E. 2005. Anterior cingulate error-related activity is modulated by predicted reward. Eur J Neurosci. 21:3447–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiez C, Kostopoulos P, Champod AS, Petrides M. 2006. Local morphology predicts functional organization of the dorsal premotor region in the human brain. J Neurosci. 26:2724–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiez C, Neveu R, Warrot D, Petrides M, Knoblauch K, Procyk E. 2013. The location of feedback-related activity in the midcingulate cortex is predicted by local morphology. J Neurosci. 33:2217–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiez C, Petrides M. 2014. Neuroimaging evidence of the anatomo-functional organization of the human cingulate motor areas. Cereb Cortex. 24:563–578. [DOI] [PubMed] [Google Scholar]
- Botvinick MM. 2007. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci. 7:356–366. [DOI] [PubMed] [Google Scholar]
- Bush G. 2009. Dorsal anterior midcingulate cortex: roles in normal cognition and disruption in attention-deficit/hyperactivity disorder. In: Vogt BA, editor. Cingulate neurobiology and disease. New York: Oxford University Press; p. 245–274. [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. 2002. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci USA. 99:523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Yeung N, Freiwald WA, Botvinick M. 2009. Cingulate cortex: diverging data from humans and monkeys. Trends Neurosci. 32:566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Pellegrino G, Ciaramelli E, Ladavas E. 2007. The regulation of cognitive control following rostral anterior cingulate cortex lesion in humans. J Cogn Neurosci. 19:275–286. [DOI] [PubMed] [Google Scholar]
- Dum RP, Levinthal DJ, Strick PL. 2009. The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. J Neurosci. 29:14223–14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. 1991. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 11:667–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. 1996. Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci. 16:6513–6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. 2005. Is anterior cingulate cortex necessary for cognitive control? Brain. 128:788–796. [DOI] [PubMed] [Google Scholar]
- Fornito A, Wood SJ, Whittle S, Fuller J, Adamson C, Saling MM, Velakoulis D, Pantelis C, Yucel M. 2008. Variability of the paracingulate sulcus and morphometry of the medial frontal cortex: associations with cortical thickness, surface area, volume, and sulcal depth. Hum Brain Mapp. 29:222–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Pandya DN, Chakravarty MM, Bailey L, Petrides M, Collins DL. 2011. An MRI based average macaque monkey stereotaxic atlas and space (MNI monkey space). Neuroimage. 55:1435–1442. [DOI] [PubMed] [Google Scholar]
- Geyer S, Matelli M, Luppino G, Schleicher A, Jansen Y, Palomero-Gallagher N, Zilles K. 1998. Receptor autoradiographic mapping of the mesial motor and premotor cortex of the macaque monkey. J Comp Neurol. 397:231–250. [DOI] [PubMed] [Google Scholar]
- Godschalk M, Mitz AR, van Duin B, van der Burg H. 1995. Somatotopy of monkey premotor cortex examined with microstimulation. Neurosci Res. 23:269–279. [DOI] [PubMed] [Google Scholar]
- Gottlieb J, Oudeyer PY, Lopes M, Baranes A. 2013. Information-seeking, curiosity, and attention: computational and neural mechanisms. Trends Cogn Sci. 17:585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka N, Nambu A, Yamashita A, Takada M, Tokuno H. 2001. Somatotopic arrangement and corticocortical inputs of the hindlimb region of the primary motor cortex in the macaque monkey. Neurosci Res. 40:9–22. [DOI] [PubMed] [Google Scholar]
- He SQ, Dum RP, Strick PL. 1995. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the medial surface of the hemisphere. J Neurosci. 15:3284–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J, Dierker D, Neil J, Inder T, Knutsen A, Harwell J, Coalson T, Van Essen D. 2010. A surface-based analysis of hemispheric asymmetries and folding of cerebral cortex in term-born human infants. J Neurosci. 30:2268–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. 2002. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 109:679–709. [DOI] [PubMed] [Google Scholar]
- Hutchins KD, Martino AM, Strick PL. 1988. Corticospinal projections from the medial wall of the hemisphere. Exp Brain Res. 71:667–672. [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Wallis JD. 2009. Evaluating choices by single neurons in the frontal lobe: outcome value encoded across multiple decision variables. Eur J Neurosci. 29:2061–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamassi M, Quilodran R, Enel P, Dominey PF, Procyk E. 2015. Behavioral regulation and the modulation of information coding in the lateral prefrontal and cingulate cortex. Cereb Cortex. 25:3197–3218. [DOI] [PubMed] [Google Scholar]
- Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, Fox PT. 2005. A comparison of label-based review and ALE meta-analysis in the Stroop task. Hum Brain Mapp. 25:6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk CH, Wallis JD. 2009. Dynamic encoding of responses and outcomes by neurons in medial prefrontal cortex. J Neurosci. 29:7526–7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matelli M, Luppino G, Rizzolatti G. 1991. Architecture of superior and mesial area 6 and the adjacent cingulate cortex in the macaque monkey. J Comp Neurol. 311:445–462. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Matsumoto K, Abe H, Tanaka K. 2007. Medial prefrontal cell activity signaling prediction errors of action values. Nat Neurosci. 10:647–656. [DOI] [PubMed] [Google Scholar]
- Mies GW, van der Molen MW, Smits M, Hengeveld MW, van der Veen FM. 2011. The anterior cingulate cortex responds differently to the validity and valence of feedback in a time-estimation task. Neuroimage. 56:2321–2328. [DOI] [PubMed] [Google Scholar]
- Misra G, Coombes SA. 2015. Neuroimaging evidence of motor control and pain processing in the human midcingulate cortex. Cereb Cortex. 25:1906–1919. [DOI] [PubMed] [Google Scholar]
- Mitz AR, Godschalk M. 1989. Eye-movement representation in the frontal lobe of rhesus monkeys. Neurosci Lett. 106:157–162. [DOI] [PubMed] [Google Scholar]
- Montague PR, Hyman SE, Cohen JD. 2004. Computational roles for dopamine in behavioural control. Nature. 431:760–767. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, McNeal DW, Stilwell-Morecraft KS, Gedney M, Ge J, Schroeder CM, van Hoesen GW. 2007. Amygdala interconnections with the cingulate motor cortex in the rhesus monkey. J Comp Neurol. 500:134–165. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Schroeder CM, Keifer J. 1996. Organization of face representation in the cingulate cortex of the rhesus monkey. Neuroreport. 7:1343–1348. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft KS, Rossing WR. 2004. The motor cortex and facial expression: new insights from neuroscience. Neurologist. 10:235–249. [DOI] [PubMed] [Google Scholar]
- Morita T, Tanabe HC, Sasaki AT, Shimada K, Kakigi R, Sadato N. 2014. The anterior insular and anterior cingulate cortices in emotional processing for self-face recognition. Soc Cogn Affect Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P. 2011. The blind executive. Neuroimage. 57:312–313. [DOI] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Mohlberg H, Zilles K, Vogt B. 2008. Cytology and receptor architecture of human anterior cingulate cortex. J Comp Neurol. 508:906–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Vogt BA, Schleicher A, Mayberg HS, Zilles K. 2009. Receptor architecture of human cingulate cortex: evaluation of the four-region neurobiological model. Hum Brain Mapp. 30:2336–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. 2001. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2:417–424. [DOI] [PubMed] [Google Scholar]
- Paus T, Tomaiuolo F, Otaky N, MacDonald D, Petrides M, Atlas J, Morris R, Evans AC. 1996. Human cingulate and paracingulate sulci: pattern, variability, asymmetry, and probabilistic map. Cereb Cortex. 6:207–214. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang XF, Petrides M, Toga A. 2009. The Rhesus monkey brain in stereotaxic coordinates. 2nd ed New York: Academic Press. [Google Scholar]
- Petrides M. 2012. The human cerebral cortex. An MRI atlas of the sulci and gyri in MNI stereotaxic space. London: Academic Press; p. 168. [Google Scholar]
- Petrides M, Pandya DN. 1994. Comparative architectonic analysis of the human and the macaque frontal cortex. In: Boller F, Grafman J, editors. Handbook of neuropsychology. Amsterdam: Elsevier; p. 17–58. [Google Scholar]
- Petrides M, Pandya DN. 1999. Dosrsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 11:1011–1036. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. 1997. Activation on the medial wall during remembered sequences of reaching movements in monkeys. J Neurophysiol. 77:2197–2201. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. 1996. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 6:342–353. [DOI] [PubMed] [Google Scholar]
- Quilodran R, Rothé M, Procyk E. 2008. Behavioral shifts and action valuation in the anterior cingulate cortex. Neuron. 57(2):314–325. [DOI] [PubMed] [Google Scholar]
- Richter EO, Davis KD, Hamani C, Hutchison WD, Dostrovsky JO, Lozano AM. 2004. Cingulotomy for psychiatric disease: microelectrode guidance, a callosal reference system for documenting lesion location, and clinical results. Neurosurgery. 54:622–628; discussion 628–. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, Rudebeck PH, Walton ME. 2007. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci. 11:168–176. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Kolling N, Sallet J, Mars RB. 2012. Valuation and decision-making in frontal cortex: one or many serial or parallel systems? Curr Opin Neurobiol. 22:946–955. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. 2004. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 8:410–417. [DOI] [PubMed] [Google Scholar]
- Sallet J, Mars RB, Quilodran R, Procyk E, Petrides M, Rushworth M. 2011. Neuroanatomical bases of motivational and cognitive control: a focus on the medial and lateral prefrontal cortex. In: Mars RB, Sallet J, Rushworth MFS, Yeung N, editors. Neural basis of motivational and cognitive control. The MIT Press; p. 5–20. [Google Scholar]
- Schall JD, Emeric EE. 2010. Conflict in cingulate cortex function between humans and macaque monkeys: more apparent than real. Brain Behav Evol. 75:237–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segalowitz SJ, Dywan J. 2009. Individual differences and developmental change in the ERN response: implications for models of ACC function. Psychol Res. 73:857–870. [DOI] [PubMed] [Google Scholar]
- Seo H, Lee D. 2009. Behavioral and neural changes after gains and losses of conditioned reinforcers. J Neurosci. 29:3627–3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H, Lee D. 2007. Temporal filtering of reward signals in the dorsal anterior cingulate cortex during a mixed-strategy game. J Neurosci. 27:8366–8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. 2011. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 12:154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. 2013. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 79:217–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K, Tanji J. 1998. Role for cingulate motor area cells in voluntary movement selection based on reward. Science. 282:1335–1338. [DOI] [PubMed] [Google Scholar]
- Tokuno H, Takada M, Nambu A, Inase M. 1997. Reevaluation of ipsilateral corticocortical inputs to the orofacial region of the primary motor cortex in the macaque monkey. J Comp Neurol. 389:34–48. [DOI] [PubMed] [Google Scholar]
- Vogt BA, editor. 2009a. Architecture, neurocytology and comparative organization of monkey and human cingulate cortices. In: Vogt BA, editor. Cingulate neurobiology and disease. New York: Oxford University Press; p. 65–93. [Google Scholar]
- Vogt BA, editor. 2009b. Cingulate neurobiology and Disease. New York: Oxford University Press. [Google Scholar]
- Vogt BA, editor. 2009c. Regions and subregions of the cingulate cortex. In: Vogt BA, editor. Cingulate neurobiology and disease. New York: Oxford University Press; p. 3–30. [Google Scholar]
- Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR. 1995. Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J Comp Neurol. 359:490–506. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Farber NB, Bush G. 2005. Architecture and neurocytology of monkey cingulate gyrus. J Comp Neurol. 485:218–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey CN, Settlage PH, Meyer DR, Sencer W, Pinto Hamuy T, Travis AM. 1952. Patterns of localization in precentral and “supplementary” motor areas and their relation to the concept of a premotor area. Res Publ Assoc Res Nerv Ment Dis. 30:238–264. [PubMed] [Google Scholar]
- Zilles K, Schlaug G, Matelli M, Luppino G, Schleicher A, Qü M, Dabringhaus A, Seitz R, Roland PE. 1995. Mapping of human and macaque sensorimotor areas by integrating architectonic, transmitter receptor, MRI and PET data. J Anat. 187:515–537. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.